Abstract
Statins are potent inhibitors of cholesterol biosynthesis and exert beneficial effects in the primary and secondary prevention of coronary artery disease. However, the overall benefits observed with statins appear to occur much earlier and to be greater than what might be expected from changes in lipid levels alone, suggesting effects beyond cholesterol lowering. Indeed, recent studies indicate that some of the cholesterol-independent or “pleiotropic” effects of statins involve improving endothelial function, enhancing the stability of atherosclerotic plaques, decreasing oxidative stress and inflammation, and inhibiting the thrombogenic response. Many of these pleiotropic effects are mediated by inhibition of isoprenoids, which serve as lipid attachments for intracellular signaling molecules. In particular, inhibition of the small guanosine triphosphate–binding proteins Rho, Ras, and Rac, whose proper membrane localization and function are dependent on isoprenylation, may play an important role in mediating the pleiotropic effects of statins.
Each of the statins is unique with regard to tissue permeability and metabolism, a characteristic that results in different potencies for extrahepatic 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibition. These variations in tissue permeability and metabolism may account for some of the observed differences in peripheral side effects.1 Lipophilic statins, such as atorvastatin and simvastatin, are more likely to enter endothelial cells by passive diffusion than are hydrophilic statins, such as pravastatin and rosuvastatin, which are primarily targeted to the liver. However, because lipophilicity does not entirely predict the ability of statins to exert extrahepatic effects in animal models and human studies, it is likely that other unidentified factors may play a role. For example, there may be specific mechanisms for hydrophilic statins to enter extrahepatic cells, such as endothelial cells. Such a mechanism is present in the liver, where the hepatic organic anion transporter OATP-C enables hydrophilic statins to enter hepatocytes.2
Until recently, all cholesterol-independent, or “pleiotropic,” effects of statins were believed to be mediated by inhibition of mevalonate synthesis. However, a recent report suggests that statins bind to a novel allosteric site within the β2-integrin leukocyte function–associated antigen–1 (LFA-1), which is independent of mevalonate production.3 LFA-1 belongs to the integrin family and plays an important role in leukocyte trafficking and T-cell activation. Random screening of chemical libraries identified the HMG-CoA reductase inhibitor lovastatin as an inhibitor of the LFA-1–intercellular adhesion molecule–1 interaction. This article reviews new data on the different pleiotropic properties of the statins and discusses the clinical implications of these findings (Figure 1).4 Additionally, a comprehensive review of the pleiotropic effects of statins has recently been published4a for readers interested in more information on the topic.
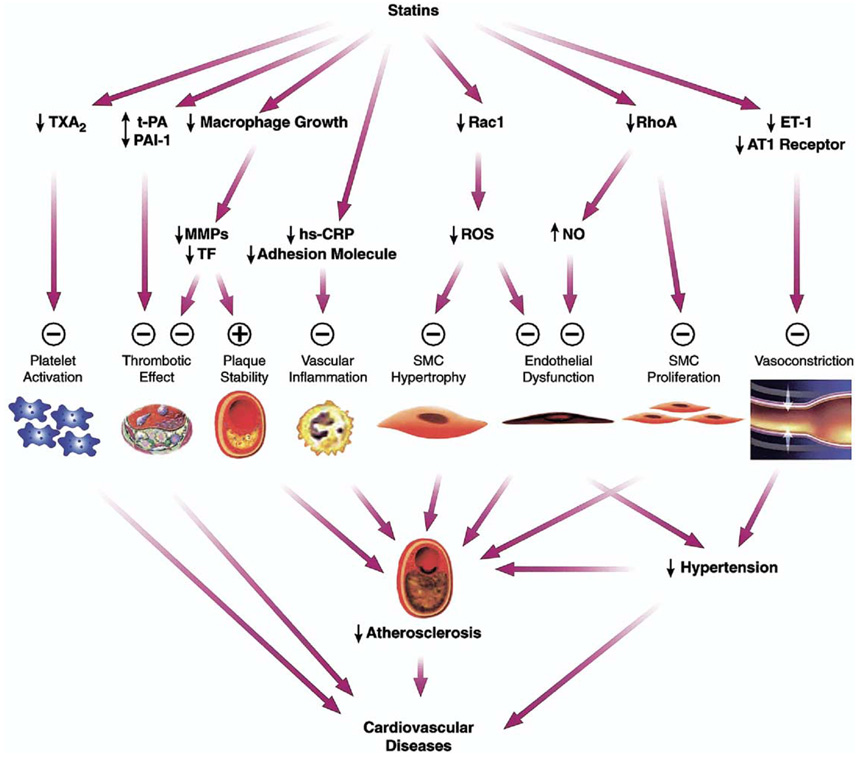
Figure 1.
Cholesterol-independent effects of statins. Plus sign = enhanced/activated; minus sign = inhibited; AT1 = angiotensin 1; ET-1 = endothelin 1; hs-CRP = high-sensitivity C-reactive protein; MMPs = matrix metalloproteinases; NO = nitric oxide; PAI-1 = plasminogen activator inhibitor-1; ROS = reactive oxygen species; SMC = smooth muscle cell; TF = tissue factor; t-PA = tissue-type plasminogen activator; TXA2 = thromboxane A2. (Adapted with permission from Arterioscler Thromb Vasc Biol.)4
Statins and Isoprenylated Proteins
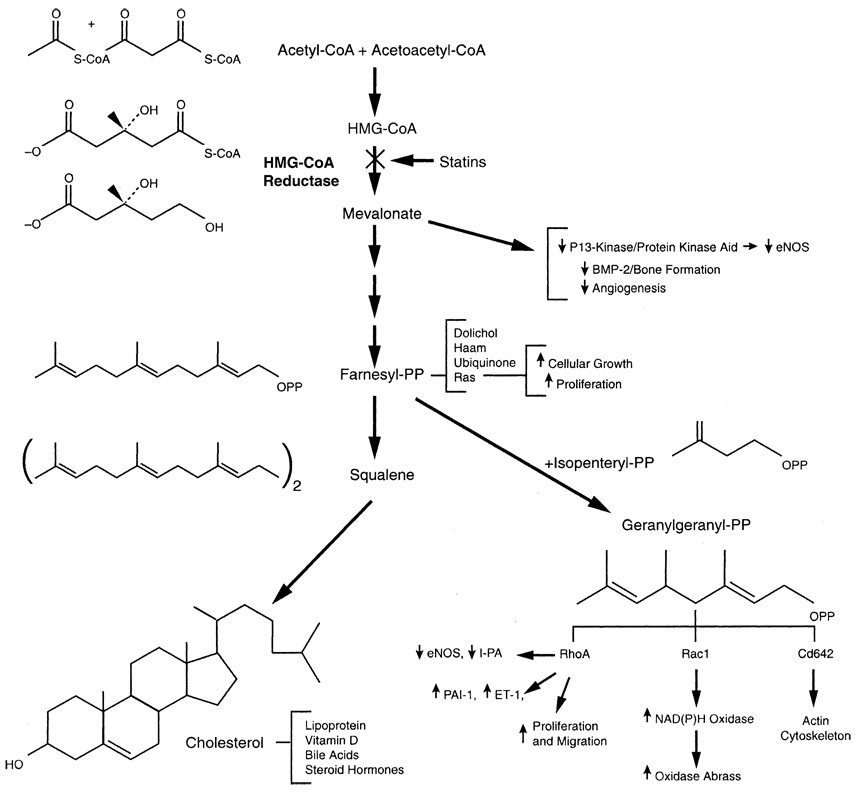
By inhibiting l-mevalonic acid synthesis, statins also prevent the synthesis of other important isoprenoid intermediates of the cholesterol biosynthetic pathway, such as farnesyl pyrophosphate and geranylgeranylpyrophosphate (GGPP)5 (Figure 2).4a These intermediates serve as important lipid attachments for the posttranslational modification of a variety of proteins, including the γ-subunit of heterotrimeric G-proteins, heme-a, nuclear lamins, and small guanosine triphosphate–binding protein Ras and Ras-like proteins, such as Rho, Rab, Rac, Ral, or Rap.6 Protein isoprenylation (Ras and Rho guanosine triphosphatase family) permits the covalent attachment, subcellular localization, and intracellular trafficking of membrane-associated proteins,6,7 which are relevant in cell-signaling pathways. Thus, by inhibiting mevalonic acid, the downstream effects of statins include reduction of inflammation, improved vasodilation, and reduced thrombogenicity.
