Abstract
Receptors coupled to the Gq and G12 families of heterotrimeric G proteins have surfaced rarely in the context of functional selectivity and always indirectly. We explore here the differential engagement of Gq and G13 (of the G12 family) by the thromboxane A2 receptor α (TPα), via agonist-effected [35S]-guanosine 5′-O-(3-thio)triphosphate binding when the G proteins themselves are used as reporters. We find for TPα introduced into human embryonic kidney 293 cells and for the receptor expressed normally in human platelets an agonist-selective engagement of Gq versus G13. Pinane thromboxane A2 (PTA2) activates Gq in preference to G13, whereas 8-iso-prostaglandin F2α activates G13 in preference to Gq. 9,11-Dideoxy-9α,11α-methanoepoxy-prosta-5Z,13E-dien-1-oic acid (U46619), in contrast, exhibits no preference. Reserve of receptor in relation to G protein and of G protein in relation to downstream events is apparent in some instances but does not have a bearing on selectivity. Activation of G proteins by PTA2 is right-shifted from binding of the ligand to receptor, a manifestation of which is a bimodal action: PTA2 is an antagonist at low concentrations and an agonist at higher concentrations. We posit two populations of TPα, or two intrinsic sites of ligand binding, with selectivity evident not only in terms of the G proteins activated but properties of antagonism versus agonism.
Functional selectivity refers to changes in the conformation of a receptor that are uniquely induced and perceived, respectively, by ligands and effectors specific to the receptor (for reviews, see Perez and Karnik, 2005; Urban et al., 2007). Selectivity is most often evident as differences in the rank-order of ligands in relation to efficacy and/or potency for distinct downstream events. In the case of 7-transmembrane domain receptors, events of interest are those set into motion by heterotrimeric G proteins, alone or in combination with those engaged by arrestins (Violin and Lefkowitz, 2007).
A great deal of information regarding selectivity has been gained through studies of receptors for serotonin (Berg et al., 1998, 2001; Kurrasch-Orbaugh et al., 2003; De Deurwaerdère et al., 2004), dopamine (Lawler et al., 1999; Kilts et al., 2002; Mottola et al., 2002; Gay et al., 2004; Ryman-Rasmussen et al., 2005), and opiates (Keith et al., 1996, 1998; Whistler et al., 1999; Alvarez et al., 2002), wherein measurements of selectivity as they pertain to G proteins are based on increases and decreases in cAMP, stimulation of phosphoinositide metabolism and consequent increases in intracellular calcium, and release of arachidonic acid. In some cases (for example, changes in cAMP and sometimes phosphoinositide metabolism), the G proteins employed are obvious; in others, they are not. Inferences of selectivity through second messengers are blurred to some extent by activities shared among G proteins, nonlinearity in signal transmission, and the potential in intact cells for cross-regulation of signaling pathways. Several studies have explored, consequently, functional selectivity more directly through measurements of G protein activation, notably using [35S]guanosine 5′-O-(3-thio)triphosphate ([35S]GTPγS) (Cordeaux et al., 2001) (Cussac et al., 2002) (Lane et al., 2007). Studies using G protein activation are important in that they provide a framework for inferences of receptor conformation that are proximal to the receptor yet operational in nature.
The G12 family of heterotrimeric G proteins in vertebrates consists of G12 and G13. The family has received considerable attention in studies of cell function, especially cell contractility and adhesion, with targets comprising the monomeric G protein Rho, cadherins, and Tec tyrosine kinases, among others (for review, see Kelly et al., 2007). The biochemical properties of the G12 family are notable (Singer et al., 1994; Kozasa and Gilman, 1995). One of the most striking aspects of receptors coupled to G12 and/or G13 is that they are invariably coupled to other G proteins as well, almost always Gq (Riobo and Manning, 2005). Events elicited through the G12 family are perceived, therefore, to be integrated with events achieved through additional families. In platelets, for example, both thrombin and thromboxane A2 (TXA2) act on receptors coupled to the G12 and Gq families. The initial response of platelets to either of the ligands is a change in shape (rounding) that requires G13 (Offermanns, 2006). The change is a prerequisite to degranulation and aggregation, which are achieved subsequently through Gq. The Gq-dependent aggregation is partly mediated through release of ADP by degranulation and subsequent engagement of Gi. Efficient activation of platelets by thrombin or TXA2, therefore, requires direct engagement of Gq and G13 and indirect engagement of Gi.
Given that receptors coupled to G12 and/or G13 invariably couple to other G proteins, and that the integration of signaling is important, a question of considerable interest is whether agonists working through these receptors exhibit functional selectivity. No studies toward this end have been conducted. A large part of the difficulty is the absence of quantifiable enzymes and second messengers uniquely regulated by the G12 family. In this study, we used [35S]GTPγS-binding to evaluate functional selectivity for agonists operating through the thromboxane A2 receptor TPα. The data reveal, for the first time, selectivity in agonists that engage the Gq and G12 families of G proteins and reveal as well an unusual bimodal action of a supposed antagonist.
Materials and Methods
Materials. U46619, SQ29548, pinane thromboxane A2 (PTA2), and 8-iso-prostaglandin F2α (8-iso-PGF2α) were purchased from Cayman Chemical Company (Ann Arbor, MI). [35S]GTPγS (1250 Ci/mmol) and [3H]SQ29548 (44 Ci/mmol) were purchased from PerkinElmer Life and Analytical Sciences, Inc. (Waltham, MA). Aprotinin, apyrase, normal rabbit serum, GDP, GTP, protein A-Sepharose, and Sepharose 2B were purchased from Sigma-Aldrich (St. Louis, MO). Pansorbin cells and Nonidet P-40 were purchased from Thermo Fisher Scientific (Waltham, MA). Rabbit antisera for Gαq and Gα13 were produced using peptides corresponding to the C-terminal 10 residues of the two proteins (Butkerait et al., 1995; Windh et al., 1999).
