Abstract
We proposed here that mobilized progenitor cells (MPCs) from the bone marrow are special cell types which carry cytoprotective proteins for cardiac repair following ischemia. Myocardial ischemia was induced by ligation of the left anterior descending coronary artery (LAD) in mice. Granulocyte colony stimulating factor (G-CSF) was used as the mobilizer of progenitor cells from the bone marrow. Progenitor cells in peripheral blood were analyzed by fluorescence-activated cell sorting (FACS). The expression of cytoprotective genes was assayed by ELISA, RT-PCR and/or real-time PCR. G-CSF was markedly up-regulated in the ischemic myocardium. A good correlation was observed between serum G-CSF and progenitor cells in circulation following LAD ligation. MPCs over-expressed cardiac transcription factor, GATA-4 and anti-apoptotic factor, Bcl-2 besides expression of the surface markers of bone marrow stem cells (BMSCs). Transplantation of cultured MPCs into the ischemic border area significantly improved cardiac function by reducing infarction size. More important, MPCs significantly protected cardiomyocytes against apoptosis when co-cultured with cardiomyocytes. The cardiac protection by MPCs was blocked by Bcl-2 neutralizing antibody and GATA-4 siRNA. In contrast, transfection of BMSCs with GATA-4 provided increased protection of myocytes against apoptosis. It is concluded that MPCs are highly cytoprotective and carry protective genes responsible for cardiac repair.
Keywords: ischemia, mobilization, progenitor cells, G-CSF, BMSCs, GATA-4, cytoprotective protein, Bcl-2
INTRODUCTION
Myocardial infarction (MI) is a leading cause of heart failure. Similar to cardiac stem cells (1) and human embryonic stem cells (2), bone marrow stem cells (BMSCs) have been used to repopulate the damaged myocardium by improving regional perfusion and systolic function in animal ischemic models (3–5). However, BMSCs transplantation strategies are limited due to low survival of transplantation cells (6) and failure to deliver large numbers of cells.
Progenitor cells with clonogenic properties have been detected in the peripheral blood. In patients with acute myocardial infarction (AMI), circulating mononuclear cells (MNCs) are significantly increased (7–9) and their homing to the infarcted myocardium are enhanced (10). Moreover, the patients with transient appearance of bone marrow cells in circulation showed significantly improved left ventricular function (11). Intracoronary transplantation of non-expanded peripheral blood-derived MNCs promotes improvement of cardiac function in patient with AMI (12,13). In animal ischemic models, intravenous infusion of cultured MNCs, obtained from peripheral blood, induces their homing to the ischemic tissue with a marked enhancement of angiogenesis (14). Upon homing to damaged myocardium, these cells differentiate into mature cardiomyocytes and endothelial cells (15,16).
The recruitment of circulating progenitors play an important role in the repair of ischemic heart. In comparison with niche bone marrow stem cells, the contents of angiogenic cytokines, including interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) are higher in mobilized cells (17). IL-1β up-regulates the expression of VEGF and VEGF receptor-2 (18). IL-1βand TNF-α have also shown to have the angiogenic activity (19). However, very low levels of progenitor cells are released into the peripheral blood under physiological condition. It has been reported that the spontaneous mobilization of CD34+ cells into the peripheral blood of patients with AMI is positively correlated to increase in endogenous G-CSF (20). Moreover, G-CSF injection induced myocardial regeneration by promoting BMSCs migration into the infarcted border areas (15,21,22). The underlying molecular mechanisms of mobilized progenitor cells mediated cardiac repair have remained obscure. We hypothesized that ischemic myocardium release G-CSF which mobilizes progenitors from bone marrow into peripheral circulation. Mobilized progenitors are special dedicated cells which protect against myocardial infarction through regeneration and paracrine effects by serving as carriers of cytoprotective proteins.
METHOD
All animals were treated in accordance with the Guidelines for the Care and Use of Laboratory Animals prepared by the National Academy of Sciences and published by the National Institutes of Health (NIH publication No. 85-23, Revised 1996).
1. Model of myocardial ischemia
Male C57BL/6J mice (24 ~ 30g) were used in all in vivo studies. Mice were anesthetized by an intraperitoneal injection (i.p.) of pentobarbital sodium (50 mg/kg) and mechanically ventilated. The heart was exposed and left anterior descending coronary artery (LAD) was ligated (23). Ischemic animals underwent the ligation of LAD. Sham animals were operated similar to ischemia except without LAD ligation.
To ascertain the effect of G-CSF injection on the mobilization of bone marrow stem cells, wild type mice were irradiated with 1200 cGy, using Cesium-137 radiation source (Gamma Cell 40 Irradiator). Two days later, about 4~5 × 105 GFP+ BMSCs which were freshly isolated from GFP transgenic mouse, were injected via tail vein. Six weeks after BMSC transplantation, MI was created by permanent ligation of LAD. G-CSF (100μg/kg/day) was subcutaneously injected once per day after 4 hrs following MI for 5 days. PBS was injected as a control. On the 6th day following ligation of LAD, the blood was taken and the circulating c-kit+/GFP+ cells were analyzed by FACS. Hearts were examined for the presence of mobilized GFP+ BMSCs.
To compare the role of MPCs and BMSCs on myocardial infarction, following LAD ligation, mice were randomly given an intramyocardial injections with a 31-gauge needle of 1) 10 μl DMEM basal medium; or 2) 5 × 105 BMSCs; or 3) 5 × 105 MPCs in 5 points (2 μl in each points) around ischemic area.
2. Assay of cardiac function and infarction size
Echocardiography was performed by using HDI 5000 SonoCT (Phillips) with 15-Mhz probe. A two-dimensional image was obtained while mice orientated on a heating pad. LV internal diastolic diameter (LVDd) and LV internal systolic diameter (LVDs) were obtained from M-mode interrogation in long-axis view. Fractional shortening (FS) and ejection fraction (EF) were calculated as: FS (%) = (LVDd−LVDs)/LVDd ×100; EF (%) = [(LVDd)3−(LVDs)3]/(LVDd)3×100.
After echocardiography was carried out, the animals were sacrificed and the infarct size of heart was measured by staining heart sections with hematoxylin-eosin and Masson’s trichome. Infarct size was defined as the sum of the epicardial and endocardial infarct circumference divided by the sum of the total LV epicardial and endocardial circumferences using computer-based planimetry. Quantitative assessments were performed with image analysis software (version 1.6065, NIH) (23).
3. In vitro ischemic model
To demonstrate the direct protection of MPCs on cardiomyocytes, MPCs were co-cultured with cardiomyocytes which were isolated from ventricles of 2-day-old neonatal Sprague-Dawley rats (Harlan, Indianapolis, IN) using the Neonatal Cardiomyocyte Isolation System (Worthington biochemical Co. NJ) as previously described (24). Cell ischemic damage was duplicated by exposure to hypoxia: low glucose DMEM (1g/l) and placed in hypoxic incubator (Sanyo, O2/CO2 incubator-MCO-18M) with 1% oxygen, 5% CO2 and 94% N2. Apoptosis was determined by Annexin-V (MBL International) binding test or DNA fragmentation (Biovision) following manufacture’s instructions.
