Abstract
Nephronophthisis (NPHP) is the most frequent genetic cause of end-stage kidney disease in children and young adults. Inv mice are a model for human nephronophthisis type 2 (NPHP2) and characterized by multiple renal cysts and situs inversus. Renal epithelial cells in inv cystic kidneys show increased cell proliferation. We studied the ERK pathway to understand the mechanisms that induce cell proliferation and renal cyst progression in inv kidneys. We studied the effects of ERK suppression by administering PD184352, an oral mitogen-activated protein kinase kinase (MEK) inhibitor on renal cyst expansion, extracellular signal-regulated protein kinase (ERK) activity, bromo-deoxyuridine (BrdU) incorporation and expression of cell-cycle regulators in invΔC kidneys. Phosphorylated ERK (p-ERK) level increased along with renal cyst enlargement. Cell-cycle regulators showed a high level of expression in invΔC kidneys. PD184352 successfully decreased p-ERK level and inhibited renal cyst enlargement. The inhibitor also decreased expression of cell-cycle regulators and BrdU incorporation in renal epithelial cells. The present results showed that ERK regulated renal cell proliferation and cyst expansion in inv mutants.
Keywords: ERK, inv, renal cyst, cell proliferation, MEK inhibitor
I. Introduction
Nephronophthisis (NPHP), the most frequent genetic cause of end-stage kidney disease in children and young adults, is characterized by a variable number of renal cysts associated with cortical tubular atrophy and interstitial fibrosis [5]. Nine genes responsible for NPHP have been identified [5]. The infantile form of NPHP progresses to terminal renal failure before the age of 5 years [4], and is found to be caused by a mutation in the Invs/NPHP2 gene [10]. The mouse homolog of the NPHP2 gene is inv that encodes 1062 amino acids with ankyrin repeats in N-terminus and two nuclear localization signals and IQ domains in C-terminal half [6]. Although the inv protein contains nuclear localization signals, the protein is found to localize in the cilia of renal epithelial cells [14, 15, 23]. The inv gene was first discovered as the gene responsible for mouse inv mutant [6, 25]. Human NPHP2 was then identified as mouse inv homolog [10].
The inv mouse shows a reversal of left-right asymmetry, jaundice and multiple renal cysts [25]. Kidneys of inv mutant exhibit dilatation of all the segments of nephrons resembling infantile NPHP2 [12]. Most inv/inv mutant mice die before 7 days of age, probably because of cardiovascular malformations caused by situs abnormalities. The invΔC mouse was created by introduction of an inv gene that lacked a C-terminus fused with GFP into an inv mouse [23]. The lacking C-terminus region contains an important sequence that regulates Inv localization in the cilia [15]. InvΔC mice (inv/inv carrying two invΔC transgenic alleles) develop renal cysts, but do not show any situs abnormality and jaundice, and can survive beyond 6 weeks of age. Furthermore, renal cysts grow slowly in this model, which makes invΔC mice suitable for study of the mechanism of renal cyst progression [23].
Augmented cell proliferation is a common feature in renal cystic diseases including human polycystic kidney diseases (PKDs) and NPHPs as well as mouse mutants with polycystic kidneys. A recent study has shown that roscovitine, a cyclin-dependent kinase (CDK) inhibitor, effectively inhibits renal cyst progression in jck and cpk mice [2]. Thus, cell proliferation is a critical component for renal cyst progression. Kidneys of inv as well as invΔC mice also are shown to increase cell proliferation [19]. Although the exact mechanisms that induce cell proliferation are unknown in renal cystic diseases, the abnormality of several intracellular signaling pathways has been reported [1, 7, 8, 17, 18, 22]. In inv mutants, lack of inv protein is proposed, but has not been shown to induce continuous activation of the canonical wnt pathway, which is assumed to lead to cyst formation [18]. In other renal cystic mutants, including bpk, pcy mice and Ham: SPRD rats, as well as renal epithelial cells from human autosomal dominant polycystic kidney disease (ADPKD) patients, activation of the ERK pathway has been reported [7, 9, 22]. Omori et al. have shown that suppression of ERK activity by administration of an oral MEK inhibitor, PD184352, inhibits renal cyst enlargement in pcy mice [9]. However, Shibasaki et al. have reported that activation of the ERK pathway did not correlate with cell proliferation, although the pathway is activated in renal cysts of conditionally Pkd1 knockout mice [16]. Thus, the relationship between renal cell proliferation and cyst development by the ERK activation is still controversial.