Cells and Membrane Preparation. Human embryonic kidney (HEK) 293 cells stably expressing TPα [4.8 pmol receptor per mg of membrane protein (Wilson et al., 2004)] were maintained in Dulbecco's modified Eagle's medium with 10% fetal bovine serum and 0.5 mg/ml G418 at 37°C with 5% CO2. Cells were harvested, washed three times with phosphate-buffered saline, and lysed in 20 mM HEPES, pH 8.0, 1 mM EDTA, 0.1% aprotinin, 0.02% leupeptin, and 0.1% phenylmethylsulfonyl fluoride by repeated passage through a 26-gauge needle. The homogenate was centrifuged at 660g for 5 min, and the resultant supernatant was centrifuged at 20,800g for 30 min at 4°C. The pellet (membrane) was resuspended at ∼3 mg/ml protein.
For platelets, human blood was obtained from healthy donors who denied ingestion of aspirin or any other drug for at least 1 week. The donated blood was collected into a one-sixth volume of a solution containing 65 mM trisodium citrate, 60 mM citric acid, and 100 mM dextrose, pH 4.4. Platelet-rich plasma was isolated by centrifugation of the citrated blood at 180g for 20 min at room temperature, and platelets were obtained thereafter by sedimentation at 880g for 15 min. Platelets were resuspended in a small volume of 10 mM triethanolamine and 5 mM EDTA, pH 6.8, and lysed by immersion in liquid nitrogen then thawing. The lysate was centrifuged at 20,800g for 30 min at 4°C to obtain a membrane pellet, which was resuspended at approximately 3 mg/ml protein in 10 mM triethanolamine, pH 6.8.
[35S]GTPγS-Binding. The assay for agonist-promoted binding of [35S]GTPγStoGαq and Gα13 was performed essentially as described previously (Windh et al., 1999; Zhang et al., 2006). Membranes (20 μg of protein/assay point) were resuspended in 50 mM Tris-HCl, pH 7.5, 2 mM EDTA, 100 mM NaCl, 20 mM MgCl2, 0.1 μM GDP, and 5 nM [35S]GTPγS in 1.5-ml microcentrifuge tubes on ice. Ligands, if any, were added, and the tubes were transferred immediately to a 30°C water bath for 2 (Gq) or 1 (G13) min. The incubation was terminated by adding 600 μl of ice-cold 50 mM Tris-HCl, pH 7.5, 20 mM MgCl2, 150 mM NaCl, 0.5% Nonidet P-40, 0.33% aprotinin, 0.1 mM GDP, and 0.1 mM GTP. The extract was transferred to a microcentrifuge tube containing 2 μl of nonimmune serum preincubated with 100 μl of a 10% suspension of Pansorbin cells. Nonspecifically bound proteins were removed after 20 min by centrifugation. The extract was incubated for 1 h at 4 °C with 10 μl of a Gα-directed antiserum or nonimmune serum, both of which had been preincubated with 100 μl of a 5% suspension of protein A-Sepharose. Immunoprecipitates were collected and washed three times in the extraction buffer, then once in the buffer without detergent, and then boiled in 0.5 ml of 0.5% SDS followed by addition of 5 ml of Ecolite+ (MP Biomedicals, Irvine, CA). The samples were analyzed directly by scintillation spectrometry. Counts obtained with nonimmune serum, representing nonspecifically bound radiolabel and generally in the range of 50 to 200 cpm, were subtracted before portrayal of the data. In experiments involving inhibition of U46619's actions by PTA2, the two ligands were added simultaneously before incubation at 30°C.
Platelet Shape-Change and Aggregation. Platelet-rich plasma was incubated with 1 mM aspirin and, for experiments relating to shape change (rounding), 0.1 unit/ml apyrase. Platelets were then isolated by gel filtration on Sepharose 2B using modified Tyrode's buffer (137 mM NaCl, 2.7 mM KCl, 1 mM MgCl2, 11.9 mM NaHCO3, 3.6 mM NaH2PO4, 10 mM HEPES, 0.2% bovine serum albumin, and 5.5 mM glucose, adjusted pH to 7.4). Agonist-induced platelet shape-change and aggregation were determined in separate experiments by measuring the transmission of light through a 0.3-ml sample of the aspirin-treated, gel-filtered platelets (2 × 108 cells/ml) with stirring in a lumi-aggregometer (CHRONO-LOG CO., Havertown, PA) at 37°C (Prevost et al., 2002). The baseline was set using 0.5 ml of Tyrode's buffer as a blank. Shape-change in response to agonists was evaluated as a decrease in transmission, which was scored as percentage maximum attained; 1 μM tirofiban was included in these experiments to prevent aggregation. Aggregation was evaluated (without tirofiban) as an increase in transmission in the presence of 1 mM CaCl2. Aggregation was scored as incidence because of its steeply graded nature.
Meaurement of Cytosolic Calcium. Platelet-rich plasma was incubated with 1 mM aspirin and 5 μM Fura-2/acetoxymethyl ester for 1 h at 37°C. Platelets were then isolated by gel filtration as described under Cells and Membrane Preparation and, after adjusting to 2 × 108 cell/ml, were placed into a luminescence spectrometer (PerkinElmer Life and Analytical Sciences). Excitation wavelength was set to 340 nm, and emission was evaluated at a wavelength of 510 nm. Calcium was expressed as concentration using a Kd for Fura-2 of 224 nM (Grynkiewicz et al., 1985).
[3H]SQ29548 Displacement. Membranes (20 μg of protein/assay point) were incubated with 20 nM [3H]SQ29548 in the presence or absence of other ligands at specified concentrations in 20 mM HEPES, pH 7.4, 2 mM EDTA, and 5 mM NaCl for 30 min at 30°C. The incubation volume was 0.1 ml. Reactions were terminated by dilution with 10 mM HEPES, pH 7.4, and 0.01% BSA at 0°C and rapid filtration over Whatman GF/C filters presoaked in the same buffer. The filters were washed three times with the same buffer at 0°C and dried. Filter-bound radioactivity was determined by scintillation spectrometry. Nonspecific binding, generally less than 10% at Kd, was defined as the binding of radioligand in the presence of 100 μM SQ29548.
