Abstract
The CI protein of bacteriophage λ(λCI) is both a repressor and activator of transcription that has served as a model for understanding how gene regulatory proteins work. A dimeric DNA-binding protein, λCI also forms higher-order oligomers that allow it to bind cooperatively to both adjacent and nonadjacent operator sites within the phage genome. The ability of phage λ to transition efficiently from one program of gene expression to another depends upon the formation of these higher-order protein-DNA complexes. A recently determined crystal structure of a DNA-bound λCI dimer reveals that the two subunits of the dimer adopt different conformations. This unexpected asymmetry helps explain how these higher-order complexes are assembled.
Introduction
Bacteriophage λ provided one of the earliest paradigms for achieving a mechanistic understanding of gene regulation [1–3]. Its CI protein and the bacterial Lac repressor were the first transcription repressors to be isolated [4, 5]. Since that time, λCI function has been probed by a combination of genetic, biochemical and structural approaches, revealing a sophisticated regulatory circuit that allows for the efficient transition from one pattern of gene expression to another [1, 2].
A lysogenic phage, λ can access two alternative developmental pathways upon infection of a bacterial host cell: the lytic pathway, which results in host cell lysis and the release of a crop of progeny phage, or the lysogenic pathway, which results in the stable integration of the phage chromosome into the bacterial chromosome and a general shut down of phage gene expression. Once established, the lysogenic state is highly stable [6, 7]; nevertheless, specific environmental conditions (those that elicit the cellular SOS response) trigger a highly efficient switch from lysogeny to lytic development (referred to as prophage induction) [1, 2].
The off position: λCI and the maintenance of lysogeny
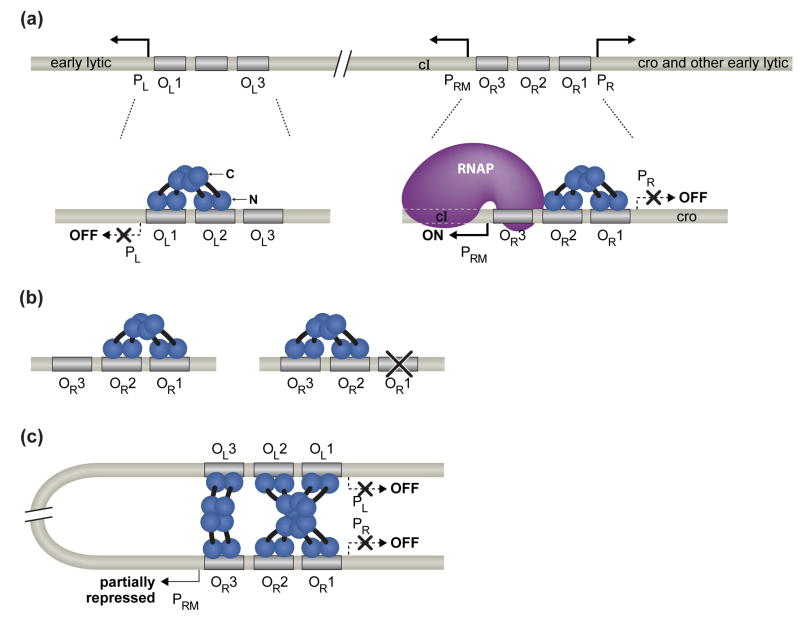
Both the maintenance of lysogeny and prophage induction depend critically on the CI protein, which functions as both a repressor and an activator of transcription [1]. A key aspect of λCI function is its ability to form higher-order oligomers, permitting it to bind cooperatively to both adjacent [8] and widely separated operator sites [9, 10]. The phage genome contains six individual operators sites, three in each of two control regions, OR and OL, which are separated by ~2.4 kilo base pairs (kb) (Fig. 1) [1]. The OR region consists of operator sites OR1, OR2, and OR3; these are flanked by promoters PR and PRM, which direct transcription of early lytic genes and the cI gene, respectively. The simpler OL region consists of operator sites OL1, OL2, and OL3 and a single promoter, PL, which, like PR, directs transcription of early lytic genes. During lysogenic growth, λCI represses transcription of the early lytic genes by occupying sites OR1 and OR2 in OR [11] and sites OL1 and OL2 in OL [1]. In addition, λCI bound at OR2 activates transcription of the cI gene from PRM [12], an autoregulatory function that also contributes to the maintenance of lysogeny [1, 13]. Finally, λCI has the potential to repress transcription of its own gene from PRM (negative autoregulation), which occurs when λCI binds to OR3 [14].
Fig 1. The interactions of λCI at the right and left operator regions in a λ lysogen.