In the present study, we studied if the ERK pathway affects cell proliferation and renal cyst development in inv mutants. We examined the ERK activation in invΔC cystic kidneys, then treated invΔC mice with an oral MEK inhibitor, PD184352 [9, 13, 20]. We examined the effect of ERK inhibition on renal cyst expansion, and cell proliferation by examination of bromo-deoxyuridine (BrdU) incorporation, Rb phosphorylation and c-myc expression in invΔC kidneys.
II. Materials and Methods
Animals
Mice were maintained in the animal facility of Kyoto Prefectural University of Medicine, Japan, as described previously [19, 23]. Studies with these mice were performed in accordance with the Guide for the Care and Use of Laboratory Animals of National Institutes of Health and approved by the Committee for Animal Research, Kyoto Prefectural University of Medicine.
Histological and immunohistochemical analysis, and cell proliferation assay
For histological evaluation, mouse kidneys were fixed in 4% paraformaldehyde (PFA) in PBS and embedded in paraffin wax. Sections (4 µm thick) were stained with hematoxylin and eosin (H&E) according to standard protocols. Immunohistochemical staining for p-ERK was performed on paraffin-embedded sections. Anti-p-ERK antibody (#4376, Cell Signaling Technology) was used. Cell proliferation was assayed by BrdU (Sigma) incorporation. BrdU incorporation was detected with the specific antibody provided in the kit (Amersham), and was visualized using 3,3'-diaminobenzidine tetrahydrochloride (DAB).
Administration of PD184352
Mice were weaned at 3 weeks of age. Male invΔC mice (n=45) and normal littermate controls (+/+ or inv/+) (n=35) were used for experiments. Sixteen invΔC and four control mice were given PD184352 [13, 20] for 3 weeks, starting from 3 weeks of age. PD184352 is an oral MEK inhibitor that was given at 300 mg/kg/day and mixed in ground food. At 6 weeks of age, animals were killed, and kidneys were subjected to immunoblot and real-time RT-PCR analysis.
Biochemical analysis
Blood samples were obtained by cardiac puncture. Blood urea nitrogen (BUN) and serum creatinine concentrations were determined by Mitsubishi Kagaku Bio-Clinical Laboratories.
Immunoblot analysis
Kidneys were homogenized with a Polytron in ice-cold Tris lysis buffer (20 mM Tris-HCl, pH 8.0, 150 mM NaCl, 100 mM NaF, 1 mM EDTA, 1 mM Na3VO4, and mammalian cell protease inhibitor mixture (1:100 v/v, P8349; Sigma), and sonicated with an Astrason ultrasonic processor for 10 s on ice. Protein concentration of the homogenates was determined using Bradford reagent (B6916; Sigma). SDS-PAGE and immunoblotting were carried out by standard procedures. Samples (10 µg) were electrophoresed by 10 or 15% SDS-PAGE and transferred onto polyvinylidene difluoride membranes (Millipore). Membranes were blocked with 2% non-fat dry milk (Difco), and were incubated with the following antibodies: anti-ERK (#9102), anti-p-ERK (#9101), anti-phospho-retinoblastoma protein (p-Rb) (#9301) (Cell Signaling Technology), and anti-actin (sc-1616; Santa Cruz Biotechnology). Ras activation was assayed by the EZ-Detect Ras activation kit (89855; Pierce Biotechnology). The signals were visualized with the ECL-plus-detection system (GE Healthcare Bio-sciences KK). Quantitation of the chemiluminescent signals was performed with a digital imaging system (VersaDoc; Bio-Rad).
Real-time RT-PCR analysis
Real-time RT-PCR was carried out on an Applied Biosystems 7300 real-time PCR system. Glyceraldehyde 3-phosphate dehydrogenase (gapdh) was used as the control gene for normalization. Each PCR amplification was performed in a 25 µl reaction mixture containing 200 ng cDNA and 2.5 ng/ml of each primer (Table 1), using the Power SYBR® Green PCR Master Mix (Applied Biosystems). Five control and experimental cystic kidneys were examined, and data were collected from three independent experiments.
