Abstract
Vectors used in gene therapy require an expression cassette. The expression cassette consists of three important components: promoter, therapeutic gene and polyadenylation signal. The promoter is essential to control expression of the therapeutic gene. A tissue-specific promoter is a promoter that has activity in only certain cell types. Use of a tissue-specific promoter in the expression cassette can restrict unwanted transgene expression as well as facilitate persistent transgene expression. Therefore, choosing the correct promoter, especially a tissue-specific promoter, is a major step towards achieving successful therapeutic transgene expression. Ideally, the elements of the natural promoter region, necessary for obtaining the required level of the gene expression while retaining tissue-specificity, should be known. Also, it is important to understand if interactions occur between the promoter region and rest of the vector genome that could affect promoter activity and specificity. To assess this, it is helpful to select a suitable vector system that will be used in further gene therapy studies. Secondly, have one or several candidate tissue-specific promoters available for use. Third, ideally have an in vitro cell model suitable to evaluate tissue-specificity. Fourth, have a convenient in vivo animal model to use. Fifth, select a good reporter gene system. Next, using conventional recombinant DNA techniques create different promoter constructs with the selected vector system. Lastly, have a suitable transfection method to test the plasmid constructs in both the in vitro and in vivo models.
Keywords: tissue specific promoter, transcription, expression cassette, gene therapy vector
1. Introduction
In mammalian cells, each gene has its own promoter, and some promoters can only be activated in a specific cell type (1). The promoter is a specific genetic region involved in the binding of a RNA polymerase to initiate transcription, and is located 5′ from the transcription start site (2). Therefore, the location of a promoter determines the template strand for each gene transcription. In eukaryotic nuclei, there are three RNA polymerases, RNA polymerase I, II and III. RNA polymerase II is involved in transcribing the most cellular genes; however, in eukaryotic cells it cannot initiate transcription on a DNA template. This requires many nuclear proteins, called general transcription factors that are designated as TFII (transcription factor for polymerase II) including TFIIA, TFIIB, etc., to assemble at the promoter region with the RNA polymerase II and initiate transcription (2). A DNA sequence called the TATA box exists in the promoter regions of most genes, and typically is located ∼25 nucleotides upstream from the transcription start site. The TATA box signals the start of transcription. TFIID recognizes and binds to the TATA box, and causes other general transcription factors to assemble at the promoter, helping to position RNA polymerase II correctly at the promoter (2). While transcription in undifferentiated mouse embryos (at the two to eight cell stages) does not require a TATA box, it becomes critical for efficient transcription in differentiated cells (3). This means that the TATA box itself also can directly be involved in the regulation of gene transcription. In eukaryotic nuclei, RNA polymerase II also requires activator, mediator and chromatin-modifying proteins to correctly transcribe DNA. The transcription activators also bind to specific sequences in DNA and help to attract RNA polymerase II to the start point of transcription. Mediators form a protein complex that allows the activator proteins to communicate properly with RNA polymerase II and with general transcription factors (2). Transcription initiation in the cell often requires the local recruitment of chromatin-modifying enzymes, including chromatin remodeling complexes and histone acetylases, which allow greater accessibility of RNA polymerase II to the DNA present in chromatin (2).
In aggregate, eukaryotic transcription initiation is a very complex process that uses many regulatory proteins. Furthermore, some regulatory proteins can bind to DNA thousands of nucleotides away from the promoter, which means that a single promoter can be controlled by an almost unlimited number of regulatory sequences scattered along the DNA. Also, each regulatory protein usually contributes to the control of many genes (2). Although some gene regulatory proteins are fairly specific and only expressed in one or a few cell types, most are found in a variety of cell types, in many tissues, and at several times during development. This type of combinatorial gene control makes it possible to generate considerable biological complexity with a relatively defined number of regulatory molecules (2).
The promoter region in eukaryotic genes is commonly a relatively large DNA fragment. Most gene therapy vectors have a size limitation in the capacity of an expression cassette, thus, only the essential part of a promoter can be used. Therefore, any use of a tissue-specific promoter requires prior careful evaluation in specific and faithful expression systems. Doing so helps to minimize toxicity, maximize efficient gene expression in the desired target cell/tissue, and optimize the gene therapy vector. Herein, we use our previous studies (4,5) evaluating salivary gland tissue-specific promoters in gene therapy vectors as an example of how to assess the tissue-specific promoters in vitro and in vivo.
