Abstract
Background
Atopic dermatitis (AD) and allergic contact dermatitis (ACD) are skin disorders triggered by epicutaneous sensitization (EC) with protein antigens and contact sensitization (CS) with haptens, respectively. Skin is colonized with bacteria, which are a source of Toll-like receptor 2 (TLR2) ligands.
Objective
To examine the role of TLR2 in murine models of AD and ACD.
Methods
TLR2-/- mice and WT littermates were EC sensitized with ovalbumin (OVA) or contact sensitized with oxazolone (OX). Skin histology was assessed by H&E staining and immunohistochemistry. Ear swelling was measured with a micrometer. Cytokine mRNA expression was examined by quantitative RT-PCR. Antibody levels and splenocyte secretion of cytokines in response to OVA stimulation were measured by ELISA. Dendritic cells (DCs) were examined for their ability to polarize TCR-OVA transgenic naïve T cells to Th1 and Th2.
Results
In response to OVA sensitization, TLR2-/- mice developed skin infiltration with eosinophils and CD4+ cells as well as upregulation of Th2 cytokine mRNAs that were comparable to WT littermates. In contrast, epidermal thickening, IFN-γ expression in the skin, IFN-γ production by splenocytes and IgG2a anti-OVA antibody levels were impaired in TLR2-/- mice. Following OX ear challenge, contact sensitized TLR2-/- mice exhibited defective ear swelling with impaired cellular infiltration, decreased epidermal thickening and local IFN-γ expression and impaired OX-specific IgG2a responses. DCs from TLR2-/- mice induced significantly lower production of IFN-γ, but normal IL-4 and IL-13 production, in naïve T cells.
Conclusions
These results indicate that TLR2 promotes the IFN-γ response to cutaneously introduced antigens.
Keywords: Toll like receptor 2, atopic dermatitis, Contact hypersensitivity, Oxazolone
Introduction
Toll-like receptors (TLRs) are a group of pathogen-associated molecular pattern (PAMP) receptors important for innate and adaptive immunity1, 2. TLR2 can heterodimerize with either TLR1 or TLR6. TLR1/2 ligands include triacyl lipopeptides of bacteria and the synthetic lipopeptide Pam3Cys. TLR2/6 ligands include bactertial diacyl lipopeptides and the synthetic lipopeptide Pam2Cys, lipoteichoic acids (LTA) and peptidoglycan (PGN) from Staphylococcus aureus and other gram positive bacteria, macrophage-activating lipopeptide (MALP) from Mycoplasma, and fungal zymosan3. TLR2 also recognizes fungal lipomannan, tGPI-mutin of trypanosomes and viral proteins such as the hemagglutinin of measles independently of TLR1 and TLR63, 4. TLR2 is also thought to bind endogenous ligands such as hyaluronan fragments, biglycan, eosinophil-derived neurotoxin and serum amyloid A5-8. TLR2 is expressed on immune cells including T cells, B cells, mast cells, eosinophils, macrophages and dendritic cells, and non-immune cells, including keratinocytes, epithelial cells, fibroblasts and smooth muscle cells2, 9. TLR2 expression is upregulated by LPS, inflammatory cytokines such as IL-1, TNF-α and IFN-γ, and by bacterial and viral infection. TLR2 signals through the adaptor MyD881.
DCs play a critical role in the polarization of naïve T cells into Th1 and Th2. Activation of TLR2 in DCs promotes IFN-γ production and Th1 responses10, 11. In addition, TLR2 can directly stimulate T cells to produce more IFN-γ 12 and TLR2-/- mice exhibit deficient Th1 dependent humoral immune response to the gram-positive extracellular bacterium S. pneumoniae13. However under different conditions, TLR2 agonists have been reported to favor a Th2 immune response in vitro and in vivo7, 14.
There are conflicting data regarding the effect of TLR2 engagement on the allergic response. A number of studies suggest that TLR2 ligation inhibits the allergic response. Co-administration of the probiotic bacterium Lactobacillus plantarum augmented Th1 responses through TLR2 and led to protection against Der p 1 induced allergic responses15. The TLR2 agonists Pam3CSK and liproprotein reduced airway inflammation, airway hyperresponsiveness (AHR) and serum levels of IgE following allergen inhalation challenge16. M. pneumoniae infection preceding allergen challenge reduces airway epithelial mucin expression in mice, partly through the TLR2- IFN-γ pathway17. Finally the TLR2/6 agonist MALP, in combination with IFN-γ strongly reduced AHR, eosinophilia and Th2 cytokines in brochoalveolar fluid18. In contrast to the above studies, intranasal immunization of mice with OVA in combination with the TLR2 ligand PGN has been reported to augment lung inflammation and AHR compared with intranasal OVA immunization alone19.
TLR2 is expressed in keratinocytes20, 21. The skin of AD patients is heavily colonized with S. aureus, a rich source of TLR2 ligands22. This adversely affects eczema severity23 Furthermore, AD skin lesions may contain endogeneous TLR2 ligands, such as eosinophil derived neurotoxin (EDN), the level of which is elevated in the blood of atopic dermatitis (AD) patients24, 25. TLR2 function, but not expression, has been reported to be impaired in monocytes and keratinocytes from AD patients26. There is conflicting data whether the TLR2 polymorphism R753Q, which impairs TLR2 function, is associated with AD27, 28.
Acute AD skin lesions are dominated by local expression of Th2 cytokines, whereas chronic AD skin lesions and ACD lesions are characterized by mixed expression of both Th2 and Th1 cytokines29-30. IFN-γ has been implicated in keratinocyte apoptosis in AD28 and in epidermal thickening in a mouse model of allergic skin inflammation31. We tested the hypothesis that TLR2 ligands could play an important role in AD by promoting a Th1 response to antigens introduced via the skin. We demonstrate that TLR2 promotes epidermal thickening and the IFN-γ response to cutaneously introduced antigen in a mouse model of AD elicited by EC sensitization with ovalbumin (OVA) as well as in a mouse model of allergic contact dermatitis (ACD) elicited by contact sensitization (CS) with the hapten oxazolone (OX).