Table 1.
Real-time RT-PCR primer list
| Primer | Sequences |
|---|---|
| gapdh | F: 5'-CAATGTGTCCGTCGTGGATCT |
| R: 5'-TTGAAGTCGCAGGAGACAACC | |
| c-fos | F: 5'-GCAGCTATCTCCTGAAGAGGAA |
| R: 5'-TGGCAATCTCAGTCTGCAAC | |
| c-myc | F: 5'-TGAGCCCCTAGTGCTGCATGA |
| R: 5'-GGGGTTTGCCTCTTCTCCACAGA | |
| VPV2R | F: 5'-AGCGTGGGATCCAGAAGCTC |
| R: 5'-CAGCAAAGCAGGCTACGCAA | |
| pkd1 | F: 5'-GCCCAGAGCTCTCACAATCCT |
| R: 5'-GCGCTCATTAAGCACTGTGTAAGT | |
| pkd2 | F: 5'-GCCAAGCTGAAGAGACGAGA |
| R: 5'-AGCTGCATCATCCGATTCCC |
Statistical analysis
Data are expressed as mean±S.E. Comparison of the data between the two groups was performed using an unpaired t-test as appropriate. Multiple group comparison of the data was performed using one-way ANOVA. p<0.05 was considered statistically significant. Statistical analysis was performed using Excel 2004 (Microsoft) and Statcel2 plug-in software (OMS Publishing).
III. Results
Renal cyst development and ERK activation in invΔC mice
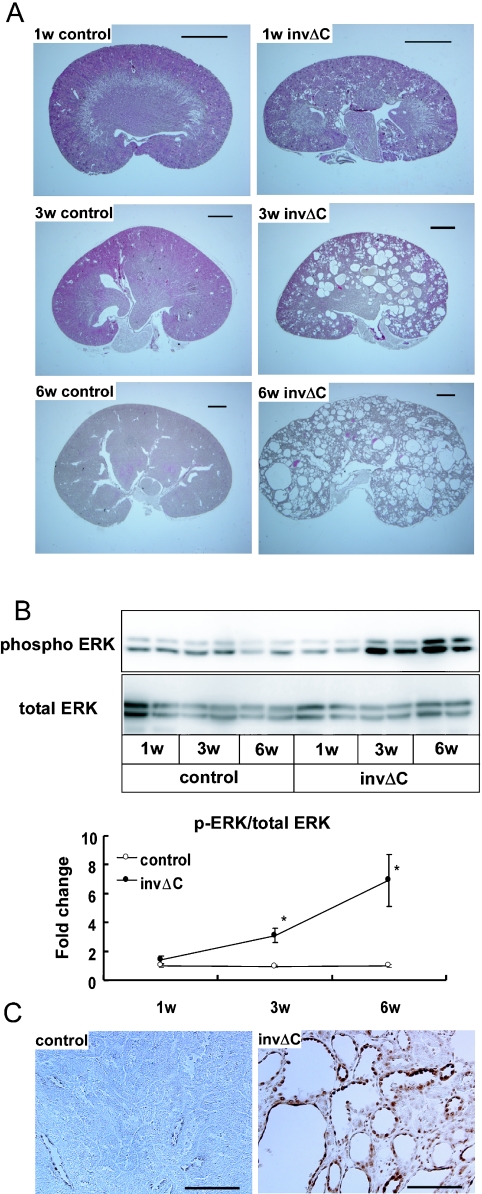
The body weight of invΔC mice was not significantly different from that of controls at 1 and 3 weeks after birth, but became significantly lower than that of controls at 6 weeks of age (p<0.05) (Table 2). The kidney weight of invΔC mice was significantly greater than that of controls (p<0.01) at 3 weeks of age, and three times larger than that of controls at 6 week of age (p<0.01) (Table 2). Although no significant difference was detected in either kidney or body weight between control and invΔC mice at 1 week of age, kidney weight to body weight ratio of invΔC mice was greater than that of control even at 1 week of age (Table 2). Histological section showed that small renal cysts were detected as early as 1 week of age in some of the invΔC kidneys (Fig. 1A). At 3 weeks of age, renal cysts were observed in all examined invΔC mice, and at 6 weeks of age the whole kidney was occupied with cysts in invΔC mice (Fig. 1A).