In salivary glands, two general epithelial cell types are present — acinar cells and ductal cells in primarily a densely packed monolayer (6). Both are involved in the formation of saliva. Acinar cells are water permeable and salt secreting, whereas ductal cells are relatively water impermeable and salt absorbing. The epithelial cells in salivary glands are easy to access in vivo in animals, as well as humans. As noted above, having a convenient in vivo animal assay for studying promoters is essential. Vector delivery is performed through local cannulation of the main excretory ducts of the targeted glands. The orifices are accessible directly in the mouth, and in animals anesthesia is required only for restraint. For these studies, we have employed mammalian plasmid expression vectors and adenoviral vectors. Replication-deficient recombinant adenoviral vectors are useful for delivering exogenous genes into both salivary epithelial cell types (7,8) in vivo. Because acinar and ductal cells can be differentially affected by salivary disorders, it is useful to target each cell type specifically (9). In our studies we have used many different promoters. Some promoters, such as human cytomegalovirus (CMV), Rous sarcoma virus (RSV), simian virus 40 (SV40) and mammalian elongation factor 1α (EF1α), are non-specific promoters and are commonly used in gene therapy vectors. Other promoters, such as cytokeratin 18 and 19, are epithelial cell-specific and should have activities in both acinar and ductal cells (10,11,12,13). The tissue kallikrein promoter is considered ductal cell specific in salivary glands (5), while the amylase 1C and aquaporin-5 (AQP5) promoters should be relatively acinar cell specific (4,14,15).
2. Materials
2.1. Serial Deletion analysis and Plasmid Construction
Restriction endonuclease enzymes, chosen according to the promoter sequence and the plasmid vector sequence.
Taq and Pfu DNA polymerase (Stratagene, La Jolla, CA) and EasyStart PCR Mix-in- a Tube (Molecular BioProducts, San Diego, CA).
Klenow Fill-In kit (Stratagene) and T4 DNA polymerase (Invitrogene, Carlsbad, CA).
Bacterial Alkaline Phosphastase (Invitrogen).
T4 DNA ligase (Invitrogen).
DH5α™ Cells (Invitrogen).
Agarose (Invitrogen).
Ethidium bromide (Invitrogen).
SOC and LB media (Invitrogen).
Wizard Plus Minipreps DNA Purification System, Wizard Plus Midipreps DNA Purification System, and Maxipreps DNA purification system (Promega, Madison, WI).
2.2 Recombinant adenoviral vector production
293 cell line (Microbix, Ontario, Canada).
pJM17 plasmid (Microbix).
Calcium Phosphate Transfection Kit (Invitrogen).
Cesium Chloride (CsCl) gradients (1.25 gm/ml, 1.33 mg/ml and 1.4 mg/ml) for adenovirus purification. CsCl (Invitrogen) is dissolved in TD buffer (140 mM NaCl, 5 mM KCl, 25 mM Tris-HCl, and 0.7 mM Na2HPO4, adjust pH to 7.4 with HCl).
Virus dialysis buffer: 100 mM Tris-HCl (pH 7.4), 10 mM MgCl2 and 10% (v/v) glycerol.
Virus dilution buffer: 5 mM MgCl2, 10 mM Tris-HCl and 20% (v/v) glycerol, pH 7.4.
SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA).
2.3 Cell Culture
McCoy’s 5A medium (Invitrogen) supplemented with 10% fetal bovine serum (HyClone, Logan, Utah), 100 u/ml penicillin G (Invitrogen) and 100 ug/ml streptomycin (invitrogen) for the A5 cell line, which is a rat submandibular ductal cell line (16).
IMEM medium (Invitrogen) supplemented with 10% fetal bovine serum (HyClone), 100 u/ml penicillin G (Invitrogen) and 100 ug/ml streptomycin (Invitrogen) for 293 cell line which is a human embryonic kidney cell line (17).
Note, there is no salivary acinar cell line available.
2.4 In vivo animal experiments
PE 10 tube for cannulation.
One cc insulin syringe U-100 28G1/2” (Becton Dickinson, Franklin Lakes,NJ)
Dexamethasone (SIGMA, St, Louis, MO)
Ketamine (100 mg/ml, Phoenix Scientific, St. Joseph, MO) and xylazine (20 mg/ml, Phoenix Scientific).
Restraint cannulation board for proper positioning of animal’s oral cavity.
2.5 Plasmid Delivery
Adcontrol which is a replication-deficient adenovirus without any transgene in the E1 region. Ad5. Null is a similar vector to Adcontrol that can be obtained from Qbiogene, Irvine, CA.
Polyethylenimine (PEI), high molecular weight, water-free (Cat. #: 40872-7, ALDRICH, Milwaukee, WI)
2.6 Protein Assay
1. BCA protein Assay Reagent A and B (PIERCE, Rockfold, IL)
2.7 Luciferase Assay
Luciferase cell Culture Lysis Reagent 5 × (Promega).
Luciferase Assay Substrate and Luciferase Assay Buffer (Promega).
2.8 Immunohistochemistry
Hydrogen peroxide 30% (w/w) solution (SIGMA)
ImmunoCruz™ Staining System for rabbit primary antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA)
3. Methods
There are many ways to evaluate tissue-specific promoters (see Note 1). The following (1 to 5) are the most common steps involved.