4. Culture of MPCs and BMSCs
Blood was directly drawn from hearts. Red blood cells and platelets were discarded using Histopaque-1083 separation. The nucleated cells were cultured with Iscove’s Modified Dulbecco’s Medium (IMDM) supplemented with 20% FBS and penicillin (100 U/ml)/streptomycin (100 μg/ml) at 37°C in humid air with 5% CO2. The adherent cells, shaped as mononuclear cells (MNCs) were considered as MPCs. BMSCs were isolated and cultured according to the method described by us previously (25).
5. Fluorescence-activated cell sorting (FACS) analysis
The nucleated cells (1~5×106cells) from circulating blood were incubated with anti-c-kit (R&D Systems) which were conjugated with PE. Cultured cells were suspended in staining medium and incubated with primary antibodies, including anti-c-kit, E-selectin (BD-Pharmingen), Sca-1, SB-10 (R&D Systems), and AC133 (eBioscience), which were conjugated with PE, FITC, or APC, respectively. Fluorescence emission of FITC, PE and APC were detected using standard filters on a FACSCalibur (Bectin Dickin, USA). Fluorescent isotype–matched antibodies were used as negative controls.
6. Immunocytochemistry
Immunostaining was performed as previously published with minor modifications (23,26). Briefly, cultured cells or heart tissues were fixed in 4% paraformaldehyde. Rabbit polyclonal anti-connexin 43 (Santa Cruz) and mouse monoclonal anti-α-sarcomere actinin (Sigma) were used. After thoroughly washing, goat anti-rabbit or anti-mouse IgG which conjugated with rhodamine or FITC was applied. Nuclei were stained with 4′, 6-diamino-2-phenylindole (DAPI) when necessary. Fluorescent images were obtained using an Olympus BX 41 microscope equipped with an Olympus U-TV0.5XC digital camera (Olympus, Japan).
7. The expression of various genes
G-CSF in serum was measured by ELISA. The mRNA of G-CSF, GATA-4 and Bcl-2 in myocardium and/or cultured cells was examined by RT-PCR and real-time PCR. Total RNA was isolated by using Trizol reagent (Invitrogen), followed by DNAse treatment and purification using RNeasy mini column kit (Qiagen). cDNA was synthesized using the SuperScript™III RNase H− Reverse Transcriptase (Invitrogen) in a 20μl reaction mixture. An aliquot of the cDNA was amplified using Taq DNA polymerase (2.5 U) (Invitrogen) in the presence of sense and anti-sense primers (1μM). The primers for different factors were shown as Table 1. Conventional PCR was performed in Mastercycler Gradient (Eppendorf, AG 22331, Humburg). Quantitative real-time PCR was carried out on the iQ5 real-time system (Bio-Rad) with the iQ SYBR Supermix (Bio-Rad). The expression of each target mRNA relative to GAPDH under experimental and control conditions was calculated based on the threshold cycle (CT) as r = 2−Δ (ΔCT), where ΔCT = CT target − CT GAPDH and Δ (ΔCT) = ΔCT experimental − ΔCT control.
Table 1.
The primers for different factors
| Primers | Forward | Reward |
|---|---|---|
| G-CSF | 5′-GTG TGC CAC C TA CAA GCT GTG TC | 5′-CAG GTG AAG CAT ATC CAA GGT GG |
| GATA-4 | 5′-CCT CTC CCA GGA ACA TCA AA | 5′-ACC CAT AGT CAC CAA GGC TG |
| Bcl-2 | 5′-AGT TCG GTG GGG TCA TGT GTG | 5′-CCA GGT ATG CAC CCA GAG TG |
| GAPDH | 5′-GGC TGC CTT CTC TTG TGA CA | 5′-GAT GTT AGC GGG ATC GCG CT |
8. MPCs transfected with GATA-4 siRNA
The sequences of rat GATA-4 siRNA (Ambion, siRNA ID: 199692) are: 5′CGGAAGCCCAAGAAUCUGAtt3′ (sense), 5′UCAGAUUCUUGGGCUUCCGtt3′ (antisense). The transfection of siRNA was performed using Lipofectamine RNAiMAX according to the manufacturer’s instructions (Invitrogen). Briefly, 3×104 MPCs/well was seeded in 2 ml of IMDM containing 20% fetal bovine serum without any antibiotics in 6-well plate. The cells were transfected with GATA-4 siRNA (75 pmol) when the cell density reached 50~60% confluence. The transfected cells were harvested 48 h later.
9. Virus-based vectors and infection of BMSCs
Retrovirus expressing GATA-4 was constructed using Clontech pMSCV retroviral expression system. IRES-EGFP (Clontech) was first cloned into pMSCV vector at Xho I and EcoR I sites. Then GATA-4 was excised from pcDNA-GATA4 (27) with HindIII and Xho I restriction enzymes and cloned into pMSCV-IRES-EGFP at Bgl II and Sal I sites. pMSCV-GATA-4-IRES-EGFP and cotransfected with pVSVG plasmid into GP2-293 cells (Contech). pMSCV-IRES-EGFP was transfected with pVSVG plasmid into GP2-293 cells and served as transfection control. After 48 hours, supernatants were filtered and incubated with BMSCs in the presence of 10 μg/ml polybrene (Sigma) for 12 hours. Stable GATA-4 and GFP expressing clones were selected with puromycin (Sigma) at 3μg/ml for 5 days. The efficiency of the infection was confirmed by analysis of GFP autofluorescence and immunofluorescence staining with anti-GATA-4 antibody.
10. Statistical analysis
All data obtained from ELISA and real-time PCR analyses were carried out on the duplicate or triplicate. Each experiment was repeated three times unless mentioned otherwise in results. Data was expressed as mean ± SEM. Difference between means was verified by using student’s t-test. For comparison, statistical differences were considered significant at a p value of less than 0.05.
RESULTS
1. Ischemia significantly increased the G-CSF in injured myocardium
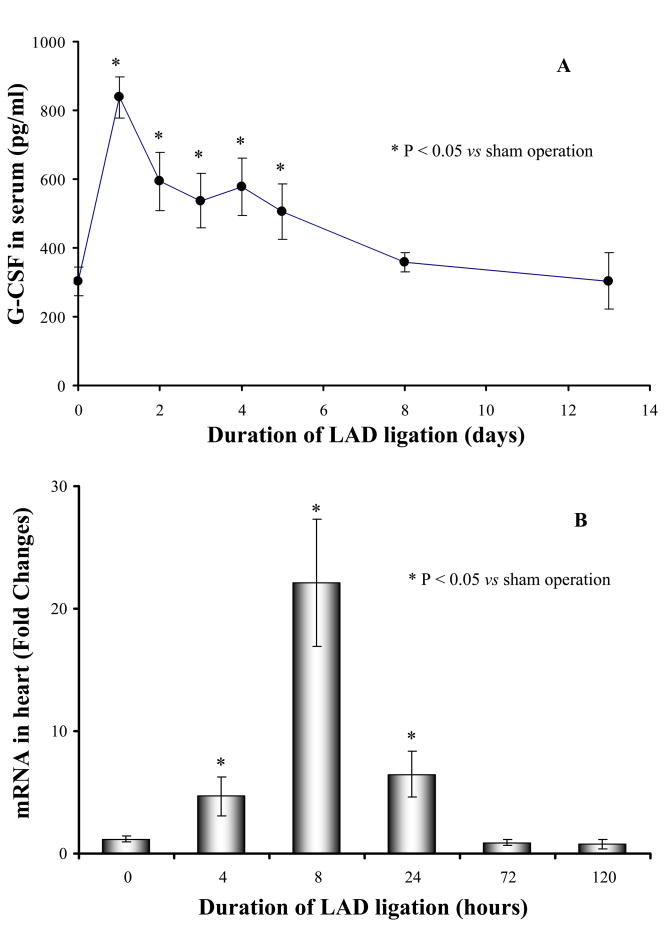
Myocardial ischemia significantly increased serum G-CSF which peaked on the 1st day (3-fold) and continued for 5 days (Figure 1A). Quantitative analysis indicated that the expression of G-CSF in heart was significantly higher after LAD ligation at 4, 8 and 24h as compared to normal control animals. However, the up-regulation of G-CSF in myocardium was significantly eliminated after heart underwent LAD ligation for 3 days.
