Abstract
The G2/M cell cycle checkpoint is regulated by a multitude of signaling pathways after genotoxic stress. Herein, we report that treatment with the 90-kDa heat shock protein (Hsp90) molecular chaperone inhibitor 17-allylamino-17-demethoxygeldanamycin (17AAG) selectively abrogates the G2/M checkpoint induced by 7-ethyl-10-hydroxycamptothecin (SN-38), an active metabolite of irinotecan, in p53-null compared with p53-intact HCT116 colon cancer cells. The basis for this selectivity can be explained in part by the lack of p21 induction in p53-null cells. In accord with published results, we could show that treatment with 17AAG resulted in depletion of Chk1, a known Hsp90 client protein. In addition, we observed a time- and dose-dependent decrease in Wee1 kinase level, a negative regulator of mitosis, after 17AAG treatment in gastrointestinal cancer cells. Depletion of Wee1 protein preceded mitotic entry induced by 17AAG, and this decrease could be partially rescued by cotreatment with a proteasome inhibitor. Coimmunoprecipitation experiments showed that Hsp90 and Wee1 interacted in whole cells, and 17AAG treatment decreased the degradative half-life of Wee1, indicating that Wee1 is another Hsp90 client in mammalian cells. Knockdown of Chk1 and Wee1 by short interfering RNA each resulted in abrogation of the G2/M checkpoint induced by SN-38. The combination of SN-38 and 17AAG was shown to be synergistic in p53-null but not in parental HCT116 cells by median effect/combination index analysis. Taken together, 17AAG specifically inhibits the G2/M checkpoint in p53-defective cells by down-regulation of two critical checkpoint kinases, Chk1 and Wee1.
The G2/M cell cycle transition in higher eukaryotic cells is controlled by a complex network of evolutionarily conserved signaling pathways that eventually converge to regulate the promitotic activity of the cyclin B/cdc2 kinase complex (Graves et al., 2000; Taylor and Stark, 2001). In the presence of genomic injury, the G2/M checkpoint is activated to delay cells from entering mitosis and thereby preventing the transmission of damaged genetic materials to daughter cells. In response to DNA damage, the ATR→ Chk1 checkpoint pathway functions to inhibit mitotic entry by down-regulating activity of the dual specificity cdc25 phosphatases (cdc25A, cdc25B, and cdc25C in mammalian cells) (Zhou and Bartek, 2004; Tse et al., 2007a)1. In a normal cell cycle, the activity of cyclin B/cdc2 during interphase is inhibited by two protein kinases that prevent premature mitosis. Myt1 catalyzes the phosphorylation of cdc2 on both inhibitory sites, whereas Wee1 phosphorylates residue Tyr15 only (Parker and Piwnica-Worms, 1992; Booher et al., 1997). Wee1 has been implicated as a downstream target of Chk1 after DNA damage in yeasts, although its functional significance in checkpoint control in higher eukaryotes is unclear (O'Connell et al., 1997).
In addition to the Chk1-dependent axis, G2/M transition is also regulated by other checkpoint-signaling pathways. Thus, the tumor suppressor p53 (and its downstream effectors p21 and 14-3-3σ) has been shown to play a key role in the maintenance of the G2/M checkpoint (Bunz et al., 1998). Initially, phosphorylation of p53 by Chk2 was believed to be the critical biochemical event leading to p53 stabilization (Chehab et al., 2000). However, more recent studies have raised questions about the role of Chk2 in p53 induction, because Chk2 knockout or depleted cells seem to retain an intact p53 response pathway after DNA damage (Ahn et al., 2003; Jallepalli et al., 2003). In addition, mitogen-activated protein kinase-activated protein kinase 2 (MAPKAPK-2 or MK2) has been implicated in controlling the G2/M checkpoint and as another UCN-01-sensitive target (Manke et al., 2005; Reinhardt et al., 2007). However, it has been shown in a recent report that MK2 was only minimally activated in response to topoisomerase I poison and was insensitive to inhibition by UCN-01, questioning the generalizability of the initial findings (Zhang et al., 2008).
Increasing evidence has indicated that abrogation of the G2/M checkpoint results in sensitization of cells to chemotherapy or radiation, especially in cells that lack functional p53. Early proof-of-concept studies were performed using the nonselective ATM/ATR inhibitor caffeine (Powell et al., 1995; Russell et al., 1995). We and others have shown that pharmacological disruption of the Chk1-mediated pathway using small-molecule inhibitors (UCN-01 and CHIR-124) can potentiate cell death induced by a variety of chemotherapeutic agents, including cisplatin, temozolomide, mitomycin C, and topoisomerase poisons (Bunch and Eastman, 1996; Wang et al., 1996; Tse et al., 2007a,b). Chk1 as a target for chemo- or radiosensitization is further validated by genetic studies, demonstrating that inactivation of Chk1 in embryonic stem cells (Liu et al., 2000) and somatic cells (Zhao et al., 2002; Sorensen et al., 2003; Xiao et al., 2003; Zachos et al., 2003) resulted in hypersensitivity to genotoxic challenges.
In addition to using kinase inhibitors, Chk1 can be targeted by other means. Hsp90 is an abundant cytoplasmic molecular chaperone that is involved in the functional maturation of a number of “client” proteins participating in signal transduction. Many of the Hsp90 clients are oncogenic: This includes Her2/Neu, Akt, Raf-1, Cdk4, Bcr-Abl, and mutant p53 (Workman, 2004; Solit and Rosen, 2006). Biochemical studies have shown that Chk1 is also an Hsp90 client protein (Arlander et al., 2003), and treatment of tumor cells with the Hsp90 inhibitor 17-allylamino-17-demethoxygeldanamycin (17AAG) results in Chk1 depletion and enhanced cytotoxicity induced by nucleoside analogs and doxorubicin (Arlander et al., 2003; Sugimoto et al., 2008).
We now report that in addition to Chk1 down-regulation, exposure of tumor cells to 17AAG causes depletion of another critical checkpoint kinase, Wee1. Decreased expression of these kinases was associated with abrogation of the G2/M checkpoint and enhancement of cytotoxicity after treatment with SN-38 in tumor cells lacking p53 function. Gene knock-down of Chk1 and/or Wee1 using siRNA showed that depletion of these two kinases resulted in G2/M checkpoint inhibition.
Materials and Methods
Chemicals. 17AAG (NSC 330507) was provided by Dr. Robert Schultz (National Cancer Institute, Bethesda, MD). SN-38 was a gift from Dr. J. Patrick McGovern (Pharmacia Upjohn Inc., Kalamazoo, MI), and MG-132 was purchased from BIOMOL Research Laboratories (Plymouth Meeting, PA). All drugs were dissolved in dimethyl sulfoxide and stored in aliquots at -20°C.
Cell Culture, Assessment of Apoptosis and Viability, and Cell Cycle Analysis. Parental HCT116 colonic carcinoma cell line and its p53-null and p21-null variants were kindly provided by Dr. Bert Vogelstein (the Johns Hopkins University, Baltimore, MD). Cultures were maintained as described previously (Tse and Schwartz, 2004). The incidence of apoptosis after drug treatment, based on the presence of condensed fragmented nuclei, was scored after counting at least 400 4′-6-diamidino-2-phenylindole (DAPI)-stained nuclei per sample under fluorescence. In experiments involving sequential therapy, floating cells were collected after incubation with the first drug and were added back to the plate for subsequent treatment. Both adherent and floating cells were collected at the end of treatment. Cell cycle distribution was analyzed by biparameter flow cytometry for both DNA content and specific labeling of mitotic cells using the MPM-2 antibody as described previously (Motwani et al., 1999).