Methods
Mice
TLR2-/- mice on C57BL/6 background were kindly provided by Biogen Idec Inc.; C57BL/6 wild-type (WT) mice were purchased from Charles River Laboratories (Wilmington, MA). All mice were kept in a pathogen-free environment and fed an OVA-free diet. All procedures performed on the mice were in accordance with the Animal Care and Use Committee of the Children's Hospital Boston.
EC sensitization with ovalbumin
Four- to six-week-old female mice were EC sensitized as described previously32. Briefly, the dorsal skin of anesthetized mice was shaved and tape stripped 6 times. 100 μg OVA (Grade V; Sigma Chemical Co.) in 100 μL of normal saline, or placebo (100 μL of normal saline), were placed on a patch of sterile gauze (1×1 cm), which was secured to dorsal skin with a transparent bio-occlusive dressing (Tegaderm, Westnet Inc.). Each mouse had a total of three one-week exposures to the patch separated by two-week intervals. Mice were euthanized immediately at the end of the third cycle of sensitization (day 49).
Hapten-Induced Contact Hypersensitivity
Mice were sensitized by the application of 100 μl of 2% oxazolone (Sigma) in ethanol to previously shaved abdominal skin. Five days later, 10 μL of 1% oxazolone were applied to the dorsal and ventral surfaces of the right ear. Ethanol was applied to the left ear. Ear thickness was measured after 24, 48, and 72 hours, using a modified spring-loaded micrometer (Mitutoyo).
Histological and immunohistochemical analysis of mouse skin
Dorsal or ear skin specimens were fixed in 10% buffered formalin and embedded in paraffin. Multiple 4-mm sections of skin were stained with hematoxylin and eosin (H&E) by HistoScientific Research Laboratories. CD4 staining of skin sections was performed as previously described32. Eosinophils and CD4+ cells were counted blinded in 10-15 high-power fields (HPFs) at a magnification of 400×. Epidermal thickness of 6 different randomly chosen sites was measured in each skin section from each mouse.
Quantitative RT-PCR for TLR-2, cytokines and chemokines
Specimens of skin were homogenized using a Polytron RT-3000 (Kinematica, AG) in lysis buffer solution provided in the RNAqueous extraction kit (Ambion Inc.). Reverse transcription was performed using iScript cDNA synthesis kit (Bio-Rad). PCR reactions were run on an ABI Prism 7300 (Applied Biosystems) sequence detection system platform. Taqman primers and probes were obtained from Applied Biosystems. The housekeeping gene β2-microglobulin was used as a control. Relative gene expression was determined using the method described by Pfaffl33.
Cell culture, proliferation assay and in vitro cytokine production
Single cell suspensions of spleen cells were cultured in complete RPMI 1640 (JRH Biosciences Inc., Lenexa, Kansas) supplemented by 10% FCS, 1 mM sodium pyruvate, 2mM L-glutamine, 0.05 mM 2-mercapto-ethanol, 100 U/ml penicillin, and 1 mg/ml streptomycin at 2×105/ml in 96 well plates or 2×106/ml in 24 well plates in the presence of OVA (50 μg/ml). Proliferation was measured in triplicate wells of 96 well plates by [3H] thymidine incorporation after 72 hrs of culture. Cytokine secretion in supernatants from 24 well plates after 96 hrs of culture was determined by ELISA following the manufacture's instructions (BD-PharMingen).
Serum antibody determination
The BD-PharMingen protocol for sandwich ELISA was used to quantify serum antibodies from EC OVA or OX-sensitized mice. OX-specific antibodies were measured as previously described30.
In vitro assessment of APC function of DCs
CD11c+ DCs were sorted from spleens using Miltenyi mouse CD11c beads and co-cultured with WT OVA specific TCR transgenic OTII CD4+ T cells at a 1:10 ratio in the presence of graded concentrations (0, 10, 100, 1000 ng/ml) of OVA323-339 peptide. After 96 hrs, T cell proliferation and cytokine production were measured as described above.
Statistical analysis
Q-PCR expression of TLR2 mRNA in mouse skin post tape stripping was assessed by one-way analysis of variance (ANOVA) with the F-test used to determine change in fold induction relative to time 0 (baseline). Comparison of TLR2 induction between OX and EtOH treated skin was evaluated by two-way ANOVA with group and time as fixed factors in the model and the interaction of group-by-time included. Two-way ANOVA was used to determine changes in epidermal thickening, skin infiltrating CD4+ T cells, eosinophils, and mRNA levels of cytokines. Generalized linear models (GLM) with two factors were used to analyze changes in ear swelling over time and to compare OX-specific levels of serum IgG1 and IgG2a. Differences in proliferation and secretion of cytokines were analyzed using ANOVA. Statistical analysis was performed with the SPSS software package (SPSS Inc., Chicago, IL). Two-tailed values of p<0.05 were considered statistically significant for all comparisons.
Results
TLR2 expression in skin is upregulated by tape-stripping and oxazolone (OX) painting
Mechanical injury induced by scratching is a hallmark of human AD. Tape stripping of mouse back skin was used to mimic mechanical injury inflicted by scratching. Fig 1A shows that tape stripping upregulated TLR2 mRNA expression in mouse skin, with a peak increase of 3.2 ± 1.5 fold over baseline (p<0.05) eight hrs after stripping. A similar increase (2.8 fold) was revealed by microarray analysis of skin 8 hrs after stripping (data not shown).
Figure 1. TLR2 mRNA expression following skin by tape stripping and painting with oxazolone.
