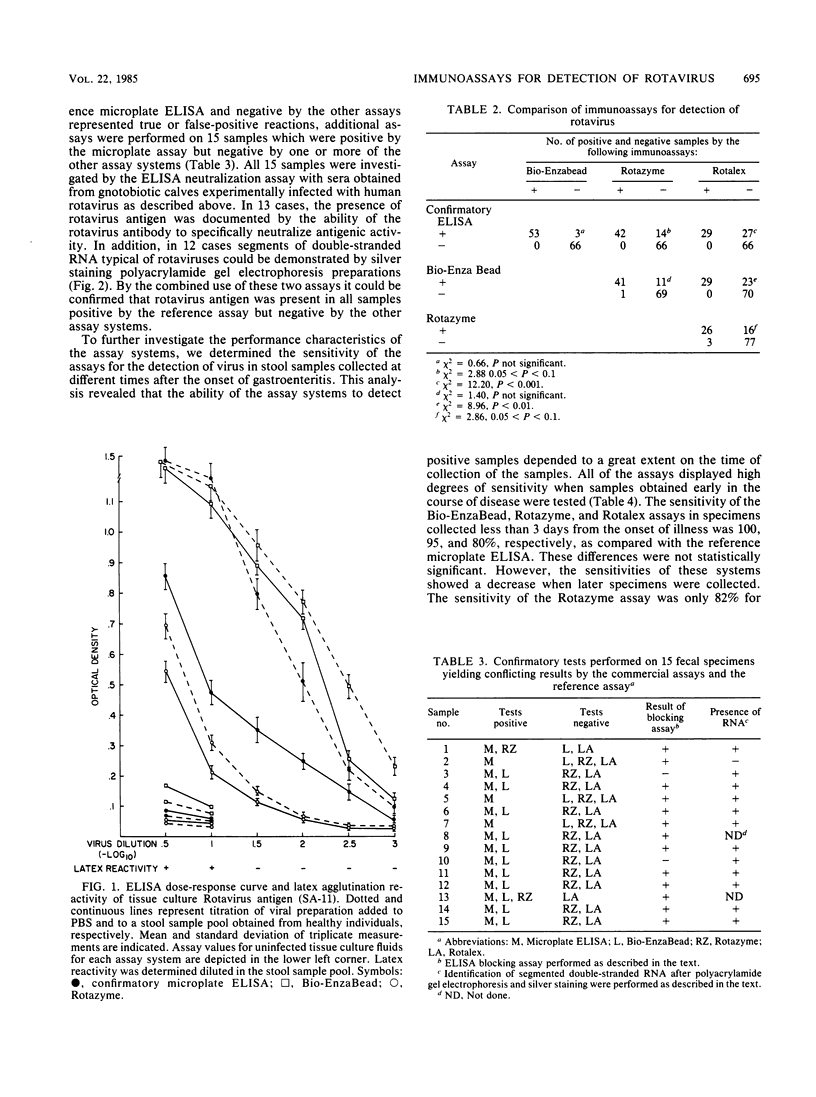
Abstract
We evaluated the performance characteristics of three commercially available immunoassays for the detection of rotavirus antigens in stool samples obtained from infants during the course of rotavirus gastroenteritis. Two of the assays, Bio-EnzaBead (Litton Bionetics, Charleston, S.C.) and Rotazyme (Abbott Laboratories, North Chicago, Ill.), are enzyme immunoassays, while the third, Rotalex (Medical Technology Corporation, Somerset, N.J.), is a latex agglutination assay. We tested a total of 122 samples obtained from 26 children with gastroenteritis; 56 samples, obtained from 21 children, were found to contain rotavirus antigen by a reference microplate enzyme immunoassay. Rotavirus antigen was found by the Bio-EnzaBead, Rotazyme, and Rotalex assays in 53, 42, and 29 samples, respectively. The true positivity of samples which were positive by the reference microplate assay but negative by the other assays was confirmed by a specific neutralization assay or by the visualization of bands of double-stranded RNA by polyacrylamide gel electrophoresis or both. No false-positive assay results were noted with any of the commercial assays. The sensitivity of the assays was determined to a great extent by the time after the onset of illness at which the specimen was collected. Thus, the sensitivity of commercial assays with specimens collected early in the course of illness did not differ significantly from that of the microplate assay. However, significantly lower degrees of sensitivity were noted later in the course of illness. Results of our studies indicate that all three commercial assays can accurately detect rotavirus in stools from children with rotavirus gastroenteritis. However, the choice of assay systems for use in the clinical laboratory will depend on the conditions in which stool specimens are collected and tested in the laboratory.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop R. F., Davidson G. P., Holmes I. H., Ruck B. J. Virus particles in epithelial cells of duodenal mucosa from children with acute non-bacterial gastroenteritis. Lancet. 1973 Dec 8;2(7841):1281–1283. doi: 10.1016/s0140-6736(73)92867-5. [DOI] [PubMed] [Google Scholar]
- Blacklow N. R., Cukor G. Viral gastroenteritis. N Engl J Med. 1981 Feb 12;304(7):397–406. doi: 10.1056/NEJM198102123040705. [DOI] [PubMed] [Google Scholar]