Figure 2.
Biologic actions of isoprenoids. Cholesterol biosynthesis pathway shows effects of inhibition of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase by statins. Decrease in isoprenylation of signaling molecules, such as Ras, Rho, and Rac, leads to modulation of various signaling pathways. BMP-2 = bone morphogenetic protein–2; CoA = coenzyme A; eNOS = endothelial nitric oxide synthase; ET-1 = endothelin-1; HMG-CoA = 3-hydroxy-3-methylglutaryl–CoA reductase inhibitor; PAI-1 = plasminogen activator inhibitor–1; PP = pyrophosphate; t-PA = tissue-type plasminogen activator. (Reprinted with permission from Annu Rev Pharmacol Toxicol.4a)
Rho is the major target of GGPP; thus, inhibition of Rho and its downstream target Rho kinase is a likely mechanism mediating some of the pleiotropic effects of statins on the vascular wall.8,9 Each member of the Rho family serves specific functions in cell shape, motility, secretion, and proliferation, although overlapping functions among the members could be observed in overexpressed systems. Activation of Rho in Swiss 3T3 fibroblasts by extracellular ligands, such as platelet-derived lysophosphatidic acid, leads to myosin light chain phosphorylation and formation of focal adhesion complexes.6,7,10 Indeed, Rho kinase increases the sensitivity of vascular smooth muscle to calcium in hypertension11 and coronary spasm.12 In contrast, activation of Rac leads to the formation of lamellipodia, membrane ruffles, and oxidative stress, whereas activation of Cdc42 induces actin-rich surface protrusions called filopodia.
Statins and Endothelial Function
Hypercholesterolemia impairs endothelial function. As an early manifestation of atherosclerosis, endothelial dysfunction occurs even in the absence of angiographic evidence of disease.13,14 An important characteristic of endothelial dysfunction is the impaired synthesis, release, and activity of endothelial-derived nitric oxide (NO). Endothelial NO inhibits several components of the atherogenic process. For example, endothelial-derived NO mediates vascular relaxation15 and inhibits platelet aggregation,16 vascular smooth muscle proliferation,17 and endothelial–leukocyte interactions.18,19 Inactivation of NO by the superoxide anion (O2·−) limits bioavailability of NO and leads to nitrate tolerance, vasoconstriction, and hypertension.20,21
Acute plasma low-density lipoprotein (LDL) cholesterol apheresis improves endothelium-dependent vasodilatation,22 which indicates that statins could restore endothelial function, in part, by lowering serum cholesterol levels. However, in some studies with statins, restoration of endothelial function occurs before significant reduction in serum cholesterol levels,23–25 suggesting that there are additional effects on endothelial function beyond cholesterol reduction. Indeed, statins increase endothelial NO production by stimulating and upregulating endothelial NO synthase.26,27 Furthermore, statins restore endothelial NO synthase activity in the presence of hypoxia28 and oxidized LDL cholesterol,26 which are conditions that lead to endothelial dysfunction. Statins also increase the expression of tissue-type plasminogen activator29 and inhibit the expression of endothelin-1, a potent vasoconstrictor and mitogen.30 Statins, therefore, exert many favorable effects on the endothelium and attenuate endothelial dysfunction in the presence of atherosclerotic risk factors.
Another important effect of statin treatment on endothelial NO synthase function is inhibition of caveolin.31,32 Statins also increase endothelial NO synthase activity via posttranslational activation of the phosphatidylinositol 3-kinase/protein kinase Akt pathway.27 Phosphorylation of Akt is an important event in several cellular activities. Indeed, endothelial production of NO can be regulated by phosphorylation and activation of endothelial NO synthase by Akt, which is increased in the presence of statins.33,34 Caveolin-1 binds to endothelial NO synthase in caveolae, thereby negatively regulating the enzyme.35 Exposure of cultured endothelial cells to hypercholesterolemic serum upregulates caveolin-1 and promotes association of caveolin-1 and endothelial NO synthase into inhibitory complexes, thereby decreasing NO production.36 Statins have been shown to reduce caveolin-1 abundance and decrease its inhibitory action on both basal and agonist-stimulated endothelial NO synthase activity.
Statins may also improve endothelial function through their antioxidant effects. For example, statins enhance endothelium-dependent relaxation by inhibiting production of reactive oxygen species, such as superoxide and hydroxy radicals, from aortas of cholesterol-fed rabbits.37 Although lipid lowering by itself can lower vascular oxidative stress,38 some of these antioxidant effects of statins appear to be cholesterol independent. For example, statins attenuate angiotensin II–induced free radical production in vascular smooth muscle cells (SMCs) by inhibiting Rac1-mediated nicotinamide adenine dinucleotide oxidase activity and downregulating angiotensin-1 receptor expression.39 Because NO is scavenged by reactive oxygen species, these findings indicate that the antioxidant properties of statins may also contribute to their ability to improve endothelial function.20,21
Statins and Endothelial Progenitor Cells
Recently, statins have also been found to increase the number of circulating endothelial progenitor cells.40 These cells augment ischemia-induced neovascularization,41 accelerate reendothelialization after carotid balloon injury,42,43 and improve postischemic cardiac function.44 Indeed, statins induce angiogenesis by promoting proliferation, migration, and survival of circulating endothelial progenitor cells.45 In patients with stable coronary artery disease (CAD), administration of atorvastatin for 4 weeks augmented the number of circulating endothelial progenitor cells and enhanced functional capacity.46 These findings are in concordance with earlier data showing that statins rapidly mobilize endothelial progenitor cells from the bone marrow and accelerate vascular structure formation via activation of phosphatidylinositol 3-kinase/protein kinase Akt and endothelial NO synthase.27,45,47 These angiogenic effects were observed at lower statin concentrations and were cholesterol independent. At higher concentrations, statins appear to have an antiangiogenic effect,48,49 suggesting a biphasic effect of statins on angiogenesis.50 However, this suggestion remains controversial because higher doses of statins also have been shown to be angiogenic.51
Statins and Smooth Muscle Proliferation
Proliferation of vascular SMCs is a central event in the pathogenesis of vascular lesions, including postangioplasty restenosis, transplant arteriosclerosis, and venous graft occlusion.52 Recent studies have shown that statins attenuate vascular proliferative disease, such as transplant-associated arteriosclerosis.52 In contrast to atherosclerosis, transplantassociated arteriosclerosis is more dependent on immunologic mechanisms as opposed to lipid disorders, although hypercholesterolemia exacerbates the immunologic process.53 Inhibition of isoprenoid synthesis, but not cholesterol synthesis, by statins decreased platelet-derived growth factor (PDGF)-induced DNA synthesis in vascular SMCs.54,55 Treatment with statins decreased PDGF-induced Rb hyperphosphorylation and cyclin-dependent kinase (CDK)-2, CDK-4, and CDK-6 activities. This correlated with increases in the level of the CDK inhibitor p27Kip1, without concomitant changes in p16INK4, p21Waf1, or p53 levels. These findings indicate that statins inhibit vascular SMC proliferation by arresting the cell cycle between the G1/S phase transition. It remains to be determined whether the upregulation of p27Kip1 is responsible for the cell-cycle arrest and whether there are differences among statins in terms of p27Kip1.
