Abstract
The relevance of emotional stimuli to threat and survival confers a privileged role in their processing. In PTSD, the ability of trauma-related information to divert attention is especially pronounced. Information unrelated to the trauma may also be highly distracting when it shares perceptual features with trauma material. Our goal was to study how trauma-related environmental cues modulate working memory networks in PTSD. We examined neural activity in participants performing a visual working memory task while distracted by task-irrelevant trauma and non-trauma material. Recent post-9/11 veterans were divided into a PTSD group (n = 22) and a trauma-exposed control group (n = 20) based on the Davidson trauma scale. Using fMRI, we measured hemodynamic change in response to emotional (trauma-related) and neutral distraction presented during the active maintenance period of a delayed-response working memory task. The goal was to examine differences in functional networks associated with working memory (dorsolateral prefrontal cortex and lateral parietal cortex) and emotion processing (amygdala, ventrolateral prefrontal cortex, and fusiform gyrus). The PTSD group showed markedly different neural activity compared to the trauma-exposed control group in response to task-irrelevant visual distractors. Enhanced activity in ventral emotion processing regions was associated with trauma distractors in the PTSD group, whereas activity in brain regions associated with working memory and attention regions was disrupted by distractor stimuli independent of trauma content. Neural evidence for the impact of distraction on working memory is consistent with PTSD symptoms of hypervigilance and general distractibility during goal-directed cognitive processing.
Keywords: PTSD, fMRI, Working memory, Emotion processing, Cognitive control
1. Introduction
Posttraumatic stress disorder (PTSD) has occurred at high rates in veterans of the military conflicts in Iraq and Afghanistan, and has been associated with marked functional impairment and cognitive deficits (Hoge et al., 2004; Vasterling et al., 2006). Understanding the nature of these deficits in PTSD patients is important, as cognitive performance is critical to effective occupational function, requiring the ability to use information flexibly, make reasonable inferences, and undertake goal-directed behavior (D'Esposito et al., 2000; McFarlane, 1997).
Several PTSD neuroimaging studies have examined emotion processing systems using symptom provocation paradigms to assess the response to trauma-related material (Liberzon and Martis, 2006), but these studies have largely ignored the significant cognitive processing deficits associated with PTSD (McNally, 2006). A few recent studies have examined neural bases of cognitive deficits in PTSD including working memory (Clark et al., 2003; Weber et al., 2005), attention (Bryant et al., 2005; Pannu Hayes et al., in press), and conflict-specific cognitive control (e.g. Stroop) (Shin et al., 2001). It has been hypothesized that the intrusive nature of trauma-related memories and goal-irrelevant environmental cues negatively impact cognitive processing in PTSD (McNally, 2006). However, the effects of trauma-related material on the neural correlates of cognitive processing have not received attention. In a prior fMRI study of PTSD, we examined the independent effects of processing trauma-related material as well as basic cognitive processing (Morey et al., 2008) on associated neural systems. However, our study did not permit the assessment of the effect of trauma-related information on neural systems for cognitive processing.
The present study examines the neural impact of trauma distraction on neural systems for working memory in PTSD. Trauma-related information was presented as task-irrelevant distraction during the delay interval of a delayed-response working memory task. Using a very similar task in a non-clinical group, we previously showed that emotional distractors evoked strong activity in ventral structures associated with emotion processing (amygdala, ventrolateral PFC, fusiform gyrus), while disrupting delay interval activity in dorsal frontoparietal brain regions (dorsolateral PFC, lateral parietal cortex) associated with active maintenance of task-relevant information in working memory (Dolcos and McCarthy, 2006). Furthermore, we established that this disruption of dorsal system activation in the presence of emotional distraction was associated with impaired working memory performance.
From the evidence summarized above, we propose three basic hypotheses concerning the response to trauma-related distractors, and their effect on cognitive processing in PTSD. First, we predicted that combat distractors presented during the working memory delay period would lead to impaired cognitive performance in the PTSD group. Second, we predicted that combat distractors would lead to increased activation of the ventral frontolimbic regions in the PTSD group. Third, we also predicted that task-irrelevant distractors would disrupt delay period activity in the frontoparietal network, and that combat distractors would lead to greater disruption of activity in these regions in the PTSD group.
2. Materials and methods
2.1. Participants
A total of 42 participants included a PTSD group (n = 22) and a trauma-exposed control group (n = 20) with comparable level of combat exposure as measured by the combat exposure scale [t(40) = 1.2, p = 0.2]. Subjects were recruited from a large registry of post-9/11 military service members and veterans and provided written informed consent to procedures approved by the Institutional Review Boards at Duke University and Durham VA Medical Center. Subjects completed a screening battery to assess comorbid neuropsychiatric disorders (see Table 1). The Davidson Trauma Scale (DTS) was administered immediately prior to fMRI scanning to assess PTSD symptomatology (Davidson et al., 1997). A cutoff score of 32 was used to divide the participants into a PTSD group with mean DTS (SD) = 74.4 (18.8)], and control group with mean DTS = 10.2 (8.8). A cut score of 32 was shown to have high diagnostic efficiency (0.94) compared against a clinician-administered interview in a larger sample of post-9/11 veterans (McDonald et al., in press). Scores on DTS were bimodally distributed with 17 subjects in the range of 0−20 and 17 subjects in the range of 60−100. At the time of the study, nine participants, one in the control group and eight in the PTSD group, were taking antidepressant drugs (see Table 4).
Table 1.