Figure 1.
G-CSF in serum and ischemic myocardium was significantly increased after LAD ligation for various periods. Panel A: G-CSF in serum (9 mice each group); Panel B: The expression of G-CSF in ischemic myocardium (4 mice each group). * p<0.05 vs sham operation (LAD ligation for 0 hours).
These data suggested that heart ischemia directly induced G-CSF up-regulation in injury myocardium, which might be responsible for the higher G-CSF in serum.
2. Mobilization of progenitor cells following heart ischemia
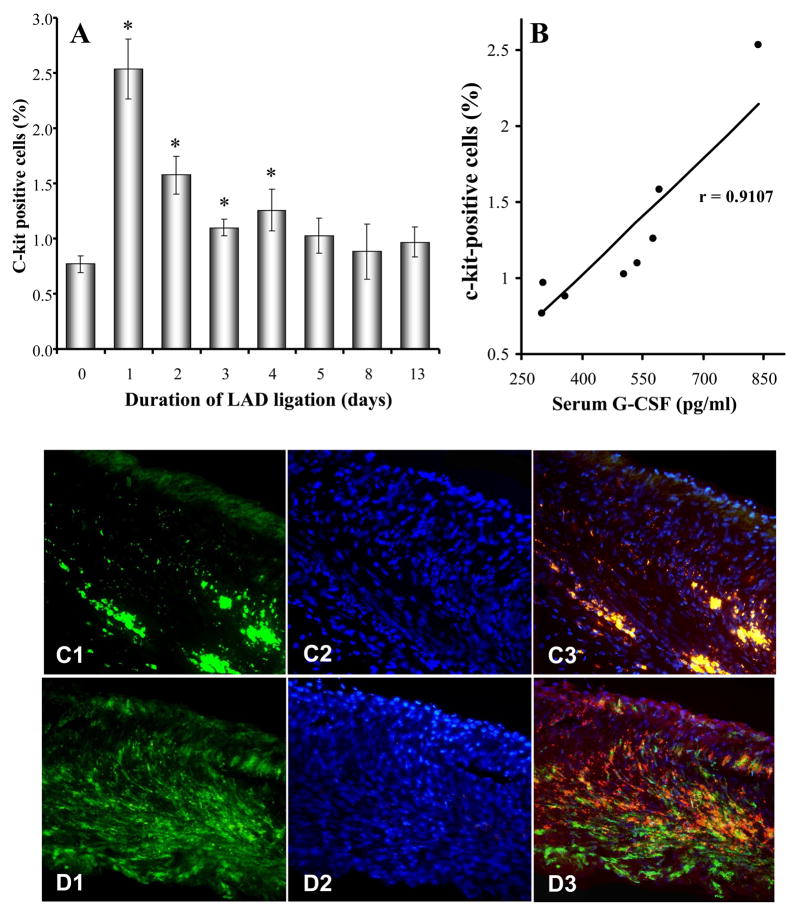
C-kit is a hallmark of progenitor cells. FACS data showed that c-kit+ cells were very few in the peripheral circulation of control mice (0.77±0.07% of nucleated cells). However, it was significantly elevated after LAD ligation with a peak on the first day (2.53±0.27%). C-kit+ cells in sham operated animals were also significantly increased by 1.8-fold on the first day after surgery compared to normal control animals (1.42±0.21 % vs 0.77±0.07% in control, p<0.05). However, it was markedly less than LAD ligation animals. The increase of c-kit+ cells (Figure 2A) induced by ischemia was positively correlated with an increase in serum G-CSF (r=0.9107, p<0.05) (Figure 2B). Injection of G-CSF (100μg/kg/day) following LAD ligation for 5 days significantly increased c-kit+ cells in peripheral blood by 4-fold compared to PBS control (2.29±0.55% vs 0.54±0.13%, p<0.05). Similar increases in GFP+ BMSCs in infarcted border zone of irradiated mice were noted (Figure 2C~D).
Figure 2.
Relationship of G-CSF and mobilization of BMSCs. Panel A: FACS analysis of c-kit+ cells which appeared in peripheral blood after heart ischemia for 1 to 13 days (9 mice each group). * p < 0.05 vs normal control. Panel B: The number of circulating c-kit+ mononuclear cells is related to serum G-CSF level (9 mice each group) (p < 0.05). Panel C–D: Injection of G-CSF after LAD ligation for 5 days, GFP+ cells homing to infarcted myocardium (n = 10 in control, n = 4 in G-CSF). GFP+ BMSCs were rarely observed in PBS control hearts (D). On the other hand, extensive localization of the GFP+ BMSC was observed in the infarct and peri-infarct regions in G-CSF treated animals (E). Green: GFP+ BMSCs, blue: nuclei stained with DAPI, red: α-sarcomere actin, yellow: cardiomyocytes derived from GFP+ BMSCs.
These observations indicate that G-CSF mobilized bone marrow progenitor cells and prompted their homing to the ischemic myocardium.
