Abstract
Objective
To characterize the relationship between advanced glycation end products (AGEs) and circulating receptors for AGEs (RAGE) with cardiovascular disease mortality.
Methods
The relationships between serum AGEs, total RAGE (sRAGE), and endogenous secretory RAGE (esRAGE), and mortality were characterized in 559 community-dwelling women, ≥65 years, in Baltimore, Maryland.
Results
During 4.5 years of follow-up, 123 (22%) women died, of whom 54 died with cardiovascular disease. The measure of serum AGEs was carboxymethyl-lysine (CML), a dominant AGE. Serum CML predicted cardiovascular disease mortality (Hazards Ratio [H.R.] for highest versus lower three quartiles 1.94, 95% Confidence Interval [C.I.] 1.08-3.48, P = 0.026), after adjusting for age, race, body mass index, and renal insufficiency. Serum sRAGE (ng/mL) and esRAGE (ng/mL) predicted cardiovascular disease mortality (H.R. per 1 Standard Deviation [S.D.] 1.27, 95% C.I. 0.98-1.65, P = 0.07; H.R. 1.28, 95% C.I. 1.02-1.63, P = 0.03), after adjusting for the same covariates. Among non-diabetic women, serum CML, sRAGE, and esRAGE, respectively, predicted cardiovascular disease mortality (H.R. for highest versus lower three quartiles, 2.29, 95% C.I. 1.21-4.34, P = 0.01; H.R. per 1 S.D., 1.24, 95% C.I. 0.92-1.65, P = 0.16; H.R. per 1 S.D. 1.45, 95% C.I. 1.08-1.93, P = 0.01), after adjusting for the same covariates.
Conclusions
High circulating AGEs and RAGE predict cardiovascular disease mortality among older community-dwelling women. AGEs are a potential target for interventions, as serum AGEs can be lowered by change in dietary pattern and pharmacological treatment.
Keywords: advanced glycation end products, aging, atherosclerosis, cardiovascular disease, mortality
INTRODUCTION
Advanced glycation end products (AGEs) are a heterogeneous group of bioactive molecules formed by the non-enzymatic glycation of proteins, lipids, and nucleic acids (1,2). AGEs have been implicated in the pathogenesis of diabetes (2), atherosclerosis (1,3), and renal disease (4). AGEs form covalent cross-links with proteins such as collagen (5), enhance the synthesis of extracellular matrix components (6), increase oxidation of low-density lipoprotein (7), and upregulate the expression of adhesion molecules (8,9). AGEs accumulate within the intimal extracellular matrix of arteries and atherosclerotic lesions (10,11) and increase glomerulosclerosis (12).
AGEs upregulate inflammation through receptor for AGEs (RAGE)(13-15). Circulating isoforms of RAGE include endogenous secretory RAGE (esRAGE), a splice variant of RAGE that is secreted into blood and lacks the transmembrane and cytoplasmic portion of the receptor (16) and truncated forms of RAGE that have been cleaved from the cell surface by matrix metalloproteinases (17). Circulating RAGE can bind AGE and prevent AGE activation of cell membrane-bound RAGE (14,18). Circulating RAGE may serve as a decoy receptor to counteract the inflammatory processes triggered by RAGE ligands such as AGEs (13,19). Total circulating RAGE (sRAGE) and esRAGE have been studied in specific groups of patients with diabetes (20-22) and end-stage renal disease (23,24).
The AGE-RAGE pathway has been the focus of growing interest because of substantial improvement in measurement technology and because experiments conducted in animal models have shown that blockage of AGE-RAGE binding reduces complications of atherosclerosis and diabetes.18 In humans, treatment with AGE-breakers and dietary restriction of AGE-containing foods improved cardiovascular function (25-27). We hypothesized that elevated levels of serum AGE, sRAGE, and esRAGE were predictive of mortality, especially cardiovascular disease mortality, in older persons. To address this hypothesis, we characterized AGE, sRAGE, and esRAGE in a prospective study of older women living in the community.
METHODS
Study Population
Subjects in this study were women, aged 65 and older, who participated in the Women’s Health and Aging Study I (WHAS I), a population-based study designed to evaluate the causes and course of physical disability in older disabled women living in the community. WHAS I participants were recruited from an age-stratified random sample of women aged 65 years and older selected from Medicare enrollees residing in 12 contiguous zip code areas in Baltimore (28). Women were screened to identify self-reported physical disability that was categorized into four domains. The domains of disability were ascertained in a 20-30 minute home interview that included questions related to (1) mobility and exercise tolerance, i.e., walking for a quarter of a mile, walking up 10 steps without resting, getting in and out of bed or chairs, (2) upper extremity function, i.e., raising your arms up over your head, using your fingers to grasp or handle, lifting or carrying something as heavy as ten pounds, (3) higher functioning tasks (a subset of instrumental activities of daily living, not including heavy housework, i.e., using the telephone, doing light housework, preparing your own meals, shopping for personal items), and (4) basic self-care tasks (a subset of non-mobility dependent activities of daily living, i.e., bathing or showering, dressing, eating, using the toilet). WHAS I enrolled the one-third most disabled women ages 65 and older, those with disability in two or more domains. Of the 1409 women who met study eligibility criteria, 1002 agreed to participate in the study in 1992. There were no major differences in sociodemographic or reported health characteristics between eligible participants and those who declined to participate (28).
Data Collection
Standardized questionnaires were administered in the participant’s home by trained interviewers. Race was assessed in a questionnaire as black, white, or other, current smoking as yes or no, and education as 0-8, 9-11, 12 years or more than 12 years as the highest level of formal education achieved. Two weeks later, a trained registered full-time study nurse conducted an examination of each study participant in her home, using a standardized protocol that included physical performance measures and a standardized physical examination. Approximately 75% of women also consented to phlebotomy performed during a separate visit by a trained phlebotomist who followed a standardized protocol. Chronic diseases were adjudicated by WHAS co-investigators based on the questionnaire, physical examination, and physician contact using standardized algorithms (28). Mini-Mental Status Examination (MMSE) was administered at enrollment (29). Further details on the methods and sampling design of the WHAS studies are published elsewhere (28).