(a) λCI dimers bound cooperatively to adjacent operator sites in OR and OL. The λCI dimers are shown in blue. Each subunit of the dimer consists of an N-terminal DNA-binding domain (N), a C-terminal oligomerization domain (C), and a linker region (black) connecting the two. The dimer pair bound cooperatively at OR1 and OR2 represses transcription from PR and the dimer bound at OR2 also activates transcription from PRM. The dimer pair bound cooperatively at OL1 and OL2 represses transcription from PL.
(b) Alternate pairwise cooperativity. No more than two adjacently bound λCI dimers can interact. Ordinarily, a dimer bound at high-affinity site OR1 stabilizes the binding of a second dimer to OR2. But if OR1 is inactivated by mutation, λCI dimers can bind cooperatively to OR2 and OR3.
(c) Higher-order looped complex that facilitates negative autoregulation. The cooperatively bound λCI dimer pair at OR1 and OR2 interacts with the cooperatively bound λCI dimer at OL1 and OL2, forming an octameric complex that loops 2.4 kb of intervening DNA. Formation of the looped complex juxtaposes OL3 and OR3, allowing another pair of λCI dimers (shown in pale blue) to bind cooperatively to these sites. When bound at OR3, λCI represses transcription from PRM (negative autoregulation); however, in a lysogen OL3 and OR3 are only partially occupied.
λCI, which binds as a dimer to individual operator sites, contains two structurally distinct domains that are connected by a protease-sensitive linker region. The N-terminal domain (NTD) contains a helix-turn-helix DNA-binding domain [15] and also a small surface-exposed patch that contacts RNA polymerase to effect transcription activation [16]. The C-terminal domain (CTD) mediates dimer formation and also the higher-order dimer-dimer interactions that enable λCI to bind cooperatively to two (or more) operator sites [17, 18].
Two parameters dictate occupancy of the correct sites during lysogeny and the proper functioning of the molecular switch that mediates the process of prophage induction: (i) the intrinsic binding affinities of the individual operator sites for λCI dimers and (ii) the ability of pairs of λCI dimers to bind cooperatively to adjacent operator sites [1, 8]. OR1 and OL1 are both high-affinity λCI binding sites, whereas the remaining sites bind λCI with intrinsically lower affinities. Thus, a λCI dimer bound at OR1 or OL1 can stabilize the binding of a second dimer to the adjacent site (OR2 or OL2), increasing the apparent binding affinity of the weaker site. The resulting cooperatively bound λCI dimer pair is, however, unable to interact with a third adjacently bound λCI dimer. As a result, higher λCI concentrations are required for the occupancy of OR3 and OL3, which ensures that lysogenic concentrations of λCI do not repress transcription from PRM. This inability of more than two adjacently bound λCI dimers to interact is known as “alternate pairwise cooperativity”, because a λCI dimer bound at OR2 normally interacts with a λCI dimer bound at the higher-affinity OR1 site, but can alternatively interact with a λCI dimer bound at OR3 provided that OR1 has been inactivated by mutation (Fig. 1b) [8].
As expected, a genetically altered λ phage bearing a mutation that specifically disrupts λCI cooperativity was found to be incapable of forming stable lysogens [19**]. Strikingly, however, Babic and Little [19**] were able to restore qualitatively wild-type behavior to the mutant phage by the introduction of two suppressor mutations (one that increased the strength of lysogenic promoter PRM and the other that strengthened OR2 as a λCI binding site). These findings suggest that λCI cooperativity may have evolved in the context of a cruder, but functional form of the regulatory circuit that governs lysogenic development. The authors note, however, that CI cooperativity likely evolved early on because, with one possible exception, all lambdoid phages examined encode a CI protein that exhibits cooperativity.
From off to on: λCI and prophage induction
During prophage induction, which is triggered under conditions that elicit the cellular SOS (DNA damage) response, an activated form of the bacterial RecA protein binds to the λCI CTD and induces a self-cleavage reaction that separates the λCI CTD from the λCI NTD [20]. As a consequence, the operator sites are vacated and transcription from PR and PL commences. The first gene that is transcribed under the control of PR encodes a second repressor (Cro) [21] that binds to the same six operator sites as does λCI [1, 22]. However, Cro, which binds noncooperatively to the individual operator sites, exhibits a different order of preference for these operators, binding with the highest affinity to OR3 [23]. When bound at OR3, Cro (like λCI) represses transcription from PRM [23]. Early on, the suggestion was made that the antagonistic effect of Cro on cI transcription likely plays a critical role during prophage induction, by preventing a recovery of λCI synthesis [24]. Although this proposal has been challenged [25, 26], a more recent study provides definitive evidence in support of the original proposal [27**].