3. MPCs improved cardiac function and formed myocardial phenotypes
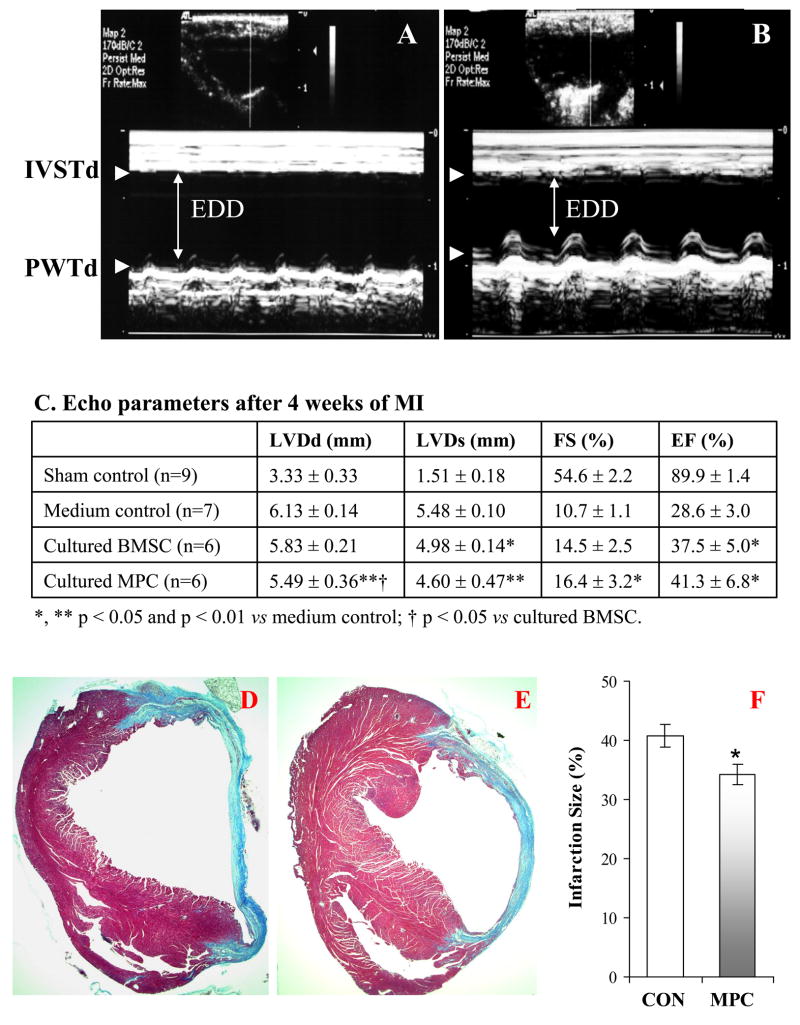
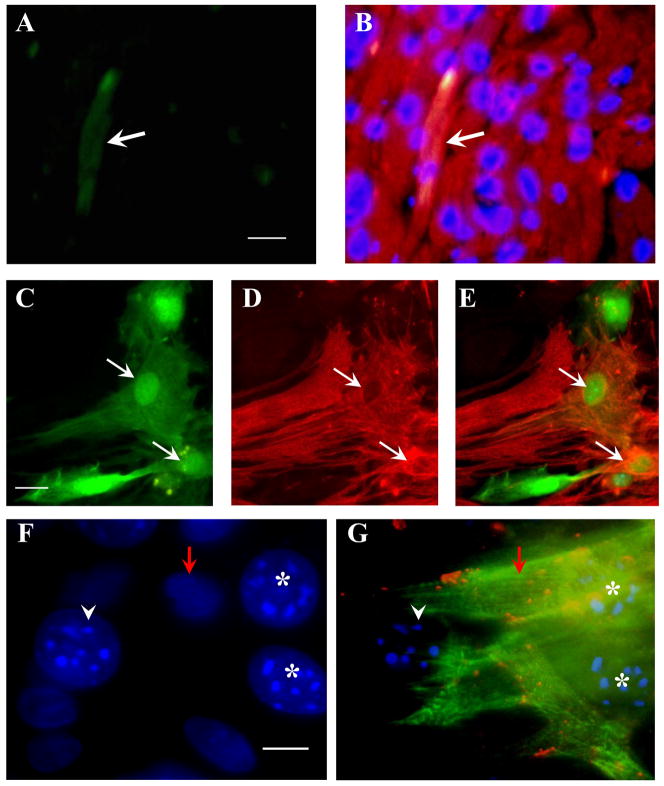
With transplantation of cultured GFP+ MPCs into the border zone of ischemic area for 4 weeks, cardiac function was markedly improved as shown by echocardiography (Figure 3A–C). The LVDd and LVDs decreased, as well as EF and FS improved in treated animals as compared to control animals which received the medium. Here, it should be emphasized that the cardiac function in mice which were transplanted with MPCs was better than that transplanted with BMSCs, especially, the improvement of LDVd and FS. The infarcted area was also significantly smaller in MPCs transplanted hearts than that in control hearts (Figure 3D–F). GFP+ cells were observed in the infarcted border area and some GFP+ cells expressed sarcomeric α-actinin (Figure 4A–B). When MPCs were co-cultured with cardiomyocytes for 7 days, about 26 % of mobilized cells were positive for both GFP and α-actinin (Figure 4C–E). These cells developed connexin 43 with native cardiomyocytes (Figure 4F–G). All results suggest that MPCs improved cardiac function and transdifferentiated into myocardial phenotype in vivo and in vitro.
Figure 3.
MPCs protect heart against ischemic injury. Panels A and B showed the M-mode echocardiograms obtained from mice in medium control (A) and in cultured MPCs transplantation (B) at 4 weeks after ligation of LAD. Panel C: Echocardiographic parameters at 4 weeks of MPC transplantation. LVDd & LVDs: left ventricular internal dimensions at end diastole and end systole; FS: fractional shortening; EF: ejection fraction. EDD indicates end-diastolic dimension. Panels D~F: The animals receiving cultured MPCs treatment (E) exhibit reduction in the infarct size compared to medium control animals (D) (Masson-Trichome staining). * p < 0.05 vs medium control.
Figure 4.
MPCs differentiated into cardiac phenotypes in vivo and in vitro. Panels A and B: GFP+ MPCs were observed in hearts after they were transplanted into ischemic border area for 4 weeks (A). Some of them were positive for sarcomeric α-actin (B, white arrow). Panels C–G, MPCs were co-cultured with native cardiomyocytes. Some GFP+ cells in Panel C were positive to α-actinin (D) (white arrows). Panel E is the overlap of “C” and “D”. Panel F and G: Two of MPCs with multinucleoli (F, stars) were positive for α-actinin staining (G, green) and gap junction protein connexin 43 (G, orange dots) which was clearly seen between MPCs and native cardiomyocytes (red arrow) (G). White arrowhead shows an undifferentiated MPCs. Bars = 25 μm.
In this study, the MPCs were obtained from mice. Considering the species difference, some in vitro co-culture studies related to DNA fragmentation and Annexin-V staining were repeated with rat MPCs. No significant differences could be seen between MPCs from mouse and rat. Neonatal cardiomyocytes began to beat spontaneously after being cultured for 24 hours as compared with adult cardiomyocytes.
4. Characterization of MPCs
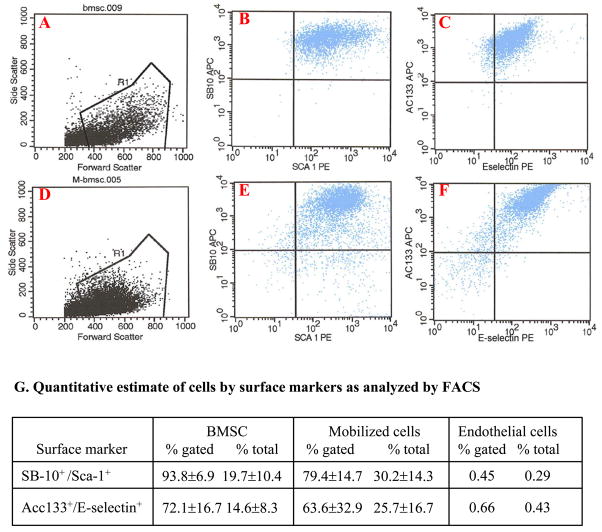
The benefit effect of MPCs on myocardial function improvement might be related to their characterization. The cultured MPCs, obtained from GFP transgenic mice, mainly contained small spindle shaped and flattened cells which displayed morphological characteristics similar to BMSC. Cell types in cultured cells were determined by using FACS. Sca-1 and SB-10 (CD166, ALCAM) were selected as the markers of mesenchymal stem cells (MSC) (28,29). AC133 (CD133) and E-selectin (CD62E) were served as the markers of endothelial progenitor cells (EPC) (28,30). Most of the mobilized cells were positive for these markers (Figure 5A–G). There were no significant differences in the expression of cell surface proteins of MSC and EPC between MPCs and niche BMSCs.