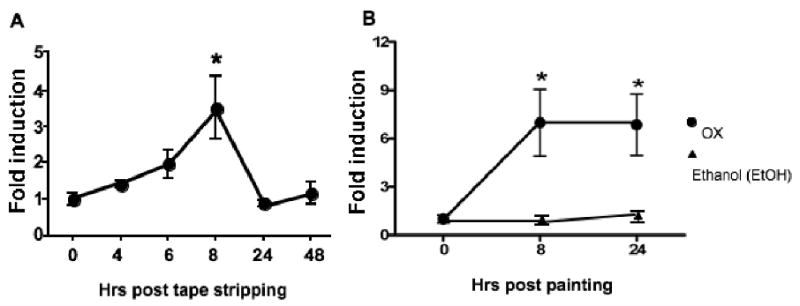
Q-PCR analysis of TLR2 mRNA expression in mouse skin following tape stripping (n=5) (A) or application of 2% oxazolone in ethanol to shaved skin (n=4) (B). Results were normalized for GAPDH expression and expressed as fold induction compared to time 0 and represent mean±S.D. *p<0.05.
We also examined whether application of the hapten OX on shaved skin of the belly upregulates TLR2 mRNA expression. Fig 1B shows that application of OX in ethanol upregulated TLR2 mRNA expression in mouse skin, with a peak increase of 7.0±4.1 fold eight hrs after stripping. This increase was sustained at 24 hrs. There was no increase in skin TLR2 mRNA expression after application of ethanol solvent (data not shown). These results show that mechanical skin injury, which is necessary for EC sensitization with protein antigen, as well as hapten application to the skin upregulate TLR2 expression in the skin.
Impaired epidermal thickening and upregulation of IFN-γ expression in EC sensitized skin of TLR2-/- mice
To determine the role of TLR2 in allergic skin inflammation elicited by EC sensitization with protein antigen, we examined the response of TLR2-/- mice to repeated EC application of OVA on tape-stripped skin. As shown previously34, EC sensitization of C57BL/6 WT mice with OVA led to epidermal thickening with foci of spongiosis (Fig 2A) and to marked infiltration of the dermis with CD4+ T cells, and modest infiltration with eosinophils (Fig 2B). OVA-sensitized TLR2-/- mice exhibited impaired epidermal thickening compared to WT mice (Fig. 2A). There was a 2.6±0.4 fold increase in epidermal thickness in OVA sensitized skin of WT mice compared to a 1.1±0.3 fold increase in TLR2-/- mice (n=3 per group, p<0.05). Spongiosis was not detected in TLR2-/- mice. WT and TLR2-/- mice had comparable levels of skin infiltration with CD4+ cells and eosinophils (Fig 2B). Expression of mRNA for the chemokines CCL17/TARC, a ligand for CCR4 expressed on skin homing T cells, and CCL11/eotaxin-1 mRNA, a ligand for CCR3 expressed on eosinophils, was comparably upregulated in OVA sensitized skin from WT and TLR2-/- mice (Fig E1).
Figure 2. Impaired epidermal thickening and IFN-γ mRNA expression but normal cellular infiltration and Th2 cytokine production in OVA sensitized skin of TLR2-/- mice.
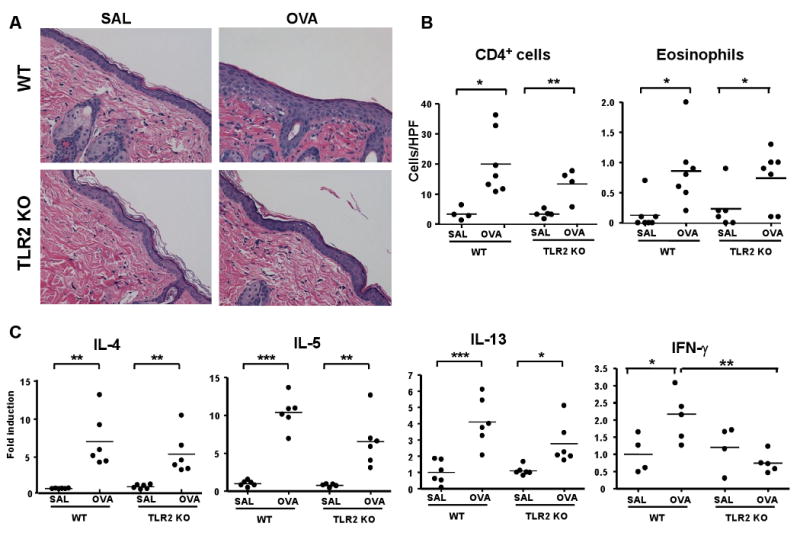
(A) Representative skin histology, Magnification 200× (B) Skin infiltrating CD4+ T cells and eosinophils. (C) Q-PCR analysis of mRNA levels of IL-4, IL-15 and IL-13 and IFN-γ. (n=6-7) *p<0.05, **p<0.01, ***p<0.001.
EC sensitization with OVA caused upregulation of mRNA for the Th2 cytokines IL-4, IL-5 and IL-13 and the Th1 cytokine IFN-γ in the skin of C57BL/6 WT mice (Fig 2C). There was no significant difference of the upregulation of IL-4, IL-5 and IL-13 between OVA sensitized skin of TLR2-/- mice and their WT littermates. In contrast, IFN- γ mRNA was not upregulated in OVA sensitized skin of TLR2-/- mice. These results demonstrate that TLR2 is important for the local Th1 response, but is dispensable for the local Th2 response to EC sensitization with protein antigen.