Miscellaneous. Data were analyzed using Prism Software (Graph Pad Software, San Diego, CA). [35S]GTPγS-binding was evaluated by nonlinear regression analysis using a logistic equation and a Hill slope of 1. Displacement of [3H]SQ29548 was fit to a one-site competition mode (a two-site model provided no better fit). Statistical differences in EC50 or Ki values were determined using a two-tailed Student's t test, p < 0.05 signifying a difference.
Results
Most receptors that are coupled to G proteins of the G12 family couple to those of the Gq family as well. Despite the importance of these receptors and the general interest in functional selectivity, differences in engagement of the two G protein families have not been investigated. Studies were initiated here with TPα, a receptor for TXA2, and the G proteins Gq and G13.G13 is a member of the G12 family, the actions of which are essential to those of TPα in platelets. The ligands employed were U46619, PTA2, and 8-iso-PGF2α.
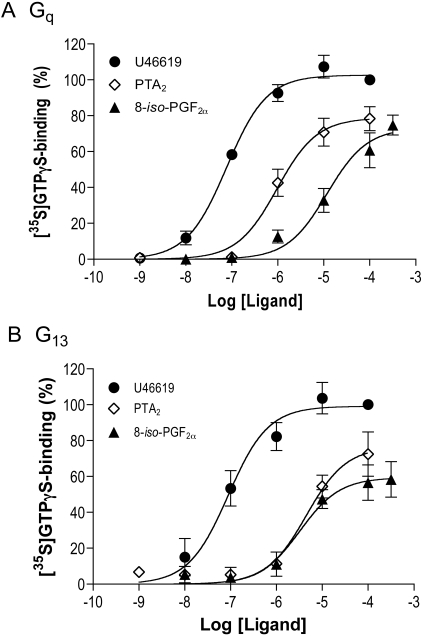
The activation of Gq and G13 through TPα was evaluated first with HEK 293 cells in which TPα was introduced and stably expressed (Wilson et al., 2004). Activation was evaluated as ligand-promoted binding of [35S]GTPγS to endogenous Gα subunits. U46619, PTA2, and 8-iso-PGF2α all activated Gq and, as we reported previously (Zhang et al., 2006), G13 (Fig. 1). U46619 was the most potent, exhibiting an EC50 of approximately 80 nM for both G proteins (Table 1). PTA2 was more potent than 8-iso-PGF2α in activating Gq, whereas the two were similar in activating G13. It is noteworthy that PTA2 activated Gq in preference to G13 (EC50 = 1 μM for Gq versus 4.5 μM for G13, p = 0.03), whereas the converse was true for 8-iso-PGF2α (EC50 = 3.4 μM for G13 versus 12 μM for Gq, p = 0.04). Activation in membranes of cells where TPα was not introduced was negligible. These data, in which opposite preferences for effectors (G proteins) by two agonists were observed, connote functional selectivity.
Fig. 1.
Activation of Gq and G13 through TPα in HEK 293 cell membranes. Membranes were prepared from HEK 293 cells stably expressing TPα and incubated with U46619, PTA2, and 8-iso-PGF2α at the concentrations indicated, together with [35S]GTPγS. Binding of [35S]GTPγStoGα subunits of endogenous Gq (A) and G13 (B) was determined by immunoprecipitation with Gα-selective antibodies and scintillation spectrometry. The data are expressed as a percentage binding obtained with 100 μM U46619, equivalent to approximately 3000 cpm for Gq and 500 cpm for G13, minus binding in the absence of agonist, approximately 150 cpm. Each point represents the mean ± S.E. of three to five independent experiments.
TABLE 1.
Potency of ligands in promoting activation of Gq and G13 through TPα
The activation of Gq and G13 was evaluated in membranes of HEK 293 cells stably (over)expressing TPα and membranes of human platelets. EC50 values for U46619, PTA2, and 8-iso-PGF2α were determined by nonlinear regression on concentration-response data. Each value is the average of three to five experiments, with 95% confidence intervals (C.I.) given in parentheses. P values for comparisons between Gq and G13 for individual ligands are also noted.
|
EC50 (95% C.I.)
|
P
|
||
|---|---|---|---|
| Gq | G13 | ||
| μM | |||
| HEK 293/TPα | |||
| U46619 | 0.078 (0.058-0.10) | 0.089 (0.049-0.16) | 0.8 |
| PTA2 | 1 (0.53-1.8) | 4.5 (1.9-10) | 0.03 |
| 8-iso-PGF2α | 12 (5.9-24) | 3.4 (1.7-7.2) | 0.04 |
| Platelets | |||
| U46619 | 0.39 (0.35-0.45) | 0.31 (0.24-0.39) | 0.1 |
| PTA2 | 3.1 (1.7-5.6) | 14 (8.1-25) | 0.008 |
| 8-iso-PGF2α | 210 (110-400) | 35 (20-64) | 0.003 |
We turned to human platelets, for which TPα and the G proteins are both endogenous (the existence of TPβ as well in platelets is a possibility, hence the term `TP' hereafter, but see Habib et al., 1999). U46619, PTA2, and 8-iso-PGF2α again activated Gq and G13 (Fig. 2). U46619 remained the most potent of the three and did not distinguish between the two G proteins (EC50 = 0.3-0.4 μM). PTA2 was more potent than 8-iso-PGF2α in activating Gq, although it was less efficacious. PTA2 and 8-iso-PGF2α activated G13 with similar potency, but here 8-iso-PGF2α was the less efficacious. Selectivity referenced to potency was again evident: PTA2 activated Gq in preference to G13 (EC50 = 3 μM for Gq versus 14 μM for G13, p = 0.008), whereas 8-iso-PGF2α activated G13 in preference to Gq (EC50 = 35 μM for G13 versus 210 μM for Gq, p = 0.003). Activation of the two G proteins by all three ligands was blocked by the TP antagonist SQ29548 (data not shown). The selectivity noted upon overexpression of TPα in HEK 293 cells, therefore, was corroborated in a setting where receptor and G proteins are expressed normally.