Table 2.
Body weight, kidney weight and kidney weight/body weight of control and invΔC mice
| Age (after birth) | 1w | 3w | 6w | |
|---|---|---|---|---|
| Body weight (g) | Control | 5.5±0.2 | 13.5±0.6 | 24.1±0.5 |
| invΔC | 5.4±0.3 | 12.6±0.4 | 19.5±0.9* | |
| Kidney weight (mg) | Control | 27.7±0.8 | 82.8±2.8 | 185.7±4.1 |
| invΔC | 32.8±1.9 | 179.7±32.8** | 560.4±33.1** | |
| Kidney weight/Body weight (%) | Control | 1.0±0.01 | 1.2±0.01 | 1.6±0.04 |
| invΔC | 1.22±0.04* | 2.9±0.59* | 5.9±0.41** | |
Examined mice are control mice (1w (n=15), 3w (n=10), and 6w (n=14)) and invΔC mice (1w (n=7), 3w (n=9) and 6w (n=10)).
* p<0.05 compared to the same age control.
** p<0.01 compared to the same age control.
Fig. 1.
Renal cyst development and ERK activation in invΔC mice. (A) H&E-stained kidneys of 1-, 3- and 6-week-old control and invΔC mice. Bars=1 mm. (B) The upper panel shows immunoblot analysis of p-ERK and total ERK proteins in 1-, 3- and 6-week-old control and invΔC mice. The lower panel shows densitometric quantitation of three independent immunoblots. Results are expressed as a ratio of the density of p-ERK to total ERK. The obtained values were further compared to the mean value from 1-week-old control mice. p-ERK level in invΔC mice started to increase at 3-week-old compared to controls of the same age (* p<0.05 compared to controls of the same age). (C) Immunohistochemical staining of p-ERK in 6-week-old invΔC kidneys (right panel) and controls (left panel). Bars=100 µm.
There was no difference in the total amount of ERK protein between control and invΔC kidneys at all ages (Fig. 1B). p-ERK level of invΔC kidneys was significantly higher in 3- and 6-week-old mice compared with that in controls (Fig. 1B). Intense p-ERK signals were detected by immunohistochemical staining of renal tissue at the epithelial cells of renal cysts in 6-week-old invΔC kidneys (Fig. 1C).
PD184352 prevented renal cyst progression, and reduced ERK phosphorylation
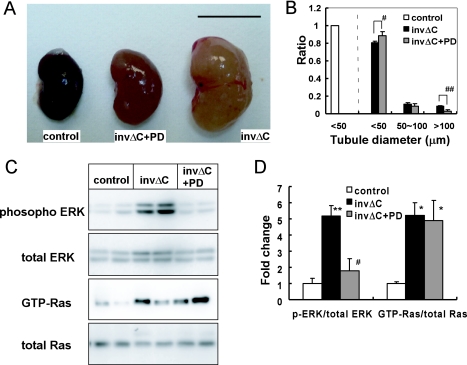
PD184352 administration reduced kidney size in invΔC mice (Fig. 2A and Table 3). The kidney weight of PD184352-treated invΔC mice was significantly lower than that of the untreated group (p<0.01). Kidney weight to body weight ratio of PD treated invΔC group was significantly lower than that of the untreated group (4.5±0.44% vs. 6.2±0.28%) (Table 3). The number of tubules >100 µm in diameter decreased and the number of tubules <50 µm in diameter increased in PD184352-treated compared to non-treated invΔC kidneys (Fig. 2B). Creatinine and blood urea nitrogen (BUN) concentration of the PD184352-treated invΔC group were significantly lower than those of the untreated group (p<0.05) (Table 3). Thus, PD184352 ameliorated both tubule structure and function of invΔC mice.
Fig. 2.