Figure 5.
Expression of cell surface markers in primary cultured BMSCs and MPCs by FACS. BMSCs (A~C) and MPCs (D~F) were positive for MSC (SB-10 and Sca-1) and EPC (E-selectin and AC133) markers. Panel G. Quantitative estimate of FACS data. Endothelial cells were used as negative control.
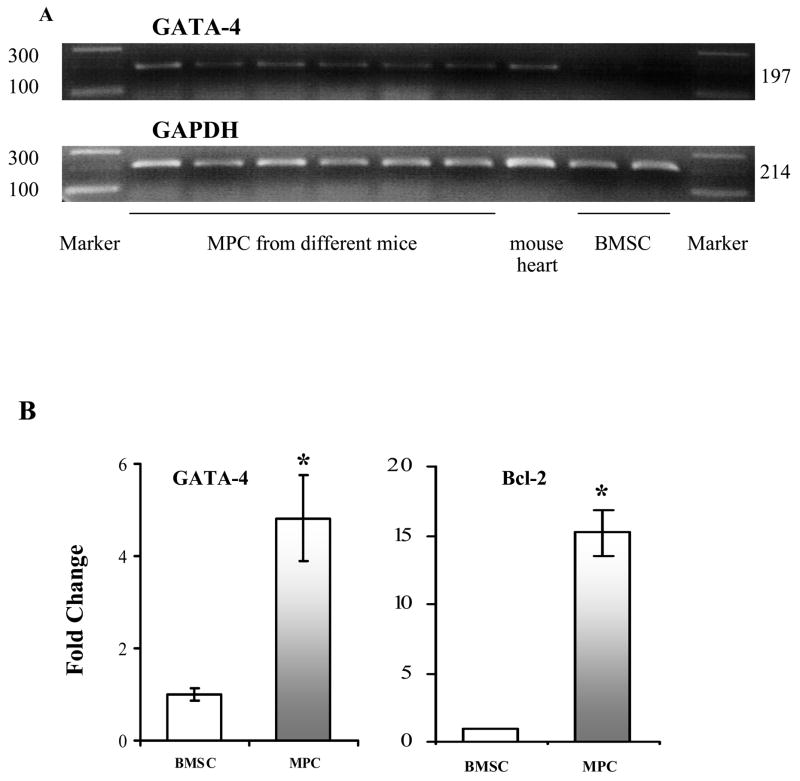
However, further investigation on the gene expression by RT-PCR and quantitative PCR indicated that MPCs were different from the niche BMSCs (Figure 6). The expression of GATA-4 and Bcl-2 were 5 and 15 fold higher in cultured MPCs than that in BMSCs, respectively.
Figure 6.
RT-PCR and Quantitative PCR for the expression of GATA-4 and Bcl-2. Panel A: RT-PCR only amplified mouse GATA-4 in 6 samples of MPCs from different mice, but did not amplify in BMSCs. Panel B,C: Real-time PCR data for GATA-4 and Bcl-2. * p<0.05 vs BMSCs, respectively.
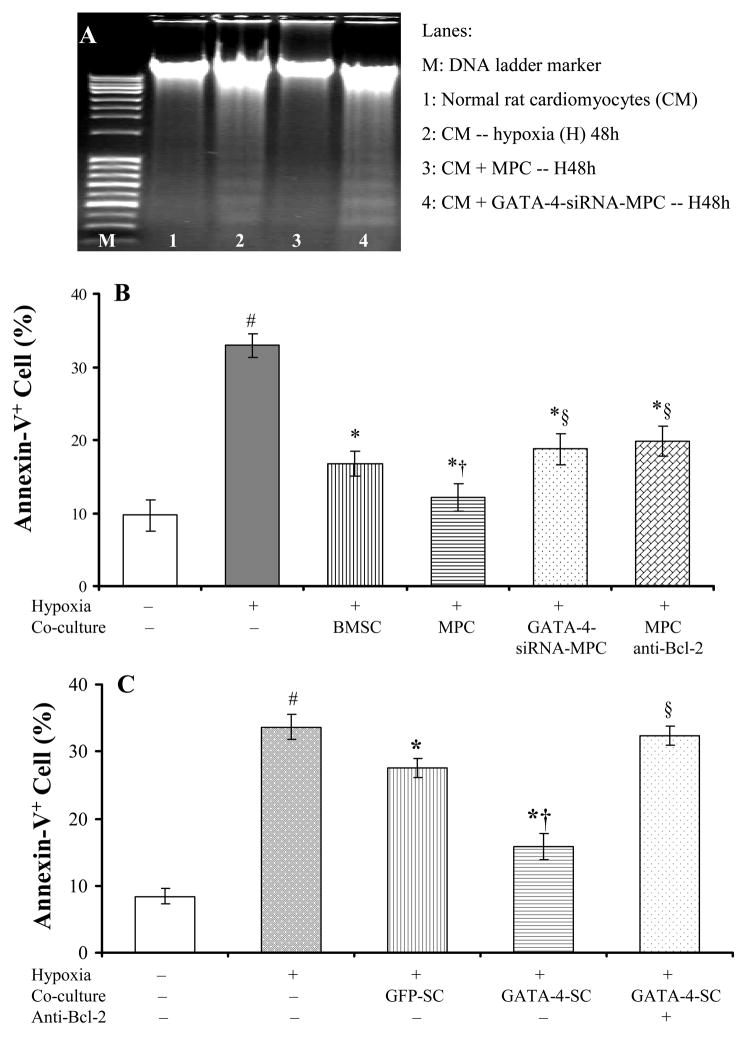
5. MPCs directly prevented cardiomyocyte apoptosis
The direct protection of MPCs on cardiomyocytes against oxidative stress was investigated in vitro. Co-culture with MPCs completely prevented cardiomyocyte DNA fragmentation induced by exposure to hypoxia for 48 hours. The role of GATA-4 and Bcl-2 in anti-apoptosis was investigated by using GATA-4 siRNA and Bcl-2 neutralizing antibody and to eliminate the effect of MPCs and by transfecting BMSCs with GATA-4 to mimic the effect of MPCs. Neutralizing antibody was added into serum-free medium prior to hypoxia for 16 hours and maintained whole hypoxia. The anti-apoptotic effect of MPCs was partially abolished by Bcl-2 neutralizing antibody and GATA-4 siRNA (Figure 7A, B). In contrary, GATA-4-transfected BMSCs (GATA-4-SCs) significantly increased the anti-apoptotic effect compared to GFP-transfected BMSCs (GFP-SCs), which is partially abolished by using Bcl-2 neutralizing antibody (Figure 7C).
Figure 7.