Cro is also required for lytic development after infection, but in this case its essential role is to turn down early lytic transcription by binding to sites OR1 and OR2 and sites OL1 and OL2 [28]. Cro’s affinity for these sites is lower than its affinity for OR3 [23], ensuring that there is a delay before Cro accumulates sufficiently to occupy these sites [29]. Presumably, Cro-mediated repression of early lytic transcription is also required for completion of the lytic program after prophage induction, but this has not been demonstrated directly [27**].
Tuning the switch: higher-order looped complex facilitates negative autoregulation
The molecular interactions depicted in Fig. 1a summarize the understanding of λCI-mediated regulation as it stood until about 10 years ago. Although this picture provided a fairly satisfactory explanation for the regulation of lysogeny, certain anomalies remained unexplained. For example, no obvious regulatory role could be ascribed to OL3. Furthermore, experiments carried out with single-copy PRM-lacZ fusions suggested that lysogenic concentrations of λCI were insufficient to mediate a significant amount of OR3-mediated repression of PRM transcription [14], suggesting that negative autoregulation might be relatively insignificant. In the meanwhile, various lines of biochemical, structural and genetic evidence pointed to the possibility of another level of regulation. In particular, biochemical studies suggested that λCI can assemble into an octamer in solution [30], and crystallographic studies of the λCI CTD revealed the structural basis for octamer formation [18, 31]. Furthermore, the striking observation that pairs of cooperatively bound λCI dimers could interact with one another over distances of several kb, forming looped protein-DNA complexes, suggested a physiological role for the λCI octamer -- namely, to link events occurring at OR with those occurring at OL [9].
A breakthrough in understanding was achieved by Dodd et al. [10, 32], who uncovered a critical function for OL3 in helping to mediate negative autoregulation. They found, unexpectedly, that mutation of OR3 in context of an otherwise wild type λ lysogen resulted in a substantial defect in prophage induction that was due specifically to the disruption of negative autoregulation by λCI. (Importantly, they used an OR3 mutation that disrupted λCI binding to OR3, but had no effect on Cro binding.) Their findings thus indicated that the fractional occupancy of OR3 in a lysogen is higher than previous experiments (performed with OR-based reporter constructs lacking the OL region) suggested. Additional experiments revealed that in a lysogen, interaction between the cooperatively bound λCI dimer pair at OR1 and OR2 and another cooperatively bound λCI dimer pair at OL1 and OL2 results in the formation of a higher-order octameric complex (Fig. 1c). The resulting 2.4 kb DNA loop juxtaposes OL3 and OR3 such that a pair of dimers can now bind cooperatively to these distant sites, bringing about the occupancy of OR3 at lower concentrations of λCI than would be predicted simply by its intrinsic λCI binding affinity. In fact, Dodd et al. [32] found that mutation of OL3, in the context of an otherwise wild-type λ phage, resulted in an ~3-fold increase in the lysogenic λCI concentration and a substantial defect in prophage induction due to the loss of negative autoregulation.
Structure-based modeling
The formation of both λCI tetramers and λCI octamers, postulated by the model depicted in Fig. 1c, is consistent with a wealth of biochemical, structural and genetic data. The crystal structure of the λCI CTD (the oligomerization domain) [18], which was determined almost 20 years after the structure of the λCI NTD (DNA-binding domain) was first reported [15], permitted direct visualization of the biologically relevant λCI tetramer (as established based on genetic data [33, 34]) and suggested how two tetramers might associate to form an octamer (Fig. 2). Subsequent crystallographic studies permitted direct visualization of the λCI CTD octamer [31]. Nevertheless, the lack of a structure for the intact λCI dimer left unexplained the structural basis for pairwise cooperativity (the ability of adjacently bound dimers to interact, forming a CTD tetramer). In addition, it was unclear why no more than two adjacently bound dimers can interact, even though up to four dimers can interact provided the two dimer pairs are bound at a distance from one another on the DNA.