Effects of MEK inhibitor (PD184352) on renal cysts in invΔC mice. (A) Gross appearance of representative kidneys from control (left), PD-treated invΔC (middle) and untreated invΔC (right) mice. Bars=1 cm. (B) Tubule diameter in control, untreated invΔC and PD-treated invΔC kidneys. At least 500 tubules in each four kidneys were examined. PD administration decreased ratio of large tubules (>100 µm) (# p<0.05 compared to untreated invΔC mice), and increased ratio of small tubules (<50 µm) (## p<0.01 compared to untreated invΔC mice). (C) Immunoblot analysis of active and total components of Ras and ERK from control, untreated invΔC and PD-treated invΔC kidney tissue. (D) Densitometric quantitation of three independent immunoblots. Results are expressed as a ratio of active to corresponding total ERK or Ras. The obtained values were further compared to the mean value from controls. p-ERK, but not Ras-GTP was reduced in PD-treated invΔC compared to untreated invΔC kidneys. ** p<0.01 compared to controls; * p<0.05 compared to controls; # p<0.05 compared to untreated invΔC mice.
Table 3.
Effect of PD184352 treatment on body and kidney weight and renal function of invΔC mice
| Control (n=29) | invΔC (n=29) | invΔC+PD (n=16) | |
|---|---|---|---|
| Body weight (g) | 25.0±0.33 | 19.5±0.52** | 18.5±0.84 |
| Kidney weight (mg) | 202.5±4.7 | 595.0±19.9** | 396.4±20.0 ## |
| Kidney weight/Body weight (%) | 1.62±0.03 | 6.25±0.28** | 4.55±0.44 ## |
| Creatinin (mg/dL) | 0.097±0.010 | 0.49±0.087** | 0.25±0.051 ## |
| BUN (mg/dL) | 27.5±1.95 | 115.1±19.2** | 58.3±0.79 ## |
6-week-old control (n=29), invΔC (n=29), and invΔC+PD (invΔC treated with PD184352) (n=16) were examined.
** p<0.01 compared to control.
## p<0.01 compared to invΔC.
As shown in Figures 2C and D, PD184352 treatment indeed decreased p-ERK level in invΔC kidneys when compared to that in untreated invΔC kidneys. GTP-Ras/total Ras ratio was unchanged between PD184352-treated and untreated animals.
Effect of PD184352 treatment on cell proliferation, nuclear transcription factors regulated by ERK and cell cycle regulators in invΔC renal cysts
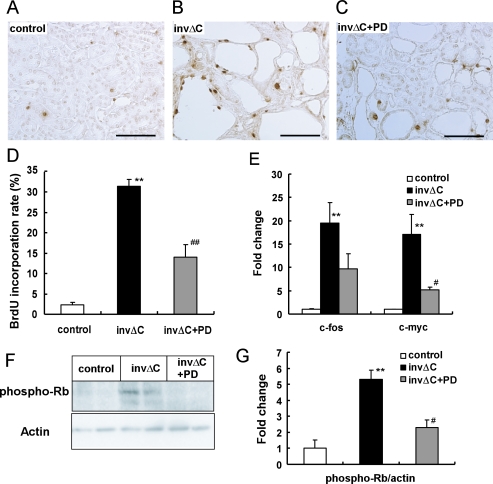
We next examined if PD184352 treatment inhibited cell proliferation. InvΔC kidneys had higher BrdU incorporation than non-cystic control kidneys (Fig. 3A–D). PD184352 treatment reduced this higher BrdU incorporation rate from 31.5% to 14.0%, p<0.01. To understand the mechanism of inhibition of cell proliferation by PD184352 treatment, we examined the expression of c-myc and c-fos, nuclear transcription factors regulated by ERK, by real-time RT-PCR analysis (Fig. 3E). Expression of these genes was increased in invΔC kidneys and reduced by PD184352 treatment. Rb phosphorylation is known to regulate G1-S transition in the cell cycle. As shown in Figures 3F and G, p-Rb level in invΔC was increased compared to that in controls. PD184352 administration reduced p-Rb level in invΔC kidneys to the control level.
Fig. 3.
Effects of PD184352 treatment on BrdU incorporation and expression of cell-cycle regulators. (A–C) BrdU incorporation in kidneys from control (A), untreated invΔC (B) and PD-treated invΔC (C) mice. Bars=100 µm (D) Percentage of tubules containing BrdU-positive cells. BrdU incorporation was increased in invΔC kidneys compared to controls (** p<0.01). PD administration reduced BrdU incorporation in invΔC kidneys (## p<0.01). At least 500 tubules in three kidneys were examined. (E) Expression of c-fos and c-myc in control and invΔC kidneys. The obtained values were compared to the mean value from the controls. Expression of both c-fos and c-myc was higher in untreated invΔC kidneys than control kidneys (** p<0.01). PD treatment decreased expression of c-myc (# p<0.05). (F and G) Immunoblot analysis of p-Rb protein in control, untreated invΔC and PD-treated invΔC kidneys. Densitometric quantitation of three independent immunoblots. Results are expressed as a ratio of p-Rb to actin protein. The obtained values were further compared to the mean value from controls. p-Rb protein was increased in untreated invΔC kidneys compared to controls (** p<0.01). PD administration reduced p-Rb level in invΔC kidneys (# p<0.05).