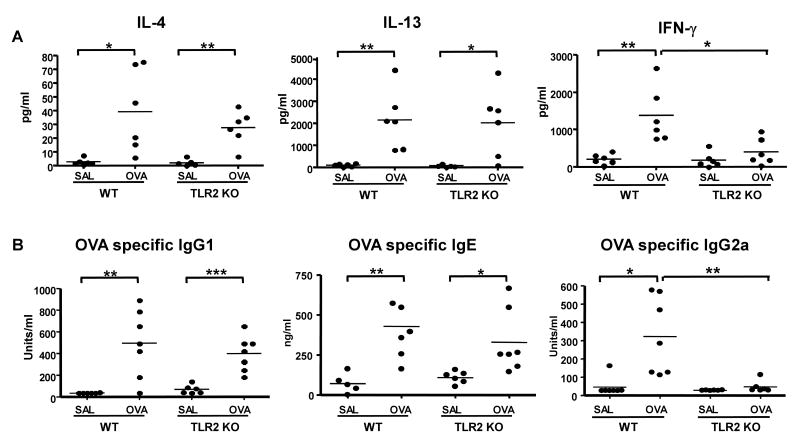
Defective systemic Th1 response to EC sensitization with OVA in TLR2-deficient mice
EC sensitization with OVA results in a mixed systemic Th2 and Th1 OVA specific response32. Splenocytes from OVA sensitized TLR2-/- mice and WT mice secreted comparable amounts of the Th2 cytokines IL-4, and IL-13 in response to in vitro stimulation with OVA (Fig 3A). In contrast, splenocytes from TLR2-/- mice secreted significantly lower amounts of IFN-γ in response to OVA stimulation than splenocytes from WT controls. TLR2-/- mice mounted normal OVA-specific IgG1 and IgE antibody responses to EC sensitization (Fig 3B). However, their OVA-specific IgG2a response was severely diminished compared to WT controls. These results suggest that TLR2 is required for the development of a systemic Th1 response to EC sensitization.
Figure 3. Impaired Th1 systemic response to EC sensitization with OVA in TLR2-/- mice.
(A) Cytokine secretion by splenocytes in response to OVA stimulation in vitro, (B) Serum levels of OVA-specific immunoglobulin isotypes. (n=6-7) *p<0.05, **p<0.01, ***p<0.001.
Impaired contact hypersensitivity (CHS) to OX in TLR2-/- mice
CHS to the hapten OX is a model of skin inflammation that involves local expression of both Th1 and Th2 cytokines. Fig 4A shows that OX challenged ears of OX sensitized WT mice exhibited swelling compared to vehicle challenged ears. Ear swelling peaked at 24 hrs remained significant at 48 hrs and resolved by 72 hrs after challenge. TLR2-/- mice developed significantly less ear swelling 24 hrs and 48 hrs post OX challenge than WT controls. Decreased ear thickening in TLR2-/- mice was confirmed by histological analysis, which revealed a marked decrease in cellular infiltration and edema (Fig 4B). There was significant epidermal thickening, in challenged skin of WT mice, but not of TLR2-/- mice (3.1±0.5 fold increase in hapten challenged vs. vehicle challenged skin in OX sensitized WT mice compared to 1.1±0.2 fold increase in TLR2-/- mice; n=6 per group, p<0.0001). Expression of mRNA for the cytokines IL-4 and IFN-γ was markedly upregulated in OX challenged ears compared to vehicle challenged ears in WT mice (Fig 4C and D). IL-4 mRNA expression was normally upregulated in OX challenged ears of TLR2-/- mice. In contrast, upregulation of IFN-γ mRNA expression was severely diminished in the ears of these mice. These results indicate that TLR2 is important for the development of CHS and for local expression of IFN-γ following hapten challenge.
Figure 4. Impaired CHS in TLR2-/- mice.
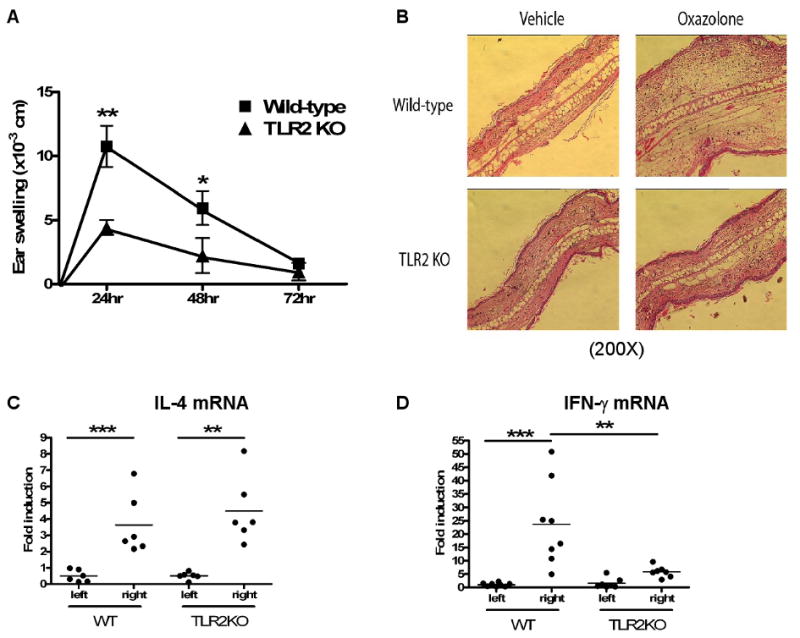
A. Ear swelling in sensitized mice challenged with OX hapten. Results represent the difference in thickness between hapten- and vehicle-challenged ears (n=10). B. Representative ear skin histology 24 hrs. post challenged. 200× magnification. C,D. IL-4 (C) and IFN-γ (D) mRNA expression as fold induction in hapten-challenged over vehicle-challenged ears. *p<0.05, **p<0.01, *** p<0.0001.
Impaired IgG2a response to OX in TLR2-/- mice
Reduced IFN-γ upregulation in OX challenged skin sites from TLR2-/- mice could be secondary to a defect in systemic Th1 response. Since IgG2a antibody responses are driven by IFN-γ35 we examined the IgG2a antibody response to OX as an indirect measure of the Th1 systemic response to OX sensitization. Fig 5 shows that WT mice developed OX specific IgG1 and IgG2a after OX contact sensitization and challenge. TLR2-/- mice developed similar levels of OX specific IgG1 as WT control. In contrast, they mounted significantly less OX specific IgG2a response. These results suggest that TLR2 is important for developing a systemic Th1 response to contact sensitization with hapten.