Vital status was determined through matching with the National Death Index from the 12 month follow-up visit, 1993-1996 through the end of 2000. Causes of death as coded by the International Classification of Diseases-9 were recorded (30). The Johns Hopkins University Institutional Review Board approved the study protocol, and written informed consent was obtained from all participants.
Laboratory Studies
There were 1002 women enrolled in the Women’s Health and Aging Study I, of whom 746 women participated in the baseline blood drawing. Eight hundred seventy-nine women participated in the 12-month follow-up visit, of whom 580 received a blood draw. Analyses of serum AGEs, sRAGE, and esRAGE were done at the 12-month follow-up visit rather than at enrollment because of a greater availability of serum aliquots in the sample repository from this visit. The 559 women involved in the present study were significantly younger, and a higher proportion had MMSE score <24, level of education <12 years, stroke, and stroke compared with the 320 women who are not included in the present analysis. Non-fasting blood samples were obtained by venipuncture between 9 AM and 2 PM. Processing, aliquoting, and freezing were carried out at the Core Genetics Laboratory of The Johns Hopkins University School of Medicine following a standardized protocol. Blood samples were stored continuously at −70° C until the time of analyses of serum AGEs, sRAGE, and esRAGE.
The measure of serum AGEs in this study was serum carboxymethyl-lysine (CML). CML is a dominant circulating AGE, the best characterized of all the AGEs, and a dominant AGE in tissue proteins (31). CML was measured using a competitive ELISA (AGE-CML ELISA, Microcoat, Penzberg, Germany) (32). This assay has been validated (33), is specific, and shows no cross-reactivity with other compounds (32).
Total sRAGE was measured using a sandwich ELISA (Quantikine Human RAGE Immunoassay, R & D Systems, Minneapolis, MN). This assay measures C-truncated RAGE that has been enzymatically cleaved from the cell surface as well as esRAGE. Serum esRAGE was measured using ELISA (B-Bridge International, Mountain View, CA) (22). Measurements were all performed in duplicate according the protocol of the manufacturers, and the results were averaged. The within assay and between assay coefficients of variation (CVs) for serum AGE, sRAGE, and esRAGE were 3% and 4%, 3% and 7%, and 6% and 8%, respectively.
Data Analysis
Continuous variables were compared using Wilcoxon rank-sum test. Categorical variables were compared using chi-square tests. Body mass index was categorized as underweight (<18.5 kg/m2), normal range (18.5-24.9 kg/m2), overweight (≥25-29.9 kg/m2) and obese (≥30 kg/m2) (34). A Mini-Mental Status Examination (MMSE) score of <24 was defined as cognitive impairment (29). Renal insufficiency was defined as estimated glomerular filtration rate of <60 mL/min/1.73 m2 using the Modification of Diet in Renal Disease equation of Levey and colleagues (35). We defined cardiovascular disease mortality by the death codes 390–459 from the 9th version of the International Classification of Diseases (ICD) (30). Cox proportional hazards models were used to examine the relationship between serum CML, sRAGE, and esRAGE, and 4.5 year all-cause and cardiovascular disease mortality. Variables that were significant in the univariate analyses were entered into the multivariate Cox proportional hazards models, except in the situation where the variables were known to be in the causal pathway, i.e., congestive heart failure and peripheral artery disease. Survival curves were compared using log-rank test. The statistical program used was SAS (SAS Institute, Cary, NC), with data analysis conducted by Kai Sun. The level of significance used in this study was P <0.05.
RESULTS
Characteristics of Study Subjects
During 4.5 years of follow-up, 123 of 559, or 22%, of women died. The main causes of death were cardiovascular disease (43.9%), cancer (17.9%), chronic obstructive pulmonary disease (5.7%), pneumonia (4.9%), urinary tract infection (3.3%), diabetes mellitus (1.6%), renal disease (1.6 %), sepsis (1.6%), and other (20.3%).
Demographic and other characteristics of women who died from all causes or survived are shown in Table 1. Median serum CML and serum esRAGE concentrations were significantly higher in women who died from all causes compared to women who survived. Serum sRAGE concentrations were higher in women who died from all causes compared to women who survived (P = 0.09). Women who died from all causes were older, had lower body mass index, and were more likely to have cognitive impairment, congestive heart failure, peripheral artery disease, depression, and renal insufficiency. There were no significant differences between women who survived or died from all causes by race, education <12 years, current smoking, triglycerides, total cholesterol, HDL cholesterol, LDL cholesterol, and prevalence of hypertension, coronary heart disease, stroke, diabetes, chronic obstructive pulmonary disease, or cancer.
Table 1.