Fig. 2.
Activation of Gq and G13 through TP in platelet membranes. Membranes were prepared from human platelets, and the activation of Gq (A) and G13 (B) in response to U46619, PTA2, and 8-iso-PGF2α was evaluated as described in the legend to Fig. 1. The data are expressed as a percentage binding obtained with 100 μM U46619, equivalent to approximately 8000 cpm for Gq and 1500 cpm for G13, minus binding in the absence of agonist, approximately 500 cpm. Each point represents the mean ± S.E. of three to five independent experiments.
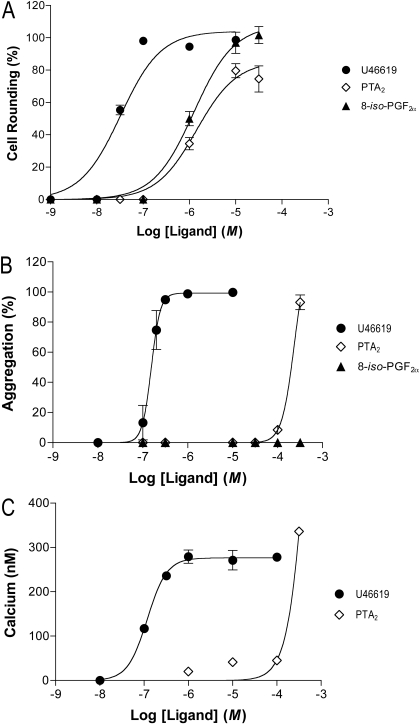
One of the reasons for measuring selectivity using G proteins is that the phenomenon is less likely to be obscured by idiosyncrasies in signal propagation and regulation attending downstream events. With this in mind, we examined platelet rounding and aggregation, respectively, as correlates of G13 and Gq activation. All three ligands, as expected, caused rounding (Fig. 3, top). The EC50 values were shifted leftward 10- to 20-fold from those for G13 activation, suggesting either a surplus of G13 in relation to downstream events (analogous to receptor reserve) or a better coupling of receptor and G protein in the intact cell. The maximal effects of the three ligands were similar. Rounding caused by the ligands was inhibited by SQ29548. Activation of G13 through one or more forms of TP by all three ligands translates, therefore, into a physiological response. This was not the case for aggregation.
Fig. 3.
Response of intact platelets to ligands for TP. The response of human platelets to U46619, PTA2, and 8-iso-PGF2α was evaluated in terms of cell rounding (top), aggregation (middle), and intracellular calcium (bottom; data for U46619 and PTA2 only). Rounding and aggregation are expressed as a percentage of that obtained with 10 μM U46619. Each point represents the mean ± S.E. of three to five independent experiments.
U46619 elicited, as expected, aggregation (Fig. 3, middle), which was steeply graded and inhibited by SQ29548. The EC50 for aggregation (135 nM) was close to that for activation of Gq in membranes, suggesting little if any Gq reserve. PTA2, however, did not effect aggregation at any but the highest concentration tested (300 μM), and the aggregation was not inhibited by SQ29548, indicating the effect to be nonspecific. 8-iso-PGF2α was without effect altogether. The activation of Gq noted for PTA2 and 8-iso-PGF2α through TP, therefore, was inconsequential to aggregation. The ability of U46619 to effect aggregation was paralleled by its ability to effect a substantial increase in intracellular calcium (Fig. 3, bottom panel). We suspect that the inability of PTA2 and 8-iso-PGF2α to effect aggregation or large increases in calcium through one or more forms of TP is due to a required threshold in Gq activation. The selectivity noted for G protein activation was, nonetheless, not evident in downstream events, underscoring the problems inherent to these events as measures of the phenomenon.
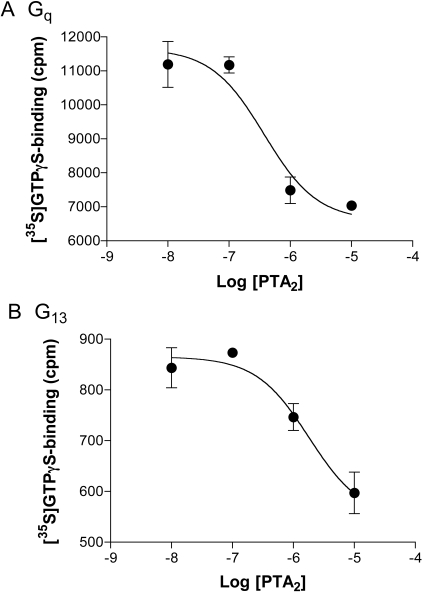
Selectivity can explain preferential engagement of one G protein or another by a ligand, but it has the ability to explain other actions as well. Contrary to the above-noted properties of PTA2 as an activator of G proteins through TPα, PTA2 is most often viewed to be an antagonist of the receptor (for example Nicolaou et al., 1979; Nie et al., 2008). We wondered whether the difference between our results and cited actions of PTA2 as an antagonist might rest with a confusion of weak agonism for antagonism, at least at the level of Gq, or with functional selectivity of some form. We found that PTA2 inhibited the activation of Gq by U46619 (Fig. 4, top), and that the activity at the highest concentration of PTA2 (10 μM) was that of PTA2 alone. This finding conforms to what one would expect for a partial agonist. Of interest, however, was what seemed to be the unusual potency of PTA2 with respect to inhibition: the IC50 was 0.5 μM when PTA2 was tested against 1 μM U46619, implying an affinity for receptor slightly greater than that of U46619 itself. This finding was in distinction to the data for G protein activation, in which the potency of PTA2 was 8-fold less than that of U46619. The distinction was even more striking when the activation of G13 was evaluated. PTA2 is a full agonist with regard to G13, hence suppression of U46619-promoted activation by PTA2 should not be discernible. This was not the case. PTA2 inhibited U46619-effected activation of G13 with an IC50 of 0.7 μM (Fig. 4, bottom), well below the EC50 (14 μM) for its activation of G13. The effects of PTA2 in the presence of U46619 were biphasic (not shown), with the inhibition at low concentrations followed by an activation analogous to that noted previously for PTA2 alone at higher concentrations. PTA2, therefore, displayed concentration-dependent opposing actions at the level of each of the two G proteins.