Figure 5. Impaired OX specific IgG2a response in TLR2-/- mice.
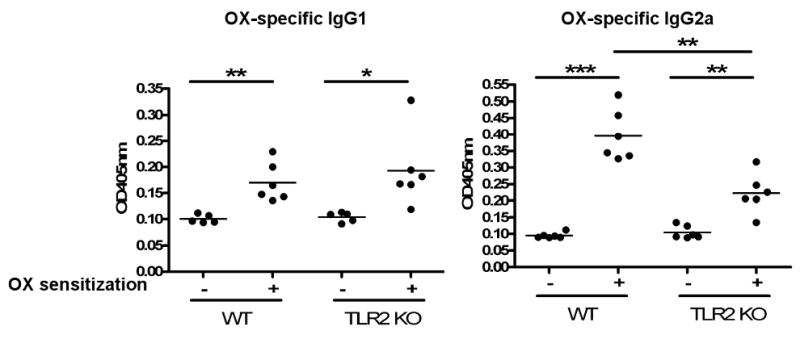
Serum anti-OX IgG1 and IgG2a levels in mice sensitized and challenged with OX. Serum was taken 24 hrs. after the challenge. *p<0.05, **p<0.01, ***p<0.001.
Impaired ability of TLR2-/- DCs to polarize the response of naïve T cells to Th1
DCs capture antigen in the skin and traffic to DLN where they prime naïve T cells to become effector cells that can home to the skin36. A possible explanation for the defective Th1 response of TLR2-/- mice to EC sensitization is that their DCs are impaired in their ability to drive a Th1 response in CD4+ T cells. To directly test this hypothesis we co-cultured splenic CD11c+ cells from WT and TLR2-/- mice with TCR-OVA transgenic CD4+ T cells from OTII mice in the presence of OVA peptide 323-339. DCs from TLR2-/- mice and WT controls were comparable in their ability to drive proliferation and production of the Th2 cytokines IL-4 and IL-13 by naïve T cells (Fig 6A-C) In contrast, DCs from TLR2-/- mice were impaired in their ability to drive IFN-γ production (Fig 6D). These results indicate that TLR2 expression by DCs is important for Th1 polarization and suggest that DCs may contribute to the impaired Th1 response to EC sensitization.
Figure 6. Impaired capacity of TLR2-/- DCs to drive a Th1 response.
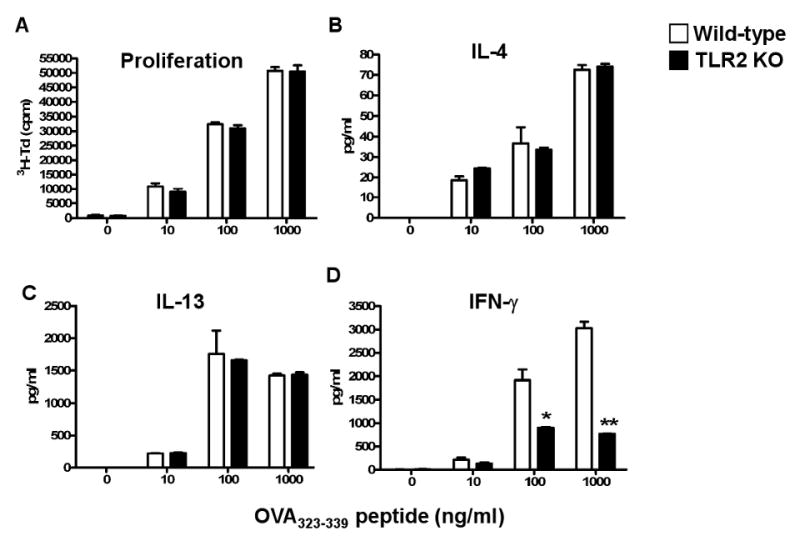
Proliferation and secretion of IL-4, IL-13 and IFN-γ by CD4+ OT II T cells stimulated with OVA323-339 peptide in the presence of CD11c+ splenic DCs derived from WT and TLR2-/- mice. Data is representative of 3 experiments. *p<0.05, **p<0.01
Discussion
This work demonstrates that TLR2 is important for the Th1 response to EC sensitization with protein antigen and contact sensitization with hapten.
TLR2 expression in the skin was upregulated by mechanical skin injury inflicted by tape stripping (Fig 1A). Inasmuch as injury by tape stripping mimics injury inflicted by scratching, this observation suggests that TLR2 might be upregulated in skin of AD patients following scratching of dry skin. TLR2 expression was also upregulated by application of the hapten oxazolone (Fig 1B). Physical injury as well as application of haptens upregulates NF-kB expression in the skin37-39, and NF-κB is known to mediate upregulation of TLR2 expression40. The mechanism by which mechanical injury and hapten application upregulate TLR2 expression in skin cells could therefore involve NF-κB. Keratinocytes, DCs, mast cells and fibroblasts all express TLR29. Immunohistologic studies are needed to determine which of these cells upregulate TLR2 in the skin following mechanical injury and application of hapten.
Infiltration with CD4+ cells and eosinophils, upregulation of mRNA expression for the Th2 cytokines IL-4, IL-5 and IL-13 and the chemokines TARC and eotaxin/CCL11 were all normal in OVA sensitized skin of TLR2-/- mice (Fig 2 and Fig E1). In contrast, TLR2-/- mice selectively failed to develop epidermal thickening and to upregulate IFN-γ mRNA expression in EC sensitized skin sites (Fig 2A and 2C). We had previously shown that IFN-γ-/- mice fail to develop epidermal thickening following EC sensitization with OVA, but have normal infiltration of the dermis by CD4+ cells and eosinophils and normal local expression of Th2 cytokines31. This suggests that the decreased IFN-γ response in TLR2-/- mice underlies their failure to develop epidermal thickening following EC sensitization. Failure of TLR2-/- mice to upregulate local expression of IFN-γ mRNA was likely due to their severely impaired Th1 systemic response to EC sensitization. This was evidenced by significantly impaired IFN-γ secretion by splenocytes from EC sensitized TLR2-/- mice following OVA stimulation in vitro and by failure of TLR2-/- mice to mount an OVA specific IgG2a antibody response to EC sensitization (Fig 3).