IV. Discussion
To search for a mechanism and a role for increased cell proliferation observed in invΔC cystic kidneys, we examined the ERK pathway. We showed for the first time that the ERK pathway was activated in invΔC cystic kidneys. p-ERK-positive staining was observed in cyst lining cells, which confirmed that cyst lining cells caused a high level of p-ERK in invΔC cystic kidneys. The activation was low or barely detectable in early-stage invΔC cystic kidneys, but high in late-stage cystic kidneys, which indicated that ERK activation correlated with renal cyst progression.
To see if ERK activation is a mere indicator or whether it has any role in cell proliferation and cyst progression in inv mutants, we suppressed ERK activity by PD184352. PD184352 is known to inhibit exclusively ERK activation in vivo [13]. Treatment with PD184352 reduced the high p-ERK/total ERK ratio in invΔC kidneys to control levels. PD184352 treatment inhibited enlargement of invΔC kidneys. Inhibition of kidney weight gain was not due to suppression of kidney development, since PD184352 did not affect development of non-cystic kidneys (data not shown). The present study showed that kidney enlargement and cell proliferation was dependent on ERK activation.
The present results also confirmed that ERK suppression inhibited activation of cell-cycle regulators such as Rb phosphorylation and c-myc expression. p-Rb protein allows E2F to become active and to push the cell through the cell cycle progression [3]. PD184352 reduced p-Rb expression that was increased in invΔC kidneys to the control level. The c-myc gene is known to stimulate cell proliferation and c-myc transgenic mice are known to produce renal cysts [21]. PD184352 administration reduced the high level of c-myc expression in invΔC cystic kidneys, which conversely suggested that ERK activation was responsible for the high level of c-myc expression. Our results suggest that ERK activation promotes renal cyst enlargement by stimulating cell proliferation. PD184352 treatment reduced increased BrdU incorporation in cystic invΔC kidneys. Combined with the recent report that roscovitine effectively inhibits renal cyst progression [2], decreased cell proliferation by ERK suppression is likely to suppress renal cyst enlargement.
PD184352 treatment reduced BrdU incorporation to about half that in non-treated invΔC kidneys (14% versus 31.5%), but the incorporation was still high compared to non-cystic controls (14% versus 2%). Oral dosing of PD184352 in the present study could not achieve a complete reduction in ERK activation. Therefore, the role of the ERK pathway might be greater than that obtained in the present study.
What stimulates ERK phosphorylation in invΔC kidneys? Ras is known to induce ERK activation [7, 11, 24]. We observed Ras activation (increased GTP-Ras) in cystic kidneys, suggesting that Ras activation induced a high level of ERK phosphorylation. It remains to be seen how mutation or loss of inv protein results in Ras activation.
V. Acknowledgments
This research was partially supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan for Young Scientists (19790587) to N.S. We wish to thank Dr. Hiroshi Hamada of Osaka University for providing the invΔC mice.
Footnotes
Conflict of interest statement: None declared.