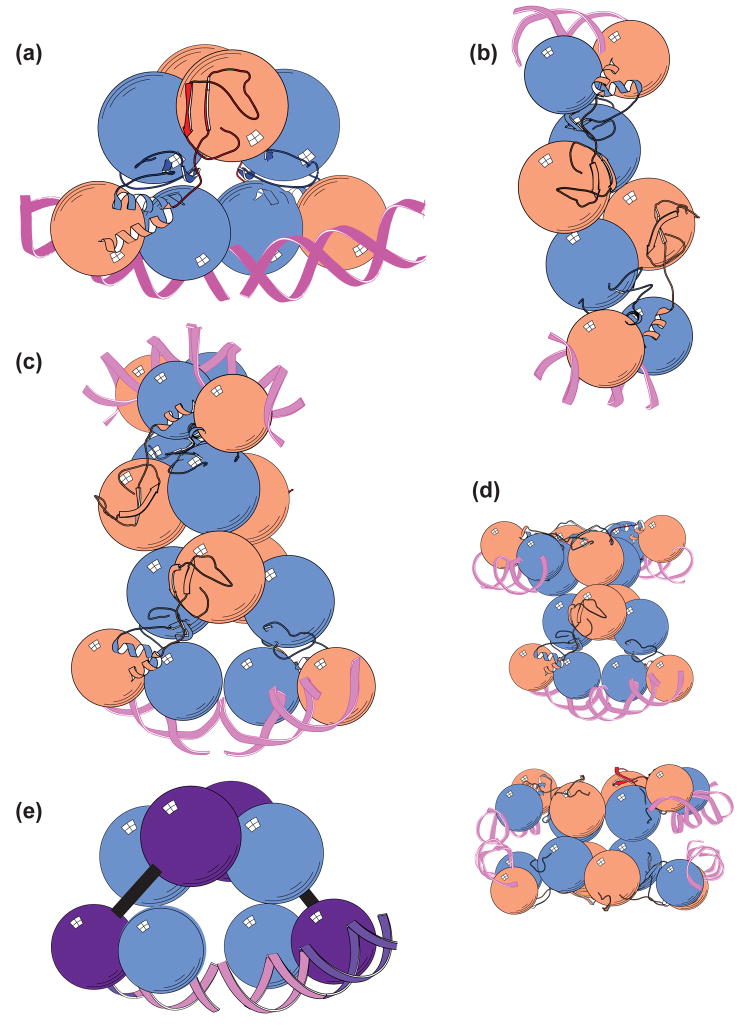
Fig 2. Formation of the λCI CTD octamer.
(a) A pair of λCI CTD dimers aligned for interaction. The structure of the λCI CTD dimer is shown in surface representation (left side), with one subunit colored salmon and the other subunit colored blue. The two dimers are related by a 180° rotation about an axis perpendicular to the plan of the page. The amino acid residues implicated in cooperativity [33, 34] form two patches on the surface of each CTD monomer, which are highlighted in red and dark blue (note that the blue patch actually consists of two neighboring but noncontiguous regions). The two dimers are also shown in cartoon representation as wedges of a cylinder (right side). The blue (B) and red (R) cooperativity patches are labeled and the dotted lines indicate how the patches pair for tetramer formation.
(b) The λCI CTD tetramer. The two dimers shown in (a) are docked to form the λCI CTD tetramer, depicted in surface representation on the left side. Notice that four cooperativity patches (two red and two blue) are still exposed on the surface of the tetramer. Shown on the right side are two tetramers in cartoon representation, each of which resembles a half-cylinder with staggered ends. Pairing of the indicated cooperativity patches results in octamer formation, as shown in (c).
(c) The λCI CTD octamer. Shown on the left is the octamer that is formed when two tetramers associate; the bottom tetramer is depicted in surface representation and the top tetramer depicted in ribbon representation so that the cooperativity patches underneath can still be seen. To form the octamer, a second tetramer is rotated approximately 180° about a horizontal axis in the plane of the page, forming a sandwich-like complex. Shown on the right is the octamer in cartoon representation (depicted as a cylinder with staggered ends). The octamer shown (left and right) has three identical dimer-dimer interfaces, those contained within each tetramer and another that is formed when the upper dimer of the bottom tetramer pairs with the upper dimer of the top tetramer. The structural details of octamer formation preclude interaction between the unpaired cooperativity patches exposed on the lower dimer of each tetramer (see, for example, the exposed blue patch at the bottom left).
The recent crystallographic analysis of a DNA-bound λCI dimer provides a plausible explanation for the rules governing higher-order complex formation, based on a striking lack of global symmetry exhibited by the dimeric complex [35**]. Rather than a single axis of symmetry as might have been expected, the dimeric complex is described by two nearly orthogonal symmetry axes, one that relates the two NTDs and another that relates the two CTDs (Fig. 3). As a result of this asymmetry, the NTD and CTD of one subunit of the dimer, but not the other, are in contact, creating an interface that is comparable in terms of buried surface area to the CTD-dimer interface. What makes this asymmetry possible is the flexibility of the short linkers that connect the N-terminal and C-terminal domains; these linker segments adopt markedly different conformations in the two subunits of the dimer. Previously reported biochemical evidence, based on the analysis of a mutant λCI protein in solution, also suggested that the intact dimer might adopt an asymmetric conformation [36].