Demographic and Health Characteristics of Women, Aged ≥65 Years, in the Women’s Health and Aging Study I in Baltimore, Maryland Who Survived or Died from All Causes during Follow-Up (n = 559)
| Characteristic1 | Lived N = 436 | Died N = 123 | P | |||
|---|---|---|---|---|---|---|
| N | % or Median (25th, 75th percentile) | N | % or Median (25th, 75th percentile) | |||
| Age, years | 65-69 | 104 | 86.7 | 16 | 13.3 | <0.0001 |
| 70-74 | 114 | 86.4 | 18 | 13.6 | ||
| 75-79 | 90 | 80.4 | 22 | 19.6 | ||
| 80-84 | 45 | 70.3 | 19 | 29.7 | ||
| 85-89 | 67 | 66.3 | 34 | 33.7 | ||
| ≥90 | 16 | 53.3 | 14 | 46.7 | ||
| Race | white | 306 | 76.9 | 92 | 23.1 | 0.32 |
| other | 130 | 80.7 | 31 | 19.3 | ||
| Education <12 years | 275 | 79.3 | 72 | 20.8 | 0.39 | |
| Current smoker | 42 | 70.0 | 18 | 30.0 | 0.11 | |
| Body mass index (kg/m2) | <18.5 | 7 | 43.8 | 9 | 56.2 | 0.0006 |
| 18.5-24.9 | 90 | 71.4 | 36 | 28.6 | ||
| 25.0-29.9 | 138 | 78.9 | 37 | 21.1 | ||
| ≥30 | 163 | 85.8 | 27 | 14.2 | ||
| Serum CML (μg/mL) | 433 | 0.54 (0.44, 0.66) | 122 | 0.59 (0.46, 0.73) | 0.017 | |
| Serum sRAGE (ng/mL) | 433 | 1.20 (0.88, 1.64) | 122 | 1.29 (0.86, 1.97) | 0.09 | |
| Serum esRAGE (ng/mL) | 436 | 0.33 (0.24, 0.45) | 123 | 0.37 (0.26, 0.51) | 0.05 | |
| Serum triglycerides (mg/dL) | 426 | 144 (99, 193) | 122 | 126 (91, 179) | 0.06 | |
| Total cholesterol (mg/dL) | 426 | 224 (200, 251) | 122 | 221 (190, 250) | 0.24 | |
| HDL cholesterol (mg/dL) | 426 | 50 (42, 60) | 121 | 51 (42, 58) | 0.95 | |
| LDL cholesterol (mg/dL) | 426 | 139 (115, 164) | 121 | 139 (113,159) | 0.46 | |
| Mini-Mental Status Exam (MMSE) score <24 | 57 | 67.1 | 28 | 32.9 | 0.008 | |
| Hypertension | 252 | 77.1 | 75 | 22.9 | 0.55 | |
| Coronary heart disease | 100 | 79.4 | 26 | 20.6 | 0.67 | |
| Congestive heart failure | 34 | 65.4 | 18 | 34.6 | 0.02 | |
| Peripheral artery disease | 69 | 61.1 | 44 | 38.9 | <0.0001 | |
| Stroke | 21 | 77.8 | 6 | 22.2 | 0.98 | |
| Diabetes mellitus | 62 | 73.8 | 22 | 26.2 | 0.32 | |
| Chronic obstructive pulmonary disease | 119 | 75.3 | 39 | 24.7 | 0.34 | |
| Depression | 58 | 69.1 | 26 | 30.9 | 0.03 | |
| Cancer | 48 | 77.4 | 14 | 22.6 | 0.91 | |
| Renal insufficiency (%) | 207 | 48.6 | 76 | 62.3 | 0.008 | |
Median (25th, 75th percentile) for continuous variables or row percentages of participants with specific characteristic as noted.
The demographic and other characteristics of women who died from cardiovascular diseases or survived are shown in Table 2. Median serum CML concentrations were significantly higher in women who died from cardiovascular disease compared to women who survived. Serum sRAGE and esRAGE concentrations were higher in women who died from cardiovascular disease compared to women who survived, a finding which did not reach statistical significance (P = 0.12, P = 0.059, respectively). Women who died from cardiovascular disease were older, less likely to be overweight and obese, and were more likely to be white and to have congestive heart failure, peripheral artery disease, and renal insufficiency. There were no significant differences between women who survived or died from all causes by education <12 years, current smoking, triglycerides, total cholesterol, HDL cholesterol, LDL cholesterol, MMSE <24, and prevalence of hypertension, coronary heart disease, stroke, diabetes, chronic obstructive pulmonary disease, depression, or cancer.
Table 2.
Demographic and Health Characteristics of Women, Aged ≥65 Years, in the Women’s Health and Aging Study I in Baltimore, Maryland Who Survived or Died from Cardiovascular Diseases during Follow-Up (n = 487)
| Characteristic1 | Lived N = 436 | Died N = 54 | P | |||
|---|---|---|---|---|---|---|
| N | % or Median (25th, 75th percentile) | N | % or Median (25th, 75th percentile) | |||
| Age, years | 65-69 | 104 | 94.6 | 6 | 5.4 | <0.0001 |
| 70-74 | 114 | 96.6 | 4 | 3.4 | ||
| 75-79 | 90 | 89.1 | 11 | 10.9 | ||
| 80-84 | 45 | 83.3 | 9 | 16.7 | ||
| 85-89 | 67 | 78.8 | 18 | 21.2 | ||
| ≥90 | 16 | 72.7 | 6 | 27.3 | ||
| Race | white | 306 | 87.2 | 45 | 12.8 | 0.04 |
| other | 130 | 93.5 | 9 | 6.5 | ||
| Education <12 years | 275 | 90.2 | 30 | 9.8 | 0.27 | |
| Current smoker | 42 | 84.0 | 8 | 16.0 | 0.23 | |
| Body mass index (kg/m2) | <18.5 | 7 | 58.3 | 5 | 41.7 | 0.003 |
| 18.5-24.9 | 90 | 86.5 | 14 | 13.5 | ||
| 25.0-29.9 | 138 | 87.9 | 19 | 12.1 | ||
| ≥30 | 163 | 92.6 | 13 | 7.4 | ||
| Serum CML (μg/mL) | 433 | 0.54 (0.44, 0.66) | 54 | 0.64 (0.47, 0.79) | 0.005 | |
| Serum sRAGE (ng/mL) | 433 | 1.20 (0.88, 1.64) | 54 | 1.30 (0.87, 2.08) | 0.12 | |
| Serum esRAGE (ng/mL) | 436 | 0.33 (0.24, 0.45) | 54 | 0.39 (0.27, 0.58) | 0.059 | |
| Serum triglycerides (mg/dL) | 426 | 144 (99, 193) | 54 | 132 (96, 189) | 0.64 | |
| Total cholesterol (mg/dL) | 426 | 224 (200, 251) | 54 | 227 (190, 252) | 0.72 | |
| HDL cholesterol (mg/dL) | 426 | 50 (42, 60) | 53 | 51 (40, 59) | 0.91 | |
| LDL cholesterol (mg/dL) | 426 | 139 (115, 164) | 53 | 139 (110, 159) | 0.43 | |
| Mini-Mental Status Exam (MMSE) score <24 | 57 | 85.1 | 10 | 14.9 | 0.27 | |
| Hypertension | 252 | 88.1 | 34 | 11.9 | 0.48 | |
| Coronary heart disease | 100 | 84.7 | 18 | 15.3 | 0.09 | |
| Congestive heart failure | 34 | 75.6 | 11 | 24.4 | 0.002 | |
| Peripheral artery disease | 69 | 75.0 | 23 | 25.0 | <0.0001 | |
| Stroke | 21 | 91.3 | 2 | 8.7 | 0.72 | |
| Diabetes mellitus | 62 | 86.1 | 10 | 13.9 | 0.40 | |
| Chronic obstructive pulmonary disease | 119 | 89.5 | 14 | 10.5 | 0.83 | |
| Depression | 58 | 86.6 | 9 | 13.4 | 0.49 | |
| Cancer | 48 | 77.4 | 14 | 22.6 | 0.91 | |
| Renal insufficiency (%) | 207 | 84.8 | 37 | 15.2 | 0.006 | |
Median (25th, 75th percentile) for continuous variables or row percentages of participants with specific characteristic as noted.