Drug Interaction by Median-Effect/Combination Index Analysis. Parental and p53-null HCT116 cells in log phase were seeded in 96-well microplates at 3000 cells/well and were allowed to attach overnight. Fresh medium (100 μl) containing the designated drug or drug combination was added for 24 h. Cells were treated with increasing concentrations of single-agent SN-38 (1, 2, 4, 10, 20, and 40 nM), 17AAG (50, 100, 200, 500, 750, and 1000 nM), or the combination in a fixed SN-38/17AAG concentration ratio of 1:20 (10:200, 12.5:250, 15:300, 20:400, and 30:600 nM). After drug washout, cells were incubated in drug-free medium for 72 h. Cell viability was measured using the Cell Counting Kit-8 (Dojindo Laboratories, Gaithersburg, MD). Ten microliters of cholecystokinin-8 solution containing the reducible salt 2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium was added to each well, and after a 4-h incubation at 37°C, absorbance was read at 450 nm using a microplate reader (Molecular Devices, Sunnyvale, CA). The dose-effect curve parameters for both SN-38 and 17AAG were used for the automated calculation for the CI values for each combination data point by the CompuSyn software (ComboSyn, Paramus, NJ) where CI <1, =1, and >1 indicate synergism, additive effect, and antagonism, respectively (Chou and Martin, 2005; Chou, 2006). Because the combination of SN-38 and 17AAG were carried out at a constant ratio (1:20), the dose-effect parameters of the mixture were used for generating the computer-simulated Fa-CI plot (Chou-Talalay plot), where Fa is the fraction affected (i.e., percentage of inhibition/100).
Immunoblotting and Immunoprecipitation. Mouse monoclonal antibodies were for Chk1 (G-4), Wee1 (B-11), p53 (Bp53-12), cdk2 (F-6), cdc25A (M-191), cyclin B (GNS1), p21 (187) (all from Santa Cruz Biotechnology, Santa Cruz, CA), and tubulin (Millipore, Billerica, MA). Rabbit polyclonal antibody was used for Myt1 and MK2 (Cell Signaling Technology, Danvers, MA). For immunoblot analysis, both floating and adherent cells were combined and lysed in radioimmunoprecipitation buffer (Tse and Schwartz, 2004). For immunoprecipitation studies, cells were lysed in a buffer containing 50 mM HEPES-KOH, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM NaF, 1 mM dithiothreitol, 2.5 mM EGTA, 0.1% Tween 20, 10% glycerol, 10 mM β-glycerophosphate, 0.1 mM Na3VO4, 0.2 mM phenylmethylsulfonyl fluoride,10 μg/ml aprotinin, and 10 μg/ml leupeptin. Five hundred micrograms of cell lysate protein was precleared by mixing with 20 μl of protein A-conjugated agarose (Millipore). The lysates were incubated with the indicated antibodies or preimmune control IgG for 1 h on ice. Immunocomplexes were then precipitated with 20 μl of protein A agarose overnight at 4°C. After three washings with lysis buffer, immunoprecipitates were boiled in the presence of 30 μl of Laemmli sample buffer. Samples were fractionated by SDS-PAGE and processed for immunoblot analysis.
Metabolic Labeling Studies. HCT116 cells in log-phase were grown in methionine/cysteine-free medium (RPMI 1640, 10% fetal calf serum, and 2 mM l-glutamine) for 30 min and pulse-labeled with 75 μCi/ml [35S]methionine (PerkinElmer Life and Analytical Sciences, Waltham, MA) for 45 min at 37°C. Cells were washed once with prewarmed phosphate-buffered saline, and fresh medium containing unlabeled l-methionine and l-cysteine was added (t = 0). Cells were lysed at the indicated time points during the chase period. For cells treated with 17AAG, the drug was present 2.5 h before, during, and after metabolic labeling. Five hundred to 1000 μg of protein lysates was precleared with protein A Sepharose for 30 min and immunoprecipitated with either control rabbit IgG or Wee1 antibody as described above. Immunoprecipitates were washed and then boiled in SDS sample buffer, fractionated by SDS-PAGE, and analyzed by autoradiography. Radioactivity of labeled proteins was also quantified using a PhosphorImager (GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK).
Real-Time PCR. Real-time PCR data are provided in Supplemental Material 1.
Cyclin B1-Associated Kinase Assays. Cyclin B1-associated kinase assays were performed as described previously (Motwani et al., 1999). In brief, 1 μg of anti-cyclin B1 (GN1) antibody (Santa Cruz Biotechnology) was added to 250 μg of cell lysate protein. Immunocomplexes were captured onto protein A-conjugated agarose beads overnight at 4°C. Immunoprecipitates were washed three times with lysis buffer and twice with kinase buffer (Motwani et al., 1999). Reactions were carried out in 30 μl of kinase buffer containing 5 μCi of [γ-32P]ATP, 15 μM ATP, and 1 μg of histone H1 (Roche Diagnostics, Indianapolis, IN) at 30°C for 20 min. Products were fractionated by SDS-PAGE, transferred onto Immobilon-P membrane, and visualized by autoradiography.
Gene Silencing by siRNA. Nontargeting control siRNA and oligoduplexes specific for Chk1 and Wee1 were purchased from Dharmacon (Chicago, IL). The sense strand sequence of the oligonucleotides for Chk1 and Wee1 were 5′-CUGAAGAAGCAGUCGCAGU and 5′-CUCCGGGGUAGUUCUCUCU, respectively. HCT116 cells seeded onto six-well plates were treated with 20 nM SN-38 for 24 h before siRNA transfection. Cells were transfected with gene-specific and/or control duplex oligonucleotides complexed in Lipofectamine RNAiMax (Invitrogen, Carlsbad, CA) to give a final concentration of 100 nM. After transfection, cells were harvested at serial time points for immunoblot and immunofluorescence analysis after labeling with MPM-2 and staining with DAPI.
Results
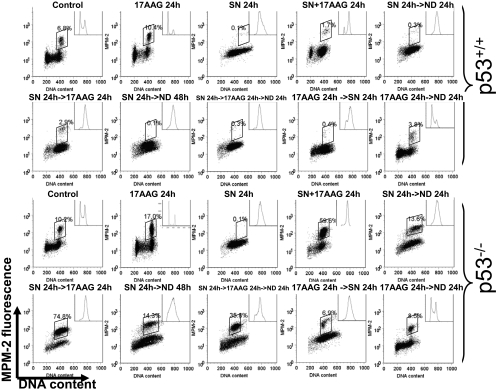
Abrogation of the G2/M Checkpoint by Treatment with 17AAG Occurs Selectively in p53-Null HCT116 Cells. It has been shown that treatment with 17AAG resulted in specific depletion of Chk1 in several tumor cell lines. Down-regulation of Chk1 resulted in an abrogation of the S-phase checkpoint induced by gemcitabine (Arlander et al., 2003). We have shown previously that sequential treatment of HCT116 colon cancer cells with SN-38, the active metabolite of irinotecan, followed by UCN-01 resulted in abrogation of the G2 arrest induced by SN-38. This checkpoint inhibitory effect is more selective in cells that lack p53 (Tse and Schwartz, 2004; Tse et al., 2007b). We therefore examined the effect of Hsp90 inhibition on regulation of the G2/M DNA damage checkpoint after treatment with SN-38 and 17AAG in both parental [p53(+/+)] and p53-null [p53(-/-)] HCT116 cells. Parental HCT116 cells bearing wild-type p53 underwent a late-S/G2 arrest with loss of mitotic cells after a 24-h treatment with 20 nM SN-38, which was expected, based on our previous results (Motwani et al., 2001; Tse and Schwartz, 2004). Treatment with 500 nM 17AAG alone resulted in an accumulation of cells in both G1 and G2, also consistent with the reported effect of this drug (Srethapakdi et al., 2000). Parental cells treated with a combination of SN-38 and 17AAG either concurrently or sequentially underwent a G2 arrest without mitosis, suggesting that 17AAG was unable to abrogate the G2/M checkpoint induced by SN-38 in this cell line. It is interesting that we consistently found that in addition to the G1 and G2 arrest seen with 17AAG treatment in parental cells, treatment of p53-null HCT116 cells with this drug resulted in an increase in mitosis (up to 17%). Examination of the nuclear morphology of these mitotic cells by fluorescent microscopy after DAPI staining revealed an increase in apparently normal metaphases compared with untreated HCT116 p53-null cells (Supplemental Fig. 1). After SN-38 treatment, p53-null cells underwent a late-S/G2 arrest in a way similar to parental HCT116 cells. However, upon removal of SN-38, approximately 14% of p53-null cells had escaped the G2/M checkpoint and entered mitosis, consistent with an intrinsic defect in maintaining the G2/M checkpoint in these cells (SN 24 h→ND 24 h; Fig. 1). This checkpoint defect was markedly enhanced by sequential treatment with 17AAG (SN→17AAG), resulting in an increase in mitotic index up to 74.8% (Fig. 1). Concurrent treatment with SN-38 and 17AAG (SN + 17AAG) also resulted in a higher level of mitotic entry than with either agent alone (Fig. 1). When cells were followed for an additional 24 h after drug washout (SN 24 h→ND 48 h and SN 24 h→17AAG 24 h→ND 24 h), p53 wild-type cells remained arrested in G2, whereas p53-null cells had begun to exit mitosis as evidenced by a decrease in MPM-2-positive cells from 74.8 to 35.8% (Fig. 1). Cells that had exited mitosis contained 4 N rather 2 N DNA, indicating a failure of cytokinesis in these cells, an observation consistent with results obtained with compounds that directly inhibit Chk1 (Tse and Schwartz, 2004; Tse et al., 2007b). Finally, abrogation of the SN-38 indued G2/M checkpoint by 17AAG is schedule-dependent because the reverse sequence (17AAG→SN) did not result in any increase in mitotic cells in both cell lines (Fig. 1).