Because the small guanosine triphosphate–binding proteins Ras and Rho require posttranslational modification for membrane localization and activity and are implicated in cell-cycle regulation, they are likely targets for the direct antiproliferative vascular effects of statins. Ras can promote cell-cycle progression via activation of the mitogen-activated protein kinase pathway,56 whereas Rho causes cellular proliferation through destabilizing the p27Kip1 protein.57 Interestingly, inhibition of vascular SMC proliferation by statins was reversed by GGPP, but not by farnesyl pyrophosphate or LDL cholesterol.54 Indeed, direct inhibition of Rho by Clostridium botulinum C3 transferase or by a dominant-negative Rho mutant increased p27Kip1 and inhibited SMC proliferation after PDGF stimulation. Taken together, these findings indicate that Rho mediates PDGF-induced SMC proliferation and that inhibition of Rho by statins is the predominant mechanism by which statins inhibit vascular SMC proliferation.
Statins and Platelet Function
Platelets play a critical role in the development of acute coronary syndromes (ACS).58 Circulating platelets are associated with mural thrombus formation at the site of plaque rupture and vascular injury,59,60 and hypercholesterolemia is associated with increases in platelet reactivity.61 These abnormalities are linked to increases in the cholesterol/phospholipid ratio in platelets. Other potential mechanisms include increases in thromboxane A2 biosynthesis,62 platelet α2-adrenergic receptor density,63 and platelet cytosolic calcium.64
Statins influence platelet function, although the precise mechanisms involved are not fully understood.65,66 Among the well-characterized effects of endothelial NO is the inhibition of platelet aggregation.16 Statin-mediated upregulation of endothelial NO synthase is associated with downregulation of markers of platelet reactivity.67 Potential additional mechanisms include a reduction in the production of thromboxane A2 and modifications in the cholesterol content of platelet membranes.68,69 The cholesterol content of platelet and erythrocyte membranes is reduced in patients receiving statin therapy, which may lead to a decrease in the thrombogenic potential of these cells. Indeed, animal studies suggest statin therapy inhibits platelet deposition on damaged vessels and reduces platelet thrombus formation.59,70 Furthermore, in vitro experiments have demonstrated that statins inhibit tissue factor expression by macrophages, thereby potentially reducing thrombotic events in the vascular wall.71
Statins and Plaque Stability
Plaque rupture is a major cause of ACS.14,72,73 Lipid lowering by statins may contribute to plaque stability by reducing plaque size or by modifying the physiochemical properties of the lipid core.74,75 However, changes in plaque size by lipid lowering tend to occur over extended time and are quite minimal as assessed by angiography. Instead, the clinical benefits from lipid lowering are probably because of decreases in macrophage accumulation in atherosclerotic lesions and inhibition of matrix metalloproteinase production by activated macrophages.71 Indeed, statins inhibit expression of matrix metalloproteinases and tissue factor by cholesterol-dependent and cholesterol-independent mechanisms,71,74,76 with the cholesterol-independent or direct macrophage effects occurring much earlier. The plaque-stabilizing properties of statins, therefore, are mediated through a combined reduction in lipids, macrophages, and matrix metalloproteinases.77 These properties of statins may reduce the incidence of ACS by decreasing the propensity for plaque to rupture and may explain the rapid time course of event reduction in patients at high risk for recurrent coronary ischemia in the Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering (MIRACL)78 and the Pravastatin or Atorvastatin Evaluation and Infection Therapy (PROVE-IT) trials.79
Statins and Vascular Inflammation
Activation of T lymphocytes and control of the immune response are mediated by the major histocompatibility complex class II (MHC-II) and CD40/CD40L. Under physiologic conditions, mostly antigen-presenting cells express MHC-II constitutively, whereas induction of interferon-γ leads to an increase of MHC-II expression in numerous cells, including human endothelial cells and monocytes. An important regulatory complex in this pathway is the class II transactivator (CIITA). Statins inhibit MHC-II expression on endothelial cells and monocyte-macrophages via inhibition of the promotor IV of the class II transactivator, and thereby repress MHC-II–mediated T-cell activation.80 In addition, statins decrease CD40 expression and CD40-related activation of vascular cells.81
A clinical marker of inflammation is high-sensitivity C-reactive protein (hs-CRP).82 hs-CRP is an acute-phase reactant that is produced by the liver in response to proinflammatory cytokines, such as interleukin-6, and reflects low-grade systemic inflammation.83 Elevated levels of hsCRP are predictive of increased risk for CAD in apparently healthy men and women.84,85 hs-CRP is elevated in patients with CAD, coronary ischemia, and myocardial infarction (MI) compared with healthy subjects.86
Statin therapy lowers hs-CRP levels in patients with hypercholesterolemia.82,87,88 In the Cholesterol and Recurrent Events (CARE) trial, statins significantly decreased plasma hs-CRP levels over a 5-year period in patients who did not have recurrent coronary events.89,90 Similarly, an analysis of baseline and 1-year follow-up from the Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS) demonstrated that hs-CRP levels were reduced in statin-treated patients who did not have acute major coronary events.82 Furthermore, preliminary data from the Pravastatin Inflammation/CRP Evaluation (PRINCE) study confirm that statin therapy can significantly reduce serum hs-CRP levels in primary and secondary prevention populations.91 After 24 weeks of statin therapy, hs-CRP levels were reduced by approximately 13% in primary and secondary prevention populations, whereas placebo treatment of subjects in the primary prevention arm of the study had no effect. These findings indicate that statins are effective in decreasing systemic and vascular inflammation. However, any potential clinical benefits conferred by lowering hs-CRP levels are difficult to separate from those of the lipid-lowering effects of statins without performing further clinical studies. Perhaps the ongoing randomized, placebo-controlled JUPITER trial, which is enrolling patients with modest LDL cholesterol (<3.4 mmol/L [130 mg/dL]) and elevated hs-CRP (>2 mg/dL) levels, will help address the question of whether hs-CRP is an additional non–lipid-associated cardiovascular risk factor that can be modified by statin therapy.
Statins and Ischemic Stroke
Although MI is closely associated with serum cholesterol levels, neither the Framingham Heart Study nor the Multiple Risk Factor Intervention Trial (MRFIT) demonstrated significant correlation between ischemic stroke and serum cholesterol levels.92,93 An intriguing result of large clinical trials with statins is the reduction in ischemic stroke.94 For example, the recent Heart Protection Study (HPS) showed a 28% reduction in ischemic strokes in >20,000 people with cerebrovascular disease or other high-risk conditions.95 The proportional reductions in stroke were about 25% in all subcategories studied, including individuals >70 years at entry and those presenting with different levels of blood pressure or lipids, even when the pretreatment LDL cholesterol was <3.0 mmol/L (116 mg/dL). Thus, the findings of these large statin trials raise the interesting question of how a class of cholesterol-lowering agents can reduce ischemic stroke when ischemic stroke is not related to cholesterol levels. It appears likely that there are cholesterol-independent effects of statins that are beneficial for ischemic stroke. Some of these beneficial effects are attributed to the effects of statins on endothelial and platelet function.
In addition to the beneficial effects of statins on endothelium and platelets, other effects of statins may reduce the severity of ischemic stroke. For example, statins attenuate P-selectin expression and leukocyte adhesion via increases in NO production in a model of cardiac ischemia and reperfusion.96,97 Others have reported that statins upregulate tissue-type plasminogen activator and downregulate plasminogen activator inhibitor–1 expression through a similar mechanism involving inhibition of Rho geranylgeranylation.29 Thus, the absence of neuroprotection in endothelial NO synthase–deficient mice emphasizes the importance of endothelium-derived NO in augmenting cerebral blood flow but also, potentially, in limiting the impact of platelet and white blood cell accumulation on tissue viability after ischemia. In humans, atherosclerosis of precerebral arteries causes stroke through plaque disruption and artery-to-artery thromboembolism, and—in contrast to the mouse models—statins exert additional stroke-protective effects in humans through their antiatherosclerotic and plaque-stabilizing effects. Furthermore, the anti-inflammatory actions and mobilization of endothelial progenitor cells of statins may also contribute to neuroprotection. Therefore, it is possible that statins contributed to the decrease in the incidence of ischemic strokes in clinical trials, in part, by reducing the size of cerebral infarcts to clinically unappreciated levels.