Serial DNA deletion (Fig.1) by either restriction endonuclease digestion or PCR.
Subclone different promoter fragments into the plasmid vector.
Check the different constructs in the cell and, ideally, animal models by transfection.
Detect reporter gene expression with an appropriate and convenient assay.
Locate site of protein expression in cells or tissue using immunohistochemistry.
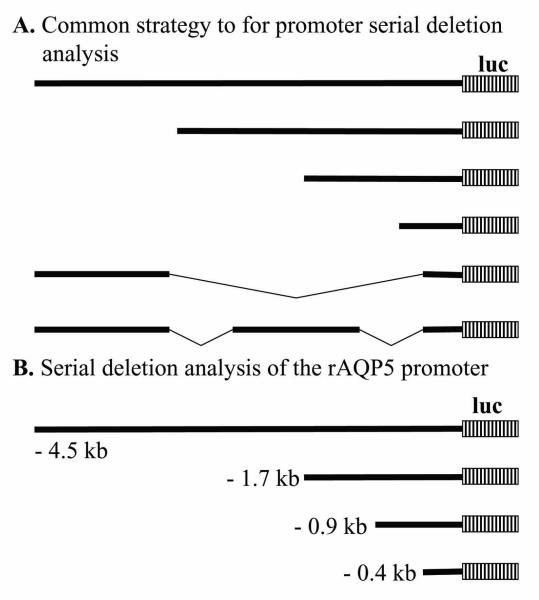
Figure 1.
Serial deletion studies for promoter analysis. (A) is a general diagram showing a common strategy for promoter serial deletion analysis. (B) is a specific example of such an analysis that we performed to evaluate the rat AQP5 (rAQP5) promoter in our vector system (5). Luc, luciferase, which is the reporter gene used.
Analysis of the rat AQP5 promoter (5) is given as an example. For this study, we selected two reporter genes. One was luciferase, which is a very sensitive reporter gene with a low background, but for which we have found no good antibodies available. The second reporter gene, encoding enhanced green fluorescence protein (EGFP), has good antibodies available. EGFP can be directly observed as well as examined by immunohistochemical staining.
3.1 Serial Deletion Analysis
Before performing a serial deletion analysis for a promoter, it is best to conduct some database searches on the sequence, e.g., PubMed, Gene Bank and tissue-specific promoter database (TiProD)(http://tiprod.cbi.pku.edu.cn:8080/index.html)(18) to determine if this promoter has previously been studied, and if so what the results were and how it was performed. If not, it is best to try to learn as much about the promoter sequence as possible from gene bank and other databases, e.g., how many and what kind of transcription factor binding sites are present, is there a TATA box, where is it, what is the guanine/cytosine content. The rAQP5 promoter had been described by Borok et al (19). They made a series of progressive unidirectional 5′ → 3′ AQP5-luciferase deletion constructs beginning at -1716 with an identical 3′-end (starting at -6 bp) by using a combination of Exonuclease III, the Erase-a-Base System (Promega) and Bal31 nuclease digestion. The Erase-a-Base System is designed for the rapid construction of a plasmid containing progressive unidirectional deletions of any inserted DNA. This system (20,21) uses Exonuclease III to specifically digest inserted DNA from a 5′ protruding or blunt end restriction site. The adjacent sequencing primer binding site is protected from digestion by a four base 3′ overhang restriction site or by an α-phosphorothioate-filled end. This method makes it rapid to construct nested deletions from plasmids, and useful to evaluate a tissue-specific promoter, especially an unknown promoter.
Based on the studies of Borok et al (19), we decided to do three serial deletions in the 4.5 kb rAQP5 promoter fragment (1.791, 0.9 and 0.391 kb) and evaluate those rAQP5 promoter fragments in our plasmid and adenoviral vector systems (see Fig. 1B). We received the plasmid pUC-AQP5-2 thanks to the generosity of Dr. David Ann, University of Southern California. After restriction endonuclease analysis of the 4.5 kb fragment, we recognized we could use Eco RI/Hind III to get the 4.5 kb rAQP5 promoter fragment, Fok I/Hind III for the 1.791 kb fragment, Sau 3AI/Hind III for the 0.9 kb fragment, and Del I/Hind III for the 0.391 kb fragment. In brief, each fragment was filled in with Klenow Fill-In kit (Stratagene), cleaned of the Klenow enzyme, precipitated with 100% of ethanol, resuspended with dd H2O, ligated with Hind III linker, cleaned again, then digested with Hind III. We used Hind III to open the plasmid vector, pAC-luc, cleaned this, precipitated with 100% of ethanol, resuspended with dd H2O, dephosphorylated, then ligated it with the above individual rAQP5 promoter fragments. Ligated plasmids were transformed into DH5α competent cells, colonies screened by preparing plasmid DNA, and restriction endonuclease digestions performed. These procedures resulted in construction of the following plasmids: pACrAQP5-4.5-luc, pACrAQP5-1.7-luc, pACrAQP5-0.9-luc and pACrAQP5-0.4-luc.