Fig. 1.
Abrogation of the SN-38-induced G2/M checkpoint by 17AAG in p53-null HCT116 p53(+/+) and p53(-/-) colon cancer cells. Asynchronous parental and p53-null HCT116 cells were treated with 20 nM SN-38 or 500 nM 17AAG alone for 24 h or in combination according to the indicated sequences. In sequential treatment, cells were incubated with the first agent for 24 h, washed with drug-free medium, and incubated with either drug-free medium (ND) or the second agent for an additional 24 h. In concurrent treatment, both drugs were present for 24 h. Cell cycle position was analyzed by biparameter flow cytometry for DNA content and mitosis by MPM-2 staining (boxed population). Insets, flow cytometry histograms. Results are representative of three experiments.
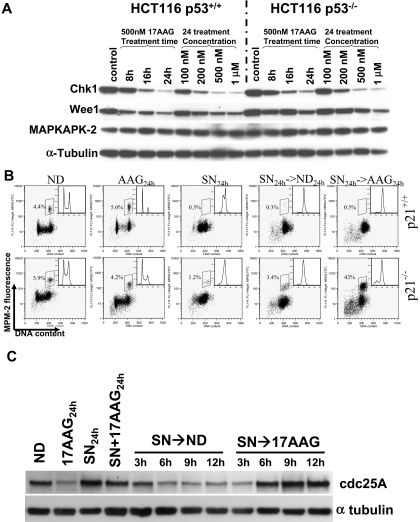
Treatment with 17AAG Depletes Cellular Chk1 in Both Parental and p53-Null HCT116 Cells. In accord with results published previously (Arlander et al., 2003), we found that treatment with 17AAG reduced the protein level of Chk1 in a time- and dose-dependent manner (Fig. 2A). It is interesting that Chk1 was similarly depleted in both parental and p53-null HCT116 cells, even though abrogation of the SN-38 induced G2/M checkpoint abrogation by 17AAG was seen only in the latter cell line. We therefore queried the basis for the selective abrogation of the G2/M checkpoint in cells that lack p53. We first studied the level of p53 and its downstream effector p21 during combination treatment. In parental cells, both p53 and p21 were up-regulated by treatment with SN-38 alone, and their protein levels continue to increase in a time-dependent fashion even upon removal of the drug (Supplemental Fig. 2). After sequential treatment with 17AAG, the up-regulation of p53 was maintained, indicating that 17AAG treatment had no effect on the level of wild-type p53 protein, which was consistent with reports in the literature showing that Hsp90 inhibition destabilized only mutated p53 proteins (Blagosklonny et al., 1995, 1996). The induction of p21 after sequential treatment with SN-38 and 17AAG seemed to be more robust than treatment with SN-38 followed by drug-free medium (Supplemental Fig. 2). As expected, p21 was not induced in p53-null cells treated with SN-38 and 17AAG (data not shown). To directly test the role of p21 in checkpoint maintenance in parental HCT116 cells after SN-38 and 17AAG treatment, we examined the checkpoint response of isogenic HCT116 p21-null cells treated by the combination. Sequential treatment with SN-38 followed by 17AAG resulted in a marked increase in mitosis that was not seen with SN-38 followed by drug-free medium (43 versus 3% at 24 h after 17AAG treatment) (Fig. 2B). We have also confirmed that treatment with 17AAG resulted in down-regulation of Chk1 in a dose-dependent fashion in these cells similar to parental cells (data not shown).
Fig. 2.
Changes in G2/M regulators and involvement of p21 in checkpoint maintenance after 17AAG treatment. A, parental and p53-null HCT116 cells were treated with increasing concentrations of 17AAG for 24 h or with 500 nM 17AAG for increasing length of time. At the end of incubation, cell lysates were prepared, fractionated by SDS-PAGE, and probed for Chk1, Wee1, MAPKAPK-2, or tubulin. B, abrogation of the SN-38-induced G2/M checkpoint in HCT116 p21(-/-) cells by 17AAG. Biparameter flow cytometry analysis was performed on HCT116 p21(-/-) cells treated with SN-38 followed by either ND or 500 nM 17AAG for 24 h. Mitotic population is boxed, and flow histograms are shown in insets. C, reversal of the SN-38 induced cdc25A down-regulation by 17AAG in HCT116 p53(-/-) cells.
To assess the effect of 17AAG treatment on Chk1-depedent signaling events, we examined the protein level of cdc25A, a dual-specificity phosphatase that is known to be destabilized after phosphorylation by Chk1 (Mailand et al., 2000; Zhao et al., 2002). Consistent with an interruption of Chk1-dependent signaling pathway, concurrent or sequential 17AAG treatment reversed the SN-38-induced down-regulation of the cdc25A (Fig. 2C). Taken together, we conclude that the selective abrogation of the SN-38 induced G2 arrest by 17AAG in p53-null HCT116 cells was caused by a concomitant loss of two G2/M checkpoint pathways in these cells: loss of p21 as a result of p53 deletion and pharmacological disruption of the Chk1-mediated checkpoint by 17AAG.