Clinical Trials with Statins: Evidence for Pleiotropy
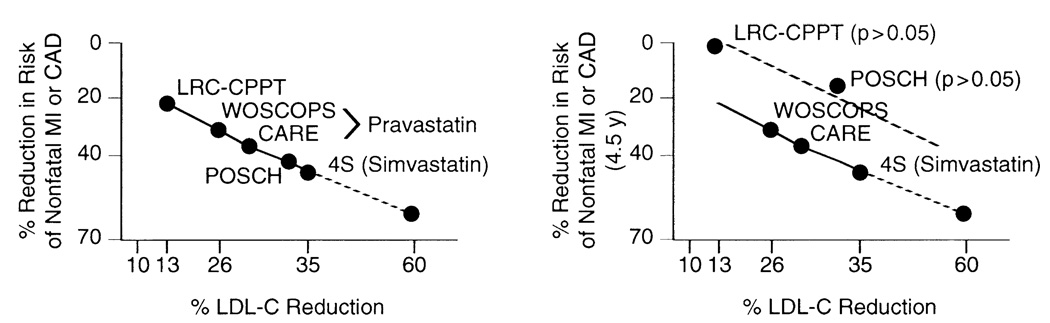
Because serum cholesterol level is strongly associated with CAD, it has been generally assumed that cholesterol reduction by statins is the predominant, if not the only, mechanism underlying their beneficial effects. Data from an analysis of lipid-lowering trials suggest lipid modification alone cannot account for all of the clinical benefits associated with statin therapy.4a Indeed, the slope of the relation between cholesterol reduction and mortality risk reduction was the same for statins and nonstatins, whereas the mortality risk reductions realized during statin treatment periods ≥2 years were found to be a consequence of cholesterol reduction alone (Figure 3, left). However, this type of analysis does not account for differences in the length of the individual trials with respect to cardiovascular benefits. Some of the nonstatin lipid-lowering trials—such as the Lipid Research Clinics–Coronary Primary Prevention Trial (LRC-CPPT), which used the bile acid resin, cholestyramine,98 and the Program on the Surgical Control of the Hyperlipidemias (POSCH) trial, which used partial ileal bypass surgery99—reported benefits after 7.4 and 9.7 years, respectively, whereas most of the statin trials showed benefits at much earlier time points (within 5 years). Thus, when benefits after 5 years for all lipid-lowering trials are compared, it is evident that the nonstatin trials are no longer on the same slope of cholesterol-to-mortality risk reduction as are all of the statin trials (Figure 3). In fact, the benefits of cholesterol lowering after ileal bypass surgery in the POSCH study were not realized at 4.5 years, despite a significant reduction in LDL cholesterol of 34% within the first 3 months after the surgical procedure. These results suggest that the beneficial effects of statins occur more rapidly and may not be entirely dependent on cholesterol reduction.
Figure 3.
Relation between low-density lipoprotein cholesterol (LDL-C) reduction and risk of cardiovascular events. (Left) Decrease in LDL-C (% reduction) is correlated with reduction in risk of nonfatal myocardial infarction (MI) or coronary artery disease (CAD) among statin (the West of Scotland Coronary Prevention Study [WOSCOPS], the Cholesterol and Recurrent Events [CARE] study, and the Scandinavian Simvastatin Survival Study [4S]) and nonstatin (the Lipid Research Clinics–Coronary Primary Prevention Trial [LRC-CPPT] and Program on the Surgical Control of the Hyperlipidemias [POSCH]) trials. Note that the relation (slope) holds between statin and nonstatin trials, suggesting that the beneficial effects of statins are likely due only to cholesterol lowering. (Right) Decrease in LDL-C (% reduction) is correlated with reduction in risk of nonfatal infarctions MI or CAD among statin (WOSCOPS, CARE, and 4S) and nonstatin (LRC-CPPT and POSCH) trials after 4.5 years of treatment. Note that the nonstatin trials (LRC-CPPT and POSCH; dashed lines) show fewer cardiovascular benefits than statin trials (WOSCOPS, CARE, and 4S) and are no longer on the same slope (solid lines). (Reprinted with permission from Annu Rev Pharmacol Toxicol.4a)
Despite the rapidity of benefits of statin therapy compared with other nonstatin lipid-lowering therapies, it is still difficult to prove that pleiotropic effects of statins translate into clinically meaningful outcomes. First, patients receiving statin therapy invariably will have reduced lipid levels, and it is often difficult to separate the lipid-lowering from the non–lipid-lowering effects of statins in clinical trials. Second, many effects of statins, such as improved endothelial function, decreased inflammation, increased plaque stability, and reduced thrombogenic response, could all be accounted for, to some extent, by lipid lowering. Third, concentrations used to demonstrate the biologic effects of statins in cell culture and animal experiments, especially in inhibition of Rho geranylgeranylation, but not phosphatidylinositol 3-kinase/Akt activation, appear to be much higher than what is prescribed clinically.100 Finally, both hydrophilic and lipophilic statins, which inhibit hepatic HMG-CoA reductase, appear to exert cholesterol-independent effects, despite the relative impermeability of hydrophilic statins in vascular tissues.2
Recently, in HPS and the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT), relative risk reduction conferred by statin treatment was independent of pretreatment lipid levels.101,102 These large, prospective trials raise the question of whether individuals with CAD can benefit from statin drugs independently of cholesterol levels. Interestingly, subgroup analyses of previous clinical trials suggested that the beneficial effects of statins could extend to mechanisms beyond cholesterol reduction. For example, subgroup analyses of the West of Scotland Coronary Prevention Study (WOSCOPS) and CARE trial indicate that despite comparable serum cholesterol levels among the statin-treated and placebo groups, statin-treated individuals had significantly lower risks for CAD compared with agematched placebo-controlled individuals.103,104 Indeed, when the statin treatment group was divided into quintiles of percentage of LDL cholesterol reduction, there was no difference in the 4.4-year coronary event rate for quintiles 2 through 5 (LDL cholesterol reductions of 23% to 41%). Hence, there was no apparent association between coronary event rate and the level of LDL cholesterol reduction. Furthermore, analyses of cholesterol-lowering trials suggest that the risk of myocardial infarction in individuals treated with statins is significantly lower compared with individuals treated with other cholesterol-lowering agents or modalities, despite comparable reduction in serum cholesterol levels in both groups.2,4a,105 For example, application of the Framingham risk score to WOSCOPS produced a coincidence between predicted and observed risk in the placebo group but underestimated the benefit of the pravastatin group by 31%.106
Finally, the lipophilic statins lovastatin, fluvastatin, simvastatin, and atorvastatin would be expected to penetrate cell membranes more effectively than the more hydrophilic statins, eliciting more pleiotropic effects. However, the observation that hydrophilic statins also have pleiotropic effects raises an important question on the role of solubility in any of the cholesterol-independent effects of statins and also on the magnitude of these effects. Indeed, recent evidence suggests that some of the cholesterol-independent effects of these agents may be mediated by inhibition of hepatic HMG-CoA reductase, leading to subsequent reduction in circulating isoprenoid levels.2 This hypothesis may help explain why hydrophilic statins, such as pravastatin and rosuvastatin, are clinically still able to demonstrate cholesterol-independent benefits2 while being less efficient at directly entering vascular wall cells.2 In this respect, the word pleiotropic probably does not reflect the hepatic versus nonhepatic effects of these agents. The clinical relevance of hepatic versus nonhepatic isoprenoid inhibition to cardiovascular outcomes is currently unclear, and future studies could explore the role of statin solubility on the differences between these cholesterol-independent effects.
Conclusion
Statins exert many pleiotropic effects in addition to lowering serum cholesterol levels. These additional properties include having beneficial effects on endothelial function and blood flow, decreasing LDL cholesterol oxidation, enhancing the stability of atherosclerotic plaques, inhibiting vascular smooth muscle proliferation and platelet aggregation, and reducing vascular inflammation (Figure 1). Recent evidence suggests that most of these effects are mediated by the inhibitory effect of statins on isoprenoid synthesis. In particular, inhibition of Rho guanosine triphosphatases in vascular wall cells by statins leads to increased expression of atheroprotective genes and inhibition of vascular SMC proliferation. It remains to be determined which and to what extent pleiotropic effects account for the early clinical benefits of statin therapy that are beyond cholesterol lowering.