VI. References
- 1.Bhunia A. K., Piontek K., Boletta A., Liu L., Qian F., Xu P. N., Germino F. J., Germino G. G. Pkd1 induces p21(waf1) and regulation of the cell cycle via direct activation of the JAK-STAT signaling pathway in a process requiring PKD2. Cell. 2002;109:157–168. doi: 10.1016/s0092-8674(02)00716-x. [DOI] [PubMed] [Google Scholar]
- 2.Bukanov N. O., Smith L. A., Klinger K. W., Ledbetter S. R., Ibraghimov-Beskrovnaya O. Long-lasting arrest of murine polycystic kidney disease with CDK inhibitor roscovitine. Nature. 2006;444:949–952. doi: 10.1038/nature05348. [DOI] [PubMed] [Google Scholar]
- 3.Chambard J. C., Lefloch R., Pouyssegur J., Lenormand P. ERK implication in cell cycle regulation. Biochim. Biophys. Acta. 2007;1773:1299–1310. doi: 10.1016/j.bbamcr.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 4.Gagnadoux M. F., Bacri J. L., Broyer M., Habib R. Infantile chronic tubulo-interstitial nephritis with cortical microcysts: Variant of nephronophthisis or new disease entity? Pediatr. Nephrol. 1989;3:50–55. doi: 10.1007/BF00859626. [DOI] [PubMed] [Google Scholar]
- 5.Hildebrandt F., Attanasio M., Otto E. Nephronophthisis: Disease mechanisms of a ciliopathy. J. Am. Soc. Nephrol. 2009;20:23–35. doi: 10.1681/ASN.2008050456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mochizuki T., Saijoh Y., Tsuchiya K., Shirayoshi Y., Takai S., Taya C., Yonekawa H., Yamada K., Nihei H., Nakatsuji N., Overbeek P. A., Hamada H., Yokoyama T. Cloning of inv, a gene that controls left/right asymmetry and kidney development. Nature. 1998;395:177–181. doi: 10.1038/26006. [DOI] [PubMed] [Google Scholar]
- 7.Nagao S., Yamaguchi T., Kusaka M., Maser R. L., Takahashi H., Cowley B. D., Grantham J. J. Renal activation of extracellular signal-regulated kinase in rats with autosomal-dominant polycystic kidney disease. Kidney Int. 2003;63:427–437. doi: 10.1046/j.1523-1755.2003.00755.x. [DOI] [PubMed] [Google Scholar]
- 8.Nishio S., Hatano M., Nagata M., Horie S., Koike T., Tokuhisa T., Mochizuki T. Pkd1 regulates immortalized proliferation of renal tubular epithelial cells through p53 induction and JNK activation. J. Clin. Invest. 2005;115:910–918. doi: 10.1172/JCI22850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Omori S., Hida M., Fujita H., Takahashi H., Tanimura S., Kohno M., Awazu M. Extracellular signal-regulated kinase inhibition slows disease progression in mice with polycystic kidney disease. J. Am. Soc. Nephrol. 2006;17:1604–1614. doi: 10.1681/ASN.2004090800. [DOI] [PubMed] [Google Scholar]
- 10.Otto E. A., Schermer B., Obara T., O’Toole J. F., Hiller K. S., Mueller A. M., Ruf R. G., Hoefele J., Beekmann F., Landau D., Foreman J. W., Goodship J. A., Strachan T., Kispert A., Wolf M. T., Gagnadoux M. F., Nivet H., Antignac C., Walz G., Drummond I. A., Benzing T., Hildebrandt F. Mutations in invs encoding inversin cause nephronophthisis type 2, linking renal cystic disease to the function of primary cilia and left-right axis determination. Nat. Genet. 2003;34:413–420. doi: 10.1038/ng1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parker E., Newby L. J., Sharpe C. C., Rossetti S., Streets A. J., Harris P. C., O’Hare M. J., Ong A. C. Hyperproliferation of PKD1 cystic cells is induced by insulin-like growth factor-1 activation of the Ras/Raf signalling system. Kidney Int. 2007;72:157–165. doi: 10.1038/sj.ki.5002229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phillips C. L., Miller K. J., Filson A. J., Nurnberger J., Clendenon J. L., Cook G. W., Dunn K. W., Overbeek P. A., Gattone V. H., 2nd and Bacallao R. L. Renal cysts of inv/inv mice resemble early infantile nephronophthisis. J. Am. Soc. Nephrol. 2004;15:1744–1755. doi: 10.1097/01.asn.0000131520.07008.b3. [DOI] [PubMed] [Google Scholar]