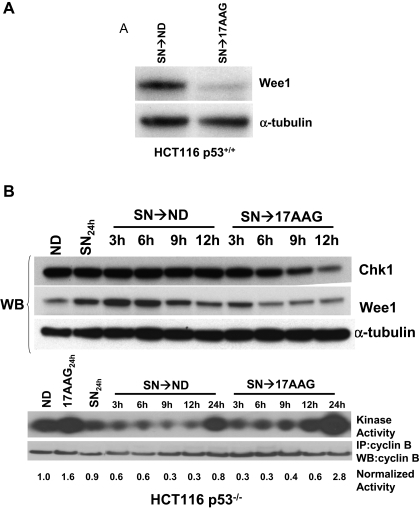
Down-Regulation of Wee1 by 17AAG Treatment. Because 17AAG treatment may lead to down-regulation of a number of Hsp90 client proteins, we examined the effect of 17AAG treatment on other checkpoint targets, besides Chk1, that may be responsible for the G2/M checkpoint abrogation. The mitogen-activated protein kinase-MK2 pathway has been implicated recently in mediating the G2/M checkpoint induced by UV damage (Manke et al., 2005). However, treatment with 17AAG resulted in no appreciable change in the level of MK2 in either cell lines (Fig. 2A). Wee1 is a tyrosine kinase that prevents premature mitosis by phosphorylating cdc2 at Tyr15 (Watanabe et al., 1995). We found that treatment with 17AAG caused a time- and dose-dependent depletion of Wee1 in both parental and p53-null HCT116 cells (Fig. 2A) and in MKN-74 gastric cancer cells (Supplemental Fig. 3). It has been reported that Wee1 activity varies according to cell cycle phases, and its activity decreases precipitously during mitosis in part from destabilization of the kinase via association with the F-box containing ubiquitin ligases (Watanabe et al., 2004). We have ruled out the possibility that the observed down-regulation of Wee1 was caused by mitosis induced by 17AAG. First, checkpoint-competent parental HCT116 cells treated sequentially with SN-38 followed by 17AAG remained arrested in G2 without mitotic entry (Fig. 1), and yet Wee1 expression declined markedly in these cells (Figs. 1 and 3A). Second, in checkpoint-defective HCT116 p53-null cells, the loss of Wee1 and Chk1 upon sequential treatment with SN-38 and 17AAG preceded the activation of the promitotic cyclin B1-associated kinase by 6 h (Fig. 3B). Together, these results strongly suggest that the depletion of Wee1 after 17AAG treatment is a direct effect of Hsp90 inhibition rather than a consequence of mitotic entry induced by the drug.
Fig. 3.
Wee1 down-regulation of 17AAG is a cause rather than a consequence of mitotic entry. A, depletion of Wee1 protein in G2-arrested parental HCT116 after SN-38 and 17AAG treatment. HCT116 cells were sequentially treated with SN-38 for 24 h followed by either ND or 17AAG for 24 h. B, decrease of Wee1 protein preceded activation of cyclin B-associated kinase. HCT116 p53-null cells were treated with SN-38 followed by either ND or 17AAG. Cell lysates were processed for immunoblotting for Chk1 and Wee1, or for immunoprecipitation and cyclin B-associated kinase assay using histone H1 as a substrate. The radioactivity of 32P-labeled histone H1 was quantitated using a PhosphorImager and normalized to the amount of immunoprecipitated cyclin B as determined by Western blot. The kinase activity from untreated control cells (ND) was set as 1.0.
In addition, we examined the level of Myt1, another key regulatory kinase of G2/M transition, after sequential treatment with SN-38 and 17AAG in HCT116 p53-null cells. In contrast to Chk1, the protein level of Myt1 was not appreciably affected by SN-38 and 17AAG (Supplemental Fig. 4). At a late time point (SN→17AAG 24 h), there was an upward motility shift of Myt1, consistent with the mitotic form of this protein reported in the literature (Booher et al., 1997) and, in our case, probably indicative of mitotic entry induced by 17AAG treatment. Thus, although Myt1 is important in regulating the cyclin B/cdc2 activity, it is unlikely to play a major role in abrogating the G2/M checkpoint by 17AAG.
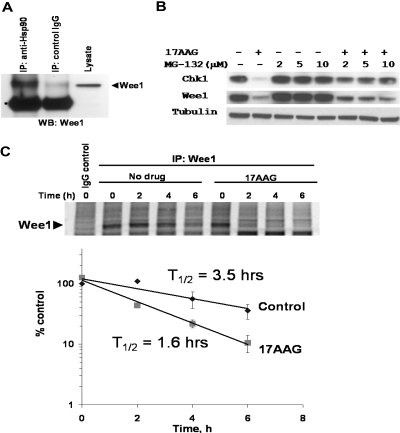
Wee1 Is an Hsp90 Client Protein in Mammalian Cells. Chk1 has been implicated as an Hsp90 client protein that physically interacts with the molecular chaperone in whole cells based on coimmunoprecipitation studies. To demonstrate that Wee1 is also an Hsp90 client, cell lysate prepared from parental HCT116 cells were incubated with an Hsp90-specific or control IgG antibody. Endogenous Wee1 coimmunoprecipitated with Hsp90 only when an anti-Hsp90 antibody was used (Fig. 4A). We next determined whether the depletion of Chk1 and Wee1 by 17AAG depends on the 26S proteasome. HCT116 parental cells were treated with 500 nM 17AAG in the presence or absence of the proteasome inhibitor MG-132 at three different concentrations (2, 5 and 10 μM). Coincubation with 17AAG and MG-132 resulted in near complete restoration of Chk1 protein level (Fig. 4B). Down-regulation of Wee1 by 17AAG was partially protected by cotreatment with MG-132, suggesting the possibility of a proteasome-independent degradative process (see below and Matsuda et al., 1999).
Fig. 4.
Wee1 is an Hsp90 client protein in mammalian cells. A, Wee1 interacts with Hsp90 in whole cells. Cell lysates prepared from parental HCT116 cells were immunoprecipitated with control IgG or anti-Hsp90 antibody. Immunoprecipitates were prepared and probed for Wee1. Position of the immunoglobulin heavy chain is denoted by an asterisk. B, depletion of Chk1 and Wee1 by 17AAG is partially reversed by proteasome inhibition. Parental HCT116 cells were treated with vehicle alone, 500 nM 17AAG, MG-132, or in combination for 24 h. Cell lysates were prepared at the end of incubation and immunoblotted for Chk1 and Wee1. C, destabilization of Wee1 protein by 17AAG. Top, HCT116 cells were grown in methionine/cysteine-free medium for 30 min, pulse-labeled with 75 μCi/ml [35S]methionine for 45 min, and chased in medium containing unlabeled methionine/cysteine for the indicated time. For cells treated with 17AAG, the drug was present 2.5 h before, during, and after metabolic labeling. Protein lysates were immunoprecipitated with either control rabbit IgG or antibody for Wee1, fractionated by SDS-PAGE, and analyzed by autoradiography. Bottom, radioactivity of labeled proteins was quantified using a PhosphorImager and expressed as the percentage of untreated control at t = 0. Each data point represents the mean of three independent experiments. t½, degradation half-life.
To examine the effect of Hsp90 inhibition on Wee1 protein stability more directly, we performed a [35S]methionine-labeled pulse-chase experiment in control or 17AAG-treated HCT116 cells. After a 30-min pulse with [35S]methionine, the level of radiolabeled Wee1 was followed during a 6-h chase period (Fig. 4C, top). In untreated cells, the half-life of newly synthesized Wee1 was estimated to be 3.5 h (Fig. 4C, top). In the presence of 500 nM 17AAG, the half-life of Wee1 was shortened to 1.6 h (Fig. 4C, bottom). It is noteworthy that the level of radiolabeled Wee1 at the beginning of the chase was not affected by 17AAG treatment, indicating that Hsp90 inhibition did not affect the translation of Wee1. To rule out an effect of Hsp90 inhibition on mRNA expression, we compared the abundance of Wee1 message (relative to glyceraldehyde 3-phosphate dehydrogenase) in HCT116 cells treated sequentially with SN-38 followed by either drug-free medium or 17AAG using real-time PCR and found no difference in Wee1 mRNA levels between the two conditions (Supplemental Fig. 5). Thus, our results indicate that Wee1 interacts with Hsp90 in vivo, and inhibition of Hsp90 by 17AAG results in accelerated degradation of Wee1, which at least partially depends on the 26S proteasome. Taken together, these data strongly suggest that Wee1 is an Hsp90 client protein in mammalian cells.