Fig. 4.
Inhibition by PTA2 of U46619's activation of Gq and G13 in platelet membranes. Membranes from human platelets were incubated with 1 μM U46619, the indicated concentrations of PTA2, and [35S]GTPγS. Binding of [35S]GTPγStoGαq and Gα13 was evaluated as described in the legend to Fig. 2. The data are from single experiments performed in duplicate, which are representative of two others each for Gq and G13, and are expressed as counts per minute.
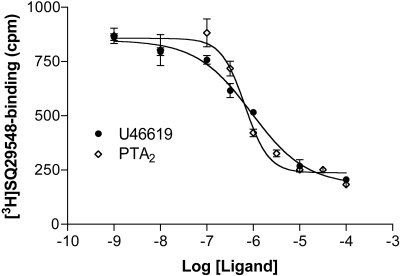
To help clarify the behavior of PTA2, we evaluated the affinity displayed by U46619 and PTA2 for receptor(s) on platelet membranes by displacement assays using [3H]-SQ29548. The Ki for SQ29548 with platelet membranes was 40 nM (Table 2). Both U46619 and PTA2 displaced the radiolabel with Ki values of 0.7 and 0.4 μM, respectively (Fig. 5 and Table 2). In the case of U46619, therefore, binding was roughly coincident with activation of Gq and G13 (EC50 = 0.3-0.4 μM, as noted above). For PTA2, binding was consistent with its antagonism of U46619 but not with its activation of G proteins—the Ki values for PTA2 were 5- and 30-fold lower than EC50 values for Gq and G13 activation (3 and 14 μM, respectively). Variations in Mg2+ and/or the inclusion of guanosine 5′-(β,γ-imido)triphosphate or GTPγS had no impact. Viewed from the perspective of concentration-response relationships, therefore, the activation of G proteins by PTA2 in platelet membranes was shifted rightward from binding. The Hill slopes for U46619 and PTA2 were -0.85 ± 0.10 (n = 3) and -1.25 ± 0.16 (n = 6), respectively. These values were not statistically different from each other or from 1; however, the power of the analysis was not so high as to completely rule out a difference in the two slopes that might connote differences in cooperativity.
TABLE 2.
Binding of ligands to TP
Ki values were determined for SQ29548, U46619, and PTA2 in displacement assays with 20 nM [3H]SQ29548 using membranes prepared from human platelets and HEK 293 cells, in the latter case stably (over)expressing TPα. Each assay was conducted with five or six concentrations of ligand in duplicate. Each reported value is the mean; 95% confidence intervals, obtained by nonlinear regression on data accumulated from three or four experiments, are in parentheses.
|
Ki
|
||
|---|---|---|
| Human Platelets | HEK 293αTPα | |
| μM | ||
| SQ29548 | 0.04 (0.03-0.06) | 0.07 (0.05-0.10) |
| U46619 | 0.7 (0.5-1.0) | 0.8 (0.7-1.1) |
| PTA2 | 0.4 (0.3-0.6) | 0.7 (0.5-1.1) |
Fig. 5.
Displacement of [3H]SQ29548 from platelet membranes. Platelet membranes were incubated with 20 nM [3H]SQ29548 in the absence or presence of increasing concentrations of U46619 and PTA2 for 30 min at 30°C. Bound radiolabel was determined subsequently by filtration. Shown is a single experiment, performed in duplicate for each displacing ligand, which is representative of several others.
A rightward shift in an effect is almost always due to the relevance of two receptors differing in affinity for the ligand, in which the effect is achieved through the one having a lower affinity, but the binding is evident only for the one having the higher affinity for reasons of selectivity in radiolabeling or disproportionate levels of receptor. We turned to HEK 293 cells, therefore, to evaluate TPα in isolation, aware that the receptor reserve implied above and elsewhere (Zhang et al., 2006), which would cause leftward shifts, might complicate the analysis. The binding data were quite similar to those of platelets. U46619 and PTA2 were comparable with each other in terms of affinities for TPα, having Ki values of 0.8 and 0.7 μM, respectively (Table 2). As anticipated for receptor reserve, the activation of Gq and G13 by U46619 (EC50 ∼ 0.08 μM in HEK 293 cell membrane) was left-shifted from binding. Despite the reserve, the activation of G proteins by PTA2 (EC50 values = 1 μM for Gq and 4.5 μM for G13) was not left-shifted; in fact, the activation of G13 remained right-shifted from binding. Therefore, two “different” receptors can be entertained as a basis for differences in binding and activation; however, the two would originate with TPα, presumably through different conformations. An alternative is that two sites for PTA2 exist on TPα, accounting for the duality in the actions of PTA2.
Discussion
The G12 family has surfaced only rarely in the context of functional selectivity and always indirectly. The family is possibly involved in the selectivity exhibited through the 5-hydroxytryptamine2C receptor in reference to phosphoinositide accumulation and arachidonic acid release (Berg et al., 1998), because RNA-edited forms of the receptor, which are uncoupled from the G12 family (Price et al., 2001), no longer exhibit selectivity (Berg et al., 2001). The family might also underlie the selectivity exhibited through the 5-hydroxytryptamine2A receptor, presuming the family again to be involved in arachidonic acid release (Berg et al., 1998; Kurrasch-Orbaugh et al., 2003). We are aware of no other possible examples. The paucity of information regarding the G12 family is not entirely unexpected, in that the actions of the family are not easily separated from those of other signaling entities nor easily quantified through regulation of second messengers, proximate enzymes, or ion channels. However, receptors coupled to the G12 family are basic to cell function and, because they are invariably coupled to G proteins of other families, provide ample potential for selectivity.