Acknowledgments
This work was supported, in part, by Grant Nos. HL-52233 and NS-10828 from the National Institutes of Health and by the American Heart Association Bugher Foundation Award.
References
- 1.Germershausen JI, Hunt VM, Bostedor RG, Bailey PJ, Karkas JD, Alberts AW. Tissue selectivity of the cholesterol-lowering agents lovastatin, simvastatin and pravastatin in rats in vivo. Biochem Biophys Res Commun. 1989;158:667–675. doi: 10.1016/0006-291x(89)92773-3. [DOI] [PubMed] [Google Scholar]
- 2.Corsini A, Bellosta S, Baetta R, Fumagalli R, Paoletti R, Bernini F. New insights into the pharmacodynamic and pharmacokinetic properties of statins. Pharmacol Ther. 1999;84:413–428. doi: 10.1016/s0163-7258(99)00045-5. [DOI] [PubMed] [Google Scholar]
- 3.Weitz-Schmidt G, Welzenbach K, Brinkmann V, Kamata T, Kallen J, Bruns C, Cottens S, Takada Y, Hommel U. Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Nat Med. 2001;7:687–692. doi: 10.1038/89058. [DOI] [PubMed] [Google Scholar]
- 4.Takemoto M, Liao JK. Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors. Arterioscler Thromb Vasc Biol. 2001;21:1712–1719. doi: 10.1161/hq1101.098486. [DOI] [PubMed] [Google Scholar]
- 4a.Liao JK, Laufs U. Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol. 2005;45:89–118. doi: 10.1146/annurev.pharmtox.45.120403.095748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343:425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 6.Van Aelst L, D’Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11:2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- 7.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 8.Takemoto M, Sun J, Hiroki J, Shimokawa H, Liao JK. Rho-kinase mediates hypoxia-induced downregulation of endothelial nitric oxide synthase. Circulation. 2002;106:57–62. doi: 10.1161/01.cir.0000020682.73694.ab. [DOI] [PubMed] [Google Scholar]
- 9.Laufs U, Endres M, Stagliano N, Amin-Hanjani S, Chui DS, Yang SX, Simoncini T, Yamada M, Rabkin E, Allen PG, et al. Neuroprotection mediated by changes in the endothelial actin cytoskeleton. J Clin Invest. 2000;106:15–24. doi: 10.1172/JCI9639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hall A. Small GTP-binding proteins and the regulation of the actin cytoskeleton. Annu Rev Cell Biol. 1994;10:31–54. doi: 10.1146/annurev.cb.10.110194.000335. [DOI] [PubMed] [Google Scholar]
- 11.Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- 12.Katsumata N, Shimokawa H, Seto M, Kozai T, Yamawaki T, Kuwata K, Egashira K, Ikegaki I, Asano T, Sasaki Y, Takeshita A. Enhanced myosin light chain phosphorylations as a central mechanism for coronary artery spasm in a swine model with interleukin-1β. Circulation. 1997;96:4357–4363. doi: 10.1161/01.cir.96.12.4357. [DOI] [PubMed] [Google Scholar]
- 13.Liao JK, Bettmann MA, Sandor T, Tucker JI, Coleman SM, Creager MA. Differential impairment of vasodilator responsiveness of peripheral resistance and conduit vessels in humans with atherosclerosis. Circ Res. 1991;68:1027–1034. doi: 10.1161/01.res.68.4.1027. [DOI] [PubMed] [Google Scholar]
- 14.Libby P. Molecular bases of the acute coronary syndromes. Circulation. 1995;91:2844–2850. doi: 10.1161/01.cir.91.11.2844. [DOI] [PubMed] [Google Scholar]
- 15.Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987;84:9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Radomski MW, Rees DD, Dutra A, Moncada S. S-nitroso-glutathione inhibits platelet activation in vitro and in vivo. Br J Pharmacol. 1992;107:745–749. doi: 10.1111/j.1476-5381.1992.tb14517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garg UC, Hassid A. Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J Clin Invest. 1989;83:1774–1777. doi: 10.1172/JCI114081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gauthier TW, Scalia R, Murohara T, Guo JP, Lefer AM. Nitric oxide protects against leukocyte-endothelium interactions in the early stages of hypercholesterolemia. Arterioscler Thromb Vasc Biol. 1995;15:1652–1659. doi: 10.1161/01.atv.15.10.1652. [DOI] [PubMed] [Google Scholar]
- 19.Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci U S A. 1991;88:4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harrison DG. Cellular and molecular mechanisms of endothelial cell dysfunction. J Clin Invest. 1997;100:2153–2157. doi: 10.1172/JCI119751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Münzel T, Sayegh H, Freeman BA, Tarpey MM, Harrison DG. Evidence for enhanced vascular superoxide anion production in nitrate tolerance: a novel mechanism underlying tolerance and cross-tolerance. J Clin Invest. 1995;95:187–194. doi: 10.1172/JCI117637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tamai O, Matsuoka H, Itabe H, Wada Y, Kohno K, Imaizumi T. Single LDL apheresis improves endothelium-dependent vasodilatation in hypercholesterolemic humans. Circulation. 1997;95:76–82. doi: 10.1161/01.cir.95.1.76. [DOI] [PubMed] [Google Scholar]
- 23.Anderson TJ, Meredith IT, Yeung AC, Frei B, Selwyn AP, Ganz P. The effect of cholesterol-lowering and antioxidant therapy on endothelium-dependent coronary vasomotion. N Engl J Med. 1995;332:488–493. doi: 10.1056/NEJM199502233320802. [DOI] [PubMed] [Google Scholar]
- 24.Treasure CB, Klein JL, Weintraub WS, Talley JD, Stillabower ME, Kosinski AS, Zhang J, Boccuzzi SJ, Cedarholm JC, Alexander RW. Beneficial effects of cholesterol-lowering therapy on the coronary endothelium in patients with coronary artery disease. N Engl J Med. 1995;332:481–487. doi: 10.1056/NEJM199502233320801. [DOI] [PubMed] [Google Scholar]
- 25.O’Driscoll G, Green D, Taylor RR. Simvastatin, an HMG-coenzyme A reductase inhibitor, improves endothelial function within 1 month. Circulation. 1997;95:1126–1131. doi: 10.1161/01.cir.95.5.1126. [DOI] [PubMed] [Google Scholar]
- 26.Laufs U, La Fata V, Plutzky J, Liao JK. Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation. 1998;97:1129–1135. doi: 10.1161/01.cir.97.12.1129. [DOI] [PubMed] [Google Scholar]
- 27.Kureishi Y, Luo Z, Shiojima I, Bialik A, Fulton D, Lefer DJ, Sessa WC, Walsh K. The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nat Med. 2000;6:1004–1010. doi: 10.1038/79510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laufs U, La Fata V, Liao JK. Inhibition of 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase blocks hypoxia-mediated down-regulation of endothelial nitric oxide synthase. J Biol Chem. 1997;272:31725–31729. doi: 10.1074/jbc.272.50.31725. [DOI] [PubMed] [Google Scholar]
- 29.Essig M, Nguyen G, Prié D, Escoubet B, Sraer JD, Friedlander G. 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors increase fibrinolytic activity in rat aortic endothelial cells: role of geranylgeranylation and Rho proteins. Circ Res. 1998;83:683–690. doi: 10.1161/01.res.83.7.683. [DOI] [PubMed] [Google Scholar]