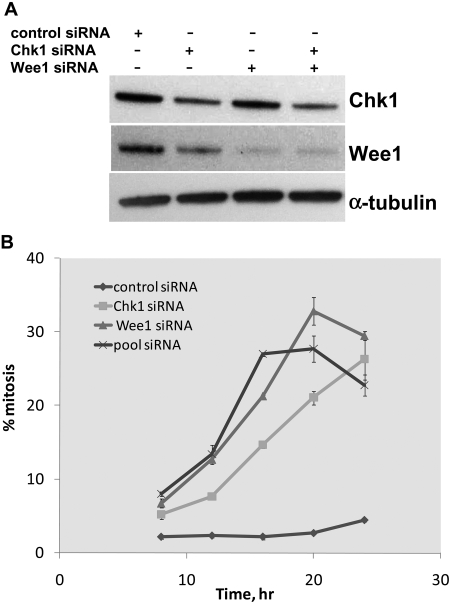
Gene Knockdown of Chk1 and Wee1 by siRNA Phenocopied the Pharmacological Effect of 17AAG. To confirm that the down-regulation of Chk1 and Wee1 upon 17AAG treatment caused the abrogation of the G2/M checkpoint rather than being part of a pleiotropic effect caused by Hsp90 inhibition, we knocked down the expression of these two checkpoint kinases by siRNA and determined the effect of their individual or combined depletion on the G2/M checkpoint. To mimic the schedule of sequential treatment with SN-38 and 17AAG, HCT116 p53-null cells were pretreated with SN-38 for 24 h to induce a G2 checkpoint arrest before siRNA transfection. As shown in Fig. 5A, transfection with siRNA oligonucleotides specific for Chk1 or Wee1, but not control siRNA, resulted in a substantial down-regulation of their respective protein targets (≥60% for Chk1 and ≥80% for Wee1). It is noteworthy that we consistently observed a slight decrease in Wee1 protein level in cells transfected with Chk1 siRNA. We postulated that this reduction in Wee1 level was caused by mitotic entry induced by Chk1 knockdown (see below and Watanabe et al., 2004) rather than an off-target effect of the Chk1-directed siRNA oligonucleotide used, because the decline in Wee1 could be reproduced with a different Chk1-specific siRNA duplex (data not shown).
Fig. 5.
Knockdown of expression of Chk1 and/or Wee1 by siRNA phenocopies the inhibitory effect of 17AAG on the G2/M checkpoint. HCT116 p53(-/-) cells grown on six-well plates were treated with 20 nM SN-38 for 24 h. Cells were washed with drug-free medium and transfected with control, Chk1-specific, Wee1-specific, or pooled (Chk1 and Wee1) siRNA oligonucleotides. Cells were processed for immunoblot (A) and cell cycle (B) analysis at various times after SN-38 removal.
We next examined the effect of gene knockdown on the G2/M DNA damage checkpoint in these cells by monitoring the percentage of mitotic cells 8, 12, 16, 20, and 24 h after siRNA transfection. Compared with SN-38-treated cells transfected with control siRNA, cells transfected with siRNA specific for Chk1 or Wee1 showed a progressive increase in mitotic index (Fig. 5B). The kinetics of mitotic entry were somewhat faster in cells transfected with both Chk1 and Wee1 siRNA than in those transfected with each individual oligonucleotide. However, the extent of checkpoint escape seen in cells transfected with the pooled oligonucleotides was lower than what one would have expected if the combined effect of down-regulating each kinase was additive, suggesting that Chk1 and Wee1 may function along the same signaling pathway in controlling the G2/M checkpoint (see Discussion). Together, gene knockdown of Chk1 and Wee1 recapitulated in part the pharmacological effects of 17AAG in causing abrogation of the G2/M checkpoint.
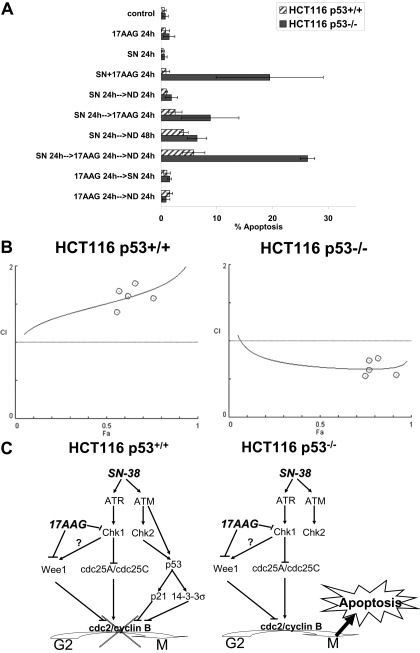
Treatment with SN-38 and 17AAG Is Selectively More Cytotoxic in HCT116 Cells Lacking p53. Finally, we explored the therapeutic potential of combining SN-38 and 17AAG to target p53-defective cells. Apoptosis was measured in parental and p53-null HCT116 after combined treatment with SN-38 and 17AAG in various schedules. As shown in Fig. 6A, single-agent treatment with 20 nM SN-38 or 500 nM 17AAG resulted in minimal apoptosis (<4%) in both cell lines. The combination of SN-38 and 17AAG was ineffective in causing apoptosis in the parental cells, regardless of the sequence of drug treatment. This result is in agreement with the flow cytometry data, which showed no abrogation of the G2/M checkpoint by 17AAG in this cell line (Fig. 1). On the other hand, in p53-null cells, concurrent treatment with SN-38 and 17AAG for 24 h (SN + 17AAG 24 h) resulted in a marked increase in apoptosis (20 ± 10%) (Fig. 6A). Sequential treatment with SN-38 followed by 17AAG (SN 24 h→AAG 24 h) also caused an increase in apoptosis (9 ± 5%), which seemed to be a delayed phenomenon as the incidence of apoptosis increased further (26 ± 1%) when sequential treatment was followed by an additional 24 h of drug washout (SN 24 h→AAG 24 h→ND) (Fig. 6A). Pretreatment with 17AAG followed by SN-38 did not result in apoptosis in both cell lines, again consistent with the results from cell cycle analysis demonstrating no abrogation of the G2/M checkpoint when the two agents were given in this sequence (Fig. 1). Examination of the nuclear morphology of cells in mitosis after concurrent or sequential SN-38 and 17AAG treatment revealed the presence of condensed but disorganized chromatin without discernible metaphases or anaphases (Supplemental Fig. 1).
Fig. 6.
Combined SN-38 and 17AAG is more active in HCT116 cells that lack p53. A, selective induction of apoptosis in p53-null HCT116 cells by concurrent or sequential treatment with SN-38 followed by 17AAG. Log-phase parental and p53-null HCT116 cells were treated with single-agent 20 nM SN-38 or 500 nM 17AAG or the combination in various sequences. Apoptosis was assessed by quantitative fluorescence microscopy after DAPI staining. Data were averages of three independent experiments (mean ± S.D.). B, CI-Fa plot of SN-38 and 17AAG in HCT116 p53(+/+) and p53(-/-) cells. Cell viability was determined after treatment with graded concentrations of SN-38, 17AAG, or the combination in a fixed concentration ratio of 1:20 (SN-38/17AAG) for 24 h followed by drug-free medium for another 72 h. CI, combination index; Fa, fraction affected. CI > 1 indicates antagonism, and CI <1 indicates synergism. Solid curves are computer-simulated CI-Fa plots. CI plots shown are representative of two independent experiments. C, model for selective cytotoxicity of SN-38 and 17AAG in p53-deficient cells. In response to DNA damage induced by SN-38, both Chk1- and p53-dependent pathways are activated to trigger the G2/M checkpoint in p53 wild-type cells. In contrast, p53-null cells are more dependent on the Chk1-mediated pathways for maintaining the G2/M checkpoint and are therefore more sensitive to the combination of SN-38 and 17AAG.
We corroborated our apoptosis studies with a viability assay and formally evaluated the nature of the interaction between SN-38 and 17AAG in both parental and p53-null cells. The IC50 values of SN-38 were comparable in both cell lines (7.5 ± 2.0 and 11 ± 4.1 nM for p53(+/+) and p53(-/-) cells, respectively; n = 3, p = 0.25 by t test). p53-null cells were more sensitive than their parental counterpart to 17AAG in this assay (510 ± 120 versus 200 ± 44 nM; n = 3, p = 0.01 by t test). Shown in Fig. 6B are CI versus Fa plots generated for the two cell lines treated with SN-38 and 17AAG in a fixed concentration ratio of 1:20 (10:200, 12.5: 250, 15:300, 20:400, and 30:600 nM) (Chou and Martin, 2005; Chou, 2006). When drug-interaction was assessed using median effect/combination index analysis, combined treatment with SN-38 and 17AAG was found to be antagonistic (CI > 1) in parental but synergistic (CI < 1) in p53-null HCT116 cells (Fig. 6B). Taken together, concomitant treatment with SN-38 and 17AAG overrode the G2/M checkpoint induced by SN-38 selectively in p53-null HCT116 cells and induced death in these cells.