Demographic and clinical characteristics of subject samplea.
| Characteristic | Control n = 20 | PTSD n = 22 | Group comparison |
|---|---|---|---|
| Age (years), (SD) | 37.6 (11.0) | 30.8 (8.8) | t(40) = 2.2, p = 0.05 |
| Gender, no. (%) of females | 7 (35.0) | 13 (59.1) | χ2(1) = 0.29, p > 0.5 |
| Handedness, no. (%) right-handed | 17 (85.0) | 19 (86.4) | χ2(2) = 0.68, p > 0.7 |
| Ethnicity, no. (%) of caucasian subjects | 8 (40.0) | 12 (54.5) | χ2(2) = 2.1, p > 0.3 |
| Education (years), (SD) | 13.9 (2.8) | 13.3 (1.8) | t(40) = 0.8, p > 0.4 |
| Davidson trauma scale (SD) | 10.2 (8.8) | 74.4 (18.8) | t(40) = 13.9, p < 0.001 |
| Combat exposure scale (SD) | 8.6 (11.0) | 12.6 (10.3) | t(40) = 1.2, p > 0.2 |
| Connor–Davidson resilience scale, (SD) | 83.4 (9.9) | 66.7 (15.8) | t(40) = 4.1, p < 0.001 |
| Beck depression inventory (SD) | 7.1 (6.1) | 20.8 (9.0) | t(40) = 5.7, p < 0.001 |
| Alcohol use disorders identification test (SD) | 2.6 (3.2) | 6.1 (6.3) | t(40) = 2.6, p < 0.05 |
| Drug abuse screening test, (SD) | 0.4 (0.8) | 2.1 (2.5) | t(40) = 2.9, p < 0.01 |
| Antidepressant medication, no. (%) prescribedb | 1 (5) | 8 (36.4) | χ2(1) = 6.1, p < 0.01 |
| DTS of subjects taking medication, (SD) | 19 | 74 (16.8) | N/A |
Data values represent means except where indicated otherwise.
Antidepressant medications taken were either selective serotonin reuptake inhibitors (SSRI) or mirtazipine.
2.2. Stimuli and working memory task design
During the fMRI scan, subjects performed a version of the working memory task with distraction as used by Dolcos and McCarthy (Dolcos and McCarthy, 2006). Each trial consisted of an encoding phase, a delay period with emotional (trauma-related) and non-emotional novel visual distractors, and a retrieval phase for an overall epoch duration of 29 s (see Fig. 1). The memoranda presented during the encoding phase consisted of three similar faces (female faces for 50% of trials) presented for 3.5 s, which subjects encoded into working memory and maintained for 11.5 s. The visual distractors consisted of (i) two combat scenes from Iraq or Afghanistan, or (ii) two non-combat scenes, or (iii) two digitally scrambled pictures (control condition), which were presented for 3 s each. The three distractor types were matched for luminance, presence of human figures/faces, and chromatic features. During the retrieval phase, a single-face probe was presented requiring a button response to indicate its presence (Old) or absence (New) in the three-face memoranda (50% probes were old and 50% were new). Subjects were instructed to attend to the memoranda, distractors, and respond with an Old or New judgment to the probes stimulus. Each probe was followed by a fixation cross for 12.5 s to allow the hemodynamic response to return to baseline. Subjects viewed 40 trials per stimulus type randomized across 10 runs, each lasting 6 min containing 180 image volumes. There were no repetitions in the memoranda, distractors, or probes.
Fig. 1.
Diagram of the working memory task showing the event order and trial types. Subjects were instructed to encode the memoranda (3 faces) and had to actively maintain them in working memory during a delay period while looking at distractors. During the retrieve period subjects were required to press a response button to indicate whether the probe (single-face) was part of the memoranda. Three categories of trials were presented during the working memory delay period, defined by the type of distractors (i) combat-related scenes from Iraq or Afghanistan, (ii) non-combat scenes, or (iii) digitally scrambled images. Each trial contained two distractors from the same category that were presented, consecutively, for 3 s each.
2.3. Picture rating task
After scanning, subjects judged the emotional intensity and distractibility of stimuli using a four-point Likert scale (1 = low/4 = high). Individual indices of distractibility and emotional intensity were calculated by averaging subject's ratings separately for combat stimuli and non-combat stimuli.
2.4. Imaging protocol
Acquisition of MR images was conducted on a 4T General Electric SIGNA scanner. A series of 34 interleaved axial functional slices were acquired for full-brain coverage (TR/TE/flip = 2000/31/60; FOV = 240 mm; 3.75 × 3.75 × 3.8 mm voxels; interslice skip = 0) using an inverse-spiral pulse sequence to reduce susceptibility artifact. High-resolution three-dimensional spin-echo co-planar structural images were acquired in 68 axial slices (TR/TE/flip = 12.2/5.3/20, voxel size = 1 × 1 × 1.9 mm, FOV = 240 mm, interslice skip = 0).