3.2 Plasmid Constructions
To construct the rAQP5 tissue-specific promoter into the plasmid expression vector, the following common recombinant DNA techniques were used. Detailed protocols for each can be obtained from commercial sources, as well as from various standard molecular biology texts (, e.g., 22, 23). Accordingly, these techniques will not be described further.
Plasmid DNA preparations using Wizard Plus Midipreps DNA Purification System, and Maxipreps DNA purification system (Promega).
Restriction endonuclease digestion of plasmid DNA (see Note 2), followed by agarose gel electrophoresis.
PCR to amplify a special fragment of the tissue-specific promoter with Taq or Pfu DNA polymerases (Stratagene).
Fill-in or removal of both 5′ or 3′ overhangs to make blunt ends using Klenow Fill-in kit (Stratagene) or T4 DNA polymerase (Invitrogen).
Plasmid DNA precipitation with 2 volumes of 100% cold ethanol, then wash twice with 70% ethanol
Dephosphorylation of 5′-phosphorylated termini of vector DNA to prevent self-ligation using bacterial alkaline phosphatase (Invitrogen).
Plasmid DNA and promoter DNA fragment ligation using T4 DNA ligation (Invitrogen).
Transformation using MAX Efficiency DH5α Chemically Competent Cells (Invitrogen), then culture in a shaker at 37°C for 1 hour, streak out competent cells on an LB agar or agarose ampcillin plate and grow the bacterial overnight at 37°C to obtain colonies
Colony screening. Pick up single colonies from the above plate and transfer to a bacterial culture tube with 3 ml LB medium, then incubate in a shaker at 37°C overnight; perform mini plasmid preparation using Wizard plus Minipreps, digest DNA samples using selected restriction endonuclease enzymes, run the digested samples in a 1% agarose gel with ethidium bromide (50ng/100 ml), then select a correct clone based upon the electrophoresis results for use in evaluating the promoter activity in vitro and in vivo.
3.3 Recombinant Adenoviral Vector Production
A common gene transfer vector system that we use is based on the serotype 5 adenovirus. We therefore also evaluated the rAQP5 promoter in the adenoviral vector system. Recombinant adenoviral vectors are a ∼36 kb, replication-deficient DNA virus. For these studies the expression cassette, including the rAQP5 promoter, was inserted into the deleted E1 gene region of the adenovirus. Since remaining portions of the adenoviral genome can possibly affect and/or interfere with the rAQP5 promoter, i.e., cis-acting effects, it was important for us to evaluate the rAQP5 promoter in an adenoviral vector context. Our in vitro and in vivo plasmid expression results demonstrated that pACrAQP5-0.4-luc was of interest for further evaluation. Therefore, we used this plasmid to make an adenoviral vector. The following is the protocol we used to produce the adenoviral vector, AdrAQP5-0.4-luc.
Make a maxipreparation using Wizard Plus Maxipreps of pACrAQP5-0.4-luc constructed based on 3.1 and 3.2.
Make a maxipreparation of the plasmid pJM17 using Wizard Plus Maxipreps (see Note 3). pJM17 consists of most of the adenovirus serotype 5 (Ad5) genome except for the E1 region. The pJM17 vector and the pAC shuttle vector contain overlap regions (0.0-1.3 and 9.3-17.0 map units) that permit homologous exchange when co-transfected into 293 cells (24).
Grow 293 cells in IMEM (see Note 4). The 293 cell line is a human embryonic kidney cell line that contains an integrated copy of the leftmost Ad5 genome, which complements the defect in E1 deficient vectors (17).
Cotransfection of pJM17 and pACrAQP5-0.4-luc into 293 cells by calcium phosphate co-precipitation using the Calcium Phosphate Transfection Kit (Invitrogen). Add15 μg of pJM17, 5 μg of pACrAQP5-0.4-luc, 36 μl of 2 M CaCl2 and dd H2O to 300 μl in a 14 ml polypropylene tube, mix well. Next, quickly add 300 μl 2× Hepes buffered saline and bubble with a pipet for 1 min, then incubate at room temperature for 30 min to form a fine precipitate. Add the entire mixture to a 100 mm plate of 293 cells, and gently rotate the plate to mix well. Put the plate back into the tissue culture incubator, and replace the growth medium every two days. After ∼10 – 12 days, plaques (holes in the 293 monolayer) are observed, which indicate the replication of the recombinant adenovirus, AdrAQP5-0.4-luc. Continually and carefully replace the medium until ∼50% of 293 cells are lysed. Harvest the entire plate (293 cells and growth medium) by pipetting into a 50 ml conical test tube.