- 13.Sebolt-Leopold J. S., Dudley D. T., Herrera R., Van Becelaere K., Wiland A., Gowan R. C., Tecle H., Barrett S. D., Bridges A., Przybranowski S., Leopold W. R., Saltiel A. R. Blockade of the MAP kinase pathway suppresses growth of colon tumors in vivo. Nat. Med. 1999;5:810–816. doi: 10.1038/10533. [DOI] [PubMed] [Google Scholar]
- 14.Shiba D., Takamatsu T., Yokoyama T. Primary cilia of inv/inv mouse renal epithelial cells sense physiological fluid flow: Bending of primary cilia and ca2+ influx. Cell Struct. Funct. 2005;30:93–100. doi: 10.1247/csf.30.93. [DOI] [PubMed] [Google Scholar]
- 15.Shiba D., Yamaoka Y., Hagiwara H., Takamatsu T., Hamada H., Yokoyama T. Localization of inv in a distinctive intraciliary compartment requires the c-terminal ninein-homolog-containing region. J. Cell Sci. 2009;122:44–54. doi: 10.1242/jcs.037408. [DOI] [PubMed] [Google Scholar]
- 16.Shibazaki S., Yu Z., Nishio S., Tian X., Thomson R. B., Mitobe M., Louvi A., Velazquez H., Ishibe S., Cantley L. G., Igarashi P., Somlo S. Cyst formation and activation of the extracellular regulated kinase pathway after kidney specific inactivation of pkd1. Hum. Mol. Genet. 2008;17:1505–1516. doi: 10.1093/hmg/ddn039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shillingford J. M., Murcia N. S., Larson C. H., Low S. H., Hedgepeth R., Brown N., Flask C. A., Novick A. C., Goldfarb D. A., Kramer-Zucker A., Walz G., Piontek K. B., Germino G. G., Weimbs T. The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc. Natl. Acad. Sci. U S A. 2006;103:5466–5471. doi: 10.1073/pnas.0509694103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simons M., Gloy J., Ganner A., Bullerkotte A., Bashkurov M., Kronig C., Schermer B., Benzing T., Cabello O. A., Jenny A., Mlodzik M., Polok B., Driever W., Obara T., Walz G. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat. Genet. 2005;37:537–543. doi: 10.1038/ng1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sugiyama N., Yokoyama T. Sustained cell proliferation of renal epithelial cells in mice with inv mutation. Genes Cells. 2006;11:1213–1224. doi: 10.1111/j.1365-2443.2006.01011.x. [DOI] [PubMed] [Google Scholar]
- 20.Tanimura S., Asato K., Fujishiro S. H., Kohno M. Specific blockade of the ERK pathway inhibits the invasiveness of tumor cells: Down-regulation of matrix metalloproteinase-3/-9/-14 and cd44. Biochem. Biophys. Res. Commun. 2003;304:801–806. doi: 10.1016/s0006-291x(03)00670-3. [DOI] [PubMed] [Google Scholar]
- 21.Trudel M., D’Agati V., Costantini F. C-myc as an inducer of polycystic kidney disease in transgenic mice. Kidney Int. 1991;39:665–671. doi: 10.1038/ki.1991.80. [DOI] [PubMed] [Google Scholar]
- 22.Veizis I. E., Cotton C. U. Abnormal EGF-dependent regulation of sodium absorption in ARPKD collecting duct cells. Am. J. Physiol. Renal. Physiol. 2005;288:F474–482. doi: 10.1152/ajprenal.00227.2004. [DOI] [PubMed] [Google Scholar]
- 23.Watanabe D., Saijoh Y., Nonaka S., Sasaki G., Ikawa Y., Yokoyama T., Hamada H. The left-right determinant inversin is a component of node monocilia and other 9+0 cilia. Development. 2003;130:1725–1734. doi: 10.1242/dev.00407. [DOI] [PubMed] [Google Scholar]
- 24.Yamaguchi T., Nagao S., Wallace D. P., Belibi F. A., Cowley B. D., Pelling J. C., Grantham J. J. Cyclic AMP activates B-Raf and ERK in cyst epithelial cells from autosomal-dominant polycystic kidneys. Kidney Int. 2003;63:1983–1994. doi: 10.1046/j.1523-1755.2003.00023.x. [DOI] [PubMed] [Google Scholar]
- 25.Yokoyama T., Copeland N. G., Jenkins N. A., Montgomery C. A., Elder F. F., Overbeek P. A. Reversal of left-right asymmetry: A situs inversus mutation. Science. 1993;260:679–682. doi: 10.1126/science.8480178. [DOI] [PubMed] [Google Scholar]