Fig 3. Views of the dimeric λCI-operator complex.
(a) Cartoon representation of the asymmetric DNA-bound λCI dimer. One subunit of the dimer (salmon colored) adopts an extended conformation and the other adopts a compact conformation (blue). The radii of the spheres were determined based on the actual size of the NTD and CTD. The NTD and CTD of the compact subunit associate through the cleavage site region (CSR) (highlighted in dark blue, but only partially visible), a pair of antiparallel β-strands and a connecting loop that contains the site of RecA-mediated autocleavage [20, 35**]. The NTD and CTD of the extended subunit do not interact. The DNA is shown in pink.
(b) Crystal structure of the DNA-bound λCI dimer in ribbon representation. The color scheme is as in (a), with the compact subunit in blue and the extended subunit in salmon. The CSR of the extended subunit is highlighted in red and that of the compact subunit is highlighted in dark blue. The symmetry axes of the individual domains are shown as green rods, and are nearly perpendicular to one another.
(c) Crystal structure of the DNA-bound λCI dimer in space-filling representation. The color scheme is as in (a) and (b). This representation emphasizes that the CSR of the blue subunit (highlighted in dark blue) forms the interface between the NTD and CTD.
Although crystal-packing forces could, in principle, be responsible for the observed asymmetry, the asymmetric dimer assembly neatly accounts for the rules that govern the cooperative binding of λCI to both adjacent and nonadjacent operator sites [35**]. To model the higher-order interactions of DNA-bound λCI dimers, the CTDs of the intact operator-bound λCI dimer were superimposed onto the structures of the λCI CTD tetramer and octamer. Consider first the superimposition of the CTDs of two intact operator-bound λCI dimers on the structure of the λCI CTD tetramer [18]. Because of the inherent asymmetry of the intact operator-bound dimer, the superimposition can be performed in two different ways, one of which allows for cooperative binding to adjacent operator sites. Specifically, when the superimposition is performed as shown in Fig. 4a, the DNA of one λCI-operator complex comes into rough alignment with that of the second λCI-operator complex, so that the two λCI dimers are adjacently bound on what is essentially a continuous DNA helix. Thus, this superimposition provides a model for the interaction of adjacently bound λCI dimers at either OR1 and OR2 or OL1 and OL2. When the superimposition is done in the other way (Fig. 4b), the DNA-binding domains of the two interacting dimers point in opposite directions, a configuration that allows for binding to nonadjacent operator sites, such as OR3 and OL3 (see Fig. 1c). λCI dimers have also been found to bind cooperatively to nonadjacent operator sites that are separated by several (e.g. five or six) turns of the DNA helix on artificial constructs [37]; the superimposition shown in Fig. 4b also accounts for these cooperativity complexes, in which the intervening DNA is bent.
Fig 4. Structural modeling of higher-order λCI complexes.
(a) Model depicting interaction of adjacently bound λCI dimers. The pairwise cooperativity complex was modeled by superimposing the CTDs of two intact DNA-bound dimers onto the structure of the CTD tetramer. The color scheme is as in Fig. 3. This superimposition brings the DNA of one λCI-operator complex into rough alignment with that of the second complex. Also shown are the C-terminal α-helices of the NTDs (visible only on the left-hand dimer), which mediate formation of a weak NTD dimer.
(b) Model depicting interaction of nonadjacently bound λCI dimers. Alternative superimposition shows how two λCI dimers might look when bound cooperatively to OL3 and OR3 in the context of the looped complex depicted in Fig. 1c.
(c) Model depicting the interaction of two adjacently bound λCI dimer pairs. The octameric complex that links OR with OL was modeled by superimposing the CTDs of four DNA-bound dimers onto the structure of the CTD octamer. In this case, the superimposition was performed by first constructing two pairwise cooperativity complexes, as shown in (a).
(d) Alternative superimpositions illustrating that no more than two adjacently bound λCI dimers can interact. The CTDs of four DNA-bound dimers can be superimposed onto the structure of the CTD octamer in three different ways, the one shown in (c) and the two alternatives shown here. One of the alternatives (top) is generated by docking a pairwise cooperativity complex with the alternative tetrameric complex shown in (b). The other alternative (bottom) is generated by docking two tetrameric complexes configured as shown in (b). Regardless of how these superimpositions are performed, no more than two DNA operators come into alignment.