We approached the need for uniquely linked and quantifiable effectors by measuring G protein activation directly, using agonist-promoted exchange of GDP for [35S]GTPγS. The assay's utility in measurements of potency and efficacy has already been documented (Barr and Manning, 1997; Windh et al., 1999; Windh and Manning, 2002; Zhang et al., 2006), and extension to selectivity was made most recently for the dopamine D2 receptor in relation to subtypes of the Gi family (Lane et al., 2007). The use of proximal effectors (i.e., G proteins) and cell membranes avoids pitfalls of nonlinearity and convergence in downstream signaling and superimposed forms of regulation that confound results in the intact cell. The choice in this study of Gq and G13 for analysis was based on the large number of receptors that engage these two G proteins specifically (Riobo and Manning, 2005) and the relevance of both to platelet function (Offermanns et al., 1997; Moers et al., 2003; Offermanns, 2006).
We found that Gq and G13 were, in fact, differentially engaged by agonists for TPα. PTA2 activated Gq at lower concentrations than it did G13, whereas the converse was true for 8-iso-PGF2α. Selectivity for the two agonists was discerned in membranes of both HEK 293 cells overexpressing TPα and platelets. U46619, in contrast, exhibited no selectivity. The opposite (PTA2 versus 8-iso-PGF2α) and nonselective (U46619) patterns of G protein engagement are important in precluding receptor reserve alone as a basis for the phenomenon.
Platelet rounding, a measure of G13 action, was elicited by all three ligands; however, aggregation, which requires Gq as well, was elicited by U46619 alone through TP. This observation is consistent with our assertion that functional selectivity cannot always be detected through events downstream of G proteins. The more proximal the effector being measured to receptor, we believe, the more likely the measure is to be faithful to receptor conformation. We suspect for platelets that aggregation requires a threshold level of activated Gq, noting that neither PTA2 nor 8-iso-PGF2α can achieve full activation of this G protein. It is noteworthy that the EC50 for U46619 in aggregation was congruent with the EC50 in activation of Gq and the Ki for binding of U46619 to platelet membranes, suggesting little or no reserve of receptor or G protein. This finding was in distinction to rounding, for which the concentration-response relationship appeared to be substantially left-shifted from G protein activation and binding, suggesting a reserve of receptor and/or receptor-activated G13. This observation is important—any nonselective ligand such as U46619 would trigger rounding at concentrations much lower than aggregation, an action that might be confused with true (functional) selectivity. Again, the choice of G proteins to evaluate selectivity is essential. The reserve here has nothing to do with, and does not impede, the deduction of selectivity using G proteins as endpoints.
In evaluating the properties of PTA2, we noted in platelets an antagonistic action for the ligand that did not conform to what would be expected for even a weak agonist; i.e., PTA2 blocked the actions of U46619 at concentrations well below those anticipated from the affinity suggested by its EC50 in G protein activation. We found that the EC50 values for activation of Gq and G13 by PTA2 were, in fact, right-shifted from the apparent binding of the ligand: the EC50 for activation of Gq was shifted from the Ki by approximately 8-fold, whereas that for activation of G13 was shifted by approximately 35-fold. Differences between the [35S]GTPγS-binding and displacement assays are unlikely to account for the shift, because buffer constituents in the activation assay (for example, Mg2+ and/or GTPγS) had no impact on displacement of [3H]SQ29548. More compellingly, no meaningful shift was evident for U46619; the shift was unique to PTA2.
The almost universal explanation for a rightward shift of function from binding is the existence of two different receptors. The relevance of any receptor apart from TPα itself, however, is doubtful. The receptor labeled with [3H]SQ29548 in platelet membranes is mostly if not solely TPα (Habib et al., 1999). The rightward shift for activation of G13, moreover, is also noted in HEK 293 cells made to express TPα. The possibility of TPβ might be entertained in platelets, but it cannot account for the results in HEK 293 cells. We suggest as one possibility, therefore, that TPα itself resolves into two populations. PTA2 would bind to one as an antagonist with relatively high affinity and to the other as an agonist with lower affinity. The latter population would not be recognized by [3H]SQ29548 binding because of low density (i.e., the population is beneath the threshold of detection by radiolabeling) or low affinity of the population for the radioligand, such that binding is not stable in the filtration assay. The existence of different populations of TPα is easily posited. Populations can arise through heterodimerization, other forms of oligomerization, or free receptor versus that complexed to the assayed G protein. Populations might also arise by virtue of the receptor interacting with other proteins, perhaps “silent” G proteins (such as Gi or G12), regulators of G protein signaling, or β-arrestin, or as a function of location within membrane (Zheng et al., 2008). Our data might also be explained by two sites for binding of ligands on TPα, one used by U46619 as an agonist and PTA2 as an antagonist, and the other by PTA2 as an agonist. We note the resemblance of our data for PTA2 with TPα to those obtained by Baker et al. (2003) for CGP12177 with the β1-adrenoreceptor, where distinct conformations or activation sites were similarly posited. Functional duality speaks to the possibility of selectivity apparent not so much at the level of one G protein or another but at basic distinctions between agonism and antagonism.
The data obtained here for TPα underscore functional selectivity in relation to the Gq and G12 families, the potential for reserves of receptor and/or G protein that can mimic selectivity, and populations of a single receptor that relate to the agonistic properties of a ligand (PTA2). All of these introduce a largely unrecognized and important complexity to the coordination of signaling noted for the Gq and G12 families. Remaining questions pertain to the basis of underlying conformations of receptor, specific links to events downstream of the G proteins, and extension to other receptors coupled conjointly to the two families.
Acknowledgments
We thank Cherisse DiLizio for her many technical contributions to this work.
This work was supported by National Institutes of Health grant GM066892.