2.5. Analysis of functional MRI data
Functional data sets were analyzed using FSL version 3.3.5 (Oxford Centre for Functional Magnetic Resonance Imaging of the Brain (FMRIB), Oxford University, UK) (Smith et al., 2004). Paradigm timing files were converted to FSL compatible format and NIFTI image data files were generated. Preprocessing was applied to individual subjects’ data using the following steps (i) motion correction with Motion Correction FMRIB Linear Image Registration Tool (MCFLIRT) (Jenkinson et al., 2002) to correct for motion within each experimental run using the middle volume of the run as reference, (ii) slice timing correction using sinc interpolation to shift each time-series by an appropriate fraction of a TR relative to the middle of the TR period based on an interleaved slice acquisition sequence (iii) brain extraction using the brain extraction tool (BET) to remove the skull prior to analysis (Smith, 2002), (iv) spatial smoothing using a Gaussian kernel of FWHM 5 mm to reduce noise and improve sensitivity; (v) intensity normalization whereby the entire 4D data set was normalized by a single scaling factor or grand mean scaling so higher-level analyses remain valid (v) high-pass temporal filtering to remove low frequency artifacts (Jenkinson et al., 2002; Smith et al., 2004). Functional images of each subject were co-registered to structural images in native space, and structural images were normalized to structural standard images, defined by the MNI standard brain supplied in FSL (Avg152, T1 2 × 2 × 2 mm). The same transformation matrices used for structural-to-standard transformations were then used for functional-to-standard space transformations of co-registered functional images. All registrations were carried out using FMRIB Linear Image Registration Tool (FLIRT) for linear (affine with 12 degrees of freedom) registration based on a multi-start, multi-resolution global optimization method of intermodal registration (Jenkinson and Smith, 2001).
After preprocessing, subsequent data analyses used whole brain voxel-wise and region of interest (ROI) approaches similar approach to our earlier report in a non-clinical sample (Dolcos and McCarthy, 2006). Custom software tools from Duke-UNC Brain Imaging and Analysis Center (Duke University, Durham NC) were used to compare brain activity associated with the contrasts of interest (e.g., combat vs. non-combat conditions). For individual subject analyses, the fMRI signal was selectively averaged in each subject as a function of trial type (i.e., combat, non-combat, and scrambled) and image volume (TR) within the trial epoch (two image volumes preceding epoch onset and 14 image volumes following epoch onset), and compared for the contrasts of interest using pairwise t-statistics. Individual subject analyses produced whole brain average and activation t-maps for each condition, contrast of interest, and sub-epochs (encoding, maintenance, and retrieval). Data for sub-epochal contrast maps was extracted from the overall time course by averaging a grouping of image volumes representing maximal change relative to the pre-memorandum onset baseline: 6−8 s for encoding, 12−14 s for maintenance, and 22 s for retrieval. Thus, no assumption was made about the shape of the hemodynamic response function. Only trials for which subjects correctly identified the probe stimulus as Old or New relative to the memory set were included in the analyses.
2.6. Determination of functional regions of interest for between-groups analyses
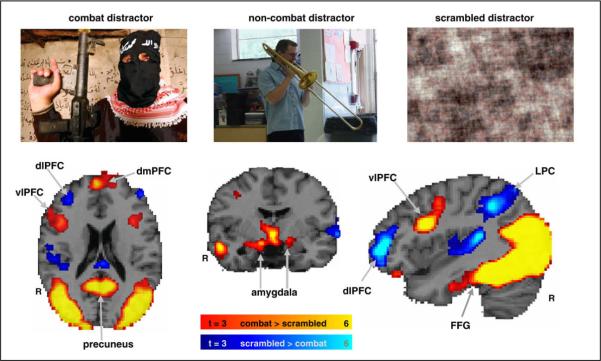
Choice of regions for analysis was guided by a priori hypotheses and consistent with results from our previous study of a non-clinical sample (Dolcos and McCarthy, 2006). We first examined activation effects for distractor type in a whole brain analysis of our entire sample of subjects, without regard to diagnosis. As expected; based on our previous findings in healthy normal subjects (Dolcos and McCarthy, 2006), combat distractors produced markedly different patterns of activity in the ventral and dorsal neural systems during the working memory delay interval (see Fig. 2). The ROIs were traced around the voxels showing the maximum effects in the contrasts of interest, as identified by the whole brain voxel-based analyses. Specifically, contrast activation maps between the most vs. least distracting conditions (i.e., combat > scrambled distractors) showed strong activation in ventral emotion processing regions, including the amygdala, vlPFC, and fusiform gyrus (see Table 2 from Supplementary material). On the other hand, activation maps for the opposite contrast (i.e., scrambled > combat distractors) showed strong deactivations (signal activity below the baseline level) in both dorsolateral PFC (dlPFC) and lateral parietal cortex (LPC), thus indicating that combat distractors disrupted activity in the canonical working memory network during the delay period (see Table 3 from Supplementary material). Given our a priori hypotheses based on findings in non-clinical participants, we used an intensity threshold of t > 3.0 (P < 0.002) and an extent threshold of ten contiguous voxels to define the functional ROIs. Note that the contrasts described were used solely for the purpose of defining functional ROIs but not for testing of main hypotheses.
Fig. 2.
Dissociable dorsal–ventral patterns of activity from subjects (n = 42) observed in the presence of combat-related distracters. Combat distracters produced the most disrupting effect on activity during the delay period in a set of dorsal brain regions associated with working memory (blue blobs) while producing the most enhancing effect on activity on ventral brain regions associated with emotion processing (red blobs). The activation maps show direct contrasts between the most versus least distracting conditions, combat > scrambled (red) and scrambled > combat (blue), superimposed on a high-resolution brain image. The colored horizontal bars at the bottom of the brain image indicate the gradients of the t values for the activation maps displayed.