(e) Cartoon illustrating the design of oriented heterodimer experiment to detect λCI dimer asymmetry. The crystal structure of the asymmetric DNA-bound λCI dimer is used to design a mutant (purple subunit) that is unable to adopt the compact conformation (due to one or more amino acid substitutions predicted to disrupt the NTD-CTD interface). In addition, the DNA-binding specificity of the mutant subunit is altered by introduction of a suitable amino acid substitution on the DNA-binding surface of the NTD. Wild-type/mutant heterodimers are assembled in vitro. Only the wild-type subunit (blue) can adopt the compact conformation. These heterodimers should bind in an oriented fashion to an asymmetric operator bearing a half-site (purple) that has been mutated so that it is specifically recognized by the mutant subunit only. Furthermore, the wild-type/mutant heterodimers should bind cooperatively to a pair of asymmetric operators, configured as shown, with the mutant half-sites on the outside. Alternative configurations of the adjacent operator sites (not shown) are predicted to preclude cooperative binding by the wild-type/mutant heterodimers.
To model the higher-order octameric complex that forms when the pair of cooperatively bound λCI dimers at OR1 and OR2 interacts with another pair of cooperatively bound λCI dimers at OL1 and OL2, the CTDs of four intact operator-bound λCI dimers were superimposed onto the structure of the λCI CTD octamer [31]. When two tetrameric complexes configured as shown in Fig. 4a are brought together, the physiologically relevant octameric complex is formed (Fig. 4c). Two alternative superimpositions are also possible (involving either one or two tetrameric complexes configured as shown in Fig. 4b), as depicted in Fig. 4d. Notably, no matter how the superimposition is done, no more than two λCI dimers can be positioned at adjacent DNA sites, providing a structural explanation for alternate pairwise cooperativity [35**].
Future directions
Genetic analysis may permit definitive confirmation that the asymmetric configuration of the DNA-bound dimer seen in the crystal structure of the λCI-operator complex is the biologically relevant configuration. A possible approach would be to use the crystal structure to identify amino acid substitutions predicted to disrupt the observed interaction between the λCI NTD and CTD in the compact subunit of the DNA-bound dimer (Fig. 3). Such a mutant, which might or might not manifest a defect in binding to a single operator site, would presumably be unable to bind cooperatively to adjacent operator sites (or in any case no longer exhibit alternate pairwise cooperativity). However, cooperative binding should be restored in the context of a wild-type/mutant heterodimer (Fig. 4e). Moreover, such heterodimers should bind in a defined orientation when the pairwise cooperativity complex is formed, with the mutant subunits bound to the outer half-sites (see Fig. 4a, e). The requirement for oriented binding could, in principle, be tested by altering the DNA-binding specificity of one or the other subunit so that it can be targeted to a specifically mutated operator half-site [38] (Fig. 4e).
Further structural work could also provide additional support for the proposed structural models. It may be possible to crystallize the pairwise cooperativity complex by using a DNA template containing a pair of appropriately spaced operator sites. The direct visualization of a pair of λCI dimers interacting as predicted by the model would provide strong confirmation for the proposed significance of the asymmetric DNA-bound λCI dimer.
Many transcription regulators bind as dimers and participate in higher order oligomeric interactions. It will be interesting to learn whether asymmetry also plays a role in other cases to facilitate formation of the biologically relevant higher-order complexes, while preventing the formation of competing (unwanted) higher-order complexes.
Acknowledgments
We are very grateful to Sean Garrity for numerous helpful discussions and to Bryce Nickels for comments on the manuscript. We thank Renate Hellmiss for expert artwork. Work in the laboratories of AH and ML is supported by NIH grants GM44025 and GM44617, respectively.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the annual period of the review, have been highlighted (**).