- Brandt C. D., Kim H. W., Rodriguez W. J., Arrobio J. O., Jeffries B. C., Stallings E. P., Lewis C., Miles A. J., Chanock R. M., Kapikian A. Z. Pediatric viral gastroenteritis during eight years of study. J Clin Microbiol. 1983 Jul;18(1):71–78. doi: 10.1128/jcm.18.1.71-78.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt C. D., Kim H. W., Rodriguez W. J., Thomas L., Yolken R. H., Arrobio J. O., Kapikian A. Z., Parrott R. H., Chanock R. M. Comparison of direct electron microscopy, immune electron microscopy, and rotavirus enzyme-linked immunosorbent assay for detection of gastroenteritis viruses in children. J Clin Microbiol. 1981 May;13(5):976–981. doi: 10.1128/jcm.13.5.976-981.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrystie I. L., Totterdell B. M., Banatvala J. E. False positive rotazyme tests on faecal samples from babies. Lancet. 1983 Oct 29;2(8357):1028–1028. doi: 10.1016/s0140-6736(83)91014-0. [DOI] [PubMed] [Google Scholar]
- Cukor G., Berry M. K., Blacklow N. R. Simplified radioimmunoassay for detection of human rotavirus in stools. J Infect Dis. 1978 Dec;138(6):906–910. doi: 10.1093/infdis/138.6.906. [DOI] [PubMed] [Google Scholar]
- Flores J., Boeggeman E., Purcell R. H., Sereno M., Perez I., White L., Wyatt R. G., Chanock R. M., Kapikian A. Z. A dot hybridisation assay for detection of rotavirus. Lancet. 1983 Mar 12;1(8324):555–558. doi: 10.1016/s0140-6736(83)92811-8. [DOI] [PubMed] [Google Scholar]
- Grauballe P. C., Jarzabek Z. Comparison of indirect double antibody and double antibody sandwich ELISA techniques with latex agglutination test for the diagnosis of human rotavirus infection. Acta Virol. 1984 Jan;28(1):59–63. [PubMed] [Google Scholar]
- Haikala O. J., Kokkonen J. O., Leinonen M. K., Nurmi T., Mäntyjärvi R., Sarkkinen H. K. Rapid detection of rotavirus in stool by latex agglutination: comparison with radioimmunoassay and electron microscopy and clinical evaluation of the test. J Med Virol. 1983;11(2):91–97. doi: 10.1002/jmv.1890110202. [DOI] [PubMed] [Google Scholar]
- Hammond G. W., Ahluwalia G. S., Barker F. G., Horsman G., Hazelton P. R. Comparison of direct and indirect enzyme immunoassays with direct ultracentrifugation before electron microscopy for detection of rotaviruses. J Clin Microbiol. 1982 Jul;16(1):53–59. doi: 10.1128/jcm.16.1.53-59.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring A. J., Inglis N. F., Ojeh C. K., Snodgrass D. R., Menzies J. D. Rapid diagnosis of rotavirus infection by direct detection of viral nucleic acid in silver-stained polyacrylamide gels. J Clin Microbiol. 1982 Sep;16(3):473–477. doi: 10.1128/jcm.16.3.473-477.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J. H., Tuomari A. V., Mann D. R., Hamparian V. V. Latex immunoassay for rapid detection of rotavirus. J Clin Microbiol. 1984 Sep;20(3):441–447. doi: 10.1128/jcm.20.3.441-447.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapikian A. Z., Kim H. W., Wyatt R. G., Cline W. L., Arrobio J. O., Brandt C. D., Rodriguez W. J., Sack D. A., Chanock R. M., Parrott R. H. Human reovirus-like agent as the major pathogen associated with "winter" gastroenteritis in hospitalized infants and young children. N Engl J Med. 1976 Apr 29;294(18):965–972. doi: 10.1056/NEJM197604292941801. [DOI] [PubMed] [Google Scholar]