2.7. Between group ROI analyses
Activity in these functionally identified ROIs was subject to further between group analyses. The group analyses involved both voxel-based and ROI-based statistics (t-tests/ANOVAs/ANCOVAs and post hoc analyses performed with Fisher's PLSD), using the individual activation t-maps and percent signal change data extracted from functional ROIs. Analyses were performed on changes in MR signal from a pre-stimulus baseline. Hypothesis testing was conducted with ANCOVA for each ROI using a 2 × 2 repeated measures design with group as a between measure (2 levels; PTSD, control) and distractor as repeated measure (2 levels; combat, non-combat). Given the rates of comorbid depression the analysis included covariates for depression scores (see Table 1) and dosage equivalents for antidepressant medication (see Table 4).
For the covariate analysis, severity of depression was assessed with the Beck Depression Inventory (Beck et al., 1988). For antidepressant medication, covariate analysis used the following dosing equivalence formula: 20 mg citalopram = 50 mg sertraline = 5 mg escitalopram = 50 mg fluvoxamine = 20 mg paroxetine = 20 mg fluoxetine. The action of mirtazipine is heterogeneous with action at serotonergic (5-HT2 and 5-HT3), adrenergic (α1), histaminergic (H1), and muscarinic receptor sites. Therefore, two different approaches were used for analysis of subjects on mirtazipine. In the first approach assigned an equivalence of mirtazipine 15 mg = citalopram 20 mg. However, this approach was limited given the adrenergic and histaminergic actions of mirtazipine. In the second approach subjects taking mirtazipine were removed from the analysis (1 control, 4 PTSD). The results of the first approach are presented in the main results with nearly identical results from the second approach. A listing of subjects’ antidepressant medication and dosing is presented in Table 4.
3. Results
3.1. Behavioral results
3.1.1. PTSD effect on working memory performance
Partially consistent with our first hypothesis, analysis of detectability scores (d-prime = Z(hit rate) – Z(false alarm rate), revealed lower scores in the PTSD group than control group: main effect of group [F(1,40) = 5.5, P < 0.05] but did not show group * distractor effect [F(1,40) = 1.17, P > 0.2] or a main effect for distractor [F(1,40) = 0.20, P > 0.6] as seen in Fig. 3. Thus, the PTSD group showed poorer performance than the control group when the combat and non-combat distractors were presented during the working memory delay period.
Fig. 3.
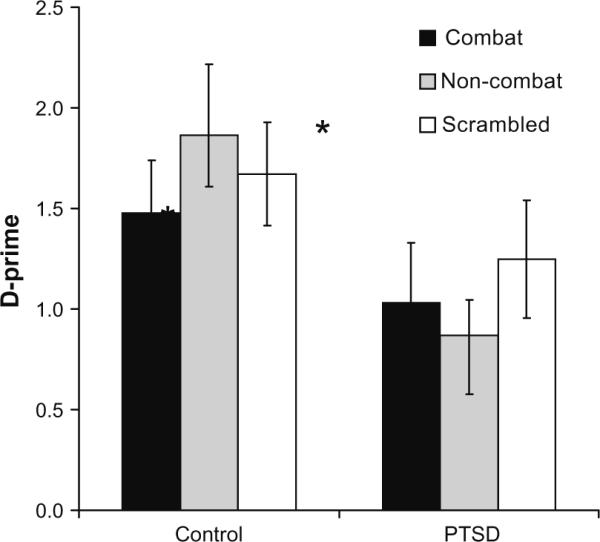
Working memory performance measured by d-prime scores for control and PTSD groups for combat, non-combat, and scrambled distractor types.
3.1.2. PTSD effect on picture rating task
Repeated measures ANOVAs were performed on emotion intensity and distractibility ratings of the two distractor types (combat vs. non-combat), using the group (PTSD vs. control) as the between subjects factor. Overall, participants rated combat distractors as more intense than non-combat distractors, as shown by a significant main effect of condition: [F(1,35) = 172.1, P < 0.0001]). The PTSD group tended to have higher emotion intensity ratings than the control group, as shown by a trend level condition*group interaction [F(1,35) = 3.5, P < 0.07]. Similar effects were found in the distractibility ratings. Overall, participants rated combat scenes as more distracting than non-combat scenes (significant main effect of condition: [F(1,35) = 116.6, P < 0.0001]), and the PTSD group tended to have higher distractibility rating than the control group (main effect of group: [F(1,35) = 3.2, P < 0.08]).
3.2. fMRI results
3.2.1. PTSD effects on ventral emotional regions
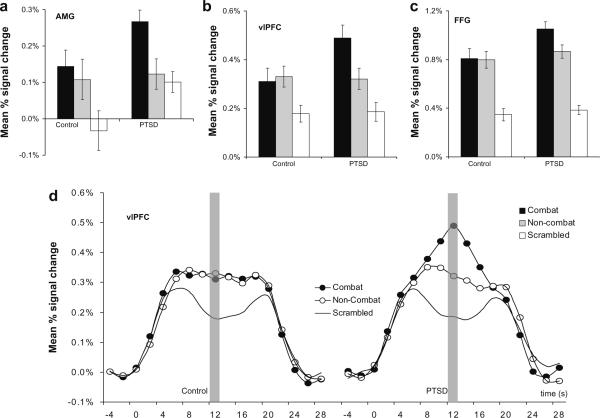
Confirming our second prediction, combat distractors were associated with increased delay interval activation in the ventral emotional structures in the PTSD group. An ANCOVA test for each ROI was conducted using a 2 × 2 repeated measures design with group as the between measure (2 levels; PTSD, control) and distractor as repeated measure (2 levels; combat, non-combat). Covariates in the ANCOVA were depression scores and dosage equivalent of antidepressant medication. A significant distractor*group interaction was found in the amygdala [F(1,40) = 4.9, P < 0.05], fusiform gyrus [F(1,40) = 9.5, P < 0.005], and ventrolateral PFC [F(1,40) = 11.1, P < 0.001]. Planned comparisons showed greater activation for the combat than non-combat distractors in the PTSD group but not in the control group for the amygdala ([t(40) = 2.2, P < 0.05] vs. [t(40) = 0.21, P > 0.8]) the ventrolateral PFC ([t(40) = 2.3, P < 0.05] vs. [t(40) = 0.15, P > 0.8]), and the fusiform gyrus (([t(40) = 2.4, P < 0.05] vs. [t(40) = 0.76, P > 0.4. Fig. 4 shows the maximal extent of percent signal change for the three ventral regions during the working memory delay interval, and for illustrative purposes Fig. 4d, shows the hemodynamic response in the vlPFC for the overall task epoch.