ABBREVIATIONS: GTPγS, guanosine 5′-O-(3-thio)triphosphate; TXA2, thromboxane A2; U46619, 9,11-dideoxy-9α,11α-methanoepoxy-prosta-5Z,13E-dien-1-oic acid; SQ29548, [1S-[1α,2α(Z),3α,4α]]-7-[3-[[2-[(phenyl amino)carbonyl]hydrazine]methyl]-7-oxabicyclo[2.2.1]hept-2-yl]-5-heptenoic acid; PTA2, pinane-thromboxane A2; 8-iso-PGF2α, 8-iso-prostaglandin F2α; HEK, human embryonic kidney; TP, receptor(s) for thromboxane A2; CGP12177, 4-[3-[(1,1-dimethylethyl)amino]-2-hydroxypropoxy]-1,3-dihydro-2H-benzimidazol-2-one.
References
- Alvarez VA, Arttamangkul S, Dang V, Salem A, Whistler JL, Von Zastrow M, Grandy DK, and Williams JT (2002) μ-Opioid receptors: ligand-dependent activation of potassium conductance, desensitization, and internalization. J Neurosci 22 5769-5776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JG, Hall IP, and Hill SJ (2003) Agonist actions of “β-blockers” provide evidence for two agonist activation sites or conformations of the human β1-adrenoreceptor. Mol Pharmacol 63 1312-1321. [DOI] [PubMed] [Google Scholar]
- Barr AJ and Manning DR (1997) Agonist-independent activation of Gz by the 5-HT1A receptor co-expressed in Sf9 cells: distinguishing inverse agonists from neutral antagonists. J Biol Chem 272 32979-32987. [DOI] [PubMed] [Google Scholar]
- Berg KA, Cropper JD, Niswender CM, Sanders-Bush E, Emeson RB, and Clarke WP (2001) RNA-editing of the 5-HT2C receptor alters agonist-receptor-effector coupling specificity. Br J Pharmacol 134 386-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg KA, Maayani S, Goldfarb J, Scaramellini C, Leff P, and Clarke WP (1998) Effector pathway-dependent relative efficacy at serotonin Type 2A and 2C receptors: Evidence for Agonist-directed trafficking of receptor stimululs. Mol Pharmacol 54 94-104. [PubMed] [Google Scholar]
- Butkerait P, Zheng Y, Hallak H, Graham TE, Miller HA, Burris KD, Molinoff PB, and Manning DR (1995) Expression of the human 5-hydroxytryptamine1A receptor in Sf9 cells. J Biol Chem 270 18691-18699. [DOI] [PubMed] [Google Scholar]
- Cordeaux Y, Nickolls SA, Flood LA, Graber SG, and Strange PG (2001) Agonist regulation of D2 domapine receptor/g protein interaction. J Biol Chem 276 28667-28675. [DOI] [PubMed] [Google Scholar]
- Cussac D, Newman-Tancredi A, Duqueyroix D, Pasteau V, and Millan MJ (2002) Differential activation of Gq/11 and Gi3 proteins at 5-hydroxytryptamine2C receptors revealed by antibody capture assays: Influence of receptor reserve and relationship to agonist-directed trafficking. Mol Pharmacol 62 578-589. [DOI] [PubMed] [Google Scholar]
- De Deurwaerdère P, Navailles S, Berg KA, Clarke WP, and Spampinato U (2004) Constitutive activity of the serotonin2C receptor inhibits in vivo dopamine release in the rat striatum and nucleus accumbens. J Neurosci 24 3235-3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay EA, Urban JD, Nichols DE, Oxford GS, and Mailman RB (2004) Functional selectivity of D2 receptor ligands in a Chinese hamster ovary hD2L cell line: evidence for induction of ligand-specific receptor states. Mol Pharmacol 66 97-105. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, and Tsien RY (1985) A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260 3440-3450. [PubMed] [Google Scholar]
- Habib A, FitzGerald GA, and Maclouf J (1999) Phosphorylation of the thromboxane receptor A, the predominant isoform expressed in human platelets. J Biol Chem 274 2645-2651. [DOI] [PubMed] [Google Scholar]
- Keith DE, Anton B, Murray SR, Zaki PA, Chu PC, Lissin DV, Monteillet-Agius G, Stewart PL, Evans CJ, and von Zastrow M (1998) μ-Opioid receptor internalization: opiate drugs have differential effects on a conserved endocytic mechanism in vitro and in the mammalian brain. Mol Pharmacol 53 377-384. [PubMed] [Google Scholar]
- Keith DE, Murray SR, Zaki PA, Chu PC, Lissin DV, Kang L, Evans CJ, and von Zastrow M (1996) Morphine activates opioid receptors without causing their rapid internalization. J Biol Chem 271 19021-19024. [DOI] [PubMed] [Google Scholar]
- Kelly P, Casey PJ, and Meigs TE (2007) Biologic functions of the G12 subfamily of heterotrimeric G proteins: Growth, migration, and metastasis. Biochemistry 46 6677-6687. [DOI] [PubMed] [Google Scholar]
- Kilts JD, Connery HS, Arrington EG, Lewis MM, Lawler CP, Oxford GS, O'Malley KL, Todd RD, Blake BL, Nichols DE, et al. (2002) Functional selectivity of dopamine receptor agonists. II. Actions of dihydrexidine in D2L receptor-transfected MN9D cells and pituitary lactotrophs. J Pharmacol Exp Ther 301 1179-1189. [DOI] [PubMed] [Google Scholar]
- Kozasa T and Gilman AG (1995) Purification of recombinant G proteins from Sf9 cells by hexahistidine tagging of associated subunits. J Biol Chem 270 1734-1741. [DOI] [PubMed] [Google Scholar]
- Kurrasch-Orbaugh DM, Parrish JC, Watts VJ, and Nichols DE (2003) A complex signaling cascade links the serotonin2A receptor to phospholipase A2 activation: the involvement of MAP kinases. J Neurochem 86 980-991. [DOI] [PubMed] [Google Scholar]