Discussion
Probing the function of Hsp90 using natural and synthetic specific inhibitors has led to an expanding list of interacting signaling proteins that rely on the molecular chaperone for proper folding and maturation. Chk1, a critical kinase involved in the S and G2/M checkpoints, has been identified as an Hsp90 client (Arlander et al., 2003). We report here that Wee1, a negative regulator of the promitotic cyclin B/cdc2 complex, is also an Hsp90 client in mammalian cells. This dependence of Wee1 on Hsp90 chaperone function for protein stability seems to be evolutionarily conserved from yeast to human. Thus, in a genetic screen for suppressor mutants of the G2 arrest phenotype caused by Wee1 overexpression in fission yeast, the suppressor of Wee1 overproduction mutant was identified, which encodes a member of the Hsp90 family of proteins (Aligue et al., 1994). Furthermore, the stability and activity of Wee1 from the fission yeast have been shown to be regulated by the Hsp90 chaperone complex (Munoz et al., 1999; Goes and Martin, 2001).
Although it has been shown that Wee1 protein level decreases rapidly as cells enter mitosis (Watanabe et al., 2004), our results indicate that the Wee1 down-regulation after 17AAG treatment is the cause rather than the consequence of mitotic entry. This is because parental HCT116 cells treated with SN-38 and 17AAG remain arrested in G2, yet there is a marked decrease in Wee1 expression in these cells. In addition, in HCT116 p53-null cells, the loss of Wee1 precedes the activation of the promitotic cyclin B1-associated kinase. Finally, Wee1 gene knockdown using siRNA is sufficient to abrogate the SN-38-induced G2/M checkpoint in HCT116 p53-null cells. However, it is interesting to note that even though individual knockdown of Chk1 or Wee1 expression results in G2/M checkpoint abrogation, a less than additive effect is observed when both siRNA oligonucleotides are combined, suggesting a functional interaction between Chk1 and Wee1 along a common signaling pathway. It has been shown that, in Xenopus laevis egg extracts, Xchk1 phosphorylates and positively regulates Xwee1 by increasing binding of 14-3-3 proteins to Xwee1 (Lee et al., 2001), although a functional link between Chk1 and Wee1 has yet to be demonstrated in intact mammalian cells (Rothblum-Oviatt et al., 2001). It is important to point out that the percentages of p53-null cells that were in mitosis after SN-38 and pooled Chk1/Wee1 siRNA treatment were substantially lower than those obtained using 17AAG. This discrepancy can be explained in part by the fact that cells treated with SN-38 and 17AAG had a longer dwell time in mitosis, whereas cells treated with SN-38 and siRNA exited mitosis more rapidly, based on time-lapse fluorescence microscopy studies (A. Tse and G. Schwartz, unpublished observations). We speculate that the delay in mitotic exit of 17AAG-treated cells is related to depletion of Plk1 kinase, a known Hsp90 client that promotes mitotic exit, by 17AAG (de Carcer, 2004). However, we cannot completely exclude the possibility that 17AAG abrogates the G2/M checkpoint by affecting other proteins in addition to Chk1 and Wee1.
Hsp90 clients seem to differ in their requirement for the molecular chaperone to maintain functionality. Some client proteins, such as the steroid receptors, require continuous chaperoning by Hsp90 until upon binding to their hormone ligands when the hormone-bound receptor dissociates from the molecular chaperone (Picard, 2006). However, for Chk1, the association with Hsp90 seems transient and might occur only shortly after translation of the kinase (Arlander et al., 2006). In the case of Wee1, we favor the latter scenario because of the following observations. First, in our coimmunoprecipitation experiments, although Wee1 could be found in the Hsp90 immunoprecipitates, despite multiple attempts, we were unable to detect Hsp90 in a reciprocal experiment in which immunoprecipitates were prepared using an anti-Wee1 or anti-Myc antibody (in the case of cells transfected with Myc-tagged-Wee1; data not shown), suggesting that only a small proportion of Wee1 is associated with Hsp90. These results are compatible with those reported by Arlander et al. (2006) in their coimmunoprecipitation experiments on Chk1. Second, in our metabolic labeling studies, we observed destabilization of radiolabeled Wee1 by 17AAG only when the drug was present both during and after the [35S]methionine pulse. When 17AAG was present only during the non-radioactive-chase portion of the experiment, the stability of newly synthesized Wee1 was not affected by the Hsp90 inhibitor, suggesting that once translated and presumably chaperoned, Wee1 does not require constitutive association with Hsp90 to maintain stability (A. N. Tse and G. K. Schwartz, unpublished observations).
Both Hsp90 and Chk1 have emerged recently as critical targets for cancer therapeutics. (Solit and Rosen, 2006; Tse et al., 2007a). Hsp90 inhibitors offer the potential for simultaneously disrupting multiple signaling events mediated by oncogenic proteins while maintaining selectivity against cancer cells compared with nontransformed cells. The basis for tumor selectivity of Hsp90-directed therapy remains elusive but seems to be related in part to the preferential retention of Hsp90 inhibitors in tumors, a phenomenon that has been demonstrated with a number of structurally unrelated compounds. Of considerable interest to the therapeutic areas of Hsp90 and checkpoint-targeting is the identification of critical checkpoint proteins such as Chk1 (Arlander et al., 2003) and Wee1 (Aligue et al., 1994; this report) as Hsp90 clients. Although an Hsp90 inhibitor can result in cytotoxicity via its pleiotropic effects of chaperone targeting, the induction of apoptosis after treatment with SN-38 and 17AAG in our system depends strictly on tumor p53 status. Thus, parental HCT116 cells with intact p53 were resistant to undergoing apoptosis induced by SN-38 and 17AAG compared with checkpoint-defective p53-null cells, despite the fact that Chk1 and Wee1 were depleted by 17AAG in both cell lines. An even more favorable therapeutic index can therefore be achieved by combining Hsp90 inhibitors with cytotoxic agents to selectively target tumors with intrinsic checkpoint defects, such as mutant p53 (Fig. 6C). A similar Wee1 depletion and p53-dependent abrogation of the G2/M checkpoint has been reported recently in cells treated with ionizing radiation and geldanamycin (Moran et al., 2008). It is interesting that the combination of SN-38 and 17AAG in p53 wild-type HCT116 cells was found to be antagonistic by median effect analysis (Fig. 6B). One possible explanation is that 17AAG results in a G1 and G2 arrest in wild-type HCT116 cells, reducing the exposure of cells in S phase to the replication-dependent cytotoxic effect of SN-38 (Fig. 1).
We found noticeable differences in the biochemistry and pharmacology of Chk1 targeting between a direct kinase inhibitor and Hsp90 inhibitor. Unlike 17AAG, which abrogates the SN-38-induced G2/M checkpoint exclusively in p53-null cells, treatment with Chk1 inhibitors such as UCN-01 or CHIR-124 results in significant checkpoint abrogation even in p53 wild-type cells, albeit to a lesser extent than in p53-null cells (Tse and Schwartz, 2004; Tse et al., 2007b). The basis for this selectivity for p53-defective cells is unclear but may be related to the slower kinetics of Chk1 inhibition attainable with 17AAG. The time course of Chk1 protein depletion from to Hsp90 inhibition is more akin to that produced by siRNA gene knockdown. In cells with an intact p53-p21 axis, the time-dependent induction of the potent cdk inhibitor p21 by SN-38 can potentially counteract the effect of a gradual Chk1 decline (Supplemental Fig. 2), rendering the cells resistant to undergoing G2/M checkpoint abrogation by Chk1 inhibition. It is noteworthy that cells that have escaped the G2/M checkpoint and entered mitosis induced by UCN-01 or CHIR-124 either undergo apoptosis during mitosis or develop micronucleation as they exit mitosis, whereas cells treated with SN-38 and 17AAG also undergo a highly aberrant mitosis, they do not show prominent micronucleation. Preliminary results from time-lapse studies on green fluorescent protein-H2B-expressing cells have shown that the majority of mitotic cells die by apoptosis before entering interphase (A. N. Tse and G. K. Schwartz, unpublished observations).