Completely lyse the 293 cells by freezing (dry ice) and thawing (37°C water bath) 5 times to release adenoviral vector from the cells. Centrifuge at ∼2,000×g for 5 min. Transfer supernatant [crude viral lysate (CVL)] to a new 50 ml tube. Use 50 μl of the CVL to infect one well of 293 cells (105 cells/well in 96-well plate). Measure the luciferase activity in the lysate of these cells after 24 hours to confirm that the CVL contained functional virus.
Large scale preparation to propagate AdrAQP5-0.4-luc. Use the above CVL to infect three fresh 100 mm plates of 293 cells. Harvest at day 3. Freeze and thaw 5 times as above, combine and again centrifuge to collect supernatant. Use this CVL to infect twenty 150 mm plates of 293 cells. Harvest the cells at day 3, centrifuge the harvested cells and use 6 ml of the CVL to resuspend the cell pellet. Freeze and thaw 5 times, then centrifuge at 3,500×g for 10 min.
Transfer supernatant to the first CsCl gradient [place 2.5 ml of density 1.25 CsCl in sterile ultra-clear centrifuge tubes (Beckman Coulter #344059, Fullerton, CA), then slowly underlay 2.5 ml of density 1.40 CsCl]. Spin the tubes in a SW41 rotor at 210,000×g, 22°C for 1 hour (balance carefully). Clean the outside of the tube with 70% alcohol, then use a 21 gauge needle and syringe to poke through the side of the tube to collect the lower opalescent band, which contains the recombinant Ad5 vector. Transfer this vector band to a second CsCl gradient [place 8 ml of density 1.33 CsCl into sterile ultra-clear centrifuge tubes (Beckman Coulter #344059)]. Spin in a SW41 rotor at 210,000×g, 22°C for 18 hours (balance carefully). As above clean the outside of the tube with 70% alcohol, and use a 21 gauge needle and syringe to collect the lower opalescent band.
Transfer the vector into a PIERCE Slide-A-Lyzer dialysis cassette (product #66425) and dialyze in 500 ml dialysis buffer for 30 min, twice, and then in 1000 ml dialysis buffer for 1 hour, 3 times. Remove the vector suspension from the dialysis cassette and aliquot in sterile Eppendorf tubes at ∼100 μl/tube (see Note 5).
Use real time PCR (QPCR) to titer the AdrAQP5-0.4-luc with primers from the E2 region of adenovius; E2q1 (5′-GCAGAACCACCAGCACAGTGT-3′ ) and E2q2 (5′-TCCACGCATTTCCTTCTAAGCTA-3′ ). Titers are expressed as particles/ml. The plasmid pACrAQP5-0.4-luc was used as a standard for QPCR, with 1 μg of the plasmid (10132 bp ) being equivalent to 9.0 × 1010 molecules. Standard curves are established from 102 molecules to 109 pACrAQP5-0.4-luc molecules, and adenoviral vectors are tested at three dilutions over a 100-fold range. QPCR assays are typically carried out with the SYBR Green PCR Master Mix from Applied Biosystems (Foster City, CA) using an ABI prism 7700 Sequence Detector (Applied Biosystems), with the following conditions: stage 1, 95°C for 2 min; stage 2, 95°C for 10 min; stage 3, 95°C for 15 sec, 60°C for 1 min, repeated 40 times.
3.4 Plasmid Delivery
It is difficult to deliver plasmid alone, or with typical in vitro transfection reagents, to rat submandibular glands in vivo because of minimal efficiency. Therefore, an adenovirus-PEI-plasmid complex is used to deliver plasmids containing the promoters being studied, both in vitro and in vivo (see Note 6). PEI is an organic macromolecule with the highest cationic-charge-density potential. PEI plays a bridge role in this complex to connect plasmid DNA and the adenovirus. Receptors on eukaryotic cell membranes recognize the adenovirus, facilitating cell entry and nuclear targeting. The adenovirus used by us is Adcontrol, as noted an E1 deleted replication deficient vector without any transgene.
PEI stock solution (10 mM): mix 9 mg of PEI with 10 ml dd H2O, adjust pH to 7.0 with HCl and filter to sterilize.
Add 2 × 1010 molecules (see Note 7) of plasmid DNA (e.g., pACrAQP5-0.4-luc) into 50 μl of 20 mM HEPES (pH 7.5), then add 50 μl of PEI solution (1 μl of PEI stock solution in 49 μl of 20 mM HEPES (pH 7.5). Mix well and incubate at room temperature for 20 min.
Add 3 × 1010 particles of Adcontrol, mix well and incubate at room temperature for 20 min.