- 30.Hernández-Perera O, Pérez-Sala D, Navarro-Antolin J, Sánchez-Pascuala R, Hernández G, Díaz C, Lamas S. Effects of the 3-hydroxy-3-methylglutaryl-CoA reductase inhibitors, atorvastatin and simvastatin, on the expression of endothelin-1 and endothelial nitric oxide synthase in vascular endothelial cells. J Clin Invest. 1998;101:2711–2719. doi: 10.1172/JCI1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Plenz GAM, Hofnagel O, Robenek H. Differential modulation of caveolin-1 expression in cells of the vasculature by statins. Circulation. 2004;109:e7–e8. doi: 10.1161/01.CIR.0000111128.83347.7A. [DOI] [PubMed] [Google Scholar]
- 32.Brouet A, Sonveaux P, Dessy C, Moniotte S, Balligand JL, Feron O. Hsp90 and caveolin are key targets for the proangiogenic nitric oxide-mediated effects of statins. Circ Res. 2001;89:866–873. doi: 10.1161/hh2201.100319. [DOI] [PubMed] [Google Scholar]
- 33.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF. Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 35.Bucci M, Gratton JP, Rudic RD, Acevedo L, Roviezzo F, Cirino G, Sessa WC. In vivo delivery of the caveolin-1 scaffolding domain inhibits nitric oxide synthesis and reduces inflammation. Nat Med. 2000;6:1362–1367. doi: 10.1038/82176. [DOI] [PubMed] [Google Scholar]
- 36.Feron O, Dessy C, Moniotte S, Desager JP, Balligand JL. Hypercholesterolemia decreases nitric oxide production by promoting the interaction of caveolin and endothelial nitric oxide synthase. J Clin Invest. 1999;103:897–905. doi: 10.1172/JCI4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rikitake Y, Kawashima S, Takeshita S, Yamashita T, Azumi H, Yasuhara M, Nishi H, Inoue N, Yokoyama M. Anti-oxidative properties of fluvastatin, an HMG-CoA reductase inhibitor, contribute to prevention of atherosclerosis in cholesterol-fed rabbits. Atherosclerosis. 2001;154:87–96. doi: 10.1016/s0021-9150(00)00468-8. [DOI] [PubMed] [Google Scholar]
- 38.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 39.Wassmann S, Laufs U, Bäumer AT, Müller K, Ahlbory K, Linz W, Itter G, Rösen R, Böhm M, Nickenig G. HMG-CoA reductase inhibitors improve endothelial dysfunction in normocholesterolemic hypertension via reduced production of reactive oxygen species. Hypertension. 2001;37:1450–1457. doi: 10.1161/01.hyp.37.6.1450. [DOI] [PubMed] [Google Scholar]
- 40.Llevadot J, Murasawa S, Kureishi Y, Uchida S, Masuda H, Kawamoto A, Walsh K, Isner JM, Asahara T. HMG-CoA reductase inhibitor mobilizes bone marrow-derived endothelial progenitor cells. J Clin Invest. 2001;108:399–405. doi: 10.1172/JCI13131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murohara T, Ikeda H, Duan J, Shintani S, Sasaki K, Eguchi H, Onitsuka I, Matsui K, Imaizumi T. Transplanted cord blood-derived endothelial precursor cells augment postnatal neovascularization. J Clin Invest. 2000;105:1527–1536. doi: 10.1172/JCI8296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walter DH, Rittig K, Bahlmann FH, Kirchmair R, Silver M, Murayama T, Nishimura H, Losordo DW, Asahara T, Isner JM. Statin therapy accelerates reendothelialization: a novel effect involving mobilization and incorporation of bone marrow-derived endothelial progenitor cells. Circulation. 2002;105:3017–3024. doi: 10.1161/01.cir.0000018166.84319.55. [DOI] [PubMed] [Google Scholar]
- 43.Werner N, Priller J, Laufs U, Endres M, Böhm M, Dirnagl U, Nickenig G. Bone marrow-derived progenitor cells modulate vascular reendothelialization and neointimal formation: effect of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibition. Arterioscler Thromb Vasc Biol. 2002;22:1567–1572. doi: 10.1161/01.atv.0000036417.43987.d8. [DOI] [PubMed] [Google Scholar]
- 44.Kawamoto A, Gwon HC, Iwaguro H, Yamaguchi JI, Uchida S, Masuda H, Silver M, Ma H, Kearney M, Isner JM, Asahara T. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation. 2001;103:634–637. doi: 10.1161/01.cir.103.5.634. [DOI] [PubMed] [Google Scholar]
- 45.Dimmeler S, Aicher A, Vasa M, Mildner-Rihm C, Adler K, Tiemann M, Rütten H, Fichtlscherer S, Martin H, Zeiher AM. HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. J Clin Invest. 2001;108:391–397. doi: 10.1172/JCI13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vasa M, Fichtlscherer S, Adler K, Aicher A, Martin H, Zeiher AM, Dimmeler S. Increase in circulating endothelial progenitor cells by statin therapy in patients with stable coronary artery disease. Circulation. 2001;103:2885–2890. doi: 10.1161/hc2401.092816. [DOI] [PubMed] [Google Scholar]
- 47.Aicher A, Heeschen C, Mildner-Rihm C, Urbich C, Ihling C, Technau-Ihling K, Zeiher AM, Dimmeler S. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med. 2003;9:1370–1376. doi: 10.1038/nm948. [DOI] [PubMed] [Google Scholar]
- 48.Vincent L, Soria C, Mirshahi F, Opolon P, Mishal Z, Vannier JP, Soria J, Hong L. Cerivastatin, an inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A reductase, inhibits endothelial cell proliferation induced by angiogenic factors in vitro and angiogenesis in in vivo models. Arterioscler Thromb Vasc Biol. 2002;22:623–629. doi: 10.1161/01.atv.0000012283.15789.67. [DOI] [PubMed] [Google Scholar]
- 49.Park HJ, Kong D, Iruela-Arispe L, Begley U, Tang D, Galper JB. 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors interfere with angiogenesis by inhibiting the geranylgeranylation of RhoA. Circ Res. 2002;91:143–150. doi: 10.1161/01.res.0000028149.15986.4c. [DOI] [PubMed] [Google Scholar]
- 50.Weis M, Heeschen C, Glassford AJ, Cooke JP. Statins have biphasic effects on angiogenesis. Circulation. 2002;105:739–745. doi: 10.1161/hc0602.103393. [DOI] [PubMed] [Google Scholar]
- 51.Sata M, Nishimatsu H, Suzuki E, Sugiura S, Yoshizumi M, Ouchi Y, Hirata Y, Nagai R. Endothelial nitric oxide synthase is essential for the HMG-CoA reductase inhibitor cerivastatin to promote collateral growth in response to ischemia. FASEB J. 2001;15:2530–2532. doi: 10.1096/fj.01-0415fje. [DOI] [PubMed] [Google Scholar]
- 52.Braun-Dullaeus RC, Mann MJ, Dzau VJ. Cell cycle progression: new therapeutic target for vascular proliferative disease. Circulation. 1998;98:82–89. doi: 10.1161/01.cir.98.1.82. [DOI] [PubMed] [Google Scholar]
- 53.Kobashigawa JA, Katznelson S, Laks H, Johnson JA, Yeatman L, Wang XM, Chia D, Terasaki PI, Sabad A, Cogert GA, et al. Effect of pravastatin on outcomes after cardiac transplantation. N Engl J Med. 1995;333:621–627. doi: 10.1056/NEJM199509073331003. [DOI] [PubMed] [Google Scholar]
- 54.Laufs U, Marra D, Node K, Liao JK. 3-hydroxy-3-methylglutaryl-CoA reductase inhibitors attenuate vascular smooth muscle proliferation by preventing Rho GTPase-induced down-regulation of p27Kip1. J Biol Chem. 1999;274:21926–21931. doi: 10.1074/jbc.274.31.21926. [DOI] [PubMed] [Google Scholar]