MPCs protected cultured cardiomyocytes against hypoxia. Panel A: DNA (30μg/lane) fragmentation induced by hypoxia for 48h in cardiomyocytes (Lane 2) was completely prevented by co-culture with MPCs (Lane 3), which was abolished by MPCs transfected with GATA-4 siRNA (Lane 4). Panel B~C: Quantitation of the anti-apoptotic effect of MPCs by annexin-V staining. Panel B: MPCs significantly decreased hypoxia-induced cardiomyocytes apoptosis, which was decreased by transfection of GATA-4 siRNA and by pre-treating cells with Bcl-2 neutralizing antibody. Panel C. BMSCs were transfected with GATA-4 to mimic the effect of MPCs. GATA-4-SCs significantly reduced the apoptosis. Apoptotic cells were completely restored by Bcl-2 neutralization antibody. GFP-SC: BMSCs transfected with GFP; GATA-4-SC: BMSCs transfected with GATA-4. #, p<0.05 vs normal culture; *, p<0.05 vs hypoxic cardiomyocytes alone; †, p<0.05 vs cardiomyocytes co-cultured with BMSCs (Panel B) or with GFP-SC (Panel C); §, p<0.05 vs cardiomyocytes co-cultured with MPCs (Panel B) or with GATA-4-SC (Panel C), respectively.
DISCUSSION
The present study suggests that: 1) cardiac ischemia up-regulated G-CSF expression in myocardium, causing mobilization of progenitor cells to the site of injury; 2) mobilized progenitor cells are special population of BMSC which are unique in higher expression the genes of cytoprotective proteins and cardiac transcription factors responsible for regeneration of damaged myocardium and protection of cardiomyocytes.
1. Ischemia up-regulates G-CSF which mobilizes progenitor cells
Myocardial infarction leads to cardiac remodeling, a complex, dynamic, and time-dependent phenomenon characterized by 1) death of both cardiomyocytes and nonmyocytes in part due to increased apoptosis; 2) fibrosis marked by increased collagen deposition in response to myocyte loss and stimulation by hormones, cytokines, and growth factors; and 3) hypertrophy of cardiomyocytes. We observed that MNCs were markedly increased in peripheral circulation following cardiac ischemia and was positively correlated with serum G-CSF levels. It has been reported that ischemia mobilized BMSC in brain, kidney and intestine and was related to the increase of G-CSF both in proteins and G-CSF mRNA levels (31–33). We hypothesized that G-CSF is released from the ischemic myocardium into circulation, and it then mobilizes bone marrow progenitor cells to the site of injury where these cells participate in the repair of ischemic hearts and prevent pathological remodeling. The expression of G-CSF in ischemic area was significantly increased and peaked at 8 hours after LAD ligation, which is consistent with the pervious report (34). However, the upregulation of G-CSF was null at 3 days after LAD ligation. The progressive increase of G-CSF in ischemic myocardium might be from either cardiac myocytes or the cellular infiltration of the necrotic tissue by fibroblasts and/or leukocytes (34) during early heart ischemia.
To support the hypothesis that the up-regulation of G-CSF mobilizes progenitor cells from the bone marrow to the heart, we injected G-CSF into mice. Indeed, there was a significant increase of progenitor cells in peripheral blood and homing of these MPCs to the border area of infarcted myocardium.
The mechanism of G-CSF-induced mobilization of BMSC into circulation is not very clear. Accumulating evidence suggests that SDF-1 plays a central role in G-CSF induced stem cell mobilization. Reduction of SDF-1 concentrations within the bone marrow may interfere with retention and facilitate the egress of cells. It has been reported that G-CSF induces gradual proteolytic degradation of human and murine bone marrow SDF-1 via elastase, accompanied by a gradual increase in SDF-1 receptor, CXCR4 expression on bone marrow cells (35). Up-regulation of CXCR4 may increase the sensitivity of cells to SDF-1 signals. Blocking CXCR4 led to a significant reduction of stem and progenitor cell mobilization in mice (36). G-CSF induced a severe reduction of SDF-1 within the bone marrow, whereas their protein expression in the blood was less affected (35). Coronary ligation induced SDF-1 mRNA and protein expression in the infarct and border zones (36). A newly formed gradient of SDF-1 following ischemia is sufficient to induce stromal cell mobilization from bone marrow to peripheral circulation and then to the injured heart.
2. MPCs improved myocardial function
Mobilized cells play a critical role in ischemic heart repair. Implantation of cultured MPCs into infarcted risk zone significantly improved cardiac function and limited infarct extension. We hypothesized that the beneficial effects of MPCs may be related to the stimulation of endogenous repair mechanisms and paracrine-induced trophic effects as these MPCs highly expressed GATA-4 and Bcl-2 as well as VEGF.
2. 1. MPCs regenerate the damaged myocardium
MPC transplantation may be associated with a significant improvement in myocardial contractile performance in the risk area. The functional improvement might partially be due to regeneration of the damaged myocardium. MPCs differentiated into cardiac phenotypes after they were transplanted into hearts. In vitro study showed that MPCs transdifferentiated into cardiomyocytes after co-culture with cardiomyocytes at a higher rate (26%) compared to bone marrow mixed stem cells (14%) (26). Although GATA-4 is one of cardiac transcription factors, it is also cardioprotective. As reviewed by Molkentin (37), GATA-4 controls cardiac structural and regulatory gene expression. Higher expression of GATA-4 in MPCs is further evidence of their increased differentiation potential into myocytes (38,39).
Neovascularization is a very important step for enhanced oxygen delivery to the ischemia-threatened risk area. Our study showed that MPCs had a higher potential in improving cardiac function than BM mixed stem cells. The improved function by MPCs could be due to better regional perfusion following acute coronary artery occlusion (3,5). Our unpublished data indicates that MPCs upregulated VEGF (10 fold higher than that in BMSC) in low oxygen environment, although there were no significant difference in normoxic condition between MPCs and BMSCs. Higher expression of VEGF in MPCs plays a very important role in improvement of regional perfusion. VEGF increases vessel density, induces endothelial cell proliferation, augment collateral perfusion and increase oxygen delivery to myocardium (22,40,41). The other possibility of cardiac function improvement could be due to reduction of wall stress consequently improving myocardial bioenergetics in the risk area (3).
2. 2. Activation of anti-apoptosis signaling system to protect ischemia-threatened cardiomyocytes from apoptosis
The loss of cardiac myocytes is the major problem in heart failure. Thus, it is important to protect cardiomyocytes against cell death. Various growth factors, including insulin like growth factor and hepatocyte growth factor have been shown to protect the heart against oxidative stress (42,43). One of the significant finding of this work is MPCs carry protective proteins which protect cardiomyocytes from ischemic injury. These mobilized cells highly expressed GATA-4 transcription factor and Bcl-2, which served as the donor of cytoprotective proteins and possessed higher potential for the repair of ischemic hearts.
This study indicates that the anti-apoptotic effect of MPCs is also related to their higher expression of Bcl-2. Bcl-2 neutralizing antibody significantly decreased the protection of MPCs. Overexpression of Bcl-2 reduced MSC death and apoptosis under hypoxic conditions. In vivo transplantation of Bcl-2-MSCs enhanced the survival rate of MSCs in the LAD ligation model (44). Moreover, Shimizu et al. reported that BMSCs induced over-expression of Bcl-2 in myocytes, increased their resistance to oxidative stress (45). Knockdown of Bcl-2 expression by siRNA in cardiac fibroblasts increased their apoptotic response to staurosporine, serum, and glucose deprivation and to simulated ischemia (46). GATA-4 positively regulated cardiac Bcl-2 gene expression in vitro and in vivo (47). Our micro-array analysis indicated that the expression of Bcl-2 in GATA-4 transfected BMSCs was 3 times higher than that in GFP transfected BMSCs (data not shown). The anti-apoptotic effect of MPCs was significantly decreased in MPCs-GATA-4-siRNA group as shown by DNA fragmentation and increase in annexin-V positive cells.