- 1.Ptashne M. A Genetic Switch: Phage λ Revisited. 3. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 2004. [Google Scholar]
- 2.Dodd IB, Shearwin KE, Egan JB. Revisited gene regulation in bacteriophage lambda. Curr Opin Genet Dev. 2005;15:145–152. doi: 10.1016/j.gde.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Murray NE, Gann A. What has phage lambda ever done for us? Curr Biol. 2007;17:R305–R312. doi: 10.1016/j.cub.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Ptashne M. Isolation of the λ phage repressor. Proc Natl Acad Sci USA. 1967;57:306–313. doi: 10.1073/pnas.57.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilbert W, Muller-Hill B. Isolation of the Lac repressor. Proc Natl Acad Sci USA. 1966;56:1891–1898. doi: 10.1073/pnas.56.6.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Little JW, Shepley DP, Wert DW. Robustness of a gene regulatory circuit. EMBO J. 1999;18:4299–4307. doi: 10.1093/emboj/18.15.4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baek D, Svenningsen S, Eisen H, Sneppen K, Brown S. Single-cell analysis of lambda immunity regulation. J Mol Biol. 2003;334:363–372. doi: 10.1016/j.jmb.2003.09.037. [DOI] [PubMed] [Google Scholar]
- 8.Johnson AD, Meyer BJ, Ptashne M. Interactions between DNA-bound repressors govern regulation by the λ phage repressor. Proc Natl Acad Sci USA. 1979;76:5061–5065. doi: 10.1073/pnas.76.10.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Revet B, von Wilcken-Bergmann B, Bessert H, Barker A, Muller-Hill B. Four dimers of λ repressor bound to two suitably spaced pairs of λ operators form octamers and DNA loops over large distances. Curr Biol. 1999;9:151–154. doi: 10.1016/s0960-9822(99)80069-4. [DOI] [PubMed] [Google Scholar]
- 10.Dodd IB, Perkins AJ, Tsemitsidis D, Egan JB. Octamerization of λ CI repressor is needed for effective repression of PRM and efficient switching from lysogeny. Genes Dev. 2001;15:3013–3022. doi: 10.1101/gad.937301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyer BJ, Maurer R, Ptashne M. Gene regulation at the right operator (OR) of bacteriophage λ: II. OR1, OR2, and OR3: Their roles in mediating the effect of repressor and cro. J Mol Biol. 1980;139:163–194. doi: 10.1016/0022-2836(80)90303-4. [DOI] [PubMed] [Google Scholar]
- 12.Meyer BJ, Ptashne M. Gene regulation at the right operator (OR) of bacteriophage λ: III. λ repressor directly activates gene transcription. J Mol Biol. 1980;139:195–205. doi: 10.1016/0022-2836(80)90304-6. [DOI] [PubMed] [Google Scholar]
- 13.Michalowski CB, Little JW. Positive autoregulation of cI is a dispensable feature of the phage λ gene regulatory circuitry. J Bacteriol. 2005;187:6430–6442. doi: 10.1128/JB.187.18.6430-6442.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maurer R, Meyer BJ, Ptashne M. Gene regulation at the right operator (OR) of bacteriophage λ: I. OR3 and autogenous negative control by repressor. J Mol Biol. 1980;139:147–161. doi: 10.1016/0022-2836(80)90302-2. [DOI] [PubMed] [Google Scholar]
- 15.Pabo CO, Lewis M. The operator-binding domain of λ repressor: structure and DNA recognition. Nature. 1982;298:443–447. doi: 10.1038/298443a0. [DOI] [PubMed] [Google Scholar]
- 16.Jain D, Nickels BE, Sun L, Hochschild A, Darst SA. Structure of a ternary transcription activation complex. Mol Cell. 2004;13:45–53. doi: 10.1016/s1097-2765(03)00483-0. [DOI] [PubMed] [Google Scholar]
- 17.Pabo CO, Sauer RT, Sturtevant JM, Ptashne M. The lambda repressor contains two domains. Proc Natl Acad Sci USA. 1979;76:1608–1612. doi: 10.1073/pnas.76.4.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bell CE, Frescura P, Hochschild A, Lewis M. Crystal structure of the λ repressor C-terminal domain provides a model for cooperative operator binding. Cell. 2000;101:801–811. doi: 10.1016/s0092-8674(00)80891-0. [DOI] [PubMed] [Google Scholar]
- 19**.Babic AC, Little JW. Cooperative DNA binding by CI repressor is dispensable in a phage λ variant. Proc Natl Acad Sci USA. 2007;104:17741–17746. doi: 10.1073/pnas.0602223104. The authors show that genetically disrupting λCI cooperativity in the context of an otherwise wild-type phage prevents the formation of stable lysogens. They then demonstrate that compensatory mutations can be introduced into this phage that restore qualitatively wild-type behavior (both stable lysogeny and inducibility) without restoring λCI cooperativity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Little JW. Autodigestion of lexA and phage λ repressors. Proc Natl Acad Sci USA. 1984;81:1375–1379. doi: 10.1073/pnas.81.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eisen H, Brachet P, Pereira da Silva L, Jacob F. Regulation of repressor expression inλ. Proc Natl Acad Sci USA. 1970;66:855–862. doi: 10.1073/pnas.66.3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takeda Y, Folkmanis A, Echols H. Cro regulatory protein specified by bacteriophage λ. J Biol Chem. 1977;252:6177–6183. [PubMed] [Google Scholar]