- Kapikian A. Z., Wyatt R. G., Dolin R., Thornhill T. S., Kalica A. R., Chanock R. M. Visualization by immune electron microscopy of a 27-nm particle associated with acute infectious nonbacterial gastroenteritis. J Virol. 1972 Nov;10(5):1075–1081. doi: 10.1128/jvi.10.5.1075-1081.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause P. J., Hyams J. S., Middleton P. J., Herson V. C., Flores J. Unreliability of Rotazyme ELISA test in neonates. J Pediatr. 1983 Aug;103(2):259–262. doi: 10.1016/s0022-3476(83)80361-8. [DOI] [PubMed] [Google Scholar]
- Morinet F., Ferchal F., Colimon R., Pérol Y. Comparison of six methods for detecting human rotavirus in stools. Eur J Clin Microbiol. 1984 Apr;3(2):136–140. doi: 10.1007/BF02014331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein A. S., Miller M. F. Comparison of an enzyme immunoassay with electron microscopic procedures for detecting rotavirus. J Clin Microbiol. 1982 May;15(5):938–944. doi: 10.1128/jcm.15.5.938-944.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambourg M., Goudeau A., Courant C., Pinon G., Denis F. Direct appraisal of latex agglutination testing, a convenient alternative to enzyme immunoassay for the detection of rotavirus in childhood gastroenteritis, by comparison of two enzyme immunoassays and two latex tests. J Clin Microbiol. 1985 Apr;21(4):622–625. doi: 10.1128/jcm.21.4.622-625.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theil K. W., McCloskey C. M., Saif L. J., Redman D. R., Bohl E. H., Hancock D. D., Kohler E. M., Moorhead P. D. Rapid, simple method of preparing rotaviral double-stranded ribonucleic acid for analysis by polyacrylamide gel electrophoresis. J Clin Microbiol. 1981 Sep;14(3):273–280. doi: 10.1128/jcm.14.3.273-280.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt R. G., James W. D., Bohl E. H., Theil K. W., Saif L. J., Kalica A. R., Greenberg H. B., Kapikian A. Z., Chanock R. M. Human rotavirus type 2: cultivation in vitro. Science. 1980 Jan 11;207(4427):189–191. doi: 10.1126/science.6243190. [DOI] [PubMed] [Google Scholar]
- Yolken R. H., Kim H. W., Clem T., Wyatt R. G., Kalica A. R., Chanock R. M., Kapikian A. Z. Enzyme-linked immunosorbent assay (ELISA) for detection of human reovirus-like agent of infantile gastroenteritis. Lancet. 1977 Aug 6;2(8032):263–267. doi: 10.1016/s0140-6736(77)90951-5. [DOI] [PubMed] [Google Scholar]
- Yolken R. H., Leister F. J. Evaluation of enzyme immunoassays for the detection of human rotavirus. J Infect Dis. 1981 Oct;144(4):379–379. doi: 10.1093/infdis/144.4.379. [DOI] [PubMed] [Google Scholar]