Take 20 μl of the above two mixtures, add to A5 cells, plated 24 hours previously (2 × 105 cells/well in 96-well plate) and allow transfection for 1 hour.
Add 180 μl of fresh growth medium, return to tissue culture incubator, and measure the luciferase activity after 24 hours.
For in vivo rat submandibular gland delivery use the following: 4.35 × 1012 molecules of plasmid DNA/gland, 0.5 mM PEI and 1 × 1011 particles of Adcontrol in a volume of 150 μl. The complexes are delivered into rat submandibular glands by local cannulation (see 3.6). After 3 days, the tissues are harvested to measure luciferase activity, and to perform histological staining (e.g., hematoxylin and eosin) or immunohistochemistry staining (for EGFP).
3.5 In Vitro Viral Infection
A5 cells in suspension are infected at 100 particles/cell, 37°C for 1 hour.
Plate A5 cells at 5 × 105 cells/200 μl/well in a 96-well plate and incubate for 24 hours.
Measure the luciferase activity after 24 hours as described below in section 3.7.
3.6 In Vivo Viral Infection (Rat Submandibular Gland Cannulation)
Using dilution buffer dilute AdrAQP5-0.4-luc to an intended dose of 109 particles/150 μl/gland.
Rat is anethetized with ketamine (60 mg/kg) + xylazine (8 mg/kg).
Place the rat on a special board to immobilize and access main excretory ducts of the submandibular glands. Insert a tapered PE 10 cannula into the main excretory duct under a microscope, and fix the cannula into position with superglue.
Inject atropine (1 mg/kg) intramuscularly to inhibit saliva secretion.
Ten min after atropine injection, use an insulin syringe to connect the other end of the PE 10 tubing to inject 150 μl of AdrAQP5-0.4-luc (or Adcontrol-PEI-plasmid complex, see 3.5 Plasmid Delivery) into the rat submandibular gland.
After 10 min, remove the cannula, wait for the rat to awake from anesthesia, and return the rat back to its cage.
After 3 days, the rat submandibular glands are harvested to measure luciferase activity and to perform histological or immunohistochemistry staining.
3.7 Luciferase Assay
To prepare the Luciferase Assay Reagent, add 10 ml of Luciferase Assay Buffer to the vial containing the lyophilized Luciferase Assay Substrate. To prepare 1 × Cell Culture Lysis Reagent, add 4 volumes of dd H2O to 1 volume of 5 × Cell Culture Lysis Reagent.
For cell culture which includes non-transfected and transfected cells in 96-well plate, add 25 μl of 5 × Cell Culture Lysis Reagent into each well and mix 3 times by pipeting, incubate at room temperature for 15 min. For animal tissue, add 500 μl of 1× Cell Culture Lysis Reagent to ∼30 mg of tissue, homogenize for ∼ 30 seconds with a polytron, incubate at room temperature for 15 min, and then centrifuge at 16,000 g for 20 seconds.
Mix 50 μl of cell or tissue lysates with 100 μl Luciferase Assay Reagent in a 12 × 75 mm glass test tube, then place this tube in an OPTOCOMP I luminometer (GEM Instruments, INC., Hamden, CT) to measure the light emitted for 10 seconds. The result is reported as relative light units.
3.8 Protein Assay
Working reagent. Add 1 part reagent B to 50 parts Reagent A. Mix well.
Pipet 0.1 ml of each standard or unknown protein sample into the appropriately labeled test tube.
Add 1 ml working reagent to each tube. Mix well.
Incubate tubes at 37°C for 30 min, cool tubes to room temperature.
Measure the absorbance at 562 nm vs. water reference. Subtract the absorbance of the blank from the value found.
3.9 Immunohistochemistry
Immunohistochemistry is done with the ImmunoCruz™ Staining System for rabbit primary antibody.
Primary antibody, anti-GFP (Rabbit polyclonal), was from Abcam, Inc (Cambridge, MA)
There are two types of controls (see Note 8): one is a reagent control using normal rabbit IgG instead of the EGFP primary antibody, and the second is a negative tissue control (i.e., does not receive any adenoviral vector containing the rAQP5 promoter and EGFP reporter gene).
Paraffin embedded tissue sections were deparaffinized with xylene. Slides containing tissue sections are put in xylene for 15 min, twice.
Deparaffinized slides are rehydrated in a graded series of ethanol, 100%, 100%, 95%, 90%, 80%, 70% for 5 min each. Wash sections in PBS for 5 min.
Peroxidase quenching. Submerge the slides in 15% hydrogen peroxide (25 ml dd H2O and 25 ml of 30% of hydrogen peroxide) for 30 min.