Impaired IFN-γ response and epidermal thickening of TLR2-/- mice to cutaneous sensitization was also observed following contact sensitization with the hapten OX. This was evidence by decreased swelling of hapten challenged ears, impaired epidermal thickening, decreased cellular infiltration and edema in these ears and selective impairment of local expression of IFN-γ mRNA, but intact local expression of IL-4 mRNA (Fig 4). Because IFN-γ is known to be important for the response to OX challenge30, it is likely that decreased local expression of IFN-γ contributed to the decreased inflammation of OX challenged ears in TLR2-/- mice. TLR2-/- mice were severely impaired in their capacity to mount on IgG2a response to OX, but retained an intact IgG1 response (Fig 5). Since IFN-γ drives IgG2a production, while IL-4 drives IgG1 production35, this result strongly suggests that the systemic Th1 response to hapten sensitization was selectively impaired in TLR2-/- mice.
Splenic DCs from TLR2-/- mice were selectively impaired in their ability to drive a Th1 response in CD4+ T cells. This was evidenced by a significant impairment in the ability of these DCs to drive IFN-γ production by naïve DO11.10 T cells in response to OVA peptide (Fig 6). In contrast, the ability of these DCs to support T cell proliferation and production of Th2 cytokines was intact. It is possible that splenic DCs in WT mice have been primed by exogenous and/or endogenous TLR ligands in vivo to drive a Th1 response. This is supported by the observation that the TLR2 ligands bacterial lipopeptides act as adjuvants for Th1 responses in an APC dependent manner10. PAMPs from the bacterial flora of mouse skin and/or endogenous TLR2 ligands may promote the Th1 polarizing capacity of skin DCs that capture antigens or haptens in the skin and traffic to DLN where they prime naïve T cells. Failure of skin DCs to respond to these ligands in TLR2-/- mice would impair their capacity to support a Th1 response to cutaneous sensitization. TLR2 agonists can also directly stimulate T cells, NK cells and DCs to produce IFN-γ12,41-42. Loss of this effect in TLR2-/- mice may contribute to their impaired Th1 response to cutaneous sensitization.
IFN-γ is expressed in chronic AD lesions and is thought to play an important role in keratinocyte apoptosis and in perpetuating skin inflammation28,43. Our results suggest that disrupting TLR2-TLR2 ligand interaction may impair the Th1 response to antigens introduced via the skin and inhibit epidermal thickening, and thus could be beneficial in treating AD. Decreased signaling via TLR2 by ligands from skin colonizing bacteria may contribute the reported efficacy of antibiotics in ameliorating AD skin lesions44.
Supplementary Material
Acknowledgments
The authors thank Drs. Robert Sidbury and Birgitta Schmidt (Department of Dermatology, Harvard Medical School, Boston) for help in the analysis of skin histology.
Declaration of all sources of funding: This work was supported by The Atopic Dermatitis and Vaccinia Immunization Network NIH/NIAID contract NO1 (AI 40030) and a USPHS grant AR-047417 to Raif Geha. Haoli Jin has received a postdoctoral fellowship from the American Heart Association.
Abbreviations used
- TLR2
Toll like receptor 2
- AD
atopic dermatitis
- ACD
allergic contact dermatitis
- CS
contact sensitization
- DCs
Dendritic cells
- EC
epicutaneous
- EDN
eosinophil derived neurotoxin
- IL-4
interleukin-4
- IL-13
interleukin-13
- IFN-γ
interferon-gamma
- OVA
ovalbumin
- OX
oxazolone
- TCR
T cell receptor
Footnotes
Clinical Implications: TLR2 triggering by ligands from skin bacterial flora may contribute to the Th1 response to cutaneous sensitization in AD and ACD.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–76. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 2.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–95. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 3.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 4.Weigt H, Muhlradt PF, Emmendorffer A, Krug N, Braun A. Synthetic mycoplasma-derived lipopeptide MALP-2 induces maturation and function of dendritic cells. Immunobiology. 2003;207:223–33. doi: 10.1078/0171-2985-00234. [DOI] [PubMed] [Google Scholar]
- 5.Shimada M, Yanai Y, Okazaki T, Noma N, Kawashima I, Mori T, et al. Hyaluronan fragments generated by sperm-secreted hyaluronidase stimulate cytokine/chemokine production via the TLR2 and TLR4 pathway in cumulus cells of ovulated COCs, which may enhance fertilization. Development. 2008;135:2001–11. doi: 10.1242/dev.020461. [DOI] [PubMed] [Google Scholar]