In summary, we show that 17AAG selectively abrogates the G2/M checkpoint in p53-defective cells by down-regulating two critical checkpoint kinases, Chk1 and Wee1. Based on the encouraging antitumor activity of Hsp90 inhibitors in general and the potential for specifically targeting checkpoint-defective tumors, a phase I clinical trial of 17AAG and irinotecan has been conducted.
Supplementary Material
Acknowledgments
We thank Caroll Pearce (Memorial Sloan-Kettering Cancer Center, New York) for editorial assistance.
The preliminary results of this work were presented previously at a mini-symposium of the 2005 American Association for Cancer Research Annual Meeting [Tse AN, Sheikh TN, and Schwartz GK (2005) The Hsp90 inhibitor, 17-allylamino-17-demethoxygeldanamycin (17AAG) abrogates the G2/M DNA damage checkpoint and induces apoptosis selectively in p53-defective colon cancer cells by down-regulating both Chk1 and Wee1. Proc Amer Assoc Cancer Res 46:Abstract 6159].
This work was supported in part by a Career Development Award from the American Society of Clinical Oncology and a K08 grant from the National Cancer Institute (to A.N.T.).
ABBREVIATIONS: MAPKAPK2 or MK2, mitogen-activated protein kinase-activated protein kinase 2; 17AAG, 17-allylamino-17-demethoxygeldanamycin; DAPI, 4′-6-diamidino-2-phenylindole; PAGE, polyacrylamide gel electrophoresis; SN-38, 7-ethyl-10-hydroxycamptothecin; Hsp90, 90-kDa heat shock protein; siRNA, short interfering RNA; PCR, polymerase chain reaction; ND, no drug; UCN-01, 7-hydroxystaurosporine; MG-132, N-benzoyloxycarbonyl (Z)-Leu-Leu-leucinal; CI, combination index; Fa, fraction affected; CHIR-124, ((S)-3-(1H-benzo(d)imidazol-2-yl)-6-chloro-4-quinuclidin-3-ylamino)quinolin-2(1H)-one.
The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
Footnotes
Early studies indicated that the ATM→ Chk2 pathway played a critical role in regulating cdc25A turnover; however, recent data suggest that Chk2, but not Chk1, may be dispensable for controlling the stability of cdc25A (Jin et al., 2008).
References
- Ahn J, Urist M, and Prives C (2003) Questioning the role of checkpoint kinase 2 in the p53 DNA damage response. J Biol Chem 278 20480-20489. [DOI] [PubMed] [Google Scholar]
- Aligue R, Akhavan-Niak H, and Russell P (1994) A role for Hsp90 in cell cycle control: Wee1 tyrosine kinase activity requires interaction with Hsp90. EMBO J 13 6099-6106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlander SJ, Eapen AK, Vroman BT, McDonald RJ, Toft DO, and Karnitz LM (2003) Hsp90 inhibition depletes Chk1 and sensitizes tumor cells to replication stress. J Biol Chem 278 52572-52577. [DOI] [PubMed] [Google Scholar]
- Arlander SJ, Felts SJ, Wagner JM, Stensgard B, Toft DO, and Karnitz LM (2006) Chaperoning checkpoint kinase 1 (Chk1), an Hsp90 client, with purified chaperones. J Biol Chem 281 2989-2998. [DOI] [PubMed] [Google Scholar]
- Blagosklonny MV, Toretsky J, Bohen S, and Neckers L (1996) Mutant conformation of p53 translated in vitro or in vivo requires functional HSP90. Proc Natl Acad Sci U S A 93 8379-8383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagosklonny MV, Toretsky J, and Neckers L (1995) Geldanamycin selectively destabilizes and conformationally alters mutated p53. Oncogene 11 933-939. [PubMed] [Google Scholar]
- Booher RN, Holman PS, and Fattaey A (1997) Human Myt1 is a cell cycle-regulated kinase that inhibits Cdc2 but not Cdk2 activity. J Biol Chem 272 22300-22306. [DOI] [PubMed] [Google Scholar]
- Bunch RT and Eastman A (1996) Enhancement of cisplatin-induced cytotoxicity by 7-hydroxystaurosporine (UCN-01), a new G2-checkpoint inhibitor. Clin Cancer Res 2 791-797. [PubMed] [Google Scholar]
- Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown JP, Sedivy JM, Kinzler KW, and Vogelstein B (1998) Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science 282 1497-1501. [DOI] [PubMed] [Google Scholar]
- Chehab NH, Malikzay A, Appel M, and Halazonetis TD (2000) Chk2/hCds1 functions as a DNA damage checkpoint in G1 by stabilizing p53. Genes Dev 14 278-288. [PMC free article] [PubMed] [Google Scholar]
- Chou TC (2006) Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev 58 621-681. [DOI] [PubMed] [Google Scholar]
- Chou TC and Martin NM (2005) CompuSyn for Drug Combinations: PC Software and User's Guide: A Computer Program for Quantitation of Synergism and Antagonism in Drug Combinations, and the Determination of IC50 and ED50 and LD50 Values. ComboSyn, Paramus, NJ.