- 55.Yang Z, Kozai T, van der Loo B, Viswambharan H, Lachat M, Turina MI, Malinski T, Luscher TF. HMG-CoA reductase inhibition improves endothelial cell function and inhibits smooth muscle cell proliferation in human saphenous veins. J Am Coll Cardiol. 2000;36:1691–1697. doi: 10.1016/s0735-1097(00)00924-4. [DOI] [PubMed] [Google Scholar]
- 56.Hughes DA. Control of signal transduction and morphogenesis by Ras. Semin Cell Biol. 1995;6:89–94. doi: 10.1016/1043-4682(95)90005-5. [DOI] [PubMed] [Google Scholar]
- 57.Hengst L, Reed SI. Translational control of p27Kip1 accumulation during the cell cycle. Science. 1996;271:1861–1864. doi: 10.1126/science.271.5257.1861. [DOI] [PubMed] [Google Scholar]
- 58.Fitzgerald DJ, Roy L, Catella F, FitzGerald GA. Platelet activation in unstable coronary disease. N Engl J Med. 1986;315:983–989. doi: 10.1056/NEJM198610163151602. [DOI] [PubMed] [Google Scholar]
- 59.Lacoste L, Lam JYT, Hung J, Letchacovski G, Solymoss CB, Waters D. Hyperlipidemia and coronary disease: correction of the increased thrombogenic potential with cholesterol reduction. Circulation. 1995;92:3172–3177. doi: 10.1161/01.cir.92.11.3172. [DOI] [PubMed] [Google Scholar]
- 60.Willerson JT, Golino P, Eidt J, Campbell WB, Buja LM. Specific platelet mediators and unstable coronary artery lesions: experimental evidence and potential clinical implications. Circulation. 1989;80:198–205. doi: 10.1161/01.cir.80.1.198. [DOI] [PubMed] [Google Scholar]
- 61.Opper C, Clement C, Schwarz H, Krappe J, Steinmetz A, Schneider J, Wesemann W. Increased number of high sensitive platelets in hypercholesterolemia, cardiovascular diseases, and after incubation with cholesterol. Atherosclerosis. 1995;113:211–217. doi: 10.1016/0021-9150(94)05448-r. [DOI] [PubMed] [Google Scholar]
- 62.Notarbartolo A, Davi G, Averna M, Barbagallo CM, Ganci A, Giammarresi C, La Placa FP, Patrono C. Inhibition of thromboxane biosynthesis and platelet function by simvastatin in type IIa hypercholesterolemia. Arterioscler Thromb Vasc Biol. 1995;15:247–251. doi: 10.1161/01.atv.15.2.247. [DOI] [PubMed] [Google Scholar]
- 63.Baldassarre D, Mores N, Colli S, Pazzucconi F, Sirtori CR, Tremoli E. Platelet α2-adrenergic receptors in hypercholesterolemia: relationship between binding studies and epinephrine-induced platelet aggregation. Clin Pharmacol Ther. 1997;61:684–691. doi: 10.1016/S0009-9236(97)90104-1. [DOI] [PubMed] [Google Scholar]
- 64.Le Quan Sang KH, Levenson J, Megnien JL, Simon A, Devynck MA. Platelet cytosolic Ca2+ and membrane dynamics in patients with primary hypercholesterolemia: effects of pravastatin. Arterioscler Thromb Vasc Biol. 1995;15:759–764. doi: 10.1161/01.atv.15.6.759. [DOI] [PubMed] [Google Scholar]
- 65.Huhle G, Abletshauser C, Mayer N, Weidinger G, Harenberg J, Heene DL. Reduction of platelet activity markers in type II hypercholesterolemic patients by a HMG-CoA-reductase inhibitor. Thromb Res. 1999;95:229–234. doi: 10.1016/s0049-3848(99)00037-7. [DOI] [PubMed] [Google Scholar]
- 66.Hale LP, Craver KT, Berrier AM, Sheffield MV, Case LD, Owen J. Combination of fosinopril and pravastatin decreases platelet response to thrombin receptor agonist in monkeys. Arterioscler Thromb Vasc Biol. 1998;18:1643–1646. doi: 10.1161/01.atv.18.10.1643. [DOI] [PubMed] [Google Scholar]
- 67.Laufs U, Gertz K, Huang P, Nickenig G, Böhm M, Dirnagl U, Endres M. Atorvastatin upregulates type III nitric oxide synthase in thrombocytes, decreases platelet activation, and protects from cerebral ischemia in normocholesterolemic mice. Stroke. 2000;31:2442–2449. doi: 10.1161/01.str.31.10.2442. [DOI] [PubMed] [Google Scholar]
- 68.Vaughan CJ, Gotto AM, Jr, Basson CT. The evolving role of statins in the management of atherosclerosis. J Am Coll Cardiol. 2000;35:1–10. doi: 10.1016/s0735-1097(99)00525-2. [DOI] [PubMed] [Google Scholar]
- 69.Lijnen P, Echevaria-Vazquez D, Petrov V. Influence of cholesterol-lowering on plasma membrane lipids and function. Methods Find Exp Clin Pharmacol. 1996;18:123–136. [PubMed] [Google Scholar]
- 70.Alfon J, Royo T, Garcia-Moll X, Badimon L. Platelet deposition on eroded vessel walls at a stenotic shear rate is inhibited by lipid-lowering treatment with atorvastatin. Arterioscler Thromb Vasc Biol. 1999;19:1812–1817. doi: 10.1161/01.atv.19.7.1812. [DOI] [PubMed] [Google Scholar]
- 71.Aikawa M, Rabkin E, Sugiyama S, Voglic SJ, Fukumoto Y, Furukawa Y, Shiomi M, Schoen FJ, Libby P. An HMG-CoA reductase inhibitor, cerivastatin, suppresses growth of macrophages expressing matrix metalloproteinases and tissue factor in vivo and in vitro. Circulation. 2001;103:276–283. doi: 10.1161/01.cir.103.2.276. [DOI] [PubMed] [Google Scholar]
- 72.Fuster V. Elucidation of the role of plaque instability and rupture in acute coronary events. Am J Cardiol. 1995;76 (suppl):24C–33C. doi: 10.1016/s0002-9149(99)80467-6. [DOI] [PubMed] [Google Scholar]
- 73.Fuster V, Stein B, Ambrose JA, Badimon L, Badimon JJ, Chesebro JH. Atherosclerotic plaque rupture and thrombosis: evolving concepts. Circulation. 1990;82 (suppl):II47–II59. [PubMed] [Google Scholar]
- 74.Fukumoto Y, Libby P, Rabkin E, Hill CC, Enomoto M, Hirouchi Y, Shiomi M, Aikawa M. Statins alter smooth muscle cell accumulation and collagen content in established atheroma of watanabe heritable hyperlipidemic rabbits. Circulation. 2001;103:993–999. doi: 10.1161/01.cir.103.7.993. [DOI] [PubMed] [Google Scholar]
- 75.Koh KK. Effects of statins on vascular wall: vasomotor function, inflammation, and plaque stability. Cardiovasc Res. 2000;47:648–657. doi: 10.1016/s0008-6363(00)00146-2. [DOI] [PubMed] [Google Scholar]
- 76.Bourcier T, Libby P. HMG CoA reductase inhibitors reduce plasminogen activator inhibitor-1 expression by human vascular smooth muscle and endothelial cells. Arterioscler Thromb Vasc Biol. 2000;20:556–562. doi: 10.1161/01.atv.20.2.556. [DOI] [PubMed] [Google Scholar]
- 77.Crisby M, Nordin-Fredriksson G, Shah PK, Yano J, Zhu J, Nilsson J. Pravastatin treatment increases collagen content and decreases lipid content, inflammation, metalloproteinases, and cell death in human carotid plaques: implications for plaque stabilization. Circulation. 2001;103:926–933. doi: 10.1161/01.cir.103.7.926. [DOI] [PubMed] [Google Scholar]
- 78.Schwartz GG, Olsson AG, Ezekowitz MD, Ganz P, Oliver MF, Waters D, Zeiher A, Chaitman BR, Leslie S, Stern T for the Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering (MIRACL) Study Investigators. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes. The MIRACL study: a randomized controlled trial. JAMA. 2001;285:1711–1718. doi: 10.1001/jama.285.13.1711. [DOI] [PubMed] [Google Scholar]
- 79.Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, Joyal SV, Hill KA, Pfeffer MA, Skene AM for the Pravastatin or Atorvastatin Evaluation and Infection Therapy–Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 80.Kwak B, Mulhaupt F, Myit S, Mach F. Statins as a newly recognized type of immunomodulator. Nat Med. 2000;6:1399–1402. doi: 10.1038/82219. [DOI] [PubMed] [Google Scholar]