Fig. 4.
Comparison of mean percent signal change in ventral emotional regions corresponding to combat, non-combat, and scrambled distractors displayed during the active maintenance period of working memory in the PTSD (n = 22) and control (n = 20) groups. Activation in the was greater for combat than non-combat distractors in the PTSD group but not in the control group in the (a) amygdala (AMG) (b) ventrolateral prefrontal cortex (vlPFC), and (c) the fusiform gyrus (FFG). (d) Mean percent signal change for the entire trial epoch by condition in the vlPFC for the control group and the PTSD group.
3.2.2. PTSD effects on dorsal executive regions
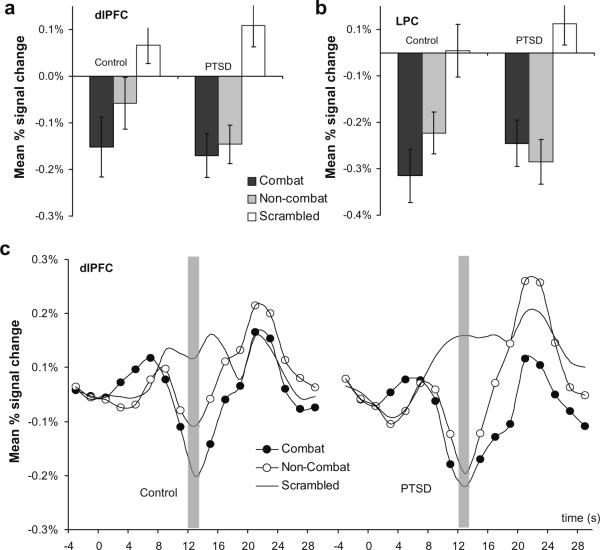
The findings in the dorsal executive regions partially confirmed our third prediction showing dlPFC activation between groups was differentially affected by distractor type. Analysis using statistical modeling with ANCOVA as described above showed a significant distractor*group interaction in the dorsolateral PFC [F(1,40) = 5.1, P < 0.05] and a trend level interaction in the lateral parietal cortex (LPC) [F(1,40) = 3.5, P = 0.07]. Planned comparisons showed there was a trend of more deactivation (signal below baseline) for combat than non-combat distractors in the control group but not in the PTSD group ([t(40) = 1.3, P = 0.11] vs. [t(40) = 0.23, P > 0.8]). Pairwise comparisons in the lateral parietal cortex were non-significant. Thus dlPFC activity in the PTSD group was disrupted regardless of distractor type (combat, non-combat) where as in the control group activation was differentially disrupted by combat distractors. The dorsal frontoparietal regions showed signal activity below the baseline during the working memory delay period. Fig. 5 shows the maximal extent of percent signal change for the two dorsal regions during the working memory delay interval, and for illustration purposes, Fig. 5c shows the hemodynamic response in the dlPFC for the overall task epoch.
Fig. 5.
Comparison of mean percent signal change in dorsal executive regions corresponding to combat, non-combat, and scrambled distractors displayed during the active maintenance period of working memory in the PTSD (n = 22) and control (n = 20) groups. (a) Combat related distractors showed differential dlPFC deactivation in the control group but not in the PTSD group. (b) There was a trend level group by distractor type interaction effect in the lateral parietal cortex (LPC). (c) Mean percent signal change for the entire trial epoch by condition in the dlPFC for the control Group and the PTSD group.
4. Discussion
The present study examined neural activity in individuals with PTSD in response to trauma-related and trauma-neutral stimuli presented as task-irrelevant distractors during the delay period of a working memory task. To our knowledge, this is the first fMRI study of PTSD to examine the effects of distracting traumatic and neutral information on the neural basis of working memory. There were three main findings from this study. First, the PTSD group showed greater activation than the control group for combat relative to non-combat distractors in three ventral emotion processing regions, namely amygdala, ventrolateral PFC, and fusiform gyrus. Second, the PTSD group showed greater concomitant disruption of activation for salient task-irrelevant distractor scenes (combat, non-combat) in the dorsal lateral PFC whereas the control group showed disruption in the dlPFC that was specific to combat distractors. This suggests a more generalized dlPFC disruption in the PTSD group than in the control group which showed disruption specific to threat-specific distractors. Finally, these neural findings complemented the behavioral results, which showed lower working memory performance for task-irrelevant distractor scenes in the PTSD group compared to the control group.