Serum CML and Mortality
The relationship between serum CML and all-cause and cardiovascular disease mortality was examined using CML as quartiles since there was a threshold at the highest quartile. The survival curves for women in each quartile of serum CML and all-cause mortality are shown in Figure 1. Quartile cut-offs for serum CML were 0.45, 0.55, and 0.68 μg/mL. The proportion of women who died from all causes in each quartile, from lowest to highest, were 19.3%, 19.3%, 20.0%, and 29.3%, respectively. Women in the highest quartile of serum CML had lower survival than women in the lower three tertiles (P = 0.013, log-rank test). Women in the highest quartile of serum CML had an increased risk of dying from all causes compared to women in the lower three quartiles (H.R. 1.47, 95% C.I. 0.97-2.22, P = 0.066) in a multivariate Cox proportional hazards model, adjusting for age, BMI, MMSE <24, depression, and renal insufficiency (Table 3).
Figure 1.
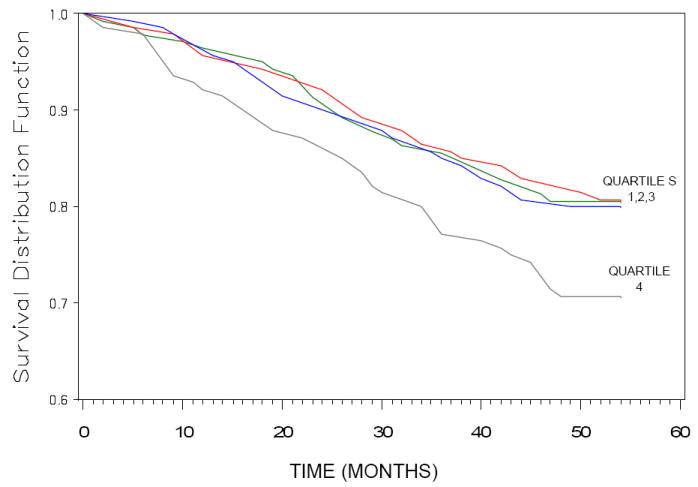
Survival curves for all-cause mortality among women, ≥65 years, in the Women’s Health and Aging Study I in Baltimore, Maryland, by quartile of serum CML. Women in the highest quartile (quartile 4) of serum CML had lower survival compared to women in the lower three tertiles together (P = 0.013, log-rank test).
Table 3.
Multivariate Cox Proportional Hazards Models of Serum CML and RAGE and All-Cause Mortality among Women ≥65 Years in the Women’s Health and Aging Study I in Baltimore, Maryland1
| Serum CML2 (μg/mL) | Model, unadjusted | Model adjusted for age | Model adjusted for age, BMI, MMSE, depression, renal insufficiency | ||||||
| H.R. | 95% C.I. | P | H.R. | 95% C.I. | P | H.R. | 95% C.I. | P | |
| 1.71 | 1.16-2.55 | 0.007 | 1.50 | 1.01-2.24 | 0.048 | 1.47 | 0.97-2.22 | 0.066 | |
| Serum sRAGE3 (ng/mL) | Model, unadjusted | Model adjusted for age | Model adjusted for age, BMI, MMSE, depression, renal insufficiency | ||||||
| H.R. | 95% C.I. | P | H.R. | 95% C.I. | P | H.R. | 95% C.I. | P | |
| 1.33 | 1.12-1.57 | 0.001 | 1.26 | 1.06-1.50 | 0.008 | 1.19 | 0.98-1.44 | 0.07 | |
| Serum esRAGE3 (ng/mL) | Model, unadjusted | Model adjusted for age | Model adjusted for age, BMI, MMSE, depression, renal insufficiency | ||||||
| H.R. | 95% C.I. | P | H.R. | 95% C.I. | P | H.R. | 95% C.I. | P | |
| 1.36 | 1.17-1.59 | <0.0001 | 1.26 | 1.07-1.49 | 0.005 | 1.20 | 1.01-1.44 | 0.047 | |
Separate Cox proportional hazards models for serum CML, sRAGE, and esRAGE for all-cause mortality as the outcome.