- 23.Johnson AD, Meyer BJ, Ptashne M. Mechanism of action of the cro protein of bacteriophage λ. Proc Natl Acad Sci USA. 1978;75:1783–1787. doi: 10.1073/pnas.75.4.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson AD, Poteete AR, Lauer G, Sauer RT, Ackers GK, Ptashne M. λ repressor and cro --Components of an efficient molecular switch. Nature. 1981;294:217–223. doi: 10.1038/294217a0. [DOI] [PubMed] [Google Scholar]
- 25.Svenningsen SL, Costantino N, Court DL, Adhya S. On the role of Cro inλ prophage induction. Proc Natl Acad Sci USA. 2005;102:4465–4469. doi: 10.1073/pnas.0409839102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Atsumi S, Little JW. Role of the lytic repressor in prophage induction of phage λ as analyzed by a module-replacement approach. Proc Natl Acad Sci USA. 2006;103:4558–4563. doi: 10.1073/pnas.0511117103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27**.Schubert R, Dodd IB, Egan JB, Shearwin KE. Cro’s role in the CI-Cro bistable switch is critical for λ’s transition from lysogeny to lytic development. Genes Dev. 2007;21:2461–2472. doi: 10.1101/gad.1584907. This study provides a rigorous test of the idea that Cro-mediated repression of PRM transcription during prophage induction is required for an efficient transition from lysogeny to lytic development. The authors accomplish this by introducing mutations into OR3 that specifically disrupt the repression of PRM transcription by Cro, without significantly affecting negative autoregulation. Their findings indicate that Cro is required primarily to prevent the recovery of λCI synthesis and a consequent disruption of lytic development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Folkmanis A, Maltzman W, Mellon P, Skalka A, Echols H. The essential role of the cro gene in lytic development by bacteriophage λ. Virology. 1977;81:352–362. doi: 10.1016/0042-6822(77)90151-9. [DOI] [PubMed] [Google Scholar]
- 29.Kobiler O, Rokney A, Friedman N, Court DL, Stavans J, Oppenheim AB. Quantitative kinetic analysis of the bacteriophage lambda genetic network. Proc Natl Acad Sci USA. 2005;102:4470–4475. doi: 10.1073/pnas.0500670102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Senear DF, Laue TM, Ross JBA, Waxman E, Eaton S, Rusinova E. The primary self-assembly reaction of bacteriophage λ cI repressor dimers is to octamer. Biochemistry. 1993;32:6179–6189. doi: 10.1021/bi00075a010. [DOI] [PubMed] [Google Scholar]
- 31.Bell CE, Lewis M. Crystal structure of the λ repressor C-terminal domain octamer. J Mol Biol. 2001;314:1127–1136. doi: 10.1006/jmbi.2000.5196. [DOI] [PubMed] [Google Scholar]
- 32.Dodd IB, Shearwin KE, Perkins AJ, Burr T, Hochschild A, Egan JB. Cooperativity in long-range gene regulation by the λ CI repressor. Genes Dev. 2004;18:344–354. doi: 10.1101/gad.1167904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whipple FW, Kuldell NH, Cheatham LA, Hochschild A. Specificity determinants for the interaction of λ repressor and P22 repressor dimers. Genes Dev. 1994;8:1212–1223. doi: 10.1101/gad.8.10.1212. [DOI] [PubMed] [Google Scholar]
- 34.Whipple FW, Hou EF, Hochschild A. Amino acid-amino acid contacts at the cooperativity interface of the bacteriophage λ and P22 repressors. Genes Dev. 1998;12:2791–2802. doi: 10.1101/gad.12.17.2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35**.Stayrook S, Jaru-Ampornpan P, Ni J, Hochschild A, Lewis M. Crystal structure of theλ repressor and a model for pairwise cooperative operator binding. Nature. 2008;452:1022–1025. doi: 10.1038/nature06831. The crystal structure of a DNA-bound λCI dimer, the first structure of full-length λCI, reveals an unexpected asymmetry, with one subunit in an extended conformation and the other in a compact conformation. This asymmetry provides an explanation for the rules governing higher-order complex formation by DNA-bound λCI dimers. [DOI] [PubMed] [Google Scholar]
- 36.Bandyopadhyay S, Deb S, Bose S, Roy S. Half-of-the-sites reactivity of F235Cλ repressor: implications for the structure of the whole repressor. Protein Engineering. 2002;15:393–401. doi: 10.1093/protein/15.5.393. [DOI] [PubMed] [Google Scholar]
- 37.Hochschild A, Ptashne M. Cooperative binding of λ repressors to sites separated by integral turns of the DNA helix. Cell. 1986;44:681–687. doi: 10.1016/0092-8674(86)90833-0. [DOI] [PubMed] [Google Scholar]
- 38.Zhou Y, Busby S, Ebright RH. Identification of the functional subunit of a dimeric transcription activator protein by use of oriented heterodimers. Cell. 1993;73:375–379. doi: 10.1016/0092-8674(93)90236-j. [DOI] [PubMed] [Google Scholar]