4.1. Findings for ventral emotional regions
In ventral emotional regions (amygdala, vlPFC, FUSIFORM GYRUS), activation for combat distractors was greater than non-combat distractors in the PTSD group, but not in the control group. Several previous studies have reported greater amygdalar activation in symptom provocation studies (Hendler et al., 2003; Rauch et al., 2000; Shin et al.,2004, 2005; Williams et al., 2006), but studies of cognitive processing have generally failed to show amygdala activation (Bremner et al., 2004; Clark et al., 2003; Morey et al., 2008; Shin et al., 2001), thus bringing into question its purported effects on cognitive processes in PTSD (Bremner et al., 2004; Shin et al., 2001). Here, we found a clear effect of greater amygdala activation for combat distractors relative to non-combat distractors in the PTSD group, but not in the control group. As expected, we also found a robust effect in the vlPFC, which is also consistent with previous reports of emotion processing in PTSD (Morey et al., 2008; Shin et al., 2001). Ventrolateral prefrontal regions serve working memory in maintenance of object information and simultaneous inhibition of distracting information (Dolcos et al., 2006; Ranganath and D'Esposito, 2005). Interestingly, although both groups rated the combat scenes as more emotionally intense, the trauma-exposed control group lacked the corresponding modulation in the neural response in the amygdala and vlPFC. However, this finding differs from our previous report of increased activity in the ventral affective regions to emotional IAPS (International Affective Picture System) distractors in a non-clinical sample (Dolcos and McCarthy, 2006).
These results for the control group may be related to differences in the perceived emotional nature and intensity of the IAPS stimuli as compared to the combat scenes used here. It is possible that the combat-related pictures were not as effective in triggering a response in the ventral affective regions for the present trauma-exposed control group as the IAPS pictures were for the non-clinical group from our previous study. Another source of discrepancy could be linked to gender-related differences (Lang et al., 1993) in the neural response to distracting emotional information, as in the present study less than half of the control group's participants (∼48%) were female, whereas all participants in our previous study were female. Yet another intriguing possibility, which should be the focus of future investigations, is that diminished response in the vlPFC in the control group may reflect enhanced resilience in maintaining focus on ongoing cognitive processing in the presence of emotional distraction. Interestingly, this latter interpretation is consistent with the difference in resilient behavior (i.e., as measured by the Connor–Davidson resilience scale – see Table 1). This idea points to the possibility that brain activation derived neural markers may be an informative resilience metric, and that the present working memory paradigm may provide a way of identifying neural markers for cognitive and affective resilience in the context of trauma exposure.
Although both groups showed strong FUSIFORM GYRUS activation to both combat and non-combat distractors, there was greater activation in the PTSD group for combat distractors. This finding is consistent with the evidence that neural structures associated sensory processing may be responding to trauma-related information (Hendler et al., 2003). It is also consistent with the interpretation that activation in these regions may be potentiated by top–down attentional effects via frontal regions such as vlPFC that are known to provide feedback regarding the salience of emotional, motivational, or threat information to sensory association areas (Corbetta and Shulman, 2002).
4.2. Findings for dorsal working memory regions
In the putative working memory brain region, dlPFC, activity in the PTSD group was disrupted regardless of distractor type (combat, non-combat) where as in the control group activation was differentially disrupted by combat distractors. In the dlPFC, deactivation in the PTSD group was not differentially modulated by combat distractors. Interestingly, this neural finding corresponds with the behavioral performance data also showing that the PTSD group had poorer working memory performance for both combat and non-combat distractors. Our findings therefore, suggest that distractor information, regardless of its trauma relevance, is more disruptive to working memory related dlPFC activity in PTSD. These results converge with previous findings of heightened responsivity to both threatening and non-threatening stimuli in PTSD (Grillon and Morgan, 1999; Peri et al., 2000), and may be consistent with the clinically observed symptom of hypervigilance.
Our findings show that ventral prefrontal regions discriminated threatening from non-threatening stimuli in PTSD, while dorsal regions did not. Our results are consistent with PET findings in PTSD of working memory for trauma-neutral information comparing a target detection task with a working memory load to one without a working memory load (Clark et al., 2003). Differential activation was reported in the inferior parietal cortex and dlPFC for the updating versus non-updating working memory condition; however, this was not found in the patient group. In the LPC, another typical working memory region (Curtis, 2006), the PTSD group showed greater deactivation than the control group to non-combat distractors but not to combat distractors. Our findings suggest that the parietal cortex in PTSD responds indiscriminately to both threat-related and threat-neutral information whereas the response of the control group may be threat-specific. In summary, the observed dorsal prefrontal deficit suggests a lack of specificity related to processing threat-related versus threat-neutral information and is consistent with the clinically observed symptom of hypervigilance.
4.3. Strengths and limitations
Two major strengths of the present study improve upon previous efforts to identify functional neuroanatomy of PTSD. First, the experimental design directly assessed the interaction between neural systems for processing trauma-related information and for working memory. Second, we used a large homogeneous sample of young post-9/11 war veterans with recent trauma, recent onset of PTSD symptoms, and relatively low level of comorbid substance use. The present study would be enhanced by more reliably ascertaining PTSD with the CAPS, and comorbid psychiatric conditions with the SCID. However, a DTS score greater 32 is highly suggestive of PTSD based on our prior assessment of DTS efficiency and validity in a larger post-9/11 sample of veterans (McDonald et al., in press). Moreover, the generous sample size of 42 subjects is expected to mitigate this limitation as well as other sources of Type I error.