Hazards Ratio for CML in top quartile versus lower three quartiles.
Hazards Ratio for sRAGE and esRAGE per increase of 1 standard deviation.
The survival curves for cardiovascular disease mortality are shown for women in each quartile of serum CML in Figure 2. The proportion of women who died from cardiovascular disease in each quartile, from lowest to highest, were 8.9%, 8.2%, 8.2%, and 18.9%, respectively. Women in the highest quartile of serum CML had lower survival than women in the lower three tertiles (P = 0.0009, log-rank test). Women in the highest quartile of serum CML had an increased risk of dying from cardiovascular disease compared to women in the lower three quartiles (Hazards Ratio [H.R.] 1.94, 95% Confidence Interval [C.I.] 1.08-3.48, P = 0.026) in a multivariate Cox proportional hazards model, adjusting for age, BMI, and renal insufficiency (Table 4). There were no significant interactions between serum CML and diabetes in relation to either all-cause or cardiovascular disease mortality.
Figure 2.

Survival curves for cardiovascular disease mortality among women, ≥65 years, in the Women’s Health and Aging Study I in Baltimore, Maryland, by quartile of serum CML. Women in the highest quartile (quartile 4) of serum CML had lower survival compared to women in the lower three tertiles together (P = 0.0009, log-rank test).
Table 4.
Multivariate Cox Proportional Hazards Models of Serum CML and RAGE and Cardiovascular Disease Mortality among Women ≥65 Years in the Women’s Health and Aging Study I in Baltimore, Maryland
| Serum CML2 (μg/mL) | Model, unadjusted | Model adjusted for age, race | Model adjusted for age, race, BMI, renal insufficiency | ||||||
| H.R. | 95% C.I. | P | H.R. | 95% C.I. | P | H.R. | 95% C.I. | P | |
| 2.31 | 1.32-4.05 | 0.003 | 1.91 | 1.08-3.38 | 0.025 | 1.94 | 1.08-3.48 | 0.026 | |
| Serum sRAGE3 (ng/mL) | Model, unadjusted | Model adjusted for age, race | Model adjusted for age, race, BMI, renal insufficiency | ||||||
| H.R. | 95% C.I. | P | H.R. | 95% C.I. | P | H.R. | 95% C.I. | P | |
| 1.43 | 1.14-1.81 | 0.003 | 1.34 | 1.06-1.70 | 0.016 | 1.27 | 0.98-1.65 | 0.07 | |
| Serum esRAGE3 (ng/mL) | Model, unadjusted | Model adjusted for age, race | Model adjusted for age, race, BMI, renal insufficiency | ||||||
| H.R. | 95% C.I. | P | H.R. | 95% C.I. | P | H.R. | 95% C.I. | P | |
| 1.52 | 1.24-1.85 | <0.0001 | 1.36 | 1.10-1.68 | 0.004 | 1.28 | 1.02-1.63 | 0.03 | |
Separate Cox proportional hazards models for serum CML, sRAGE, and esRAGE for cardiovascular disease mortality as the outcome.
Hazards Ratio for CML in top quartile versus lower three quartiles.
Hazards Ratio for sRAGE and esRAGE per increase of 1 standard deviation.
Total Secretory RAGE and Mortality
The relationship between circulating RAGE (esRAGE, sRAGE) was examined as a continuous variable only, as exploratory analyses of quartiles did not show that the highest quartile or quartiles had strong relationships with mortality as found with the analysis of serum CML. Serum sRAGE (ng/mL) was predictive of all-cause mortality (H.R. per 1 SD increase, 1.19, 95% C.I. 0.98-1.44, P = 0.07) in a multivariate Cox proportion hazards model after adjusting for age, body mass index, MMSE <24, depression, and renal insufficiency (Table 3). Total sRAGE (ng/mL) predicted cardiovascular disease mortality (H.R. per 1 S.D. increase, 1.27, 95% C.I. 0.98-1.65, P = 0.07), adjusting for age, BMI, and renal insufficiency (Table 4). There were no significant interactions between serum sRAGE and diabetes in relation to either all-cause or cardiovascular disease mortality.
Serum Endogenous Secretory RAGE and Mortality
Serum esRAGE (ng/mL) was predictive of all-cause mortality (H.R. per 1 S.D. increase, 1.20, 95% C.I. 1.01-1.44, P = 0.047), adjusting for age, body mass index, MMSE <24, depression, and renal insufficiency (Table 3). Serum esRAGE (ng/mL) was predictive of cardiovascular disease mortality (H.R. per 1 S.D. increase, 1.28, 95% C.I. 1.02-1.63, P = 0.03), after adjusting for age, race, BMI, and renal insufficiency (Table 4). There were no significant interactions between serum esRAGE and diabetes in relation to either all-cause or cardiovascular disease mortality.
Serum CML and RAGE in Women with and without Diabetes
There were 84 women with diabetes and 475 women without diabetes. Median (25th, 75th percentile) serum CML among women with and women without diabetes was 0.52 (0.42, 0.67) and 0.55 (0.45, 0.68) μg/mL, respectively (P = 0.06). Median (25th, 75th percentile) serum sRAGE among women with and women without diabetes was 1.20 (0.80, 1.67) and 1.20 (0.80, 1.67) ng/mL, respectively (P = 0.58). Median (25th, 75th percentile) serum esRAGE among women with and without diabetes was 0.32 (0.23, 0.46) and 0.35 (0.25, 0.46) ng/mL, respectively (P = 0.32).