- Lane JR, Powney B, Wise A, Rees S, and Milligan G (2007) Protean agonism at the dopamine D2 receptor: (S)-3-(3-hydroxyphenyl)-N-propylpiperidine is an agonist for activation of Go1 but an antagonist/inverse agonist for Gi1, Gi2, and Gi3. Mol Pharmacol 71 1349-1359. [DOI] [PubMed] [Google Scholar]
- Lawler CP, Prioleau C, Lewis MM, Mak C, Jiang D, Schetz JA, Gonzalez AM, Sibley DR, and Mailman RB (1999) Interactions of the novel antipsychotic aripiprazole (OPC-14597) with dopamine and serotonin receptor subtypes. Neuropsychopharmacology 20 612-627. [DOI] [PubMed] [Google Scholar]
- Moers A, Nieswandt B, Massberg S, Wettschureck N, Grüner S, Konrad I, Schulte V, Aktas B, Gratacap MP, Simon MI, et al. (2003). G13 is an essential mediator of platelet activation in hemostasis and thrombosis. Nature Med 9 1418-1422. [DOI] [PubMed] [Google Scholar]
- Mottola DM, Kilts JD, Lewis MM, Connery HS, Walker QD, Jones SR, Booth RG, Hyslop DK, Piercey M, Wightman RM, et al. (2002) Functional selectivity of dopamine receptor agonists. I. Selective activation of postsynaptic dopamine D2 receptors linked to adenylate cyclase. J Pharmacol Exp Ther 301 1166-1178. [DOI] [PubMed] [Google Scholar]
- Nicolaou KC, Magolda RL, Smith JB, Aharony D, Smith EF, and Lefer AM (1979) Synthesis and biological properties of Pinane-Thromboxane A2, a selective inhibitor of coronary artery constriction, platelet aggregation, and thromboxane formation. Proc Natl Acad Sci U S A 76 2566-2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie D, Guo Y, Yang D, Tang Y, Chen Y, Wang MT, Zacharek A, Qiao Y, Che M, and Honn KV (2008) Thromboxane A2 receptors in prostate carcinoma: Expression and its role in regulating cell motility via small GTPase Rho. Cancer Res 68 115-121. [DOI] [PubMed] [Google Scholar]
- Offermanns S (2006) Activation of platelet function through G protein coupled receptors. Circ Res 99 1293-1304. [DOI] [PubMed] [Google Scholar]
- Offermanns S, Toombs CF, Hu YH, and Simon MI (1997) Defective platelet activation in Gαq-deficient mice. Nature 389 183-186. [DOI] [PubMed] [Google Scholar]
- Perez DM and Karnik SS (2005) Multiple signaling states of G-protein-coupled receptors. Pharmacol Rev 57 147-161. [DOI] [PubMed] [Google Scholar]
- Prevost N, Woulfe D, Tanaka T, and Brass LF (2002) Interactions between Eph kinases and ephrins provide a mechanism to support platelet aggregation once cell-to-cell contact has occurred. Proc Natl Acad Sci U S A 99 9219-9224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RD, Weiner DM, Chang MS, and Sanders-Bush E (2001) RNA editing of the human serotonin 5-HT2C receptor alters receptor mediated activation of G13 protein. J Biol Chem 276 44663-44668. [DOI] [PubMed] [Google Scholar]
- Riobo NA and Manning DR (2005) Receptors coupled to heterotrimeric G proteins of the G12 family. Trends Pharmacol Sci 26 146-154. [DOI] [PubMed] [Google Scholar]
- Ryman-Rasmussen JP, Nichols DE, and Mailman RB (2005) Differential activation of adenylate cyclase and receptor internalization by novel dopamine D1 receptor agonists. Mol Pharmacol 68 1039-1048. [DOI] [PubMed] [Google Scholar]
- Singer WD, Miller RT, and Sternweis PC (1994) Purification and characterization of the a subunit of G13. J Biol Chem 269 19796-19802. [PubMed] [Google Scholar]
- Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, et al. (2007) Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther 320 1-13. [DOI] [PubMed] [Google Scholar]
- Violin JD and Lefkowitz RJ (2007) β-arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol Sci 28 416-422. [DOI] [PubMed] [Google Scholar]
- Whistler JL, Chuang HH, Chu P, Jan LY, and von Zastrow M (1999) Functional dissociation of m opioid receptor signaling and endocytosis: Implications for the biology of opiate tolerance and addiction. Neuron 23 737-746. [DOI] [PubMed] [Google Scholar]
- Wilson SJ, Roche AM, Kostetskaia E, and Smyth EM (2004) Dimerization of the human receptors for prostacyclin and thromboxane facilitates thromboxane receptor-mediated cAMP generation. J Biol Chem 279 53036-53047. [DOI] [PubMed] [Google Scholar]
- Windh RT, Lee MJ, Hla T, An S, Barr AJ, and Manning DR (1999) Differential coupling of the sphingosine 1-phosphate receptors Edg-1, Edg-3, and H218/Edg-5 to the Gi, Gq, and G12 families of heterotrimeric G proteins. J Biol Chem 274 27351-27358. [DOI] [PubMed] [Google Scholar]
- Windh RT and Manning DR (2002) Analysis of G protein activation in Sf9 and mammalian cells by agonist-promoted [35S]GTPγS binding. Meth Enzymol 344 3-14. [DOI] [PubMed] [Google Scholar]
- Zhang L, DiLizio C, Kim D, Smyth EM, and Manning DR (2006) The G12 family of G proteins as a reporter of thromboxane A2 receptor activity. Mol Pharmacol 69 1433-1440. [DOI] [PubMed] [Google Scholar]
- Zheng H, Chu J, Qiu Y, Loh HH, and Law PY (2008) Agonist-selective signaling is determined by the receptor location within the membrane domains. Proc Natl Acad Sci U S A 105 9421-9426. [DOI] [PMC free article] [PubMed] [Google Scholar]