In conclusion, the present study demonstrates that an up-regulation of G-CSF in response ischemia lead to rapid mobilization and recruitment of bone marrow progenitor cells to the injury area. These mobilized progenitor cells served as carriers of cytoprotective proteins and genes responsible for cardiac repair and protection.
Acknowledgments
This work was supported by National Institutes of Health grants HL083236 (M. Xu), R37-HL074272, HL23597, HL080686, HL70062 (M. Ashraf) and HL081859 (Y. Wang).
Footnotes
There are no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Urbanek K, Torella D, Sheikh F, et al. Myocardial regeneration by activation of multipotent cardiac stem cells in ischemic heart failure. Proc Natl Acad Sci U S A. 2005;102:8692–7. doi: 10.1073/pnas.0500169102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xue T, Cho HC, Akar FG, et al. Functional integration of electrically active cardiac derivatives from genetically engineered human embryonic stem cells with quiescent recipient ventricular cardiomyocytes: insights into the development of cell-based pacemakers. Circulation. 2005;111:11–20. doi: 10.1161/01.CIR.0000151313.18547.A2. [DOI] [PubMed] [Google Scholar]
- 3.Zeng L, Hu Q, Wang X, et al. Bioenergetic and functional consequences of bone marrow-derived multipotent progenitor cell transplantation in hearts with postinfarction left ventricular remodeling. Circulation. 2007;115:1866–75. doi: 10.1161/CIRCULATIONAHA.106.659730. [DOI] [PubMed] [Google Scholar]
- 4.Nagaya N, Kangawa K, Itoh T, et al. Transplantation of mesenchymal stem cells improves cardiac function in a rat model of dilated cardiomyopathy. Circulation. 2005;112:1128–35. doi: 10.1161/CIRCULATIONAHA.104.500447. [DOI] [PubMed] [Google Scholar]
- 5.Kudo M, Wang Y, Wani MA, Xu M, Ayub A, Ashraf M. Implantation of bone marrow stem cells reduces the infarction and fibrosis in ischemic mouse heart. Journal Of Molecular And Cellular Cardiology. 2003;35:1113–9. doi: 10.1016/s0022-2828(03)00211-6. [DOI] [PubMed] [Google Scholar]
- 6.Muller-Ehmsen J, Krausgrill B, Burst V, et al. Effective engraftment but poor mid-term persistence of mononuclear and mesenchymal bone marrow cells in acute and chronic rat myocardial infarction. Journal Of Molecular And Cellular Cardiology. 2006;41:876–84. doi: 10.1016/j.yjmcc.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 7.Leone AM, Rutella S, Bonanno G, et al. Mobilization of bone marrow-derived stem cells after myocardial infarction and left ventricular function. European Heart Journal. 2005;26:1196–204. doi: 10.1093/eurheartj/ehi164. [DOI] [PubMed] [Google Scholar]
- 8.Shintani S, Murohara T, Ikeda H, et al. Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation. 2001;103:2776–9. doi: 10.1161/hc2301.092122. [DOI] [PubMed] [Google Scholar]
- 9.Spevack DM, Cavaleri S, Zolotarev A, et al. Increase in circulating bone marrow progenitor cells after myocardial infarction. Coronary Artery Disease. 2006;17:345–9. doi: 10.1097/00019501-200606000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Aicher A, Brenner W, Zuhayra M, et al. Assessment of the tissue distribution of transplanted human endothelial progenitor cells by radioactive labeling. Circulation. 2003;107:2134–9. doi: 10.1161/01.CIR.0000062649.63838.C9. [DOI] [PubMed] [Google Scholar]
- 11.Tomoda H, Aoki N. Bone marrow stimulation and left ventricular function in acute myocardial infarction. Clin Cardiol. 2003;26:455–7. doi: 10.1002/clc.4960261005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tatsumi T, Ashihara E, Yasui T, et al. Intracoronary transplantation of non-expanded peripheral blood-derived mononuclear cells promotes improvement of cardiac function in patients with acute myocardial infarction. Circulation Journal: Official Journal Of The Japanese Circulation Society. 2007;71:1199–207. doi: 10.1253/circj.71.1199. [DOI] [PubMed] [Google Scholar]
- 13.Bartunek J, Vanderheyden M, Vandekerckhove B, et al. Intracoronary injection of CD133-positive enriched bone marrow progenitor cells promotes cardiac recovery after recent myocardial infarction: feasibility and safety. Circulation. 2005;112:I178–83. doi: 10.1161/CIRCULATIONAHA.104.522292. [DOI] [PubMed] [Google Scholar]
- 14.Kawamoto A, Gwon HC, Iwaguro H, et al. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation. 2001;103:634–7. doi: 10.1161/01.cir.103.5.634. [DOI] [PubMed] [Google Scholar]
- 15.Fukuhara S, Tomita S, Nakatani T, et al. G-CSF promotes bone marrow cells to migrate into infarcted mice heart, and differentiate into cardiomyocytes. Cell Transplant. 2004;13:741–8. doi: 10.3727/000000004783983486. [DOI] [PubMed] [Google Scholar]
- 16.Kalka C, Masuda H, Takahashi T, et al. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci U S A. 2000;97:3422–7. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamihata H, Matsubara H, Nishiue T, et al. Improvement of collateral perfusion and regional function by implantation of peripheral blood mononuclear cells into ischemic hibernating myocardium. Arterioscler Thromb Vasc Biol. 2002;22:1804–10. doi: 10.1161/01.atv.0000039168.95670.b9. [DOI] [PubMed] [Google Scholar]
- 18.Maruyama K, Mori Y, Murasawa S, et al. Interleukin-1 beta upregulates cardiac expression of vascular endothelial growth factor and its receptor KDR/flk-1 via activation of protein tyrosine kinases. J Mol Cell Cardiol. 1999;31:607–17. doi: 10.1006/jmcc.1998.0895. [DOI] [PubMed] [Google Scholar]
- 19.Giulian D, Woodward J, Young DG, Krebs JF, Lachman LB. Interleukin-1 injected into mammalian brain stimulates astrogliosis and neovascularization. J Neurosci. 1988;8:2485–90. doi: 10.1523/JNEUROSCI.08-07-02485.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leone AM, Rutella S, Bonanno G, et al. Endogenous G-CSF and CD34+ cell mobilization after acute myocardial infarction. International Journal Of Cardiology. 2006;111:202–8. doi: 10.1016/j.ijcard.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 21.Kawada H, Fujita J, Kinjo K, et al. Nonhematopoietic mesenchymal stem cells can be mobilized and differentiate into cardiomyocytes after myocardial infarction. Blood. 2004;104:3581–7. doi: 10.1182/blood-2004-04-1488. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Haider HK, Ahmad N, Xu M, Ge R, Ashraf M. Combining pharmacological mobilization with intramyocardial delivery of bone marrow cells over-expressing VEGF is more effective for cardiac repair. Journal Of Molecular And Cellular Cardiology. 2006;40:736–45. doi: 10.1016/j.yjmcc.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Uemura R, Xu M, Ahmad N, Ashraf M. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circulation Research. 2006;98:1414–21. doi: 10.1161/01.RES.0000225952.61196.39. [DOI] [PubMed] [Google Scholar]