Rinse the slides with dd H2O, then PBS three times. Drain all PBS from the slides. Draw a circle outside of the tissue on the slide with a PAP Pen (Research Products International Corp; Mount Prospect, Illinois)
Add serum blocking solution (PBS + 30% goat serum) into each circle and block for 20 min.
Drain the serum blocking solution from the slide, and add ∼ 200 μl of primary antibody at a 1:200 dilution [antibody dilution buffer: 2% bovine albumin (SIGMA) in PBS] into the circles for 1 hour.
Wash the slides with PBS for 2 min, 3 times.
Incubate the slides in biotinylated second antibody for 30 min.
Wash the slides with PBS for 2 min, 3 times.
Incubate the slides in HRP-streptavidin complex for 30 min.
Wash the slides with PBS for 2 min, 3 times.
Add 1 - 3 drops of HRP substrate to each circle on the slides for 20 min.
Rinse with dd H2O and then wash the slides in dd H2O for 2 min.
Hematoxylin counterstain and mount slides.
4. Notes
Serial deletion analysis is a very powerful method to evaluate tissue-specific promoters, but, it is not only way to do this. The gene trap method can be also used. This system is especially helpful to evaluate a tissue-specific promoter in vivo. Gene trap constructs utilize the lacZ gene, encoding beta-galactosidease, or GFP both to disrupt a natural gene’s function and monitor gene expression simultaneously. Gene trapping is rapid, cost-effective, and produces a large variety of insertional mutations throughout genome (25). Transgenic mouse models can also be used to evaluate tissue-specific promoters in vivo. Note that promoter methylation often is involved in the regulation of tissue-specific promoters (26).
For restriction endonuclease digestion, keep the enzyme volume to ≤ 10% in the reaction mixture.
pJM17 is an ∼40 kb plasmid. Maxipreparation of this plasmid is not easy. Make fresh ampcillin and add 100 ng/ml in the bacterial culture. During the plasmid DNA extraction, carefully resuspend DNA pellet with TE buffer (pH 8.0).
Use lower passage 293 cells (<50) for the cotransfection to make adenoviral vectors.
For aliquots of adenoviral vectors, minimize the number of freeze and thaw episodes to prevent loss of vector activity. Keep vector aliquots in a -80°C freezer.
Make fresh PEI stock solution each time to obtain reproducible results. PEI activity is decreased if kept as a stock solution at 4°C.
To calculate the number of plasmid molecules use the following: 1 μg of 1000 bp DNA = 9.1 × 1011 molecules.
For the immunohistochemistry, control groups are very important.
Acknowledgments
We thank Drs. Biman Paria and Gabor Racz for their careful reading of, and helpful comments on, an earlier version of this manuscript. Our research is supported by the Division of Intramural Research, National Institute of Dental and Craniofacial Research.
References
- 1.Eloranta JJ, Goodbourn S. Positive and negative regulation of RNA polymerase II transcription. In: Goodbourn JJ, editor. Eukaryotic Gene Transcription. Oxford University Press; Walton Street, Oxford: 1996. pp. 1–33. [Google Scholar]
- 2.Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. Garland Science; New York, NY: 2002. [Google Scholar]
- 3.Majumder S, DePamphilis ML. TATA-dependent enhancer stimulation of promoter activity in mice is developmentally acquired. Mol. Cell. Biol. 1994;14:4258–4268. doi: 10.1128/mcb.14.6.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng C, Hoque AT, Braddon VR, Baum BJ, O’Connell BC. Evaluation of salivary gland acinar and ductal cell-specific promoters in vivo with recombinant adenoviral vectors. Hum. Gene. Ther. 2001;12:2215–2223. doi: 10.1089/10430340152710559. [DOI] [PubMed] [Google Scholar]
- 5.Zheng C, Baum BJ. Evaluation of viral and mammalian promoters for use in gene delivery to salivary glands. Mol. Ther. 2005;12:528–536. doi: 10.1016/j.ymthe.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Baum BJ, Wellner RB, Zheng C. Gene transfer to salivary gland. Int. Rev. Cytol. 2002;213:93–146. doi: 10.1016/s0074-7696(02)13013-0. [DOI] [PubMed] [Google Scholar]
- 7.Mastrangeli A, O’Connell B, Aladib W, Fox PC, Baum BJ, Crystal RG. Direct in vivo adenovirus-mediated gene transfer to salivary glands. Am. J. Physiol. 1994;266:G1146–G1155. doi: 10.1152/ajpgi.1994.266.6.G1146. [DOI] [PubMed] [Google Scholar]