- de Cárcer G (2004) Heat shock protein 90 regulates the metaphase-anaphase transition in a polo-like kinase-dependent manner. Cancer Res 64 5106-5112. [DOI] [PubMed] [Google Scholar]
- Goes FS and Martin J (2001) Hsp90 chaperone complexes are required for the activity and stability of yeast protein kinases Mik1, Wee1 and Swe1. Eur J Biochem 268 2281-2289. [DOI] [PubMed] [Google Scholar]
- Graves PR, Yu L, Schwarz JK, Gales J, Sausville EA, O'Connor PM, and Piwnica-Worms H (2000) The Chk1 protein kinase and the Cdc25C regulatory pathways are targets of the anticancer agent UCN-01. J Biol Chem 275 5600-5605. [DOI] [PubMed] [Google Scholar]
- Jallepalli PV, Lengauer C, Vogelstein B, and Bunz F (2003) The Chk2 tumor suppressor is not required for p53 responses in human cancer cells. J Biol Chem 278 20475-20479. [DOI] [PubMed] [Google Scholar]
- Jin J, Ang XL, Ye X, Livingstone M, and Harper JW (2008) Differential roles for checkpoint kinases in DNA damage-dependent degradation of the Cdc25A protein phosphatase. J Biol Chem 283 19322-19328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Kumagai A, and Dunphy WG (2001) Positive regulation of Wee1 by Chk1 and 14-3-3 proteins. Mol Biol Cell 12 551-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Guntuku S, Cui XS, Matsuoka S, Cortez D, Tamai K, Luo G, Carattini-Rivera S, DeMayo F, Bradley A, et al. (2000) Chk1 is an essential kinase that is regulated by Atr and required for the G2/M DNA damage checkpoint. Genes Dev 14 1448-1459. [PMC free article] [PubMed] [Google Scholar]
- Mailand N, Falck J, Lukas C, Syljuâsen RG, Welcker M, Bartek J, and Lukas J (2000) Rapid destruction of human Cdc25A in response to DNA damage. Science 288 1425-1429. [DOI] [PubMed] [Google Scholar]
- Manke IA, Nguyen A, Lim D, Stewart MQ, Elia AE, and Yaffe MB (2005) MAPKAP kinase-2 is a cell cycle checkpoint kinase that regulates the G2/M transition and S phase progression in response to UV irradiation. Mol Cell 17 37-48. [DOI] [PubMed] [Google Scholar]
- Matsuda S, Suzuki-Fujimoto T, Minowa A, Ueno H, Katamura K, and Koyasu S (1999) Temperature-sensitive ZAP70 mutants degrading through a proteasome-independent pathway. Restoration of a kinase domain mutant by Cdc37. J Biol Chem 274 34515-34518. [DOI] [PubMed] [Google Scholar]
- Moran DM, Gawlak G, Jayaprakash MS, Mayar S, and Maki CG (2008) Geldanamycin promotes premature mitotic entry and micronucleation in irradiated p53/p21 deficient colon carcinoma cells. Oncogene 27 5567-5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motwani M, Delohery TM, and Schwartz GK (1999) Sequential dependent enhancement of caspase activation and apoptosis by flavopiridol on paclitaxel-treated human gastric and breast cancer cells. Clin Cancer Res 5 1876-1883. [PubMed] [Google Scholar]
- Motwani M, Jung C, Sirotnak FM, She Y, Shah MA, Gonen M, and Schwartz GK (2001) Augmentation of apoptosis and tumor regression by flavopiridol in the presence of CPT-11 in Hct116 colon cancer monolayers and xenografts. Clin Cancer Res 7 4209-4219. [PubMed] [Google Scholar]
- Muñoz MJ, Bejarano ER, Daga RR, and Jimenez J (1999) The identification of Wos2, a p23 homologue that interacts with Wee1 and Cdc2 in the mitotic control of fission yeasts. Genetics 153 1561-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell MJ, Raleigh JM, Verkade HM, and Nurse P (1997) Chk1 is a wee1 kinase in the G2 DNA damage checkpoint inhibiting cdc2 by Y15 phosphorylation. EMBO J 16 545-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker LL and Piwnica-Worms H (1992) Inactivation of the p34cdc2-cyclin B complex by the human WEE1 tyrosine kinase. Science 257 1955-1957. [DOI] [PubMed] [Google Scholar]
- Picard D (2006) Chaperoning steroid hormone action. Trends Endocrinol Metab 17 229-235. [DOI] [PubMed] [Google Scholar]
- Powell SN, DeFrank JS, Connell P, Eogan M, Preffer F, Dombkowski D, Tang W, and Friend S (1995) Differential sensitivity of p53(-) and p53(+) cells to caffeine-induced radiosensitization and override of G2 delay. Cancer Res 55 1643-1648. [PubMed] [Google Scholar]
- Reinhardt HC, Aslanian AS, Lees JA, and Yaffe MB (2007) p53-deficient cells rely on ATM- and ATR-mediated checkpoint signaling through the p38MAPK/MK2 pathway for survival after DNA damage. Cancer Cell 11 175-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothblum-Oviatt CJ, Ryan CE, and Piwnica-Worms H (2001) 14-3-3 binding regulates catalytic activity of human Wee1 kinase. Cell Growth Differ 12 581-589. [PubMed] [Google Scholar]
- Russell KJ, Wiens LW, Demers GW, Galloway DA, Plon SE, and Groudine M (1995) Abrogation of the G2 checkpoint results in differential radiosensitization of G1 checkpoint-deficient and G1 checkpoint-competent cells. Cancer Res 55 1639-1642. [PubMed] [Google Scholar]
- Solit DB and Rosen N (2006) Hsp90: a novel target for cancer therapy. Curr Top Med Chem 6 1205-1214. [DOI] [PubMed] [Google Scholar]
- Sørensen CS, Syljuåsen RG, Falck J, Schroeder T, Rönnstrand L, Khanna KK, Zhou BB, Bartek J, and Lukas J (2003) Chk1 regulates the S phase checkpoint by coupling the physiological turnover and ionizing radiation-induced accelerated proteolysis of Cdc25A. Cancer Cell 3 247-258. [DOI] [PubMed] [Google Scholar]
- Srethapakdi M, Liu F, Tavorath R, and Rosen N (2000) Inhibition of Hsp90 function by ansamycins causes retinoblastoma gene product-dependent G1 arrest. Cancer Res 60 3940-3946. [PubMed] [Google Scholar]
- Sugimoto K, Sasaki M, Isobe Y, Tsutsui M, Suto H, Ando J, Tamayose K, Ando M, and Oshimi K (2008) Hsp90-inhibitor geldanamycin abrogates G2 arrest in p53-negative leukemia cell lines through the depletion of Chk1. Oncogene 27 3091-3101. [DOI] [PubMed] [Google Scholar]
- Taylor WR and Stark GR (2001) Regulation of the G2/M transition by p53. Oncogene 20 1803-1815. [DOI] [PubMed] [Google Scholar]
- Tse AN, Carvajal R, and Schwartz GK (2007a) Targeting checkpoint kinase 1 in cancer therapeutics. Clin Cancer Res 13 1955-1960. [DOI] [PubMed] [Google Scholar]
- Tse AN, Rendahl KG, Sheikh T, Cheema H, Aardalen K, Embry M, Ma S, Moler EJ, Ni ZJ, Lopes de Menezes DE, et al. (2007b) CHIR-124, a novel potent inhibitor of Chk1, potentiates the cytotoxicity of topoisomerase I poisons in vitro and in vivo. Clin Cancer Res 13 591-602. [DOI] [PubMed] [Google Scholar]
- Tse AN and Schwartz GK (2004) Potentiation of cytotoxicity of topoisomerase I poison by concurrent and sequential treatment with the checkpoint inhibitor 7-hydroxystaurosporine involves disparate mechanisms resulting in either p53-independent clonogenic suppression or p53-dependent mitotic catastrophe. Cancer Res 64 6635-6644. [DOI] [PubMed] [Google Scholar]
- Wang Q, Fan S, Eastman A, Worland PJ, Sausville EA, and O'Connor PM (1996) UCN-01: a potent abrogator of G2 checkpoint function in cancer cells with disrupted p53. J Natl Cancer Inst 88 956-965. [DOI] [PubMed] [Google Scholar]
- Watanabe N, Arai H, Nishihara Y, Taniguchi M, Watanabe N, Hunter T, and Osada H (2004) M-phase kinases induce phospho-dependent ubiquitination of somatic Wee1 by SCFβ-TrCP. Proc Natl Acad Sci U S A 101 4419-4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N, Broome M, and Hunter T (1995) Regulation of the human WEE1Hu CDK tyrosine 15-kinase during the cell cycle. EMBO J 14 1878-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman P (2004) Combinatorial attack on multistep oncogenesis by inhibiting the Hsp90 molecular chaperone. Cancer Lett 206 149-157. [DOI] [PubMed] [Google Scholar]
- Xiao Z, Chen Z, Gunasekera AH, Sowin TJ, Rosenberg SH, Fesik S, and Zhang H (2003) Chk1 mediates S and G2 arrests through Cdc25A degradation in response to DNA-damaging agents. J Biol Chem 278 21767-21773. [DOI] [PubMed] [Google Scholar]
- Zachos G, Rainey MD, and Gillespie DA (2003) Chk1-deficient tumour cells are viable but exhibit multiple checkpoint and survival defects. EMBO J 22 713-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WH, Poh A, Fanous AA, and Eastman A (2008) DNA damage-induced S phase arrest in human breast cancer depends on Chk1, but G2 arrest can occur independently of Chk1, Chk2 or MAPKAPK2. Cell Cycle 7 1668-1677. [DOI] [PubMed] [Google Scholar]
- Zhao H, Watkins JL, and Piwnica-Worms H (2002) Disruption of the checkpoint kinase 1/cell division cycle 25A pathway abrogates ionizing radiation-induced S and G2 checkpoints. Proc Natl Acad Sci U S A 99 14795-14800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou BB and Bartek J (2004) Targeting the checkpoint kinases: chemosensitization versus chemoprotection. Nat Rev Cancer 4 216-225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.