- 24.Xu M, Wang Y, Ayub A, Ashraf M. Mitochondrial K(ATP) channel activation reduces anoxic injury by restoring mitochondrial membrane potential. American journal of physiology Heart and circulatory physiology. 2001;281:H1295–303. doi: 10.1152/ajpheart.2001.281.3.H1295. [DOI] [PubMed] [Google Scholar]
- 25.Dai Y, Xu M, Wang Y, Pasha Z, Li T, Ashraf M. HIF-1alpha induced-VEGF overexpression in bone marrow stem cells protects cardiomyocytes against ischemia. Journal Of Molecular And Cellular Cardiology. 2007;42:1036–44. doi: 10.1016/j.yjmcc.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu M, Wani M, Dai Y-S, et al. Differentiation of bone marrow stromal cells into the cardiac phenotype requires intercellular communication with myocytes. Circulation. 2004;110:2658–65. doi: 10.1161/01.CIR.0000145609.20435.36. [DOI] [PubMed] [Google Scholar]
- 27.Dai YS, Markham BE. p300 Functions as a coactivator of transcription factor GATA-4. The Journal Of Biological Chemistry. 2001;276:37178–85. doi: 10.1074/jbc.M103731200. [DOI] [PubMed] [Google Scholar]
- 28.Gindraux F, Selmani Z, Obert L, et al. Human and rodent bone marrow mesenchymal stem cells that express primitive stem cell markers can be directly enriched by using the CD49a molecule. Cell and tissue research. 2007;327:471–83. doi: 10.1007/s00441-006-0292-3. [DOI] [PubMed] [Google Scholar]
- 29.Sabatini F, Petecchia L, Tavian M, Jodon de Villeroche V, Rossi GA, Brouty-Boye D. Human bronchial fibroblasts exhibit a mesenchymal stem cell phenotype and multilineage differentiating potentialities. Laboratory Investigation; A Journal Of Technical Methods And Pathology. 2005;85:962–71. doi: 10.1038/labinvest.3700300. [DOI] [PubMed] [Google Scholar]
- 30.Peichev M, Naiyer AJ, Pereira D, et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–8. [PubMed] [Google Scholar]
- 31.Kleinschnitz C, Schroeter M, Jander S, Stoll G. Induction of granulocyte colony-stimulating factor mRNA by focal cerebral ischemia and cortical spreading depression. Brain Res Mol Brain Res. 2004;131:73–8. doi: 10.1016/j.molbrainres.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y, Woodward VK, Shelton JM, et al. Ischemia-reperfusion induces G-CSF gene expression by renal medullary thick ascending limb cells in vivo and in vitro. Am J Physiol Renal Physiol. 2004;286:F1193–201. doi: 10.1152/ajprenal.00379.2002. [DOI] [PubMed] [Google Scholar]
- 33.Hierholzer C, Kalff JC, Audolfsson G, Billiar TR, Tweardy DJ, Bauer AJ. Molecular and functional contractile sequelae of rat intestinal ischemia/reperfusion injury. Transplantation. 1999;68:1244–54. doi: 10.1097/00007890-199911150-00006. [DOI] [PubMed] [Google Scholar]
- 34.Vandervelde S, van Luyn MJ, Rozenbaum MH, Petersen AH, Tio RA, Harmsen MC. Stem cell-related cardiac gene expression early after murine myocardial infarction. Cardiovascular Research. 2007;73:783–93. doi: 10.1016/j.cardiores.2006.11.030. [DOI] [PubMed] [Google Scholar]
- 35.Petit I, Szyper-Kravitz M, Nagler A, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nature Immunology. 2002;3:687–94. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- 36.Abbott JD, Huang Y, Liu D, Hickey R, Krause DS, Giordano FJ. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004;110:3300–5. doi: 10.1161/01.CIR.0000147780.30124.CF. [DOI] [PubMed] [Google Scholar]
- 37.Molkentin JD. The zinc finger-containing transcription factors GATA-4, -5, and -6. Ubiquitously expressed regulators of tissue-specific gene expression. J Biol Chem. 2000;275:38949–52. doi: 10.1074/jbc.R000029200. [DOI] [PubMed] [Google Scholar]
- 38.Grepin C, Nemer G, Nemer M. Enhanced cardiogenesis in embryonic stem cells overexpressing the GATA-4 transcription factor. Development. 1997;124:2387–95. doi: 10.1242/dev.124.12.2387. [DOI] [PubMed] [Google Scholar]
- 39.Grepin C, Robitaille L, Antakly T, Nemer M. Inhibition of transcription factor GATA-4 expression blocks in vitro cardiac muscle differentiation. Mol Cell Biol. 1995;15:4095–102. doi: 10.1128/mcb.15.8.4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fuchs S, Baffour R, Zhou YF, et al. Transendocardial delivery of autologous bone marrow enhances collateral perfusion and regional function in pigs with chronic experimental myocardial ischemia. Journal Of The American College Of Cardiology. 2001;37:1726–32. doi: 10.1016/s0735-1097(01)01200-1. [DOI] [PubMed] [Google Scholar]
- 41.Tang YL, Zhao Q, Zhang YC, et al. Autologous mesenchymal stem cell transplantation induce VEGF and neovascularization in ischemic myocardium. Regul Pept. 2004;117:3–10. doi: 10.1016/j.regpep.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 42.Torella D, Rota M, Nurzynska D, et al. Cardiac stem cell and myocyte aging, heart failure, and insulin-like growth factor-1 overexpression. Circulation Research. 2004;94:514–24. doi: 10.1161/01.RES.0000117306.10142.50. [DOI] [PubMed] [Google Scholar]
- 43.Kitta K, Day RM, Ikeda T, Suzuki YJ. Hepatocyte growth factor protects cardiac myocytes against oxidative stress-induced apoptosis. Free Radic Biol Med. 2001;31:902–10. doi: 10.1016/s0891-5849(01)00663-3. [DOI] [PubMed] [Google Scholar]
- 44.Li W, Ma N, Ong LL, et al. Bcl-2 engineered MSCs inhibited apoptosis and improved heart function. Stem Cells (Dayton, Ohio) 2007;25:2118–27. doi: 10.1634/stemcells.2006-0771. [DOI] [PubMed] [Google Scholar]
- 45.Xu M, Uemura R, Dai Y, Wang Y, Pasha Z, Ashraf M. In vitro and in vivo effects of bone marrow stem cells on cardiac structure and function. Journal Of Molecular And Cellular Cardiology. 2007;42:441–8. doi: 10.1016/j.yjmcc.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mayorga M, Bahi N, Ballester M, Comella JX, Sanchis D. Bcl-2 is a key factor for cardiac fibroblast resistance to programmed cell death. The Journal Of Biological Chemistry. 2004;279:34882–9. doi: 10.1074/jbc.M404616200. [DOI] [PubMed] [Google Scholar]
- 47.Kobayashi S, Lackey T, Huang Y, et al. Transcription factor gata4 regulates cardiac BCL2 gene expression in vitro and in vivo. The FASEB Journal: Official Publication Of The Federation Of American Societies For Experimental Biology. 2006;20:800–2. doi: 10.1096/fj.05-5426fje. [DOI] [PubMed] [Google Scholar]