Another possible limitation was the use antidepressant medication in several subjects. Investigations on the effects of medications commonly used for anxiety and depression (SSRIs and benzodiazepines) have shown mixed results on fMRI activation in the amygdala and lateral prefrontal cortex (Fu et al., 2004; Harmer et al., 2006; Paulus et al., 2005; Rose et al., 2006). Finally, as expected, there was a high level of comorbid depressive symptoms. Two considerations are important in this regard, (i) there is a clear overlap between criteria for PTSD and depression, and (ii) symptoms of dysphoria are part of the phenomenon of PTSD not formally captured in the current DSM nosology (Simms et al., 2002). To mitigate the effects of these variables upon our results, we included both antidepressant medication level and depression scores as covariates in our analyses.
In summary, these results highlight that for individuals with PTSD, enhanced activity in ventral emotion processing regions is associated with trauma distractors, whereas activity in brain regions associated with working memory and attention regions is disrupted by distractor stimuli independent of trauma content. Neural evidence for the impact of distraction on working memory is consistent with PTSD symptoms of hypervigilance and general distractibility during goal-directed cognitive processing. These findings call attention to the need for careful investigation of the neural basis of interaction effects of re-experiencing symptoms on goal-directed cognitive processing in future PTSD research.
Acknowledgment
We gratefully acknowledge the contributions of Srishti Seth, Jeffrey Hoerle, and Syam Gadde.
Role of funding source
Funding for this research was provided by the Department of Veterans Affairs (VA) Mental Illness Research Education and Clinical Center Grant for Post Deployment Mental Health. RM was supported by National Institute of Mental Health (NIMH) Grant K23 MH073091. FD was supported by a NARSAD Young Investigator Award, a NSERC Postdoctoral Fellowship and a CPRF Award (Canada). The VA, NIMH, NARSAD, or NSERC had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues. Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited. In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier's archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright
Conflict of interest statement
The authors have no actual or potential conflict of interest including financial or personal relationships that could influence or could be perceived to influence the work in this manuscript.
Appendix A. Supplementary material
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.jpsychires.2008.10.014.
References
- Beck AT, Steer RA, Garbin MG. Psychometric properties of the beck depression inventory: twenty-five years of evaluation. Clinical Psychology Review. 1988;8(1):77–100. [Google Scholar]
- Bremner J, Vermetter E, Vythilingam M, Afzal N, Schmahl C, Bernet Elzinga, et al. Neural correlates of the classic color and emotional stroop in women with abuse-related posttraumatic stress disorder. Biological Psychiatry. 2004;55:612–20. doi: 10.1016/j.biopsych.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Bryant RA, Felmingham KL, Kemp AH, Barton M, Peduto AS, Rennie C, et al. Neural networks of information processing in posttraumatic stress disorder: a functional magnetic resonance imaging study. Biological Psychiatry. 2005;58(2):111–8. doi: 10.1016/j.biopsych.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Clark CR, McFarlane AC, Morris P, Weber DL, Sonkkilla C, Shaw M, et al. Cerebral function in posttraumatic stress disorder during verbal working memory updating: a positron emission tomography study. Biological Psychiatry. 2003;53:474–81. doi: 10.1016/s0006-3223(02)01505-6. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3(3):201–15. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Curtis CE. Prefrontal and parietal contributions to spatial working memory. Neuroscience. 2006;139(1):173–80. doi: 10.1016/j.neuroscience.2005.04.070. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Postle BR, Rypma B. Prefrontal cortical contributions to working memory: evidence from event-related fMRI studies. Experimental Brain Research. 2000;133(1):3–11. doi: 10.1007/s002210000395. [DOI] [PubMed] [Google Scholar]
- Davidson JR, Book SW, Colket JT, Tupler LA, Roth S, David D, et al. Assessment of a new self-rating scale for posttraumatic stress disorder. Psychological Medicine. 1997;27(1):153–60. doi: 10.1017/s0033291796004229. [DOI] [PubMed] [Google Scholar]
- Dolcos F, Kragel P, Wang L, McCarthy G. Role of the inferior frontal cortex in coping with distracting emotions. Neuroreport. 2006;17(15):1591–4. doi: 10.1097/01.wnr.0000236860.24081.be. [DOI] [PubMed] [Google Scholar]
- Dolcos F, McCarthy G. Brain systems mediating cognitive interference by emotional distraction. Journal of Neuroscience. 2006;26(7):2072–9. doi: 10.1523/JNEUROSCI.5042-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu CHY, Williams SCR, Cleare AJ, Brammer MJ, Walsh ND, Kim J, et al. Attenuation of the neural response to sad faces in major depression by antidepressant treatment: a prospective, event-related functional magnetic resonance imaging study. Archives of General Psychiatry. 2004;61(9):877–89. doi: 10.1001/archpsyc.61.9.877. [DOI] [PubMed] [Google Scholar]