- 81.Mulhaupt F, Matter CM, Kwak BR, Pelli G, Veillard NR, Burger F, Graber P, Luscher TF, Mach F. Statins (HMG-CoA reductase inhibitors) reduce CD40 expression in human vascular cells. Cardiovasc Res. 2003;59:755–766. doi: 10.1016/s0008-6363(03)00515-7. [DOI] [PubMed] [Google Scholar]
- 82.Ridker PM, Rifai N, Clearfield M, Downs JR, Weis SE, Miles JS, Gotto AM, Jr for the Air Force/Texas Coronary Atherosclerosis Prevention Study Investigators. Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N Engl J Med. 2001;344:1959–1965. doi: 10.1056/NEJM200106283442601. [DOI] [PubMed] [Google Scholar]
- 83.Baumann H, Gauldie J. The acute phase response. Immunol Today. 1994;15:74–80. doi: 10.1016/0167-5699(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 84.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–979. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 85.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 86.Liuzzo G, Biasucci LM, Gallimore JR, Grillo RL, Rebuzzi AG, Pepys MB, Maseri A. The prognostic value of C-reactive protein and serum amyloid A protein in severe unstable angina. N Engl J Med. 1994;331:417–424. doi: 10.1056/NEJM199408183310701. [DOI] [PubMed] [Google Scholar]
- 87.Musial J, Undas A, Gajewski P, Jankowski M, Sydor W, Szczeklik A. Anti-inflammatory effects of simvastatin in subjects with hypercholesterolemia. Int J Cardiol. 2001;77:247–253. doi: 10.1016/s0167-5273(00)00439-3. [DOI] [PubMed] [Google Scholar]
- 88.Ridker PM, Rifai N, Lowenthal SP. Rapid reduction in C-reactive protein with cerivastatin among 785 patients with primary hypercholesterolemia. Circulation. 2001;103:1191–1193. doi: 10.1161/01.cir.103.9.1191. [DOI] [PubMed] [Google Scholar]
- 89.Ridker PM, Rifai N, Pfeffer MA, Sacks F, Braunwald E for the Cholesterol and Recurrent Events (CARE) Investigators. Long-term effects of pravastatin on plasma concentration of C-reactive protein. Circulation. 1999;100:230–235. doi: 10.1161/01.cir.100.3.230. [DOI] [PubMed] [Google Scholar]
- 90.Ridker PM, Rifai N, Pfeffer MA, Sacks FM, Moye LA, Goldman S, Flaker GC, Braunwald E for the Cholesterol and Recurrent Events (CARE) Investigators. Inflammation, pravastatin, and the risk of coronary events after myocardial infarction in patients with average cholesterol levels. Circulation. 1998;98:839–844. doi: 10.1161/01.cir.98.9.839. [DOI] [PubMed] [Google Scholar]
- 91.Albert MA, Staggers J, Chew P, Ridker PM for the PRINCE Investigators. The pravastatin inflammation CRP evaluation (PRINCE): rationale and design. Am Heart J. 2001;141:893–898. doi: 10.1067/mhj.2001.115297. [DOI] [PubMed] [Google Scholar]
- 92.Kannel WB, Castelli WP, Gordon T, McNamara PM. Serum cholesterol, lipoproteins, and the risk of coronary heart disease: the Framingham study. Ann Intern Med. 1971;74:1–12. doi: 10.7326/0003-4819-74-1-1. [DOI] [PubMed] [Google Scholar]
- 93.Multiple Risk Factor Intervention Trial Research Group. Multiple risk factor intervention trial: risk factor changes and mortality results. JAMA. 1982;248:1465–1477. [PubMed] [Google Scholar]
- 94.Crouse JR, III, Byington RP, Furberg CD. HMG-CoA reductase inhibitor therapy and stroke risk reduction: an analysis of clinical trials data. Atherosclerosis. 1998;138:11–24. doi: 10.1016/s0021-9150(98)00014-8. [DOI] [PubMed] [Google Scholar]
- 95.Collins R, Armitage J, Parish S, Sleight P, Peto R for the Heart Protection Study Collaborative Group. Effects of cholesterol-lowering with simvastatin on stroke and other major vascular events in 20536 people with cerebrovascular disease or other high-risk conditions. Lancet. 2004;363:757–767. doi: 10.1016/S0140-6736(04)15690-0. [DOI] [PubMed] [Google Scholar]
- 96.Scalia R, Lefer DJ, Lefer AM. Simvastatin inhibits leukocyte-endothelium interaction in vivo under normocholesterolemic conditions: essential role of endothelial nitric oxide synthase [abstract] Circulation. 1999;100 (suppl):I–409. [Google Scholar]
- 97.Lefer AM, Scalia R, Lefer DJ. Vascular effects of HMG CoA-reductase inhibitors (statins) unrelated to cholesterol lowering: new concepts for cardiovascular disease. Cardiovasc Res. 2001;49:281–287. doi: 10.1016/s0008-6363(00)00247-9. [DOI] [PubMed] [Google Scholar]
- 98.The Lipid Research Clinics Coronary Primary Prevention Trial results. II. The relationship of reduction in incidence of coronary heart disease to cholesterol lowering. JAMA. 1984;251:365–374. [PubMed] [Google Scholar]
- 99.Buchwald H, Varco RL, Matts JP, Long JM, Fitch LL, Campbell GS, Pearce MB, Yellin AE, Edmiston WA, Smink RD, Jr, et al. Effect of partial ileal bypass surgery on mortality and morbidity from coronary heart disease in patients with hypercholesterolemia: report of the Program on the Surgical Control of the Hyperlipidemias (POSCH) N Engl J Med. 1990;323:946–955. doi: 10.1056/NEJM199010043231404. [DOI] [PubMed] [Google Scholar]
- 100.Urbich C, Dernbach E, Zeiher AM, Dimmeler S. Double-edged role of statins in angiogenesis signaling. Circ Res. 2002;90:737–744. doi: 10.1161/01.res.0000014081.30867.f8. [DOI] [PubMed] [Google Scholar]
- 101.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. [Google Scholar]
- 102.Sever PS, Dahlof B, Poulter NR, Wedel H, Beevers G, Caulfield M, Collins R, Kjeldsen SE, Kristinsson A, McInnes GT, et al. for the ASCOT Investigators. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial–Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361:1149–1158. doi: 10.1016/S0140-6736(03)12948-0. [DOI] [PubMed] [Google Scholar]
- 103.Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, MacFarlane PW, McKillop JH, Packard CJ for the West of Scotland Coronary Prevention Study Group. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. N Engl J Med. 1995;333:1301–1307. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 104.Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, Brown L, Warnica JW, Arnold JMO, Wun CC, Davis BR, Braunwald E for the Cholesterol and Recurrent Events Trial Investigators. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med. 1996;335:1001–1009. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 105.Pekkanen J, Linn S, Heiss G, Suchindran CM, Leon A, Rifkind BM, Tyroler HA. Ten-year mortality from cardiovascular disease in relation to cholesterol level among men with and without preexisting cardiovascular disease. N Engl J Med. 1990;322:1700–1707. doi: 10.1056/NEJM199006143222403. [DOI] [PubMed] [Google Scholar]
- 106.West of Scotland Coronary Prevention Study Group. Influence of pravastatin and plasma lipids on clinical events in the West of Scotland Coronary Prevention Study (WOSCOPS) Circulation. 1998;97:1440–1445. doi: 10.1161/01.cir.97.15.1440. [DOI] [PubMed] [Google Scholar]