- 6.Schaefer L, Babelova A, Kiss E, Hausser HJ, Baliova M, Krzyzankova M, et al. The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages. J Clin Invest. 2005;115:2223–33. doi: 10.1172/JCI23755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang D, Chen Q, Su SB, Zhang P, Kurosaka K, Caspi RR, et al. Eosinophil-derived neurotoxin acts as an alarmin to activate the TLR2-MyD88 signal pathway in dendritic cells and enhances Th2 immune responses. J Exp Med. 2008;205:79–90. doi: 10.1084/jem.20062027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng N, He R, Tian J, Ye PP, Ye RD. Cutting edge: TLR2 is a functional receptor for acute-phase serum amyloid A. J Immunol. 2008;181:22–6. doi: 10.4049/jimmunol.181.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marshall JS, McCurdy JD, Olynych T. Toll-like receptor-mediated activation of mast cells: implications for allergic disease? Int Arch Allergy Immunol. 2003;132:87–97. doi: 10.1159/000073709. [DOI] [PubMed] [Google Scholar]
- 10.Sieling PA, Chung W, Duong BT, Godowski PJ, Modlin RL. Toll-like receptor 2 ligands as adjuvants for human Th1 responses. J Immunol. 2003;170:194–200. doi: 10.4049/jimmunol.170.1.194. [DOI] [PubMed] [Google Scholar]
- 11.Daehnel K, Gillette-Ferguson I, Hise AG, Diaconu E, Harling MJ, Heinzel FP, et al. Filaria/Wolbachia activation of dendritic cells and development of Th1-associated responses is dependent on Toll-like receptor 2 in a mouse model of ocular onchocerciasis (river blindness) Parasite Immunol. 2007;29:455–65. doi: 10.1111/j.1365-3024.2007.00962.x. [DOI] [PubMed] [Google Scholar]
- 12.Imanishi T, Hara H, Suzuki S, Suzuki N, Akira S, Saito T. Cutting edge: TLR2 directly triggers Th1 effector functions. J Immunol. 2007;178:6715–9. doi: 10.4049/jimmunol.178.11.6715. [DOI] [PubMed] [Google Scholar]
- 13.Khan AQ, Chen Q, Wu ZQ, Paton JC, Snapper CM. Both innate immunity and type 1 humoral immunity to Streptococcus pneumoniae are mediated by MyD88 but differ in their relative levels of dependence on toll-like receptor 2. Infect Immun. 2005;73:298–307. doi: 10.1128/IAI.73.1.298-307.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Re F, Strominger JL. Toll-like receptor 2 (TLR2) and TLR4 differentially activate human dendritic cells. J Biol Chem. 2001;276:37692–9. doi: 10.1074/jbc.M105927200. [DOI] [PubMed] [Google Scholar]
- 15.Hisbergues M, Magi M, Rigaux P, Steuve J, Garcia L, Goudercourt D, et al. In vivo and in vitro immunomodulation of Der p 1 allergen-specific response by Lactobacillus plantarum bacteria. Clin Exp Allergy. 2007;37:1286–95. doi: 10.1111/j.1365-2222.2007.02792.x. [DOI] [PubMed] [Google Scholar]
- 16.Patel M, Xu D, Kewin P, Choo-Kang B, McSharry C, Thomson NC, et al. TLR2 agonist ameliorates established allergic airway inflammation by promoting Th1 response and not via regulatory T cells. J Immunol. 2005;174:7558–63. doi: 10.4049/jimmunol.174.12.7558. [DOI] [PubMed] [Google Scholar]
- 17.Wu Q, Martin RJ, Rino JG, Jeyaseelan S, Breed R, Chu HW. A deficient TLR2 signaling promotes airway mucin production in Mycoplasma pneumoniae-infected allergic mice. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1064–72. doi: 10.1152/ajplung.00301.2006. [DOI] [PubMed] [Google Scholar]
- 18.Weigt H, Nassenstein C, Tschernig T, Muhlradt PF, Krug N, Braun A. Efficacy of macrophage-activating lipopeptide-2 combined with interferon-gamma in a murine asthma model. Am J Respir Crit Care Med. 2005;172:566–72. doi: 10.1164/rccm.200411-1490OC. [DOI] [PubMed] [Google Scholar]
- 19.Chisholm D, Libet L, Hayashi T, Horner AA. Airway peptidoglycan and immunostimulatory DNA exposures have divergent effects on the development of airway allergen hypersensitivities. J Allergy Clin Immunol. 2004;113:448–54. doi: 10.1016/j.jaci.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 20.Baker BS, Ovigne JM, Powles AV, Corcoran S, Fry L. Normal keratinocytes express Toll-like receptors (TLRs) 1, 2 and 5: modulation of TLR expression in chronic plaque psoriasis. Br J Dermatol. 2003;148:670–9. doi: 10.1046/j.1365-2133.2003.05287.x. [DOI] [PubMed] [Google Scholar]
- 21.Sumikawa Y, Asada H, Hoshino K, Azukizawa H, Katayama I, Akira S, et al. Induction of beta-defensin 3 in keratinocytes stimulated by bacterial lipopeptides through toll-like receptor 2. Microbes Infect. 2006;8:1513–21. doi: 10.1016/j.micinf.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 22.Lin YT, Wang CT, Chiang BL. Role of bacterial pathogens in atopic dermatitis. Clin Rev Allergy Immunol. 2007;33:167–77. doi: 10.1007/s12016-007-0044-5. [DOI] [PubMed] [Google Scholar]
- 23.Abeck D, Mempel M. Staphylococcus aureus colonization in atopic dermatitis and its therapeutic implications. Br J Dermatol. 1998;139 53:13–6. doi: 10.1046/j.1365-2133.1998.1390s3013.x. [DOI] [PubMed] [Google Scholar]
- 24.Cheng JF, Ott NL, Peterson EA, George TJ, Hukee MJ, Gleich GJ, et al. Dermal eosinophils in atopic dermatitis undergo cytolytic degeneration. J Allergy Clin Immunol. 1997;99:683–92. doi: 10.1016/s0091-6749(97)70031-9. [DOI] [PubMed] [Google Scholar]
- 25.Taniuchi S, Chihara J, Kojima T, Yamamoto A, Sasai M, Kobayashi Y. Serum eosinophil derived neurotoxin may reflect more strongly disease severity in childhood atopic dermatitis than eosinophil cationic protein. J Dermatol Sci. 2001;26:79–82. doi: 10.1016/s0923-1811(00)00151-1. [DOI] [PubMed] [Google Scholar]