Serum CML and RAGE and Mortality in Non-Diabetic Women
In order to examine the relationship between serum CML and RAGE with mortality in non-diabetic women, we conducted analyses in which the 84 women with diabetes were excluded. Among non-diabetic women, serum CML, sRAGE, and esRAGE, respectively, predicted all-cause mortality (H.R. for highest versus lower three quartiles, 1.81, 95% C.I. 1.17-2.82, P = 0.008; H.R. per 1 S.D., 1.14, 95% C.I. 0.91-1.42, P = 0.26; H.R. per 1 S.D. 1.22, 95% C.I. 0.097-1.53, P = 0.09, after adjusting for age, body mass index, MMSE <24, depression, and renal insufficiency. Among non-diabetic women, serum CML, sRAGE, and esRAGE, respectively, predicted cardiovascular disease mortality (H.R. for highest versus lower three quartiles, 2.29, 95% C.I. 1.21-4.34, P = 0.01; H.R. per 1 S.D., 1.24, 95% C.I. 0.92-1.65, P = 0.16; H.R. per 1 S.D. 1.45, 95% C.I. 1.08-1.93, P = 0.01, after adjusting for age, race, body mass index, and renal insufficiency.
DISCUSSION
This study suggests that moderately to severely disabled older, community-dwelling women with elevated serum AGEs are at a greater risk of dying, especially from cardiovascular diseases. In addition, women with elevated circulating RAGE were at an increased risk of cardiovascular disease mortality. The two major sources of systemic AGEs are thought to be endogenous AGEs, generated by abnormal glucose metabolism, and exogenous AGEs found in foods. AGEs are especially high in Western diets where foods are processed under elevated temperatures such as by broiling, roasting, deep frying, oven frying, or grilling (36). The AGE content of the same food item can be increased 10-200 fold by increasing the temperature and conditions used in cooking (37). About 10% of dietary AGEs are absorbed, of which about one-third is excreted and two-thirds deposited in tissues (37,38). Restriction of dietary AGE intake reduces the expression of C-reactive protein and adhesion molecules and improve endothelial function (27,39). In animals, dietary restriction of AGEs increases longevity in a magnitude comparable to caloric restriction (40).
Most studies of AGEs and their circulating receptors have been limited to patients with specific diseases, mainly diabetes, atherosclerosis, and end-stage renal disease. The strengths of this study were a relatively large population-based sample of community-dwelling older women and measurements of both serum AGE and circulating RAGE. These results are consistent with previous studies showing that elevated serum AGEs predicted mortality in hemodialysis patients (41) and cardiovascular mortality in women with type 2 diabetes (3). In the present study, elevated serum AGEs predicted both all-cause and cardiovascular disease mortality in women without diabetes. The biological mechanisms by which elevated AGEs could increase the risk of dying cannot be specifically determined from this epidemiological study. However, there is potential for elevated AGEs to cause widespread damage to multiple systems, as AGEs are known to alter the structural quality of blood vessels, bone, skeletal muscle, and other tissues through cross-linking with collagen (2,5,6) and to accelerate inflammation, atherosclerosis, and renal damage through the AGE-RAGE pathway (1).
In the present study, women with elevated circulatory RAGE, both total sRAGE and esRAGE, were at an increased risk of cardiovascular death. In contrast, previous studies showed that low plasma esRAGE was a predictor of cardiovascular mortality in patients with end-stage renal disease (41). The differences in these findings may be due to the contrasting clinical characteristics of the two study populations. Whether elevated circulating RAGE concentrations are a biological response that allows circulating RAGE to bind circulating AGE and thus prevent AGE from binding with membrane-bound RAGE is not known. Circulating RAGE may be insufficient to antagonize AGE-RAGE interactions because RAGE concentrations in plasma are ~1000 times lower than circulating AGEs (43). However, the ratio of circulating RAGE to AGE may be much different at the localized sites where RAGE is upregulated than in the general circulation. Although sRAGE was a significant predictor of mortality, sRAGE consists of both es-RAGE and cleaved isoforms of RAGE. The cleaved isoforms of RAGE alone were not significantly predictive of mortality, suggesting that circulating esRAGE may be a more important biological marker for mortality than cleaved isoforms of RAGE.
In this study, elevated serum AGEs, sRAGE, and esRAGE were predictive of cardiovascular disease mortality, and appeared to be predictive of all-cause mortality at a level of marginal significance. The magnitude of the hazards ratios for mortality was greater for cardiovascular disease mortality than all-cause mortality for serum AGE, sRAGE, and esRAGE. These findings suggest that elevated AGE and its receptors may be more specifically involved in cardiovascular disease mortality. The association between serum CML and both all-cause and cardiovascular disease mortality appeared to be non-linear, with a threshold for the highest quartile. These findings suggest that there may be a critical threshold for AGEs, above which the risk of mortality increases greatly. The search for this threshold is a priority for future research, as it is essential for clinical translation of these findings.
The study involved moderately to severely disabled women living in the community, and it is not known whether elevated serum AGEs and esRAGE are predictive of mortality in less disabled women or in men. Further studies are needed to characterize the relationship between AGEs and their circulating receptors with cardiovascular disease mortality in large populations of older men and women. It will be important to determine whether serum AGEs and circulating RAGE increase the risk of atherosclerosis and are predictive of cardiovascular events such as congestive heart failure, stroke, and myocardial infarction, and cardiovascular mortality in the general population.
Acknowledgments
Grant Support: This work was supported by National Institute on Aging Grant R01 AG027012, R01 AG029148, NIH-NCRR, OPD-GCRC grant RR00722, and NIA Contract N01-AG12112, the Johns Hopkins Older Americans’ Independence Center, and the Intramural Research Program, National Institute on Aging, NIH.