- Grillon C, Morgan CA. Fear-potentiated startle conditioning to explicit and contextual cues in gulf war veterans with posttraumatic stress disorder. Journal of Abnormal Psychology. 1999;108(1):134–42. doi: 10.1037//0021-843x.108.1.134. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Mackay CE, Reid CB, Cowen PJ, Goodwin GM. Antidepressant drug treatment modifies the neural processing of non-conscious threat cues. Biological Psychiatry. 2006;59(9):816–20. doi: 10.1016/j.biopsych.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Hendler T, Rotshtein P, Yeshurun Y, Weizmann T, Kahn I, Ben-Bashat D, et al. Sensing the invisible: differential sensitivity of visual cortex and amygdala to traumatic context. Neuroimage. 2003;19(3):587–600. doi: 10.1016/s1053-8119(03)00141-1. [DOI] [PubMed] [Google Scholar]
- Hoge CW, Castro CA, Messer SC, McGurk D, Cotting DI, Koffman RL. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. New England Journal of Medicine. 2004;351(1):13–22. doi: 10.1056/NEJMoa040603. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–41. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Medical Image Analysis. 2001;5(2):143–516. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Greenwald MK, Bradley MM, Hamm AO. Looking at pictures: affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30(3):261–73. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Martis B. Neuroimaging studies of emotional responses in PTSD. Annals of the New York Academy of Sciences. 2006;1071:87–109. doi: 10.1196/annals.1364.009. [DOI] [PubMed] [Google Scholar]
- McDonald S, Beckham J, Morey RA, Calhoun P. The validity and diagnostic efficiency of the Davidson trauma scale with veterans who have served since September 11th, 2001. Journal of Anxiety Disorders. doi: 10.1016/j.janxdis.2008.07.007. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane AC. The prevalence and longitudinal course of PTSD. Implications for the neurobiological models of PTSD. Annals of the New York Academy of Sciences. 1997;821:10–23. doi: 10.1111/j.1749-6632.1997.tb48265.x. [DOI] [PubMed] [Google Scholar]
- McNally RJ. Cognitive abnormalities in posttraumatic stress disorder. Trends in Cognitive Sciences. 2006;10(6):271–7. doi: 10.1016/j.tics.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Morey RA, Petty CM, Cooper DA, LaBar KS, McCarthy G. Neural systems for executive and emotional processing are modulated by symptoms of posttraumatic stress disorder in Iraq war veterans. Psychiatry Research: Neuroimaging. 2008;162:59–72. doi: 10.1016/j.pscychresns.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannu Hayes J, LaBar KS, Petty C, McCarthy G, Morey R. Alterations in the neural circuitry for emotion and attention associated with posttraumatic stress symptomatology. Psychiatry Research: Neuroimaging. doi: 10.1016/j.pscychresns.2008.05.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Feinstein JS, Castillo G, Simmons AN, Stein MB. Dose-dependent decrease of activation in bilateral amygdala and insula by lorazepam during emotion processing. Archives of General Psychiatry. 2005;62(3):282–8. doi: 10.1001/archpsyc.62.3.282. [DOI] [PubMed] [Google Scholar]
- Peri T, Ben-Shakhar G, Orr SP, Shalev AY. Psychophysiologic assessment of aversive conditioning in posttraumatic stress disorder. Biological Psychiatry. 2000;47(6):512–9. doi: 10.1016/s0006-3223(99)00144-4. [DOI] [PubMed] [Google Scholar]
- Ranganath C, D'Esposito M. Directing the mind's eye: prefrontal, inferior and medial temporal mechanisms for visual working memory. Current Opinion in Neurobiology. 2005;15(2):175–82. doi: 10.1016/j.conb.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, et al. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biological Psychiatry. 2000;47(9):769–76. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- Rose EJ, Simonotto E, Spencer EP, Ebmeier KP. The effects of escitalopram on working memory and brain activity in healthy adults during performance of the n-back task. Psychopharmacology. 2006;185(3):339–47. doi: 10.1007/s00213-006-0334-2. [DOI] [PubMed] [Google Scholar]
- Shin LM, Orr SP, Carson MA, Rauch SL, Macklin ML, Lasko NB, et al. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Archives of General Psychiatry. 2004;61(2):168–76. doi: 10.1001/archpsyc.61.2.168. [DOI] [PubMed] [Google Scholar]
- Shin LM, Whalen PJ, Pitman RK, Bush G, Macklin ML, Lasko NB, et al. An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biological Psychiatry. 2001;50(12):932–42. doi: 10.1016/s0006-3223(01)01215-x. [DOI] [PubMed] [Google Scholar]
- Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, et al. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Archives of General Psychiatry. 2005;62(3):273–81. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- Simms LJ, Watson D, Doebbeling BN. Confirmatory factor analyses of posttraumatic stress symptoms in deployed and non-deployed veterans of the Gulf war. Journal of Abnormal Psychology. 2002;111(4):637–47. doi: 10.1037//0021-843x.111.4.637. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17(3):143–55. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Vasterling JJ, Proctor SP, Amoroso P, Kane R, Heeren T, White RF. Neuropsychological outcomes of army personnel following deployment to the Iraq war. JAMA. 2006;296(5):519–29. doi: 10.1001/jama.296.5.519. [DOI] [PubMed] [Google Scholar]
- Weber DL, Clark CR, McFarlane AC, Moores KA, Morris P, Egan GF. Abnormal frontal and parietal activity during working memory updating in posttraumatic stress disorder. Psychiatry Research. 2005;140(1):27–44. doi: 10.1016/j.pscychresns.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Williams LM, Kemp AH, Felmingham K, Barton M, Olivieri G, Peduto A, et al. Trauma modulates amygdala and medial prefrontal responses to consciously attended fear. Neuroimage. 2006;29(2):347–57. doi: 10.1016/j.neuroimage.2005.03.047. [DOI] [PubMed] [Google Scholar]