- 26.Hasannejad H, Takahashi R, Kimishima M, Hayakawa K, Shiohara T. Selective impairment of Toll-like receptor 2-mediated proinflammatory cytokine production by monocytes from patients with atopic dermatitis. J Allergy Clin Immunol. 2007;120:69–75. doi: 10.1016/j.jaci.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 27.Ahmad-Nejad P, Mrabet-Dahbi S, Breuer K, Klotz M, Werfel T, Herz U, et al. The toll-like receptor 2 R753Q polymorphism defines a subgroup of patients with atopic dermatitis having severe phenotype. J Allergy Clin Immunol. 2004;113:565–7. doi: 10.1016/j.jaci.2003.12.583. [DOI] [PubMed] [Google Scholar]
- 28.Trautmann A, Akdis M, Kleemann D, Altznauer F, Simon HU, Graeve T, et al. T cell-mediated Fas-induced keratinocyte apoptosis plays a key pathogenetic role in eczematous dermatitis. J Clin Invest. 2000;106:25–35. doi: 10.1172/JCI9199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tamura T, Matsubara M, Takada C, Hasegawa K, Suzuki K, Ohmori K, et al. Effects of olopatadine hydrochloride, an antihistamine drug, on skin inflammation induced by repeated topical application of oxazolone in mice. Br J Dermatol. 2004;151:1133–42. doi: 10.1111/j.1365-2133.2004.06172.x. [DOI] [PubMed] [Google Scholar]
- 30.Thomson JA, Troutt AB, Kelso A. Contact sensitization to oxazolone: involvement of both interferon-gamma and interleukin-4 in oxazolone-specific Ig and T-cell responses. Immunology. 1993;78:185–92. [PMC free article] [PubMed] [Google Scholar]
- 31.Spergel JM, Mizoguchi E, Oettgen H, Bhan AK, Geha RS. Role of TH1 and TH2 cytokines in a murine model of allergic dermatitis. J Clin Invest. 1999;103:1103–1111. doi: 10.1172/JCI5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spergel JM, Mizoguchi E, Brewer JP, Martin TR, Bhan AK, Geha RS. Epicutaneous sensitization with protein antigen induces localized allergic dermatitis and hyperresponsiveness to methacholine after single exposure to aerosolized antigen in mice. Journal of Clinical Investigation. 1998;101:1614–22. doi: 10.1172/JCI1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:2003–7. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woodward AL, Spergel JM, Alenius H, Mizoguchi E, Bhan AK, Castigli E, et al. An obligate role for T-cell receptor alphabeta+ T cells but not T-cell receptor gammadelta+ T cells, B cells, or CD40/CD40L interactions in a mouse model of atopic dermatitis. J Allergy Clin Immunol. 2001;107:359–66. doi: 10.1067/mai.2001.112695. [DOI] [PubMed] [Google Scholar]
- 35.Stevens TL, Bossie A, Sanders VM, Fernandez-Botran R, Coffman RL, Mosmann TR, et al. Regulation of antibody isotype secretion by subsets of antigen-specific helper T cells. Nature. 1988;334:255–8. doi: 10.1038/334255a0. [DOI] [PubMed] [Google Scholar]
- 36.Robert C, Kupper TS. Review Articles: Mechanisms of Disease: Inflammatory Skin Diseases, T Cells, and Immune Surveillance. N Engl J Med. 1999;341:1817–28. doi: 10.1056/NEJM199912093412407. [DOI] [PubMed] [Google Scholar]
- 37.Karimipour DJ, Kang S, Johnson TM, Orringer JS, Hamilton T, Hammerberg C, et al. Microdermabrasion: a molecular analysis following a single treatment. J Am Acad Dermatol. 2005;52:215–23. doi: 10.1016/j.jaad.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 38.Schmidtko A, Burian M, Altis K, Hardt K, Angioni C, Schmidt R, et al. Pharmacological and histopathological characterization of a hyperalgesia model induced by freeze lesion. Pain. 2007;127:287–95. doi: 10.1016/j.pain.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 39.Goebeler M, Roth J, Brocker EB, Sorg C, Schulze-Osthoff K. Activation of nuclear factor-kappa B and gene expression in human endothelial cells by the common haptens nickel and cobalt. J Immunol. 1995;155:2459–67. [PubMed] [Google Scholar]
- 40.Johnson CM, Tapping RI. Microbial products stimulate human Toll-like receptor 2 expression through histone modification surrounding a proximal NF-kappaB-binding site. J Biol Chem. 2007;282:31197–205. doi: 10.1074/jbc.M705151200. [DOI] [PubMed] [Google Scholar]
- 41.Sawaki J, Tsutsui H, Hayashi N, Yasuda K, Akira S, Tanizawa T, et al. Type 1 cytokine/chemokine production by mouse NK cells following activation of their TLR/MyD88-mediated pathways. Int Immunol. 2007;19:311–20. doi: 10.1093/intimm/dxl148. [DOI] [PubMed] [Google Scholar]
- 42.Fricke I, Mitchell D, Mittelstadt J, Lehan N, Heine H, Goldmann T, et al. Mycobacteria induce IFN-gamma production in human dendritic cells via triggering of TLR2. J Immunol. 2006;176:5173–82. doi: 10.4049/jimmunol.176.9.5173. [DOI] [PubMed] [Google Scholar]
- 43.Carroll JM, Crompton T, Seery JP, Watt FM. Transgenic mice expressing IFN-gamma in the epidermis have eczema, hair hypopigmentation, and hair loss. J Invest Dermatol. 1997;108:412–22. doi: 10.1111/1523-1747.ep12289702. [DOI] [PubMed] [Google Scholar]
- 44.Breuer K, Haussler S, Kapp A, Werfer T. Staphylococcus aureus: colonizing features and influence of an antibacterial treatment in adults with atopic dermatitis. Br J Dematol. 2002;147(1):55–61. doi: 10.1046/j.1365-2133.2002.04872.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.