- 8.Delporte, Redman RS, Baum BJ. Relationship between the cellular distribution of the alpha(v)beta3/5 integrins and adenoviral infection in salivary glands. Lab. Invest. 1997;77:167–173. [PubMed] [Google Scholar]
- 9.Baum BJ, Wang S, Cukierman E, Delporte C, Kagami H, Marmary Y, Fox PC, Mooney DJ, Yamada KM. Re-engineering the functions of a terminally differentiated epithelial cell in vivo. Ann. N. Y. Acad. Sci. 1999;875:294–300. doi: 10.1111/j.1749-6632.1999.tb08512.x. [DOI] [PubMed] [Google Scholar]
- 10.Moll R, Franke WW, Schiller DL, Geiger B, Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982;31:11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- 11.Chow YH, O’Brodovich H, Plumb J, Wen Y, Sohn KJ, Lu Z, Zhang F, Lukacs GL, Tanswell AK, Hui CC, Buchwald M, Hu J. Development of an epithelium-specific expression cassette with human DNA regulatory elements for transgene expression in lung airways. Proc. Natl. Acad. Sci. U S A. 1997;94:14695–14700. doi: 10.1073/pnas.94.26.14695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brembeck FH, Rustgi AK. The tissue-dependent keratin 19 gene transcription is regulated by GKLF/KLF4 and Sp1. J. Biol. Chem. 2000;275:28230–28239. doi: 10.1074/jbc.M004013200. [DOI] [PubMed] [Google Scholar]
- 13.Kagaya M, Kaneko S, Ohno H, Inamura K, Kobayashi K. Cloning and characterization of the 5′-flanking region of human cytokeratin 19 gene in human cholangiocarcinoma cell line. J. Hepatol. 2001;35:504–511. doi: 10.1016/s0168-8278(01)00167-2. [DOI] [PubMed] [Google Scholar]
- 14.Ting CN, Rosenberg MP, Snow CM, Samuelson LC, Meisler MH. Endogenous retroviral sequences are required for tissue-specific expression of a human salivary amylase gene. Genes Dev. 1992;6:1457–1465. doi: 10.1101/gad.6.8.1457. [DOI] [PubMed] [Google Scholar]
- 15.He X, Tse CM, Donowitz M, Alper SL, Gabriel SE, Baum BJ. Polarized distribution of key membrane transport proteins in the rat submandibular gland. Pflugers Arch. 1997;433:260–268. doi: 10.1007/s004240050276. [DOI] [PubMed] [Google Scholar]
- 16.Brown AM, Rusnock EJ, Sciubba JJ, Baum BJ. Establishment and characterization of an epithelial cell line from the rat submandibular gland. J. Oral. Pathol. Med. 1989;18:206–213. doi: 10.1111/j.1600-0714.1989.tb00764.x. [DOI] [PubMed] [Google Scholar]
- 17.Graham FL, Smiley J, Russell WC, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J. Gen. Virol. 1977;36:59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- 18.Chen X, Wu JM, Hornischer K, Kel A, Wingender E. TiProD: the Tissue-specific promoter database. Nucleic Acids Res. 2006;34:D104–D107. doi: 10.1093/nar/gkj113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borok Z, Li X, Fernandes VF, Zhou B, Ann DK, Crandall ED. Differential regulation of rat aquaporin-5 promoter/enhancer activities in lung and salivary epithelial cells. J. Biol. Chem. 2000;275:26507–26514. doi: 10.1074/jbc.M910007199. [DOI] [PubMed] [Google Scholar]
- 20.Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984;28:351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- 21.Putney SD, Benkovic SJ, Schimmel PR. A DNA fragment with an alpha-phosphorothioate nucleotide at one end is asymmetrically blocked from digestion by exonuclease III and can be replicated in vivo. Proc. Natl. Acad. Sci. U S A. 1981;78:7350–7354. doi: 10.1073/pnas.78.12.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sambrook J, Russell DW, editors. Molecular Cloning A Laboratory Manual. Cold Spring Harbor Laboratory; New York: 2001. [Google Scholar]
- 23.Ausubel FM, Brent R, Kingston RE, Moore DD, Scidman JG, Smith JA, Struhl K, editors. Current Protocols in Molecular Biology. Harvard Medical School. John Wiley & Sons, Inc.; 2006. [Google Scholar]
- 24.McGrory WJ, Bautista DS, Graham FL. A simple technique for the rescue of early region I mutations into infectious human adenovirus type 5. Virology. 1988;163:614–617. doi: 10.1016/0042-6822(88)90302-9. [DOI] [PubMed] [Google Scholar]
- 25.Bundschu K, Gattenlohner S, Knobeloch KP, Walter U, Schuh K. Tissue-specific Spred-2 promoter activity characterized by a gene trap approach. Gene Expr. Patterns. 2006;6:247–255. doi: 10.1016/j.modgep.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Bohwan J, Seong JK, Ryu DY. Tissue-specific and De Novo promoter methylation of the mouse glucose transporter 2. Biol. Pham. Bull. 2005;28:2054–2057. doi: 10.1248/bpb.28.2054. [DOI] [PubMed] [Google Scholar]