References
- 1.Basta G, Schmidt AM, de Caterina R. Advanced glycation end products and vascular inflammation: implications for accelerated atherosclerosis in diabetes. Cardiovasc Res. 2004;63:582–592. doi: 10.1016/j.cardiores.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Vlassara H, Striker G. Glycotoxins in the diet promote diabetes and diabetic complications. Curr Diabetes Rep. 2007;7:235–241. doi: 10.1007/s11892-007-0037-z. [DOI] [PubMed] [Google Scholar]
- 3.Kilhovd BK, Juutilainen A, Lehto S, et al. Increased serum levels of advanced glycation endproducts predict total, cardiovascular and coronary mortality in women with type 2 diabetes: a population-based 18 year follow-up study. Diabetologia. 2002;50:1409–1417. doi: 10.1007/s00125-007-0687-z. [DOI] [PubMed] [Google Scholar]
- 4.Bohlender JM, Franke S, Stein G, Wolf G. Advanced glycation end products and the kidney. Am J Renal Physiol. 2005;289:F645–F659. doi: 10.1152/ajprenal.00398.2004. [DOI] [PubMed] [Google Scholar]
- 5.Fu MX, Wells-Knecht KJ, Blackledge JA, Lyons TJ, Thorpe SR, Baynes JW. Glycation, glycoxidation, and cross-linking of collagen by glucose. Kinetics, mechanisms, and inhibition of late stages of the Maillard reaction. Diabetes. 1994;43:676–683. doi: 10.2337/diab.43.5.676. [DOI] [PubMed] [Google Scholar]
- 6.Sims TJ, Rasmussen LM, Oxlund H, Bailey AJ. The role of glycation cross-links in diabetic vascular stiffening. Diabetologia. 1996;39:946–951. doi: 10.1007/BF00403914. [DOI] [PubMed] [Google Scholar]
- 7.Bucala R, Mitchell R, Arnold K, Innerarity T, Vlassara H, Cerami A. Identification of the major site of apolipoprotein B modification by advanced glycosylation end products blocking uptake by the low density lipoprotein receptor. J Biol Chem. 1995;270:10828–10832. doi: 10.1074/jbc.270.18.10828. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt AM, Hori O, Chen JX, et al. Advanced glycation endproducts interacting with their endothelial receptor induce expression of vascular cell adhesion molecule-1 (VCAM-1) in cultured human endothelial cells and in mice. A potential mechanism for the accelerated vasculopathy of diabetes. J Clin Invest. 1995;96:1395–1403. doi: 10.1172/JCI118175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Basta G, Lazzerini G, Massaro M, et al. Advanced glycation end products activate endothelium through signal-transduction receptor RAGE: a mechanism for amplification of inflammatory responses. Circulation. 2002;105:816–822. doi: 10.1161/hc0702.104183. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura Y, Horii Y, Nishino T, et al. Immunohistochemical localization of advanced glycosylation endproducts in coronary atheroma and cardiac tissue in diabetes mellitus. Am J Pathol. 1993;143:1649–1656. [PMC free article] [PubMed] [Google Scholar]
- 11.Kume S, Takeya M, Mori T, et al. Immunohistochemical and ultrastructural detection of advanced glycation endproducts in atherosclerotic lesions of human aorta with a novel specific monoclonal antibody. Am J Pathol. 1995;147:654–667. [PMC free article] [PubMed] [Google Scholar]
- 12.Vlassara H, Striker LJ, Teichberg S, Fuh H, Li YM, Steffes M. Advanced glycation end products induce glomerular sclerosis and albuminuria in normal rats. Proc Natl Acad Sci USA. 1994;91:11704–11708. doi: 10.1073/pnas.91.24.11704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Basta G. Receptor for advanced glycation endproducts and atherosclerosis: from basic mechanisms to clinical implications. Atherosclerosis. 2007 doi: 10.1016/j.atherosclerosis.2007.07.025. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt AM, Vianna M, Gerlach M, et al. Isolation and characterization of two binding proteins for advanced glycosylation end products from bovine lung which are present on the endothelial cell surface. J Biol Chem. 1992;267:14987–14997. [PubMed] [Google Scholar]
- 15.Neeper M, Schmidt AM, Brett J, et al. Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J Biol Chem. 1992;267:14998–15004. [PubMed] [Google Scholar]
- 16.Yonekura H, Yamamoto Y, Sakurai S, et al. Novel splice variants of the receptor for advanced glycation end-products expressed in human vascular endothelial cells and pericytes, and their putative roles in diabetes-induced vascular injury. Biochem J. 2003;370:1097–1109. doi: 10.1042/BJ20021371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hudson BI, Harja E, Moser B, Schmidt AM. Soluble levels of receptor for advanced glycation endproducts (sRAGE) and coronary artery disease: the next C-reactive protein? Arterioscler Thromb Vasc Biol. 2005;24:879–882. doi: 10.1161/01.ATV.0000164804.05324.8b. [DOI] [PubMed] [Google Scholar]
- 18.Wautier JL, Zoukourian C, Chappey O, et al. Receptor-mediated endothelial cell dysfunction in diabetic vasculopathy: soluble receptor for advanced glycation end products blocks hyperpermeability in diabetic rats. J Clin Invest. 1996;97:238–243. doi: 10.1172/JCI118397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geroldi D, Falcone C, Emanuele E. Soluble receptor for advanced glycation end products: from disease marker to potential therapeutic target. Curr Med Chem. 2006;13:1971–1978. doi: 10.2174/092986706777585013. [DOI] [PubMed] [Google Scholar]
- 20.Challier M, Jacqueminet S, Benabdesselam O, Grimaldi A, Beaudeux JL. Increased serum concentrations of soluble receptor for advanced glycation endproducts in patients with type 2 diabetes. Clin Chem. 2005;51:1749–1750. doi: 10.1373/clinchem.2005.051961. [DOI] [PubMed] [Google Scholar]
- 21.Tan KCB, Shiu SWM, Chow WS, Leng L, Bucala R, Betteridge DJ. Association between serum levels of soluble receptor for advanced glycation end products and circulating advanced glycation end products in type 2 diabetes. Diabetologia. 2006;49:2756–2762. doi: 10.1007/s00125-006-0394-1. [DOI] [PubMed] [Google Scholar]
- 22.Sakurai S, Yamamoto Y, Tamei H, et al. Development of an ELISA for esRAGE and its application to type 1 diabetic patients. Diabetes Res Clin Pract. 2006;73:158–165. doi: 10.1016/j.diabres.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 23.Kalousová M, Hodková M, Kazderová M, et al. Soluble receptor for advanced glycation end products in patients with decreased renal function. Am J Kidney Dis. 2006;47:406–411. doi: 10.1053/j.ajkd.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 24.Koyama H, Shoji T, Fukumoto S, et al. Low circulating endogenous secretory receptor for AGEs predicts cardiovascular mortality in patients with end-stage renal disease. Arterioscler Thromb Vasc Biol. 2007;27:147–153. doi: 10.1161/01.ATV.0000251502.88818.4b. [DOI] [PubMed] [Google Scholar]
- 25.Kass DA, Shapiro EP, Kawaguchi M, et al. Improved arterial compliance by a novel advanced glycation end-product crosslink breaker. Circulation. 2001;104:1464–1470. doi: 10.1161/hc3801.097806. [DOI] [PubMed] [Google Scholar]
- 26.Little WC, Zile MR, Kitzman DW, Hundley WG, O’Brien TX, deGroof RC. The effect of alagebrium chloride (ALT-711), a novel glucose cross-link breaker, in the treatment of elderly patients with diastolic heart failure. J Card Fail. 2005;11:191–195. doi: 10.1016/j.cardfail.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 27.Negrean M, Stirban A, Stratmann B, et al. Effects of low-and high-advanced glycation endproduct meals on macro-and microvascular endothelial function and oxidative stress in patients with type 2 diabetes mellitus. Am J Clin Nutr. 2007;85:1236–1243. doi: 10.1093/ajcn/85.5.1236. [DOI] [PubMed] [Google Scholar]
- 28.Guralnik JM, Fried LP, Simonsick EM, Kasper D, Lafferty ME. The Women’s Health and Aging Study: Health and Social Characteristics of Older Women with Disability. Bethesda, MD: National Institute on Aging; 1995. NIH Publication No. 95-4009. [Google Scholar]
- 29.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 30.International Classification of Diseases. Ninth revision, clinical modification. Washington, D.C: U. S. Health and Human Services, Centers for Disease Control and Prevention, Centers for Medicare and Medicaid Services; 2006. [Google Scholar]
- 31.Reddy S, Bichler J, Wells-Knecht KJ, Thorpe SR, Baynes JW. N epsilon-(carboxymethyl)lysine is a dominant advanced glycation end product (AGE) antigen in tissue proteins. Biochemistry. 1995;34:10872–10878. doi: 10.1021/bi00034a021. [DOI] [PubMed] [Google Scholar]
- 32.Boehm BO, Schilling S, Rosinger S, et al. Elevated serum levels of Nε-carboxymethyllysine, an advanced glycation end product, are associated with proliferative diabetic retinopathy and macular oedema. Diabetologia. 2004;47:1376–1379. doi: 10.1007/s00125-004-1455-y. [DOI] [PubMed] [Google Scholar]
- 33.Zhang X, Frischmann M, Kientsch-Engel R, et al. Two immunochemical assays to measure advanced glycation end-products in serum from dialysis patients. Clin Chem Lab Med. 2005;43:503–511. doi: 10.1515/CCLM.2005.089. [DOI] [PubMed] [Google Scholar]
- 34.James PT, Leach R, Kalamara E, Shayeghi M. The worldwide obesity epidemic. Obes Res. 2001;9(suppl 4):228S–233S. doi: 10.1038/oby.2001.123. [DOI] [PubMed] [Google Scholar]
- 35.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 36.Goldberg T, Cai W, Peppa M, et al. Advanced glycoxidation end products in commonly consumed foods. J Am Diet Assoc. 2004;104:1287–1291. doi: 10.1016/j.jada.2004.05.214. [DOI] [PubMed] [Google Scholar]
- 37.Koschinsky T, He CJ, Mitsuhashi T, et al. Orally absorbed reactive glycation products (glycotoxins): an environmental risk factor in diabetic nephropathy. Proc Natl Acad Sci USA. 1997;94:6474–6479. doi: 10.1073/pnas.94.12.6474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vlassara H, Palace MR. Diabetes and advanced glycation endproducts. J Intern Med. 2002;251:87–101. doi: 10.1046/j.1365-2796.2002.00932.x. [DOI] [PubMed] [Google Scholar]
- 39.Vlassara H, Cai W, Crandall J, et al. Inflammatory mediators are induced by dietary glycotoxins, a major risk factor for diabetic angiopathy. Proc Natl Acad Sci USA. 2002;99:15596–15601. doi: 10.1073/pnas.242407999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cai W, He JC, Zhu L, et al. Reduced oxidant stress and extended lifespan in mice exposed to a low glycotoxin diet: association with increased AGER1 expression. Am J Pathol. 2007;170:1893–1902. doi: 10.2353/ajpath.2007.061281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wagner Z, Molnár M, Molnár GA, et al. Serum carboxymethyllysine predicts mortality in hemodialysis patients. Am J Kidney Dis. 2006;47:294–300. doi: 10.1053/j.ajkd.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 42.Koyama Y, Takeishi Y, Arimoto T, et al. High serum level of pentosidine, an advanced glycation end product (AGE), is a risk factor of patients with heart failure. J Cardiac Fail. 2007;13:199–206. doi: 10.1016/j.cardfail.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 43.Humpert PM, Djuric Z, Kopf S, et al. Soluble RAGE but not endogenous secretory RAGE is associated with albuminuria in patients with type 2 diabetes. Cardiovasc Diabetol. 2007;6:9. doi: 10.1186/1475-2840-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


