Abstract
This work seeks to establish comparisons of the physical properties of rat and human cementum, root dentin and their interface, including the cementum–dentin junction (CDJ), as a basis for future studies of the entire periodontal complex using rats as animal models. In this study the structure, site-specific chemical composition and mechanical properties of cementum and its interface with root dentin taken from 9- to 12-month-old rats were compared to the physiologically equivalent 40- to 55-year-old human age group using qualitative and quantitative characterization techniques, including histology, atomic force microscopy (AFM), micro-X-ray computed tomography, Raman microspectroscopy and AFM-based nanoindentation. Based on results from this study, cementum taken from the apical third of the respective species can be represented as a woven fabric with radially and circumferentially oriented collagen fibers. In both species the attachment of cementum to root dentin is defined by a stiffness-graded interface (CDJ/cementum–dentin interface). However, it was concluded that cementum and the cementum–dentin interface from a 9- to 12-month-old rat could be more mineralized, resulting in noticeably decreased collagen fiber hydration and significantly higher modulus values under wet conditions for cementum and CDJ (Erat-cementum = 12.7 ± 2.62 GPa; Erat-CDJ = 11.6 ± 3.20 GPa) compared to a 40- to 55-year-old human (Ehuman-cementum = 3.73 ± 1.81 GPa; Ehuman-CDJ = 1.5 ± 0.71 GPa). The resulting data illustrated that the extensions of observations made from animal models to humans should be justified with substantial and equivalent comparison of data across age ranges (life spans) of mammalian species.
Keywords: Cementum, Interfaces, Structure, Chemical composition, Animal model
1. Introduction
Destruction of tissues, including the periodontal ligament (PDL), cementum and bone, can cause loss of teeth as a result of periodontitis [1]. Key challenges in regeneration of attachment include (i) understanding degradation of the tissues associated with disease progression and (ii) regeneration of the interfaces that bind the oral tissues together. Due to the difficulty in obtaining well-preserved block sections of human periodontal tissues, including alveolar bone, PDL and cementum, this work addresses the first challenge by defining an animal model and establishing comparisons between the physical properties of rat and human cementum, root dentin and the cementum–dentin interface. Subsequently, periodontitis can be induced and the sequential degeneration of structure, chemical composition and mechanical properties of periodontal tissues in the animal model can be studied. For the second challenge, tissue engineering can be used to create novel scaffolds.
Rats are considered to be good experimental models because the periodontal anatomy of a rat molar is very similar to that of a human [2]. Additionally the genetic, clinical radiographic and histological aspects of the rat periodontium are similar to the human periodontium [2,3]. The similarities published to date include the cementum structure [4–7], accumulation of significant amounts of cementum at the apical end with age, and localization of proteoglycans within cementum, dentin [5,6,8–12] and the cementum–dentin interface [13]. Although there is some information about cementum structure and its attachment to root dentin [5,6], little is known about the correlation between structure, chemical composition and mechanical properties of cementum and the cementum–dentin interface with root dentin in rat molars.
The objective of this study was to compare the structure, defined by collagen fiber orientation using histology and atomic force microscopy (AFM); chemical composition, defined by spatial distribution of organic (C-H stretch at 2940 cm−1) and inorganic ( ν1 mode at 960 cm−1) contents; and the elastic modulus values of human and rat cementums and the respective cementum–dentin interfaces taken from physiologically equivalent age groups. The structure was studied using histology, atomic force microscopy (AFM), and micro-X-ray computed tomography (MicroXCT™). The site-specific elastic modulus values were determined using AFM-based nanoindentation technique (Triboscope, Hysitron, Minneapolis, MN). Some comparisons were made with previously published results using human specimens especially with regards to the human cementum–dentin junction (CDJ) [14].
2. Materials and methods
2.1. Specimen preparation for AFM, AFM-based nanoindentation and Raman microspectroscopy
2.1.1. Human specimens
Mandibular molars from 40- to 55-year-old males (n= 6) requiring extractions as a part of dental treatment were collected following a protocol approved by the UCSF Committee on Human Research. The teeth were sterilized using 0.31 Mrad of γ-radiation [15]. Three-millimeter-thick transversely cut blocks were taken from the apical third of the root (Fig. 1a). The blocks were mounted on AFM steel stubs (Ted Pella, Inc., Redding, CA) using cyanoacrylate adhesive (MDS Adhesive QX-4, MDS Products, Inc., Anaheim, CA) and ultrasectioned as described below.
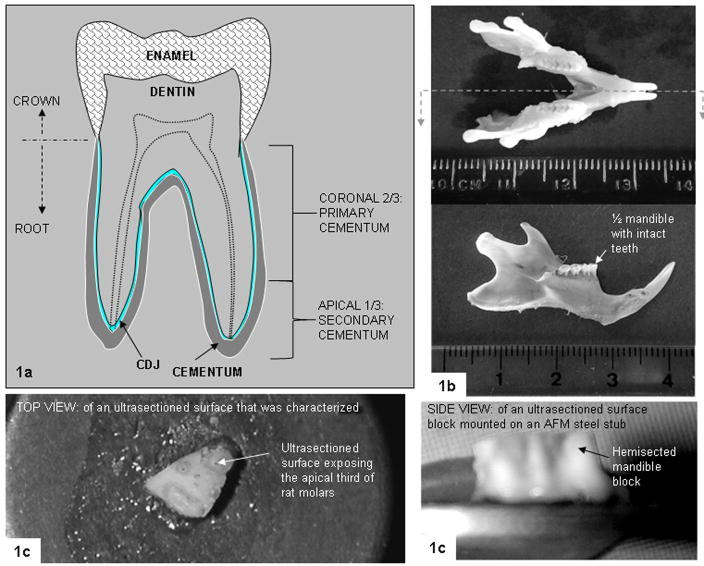
Fig. 1.
(a) Schematic of a molar (not drawn to scale). (b) Top: mandible from a Sprague Dawley rat; bottom: half-mandible with flesh removed. The units on the ruler are in centimeters. (c) Top and side views of the jaw illustrating the ultrasectioned surface and sectioned jaw mounted on an AFM stub.
2.1.2. Rat specimens
Male RA3 Sprague-Dawley rats, 9–12 months old (n=5), physiologically equivalent to 40- to 55-year-old human males, were obtained using animal tissue transfer according to guidelines by Committee on Animal Research, UCSF. Due to size limitations of rat teeth, the rat mandibles were hemisected, and the half-mandibles with teeth intact (Fig. 1b) were used. Three-millimeter-thick transverse sectioned blocks were prepared from half-mandibles using a low-speed saw (Isomet, Buehler, Lake Bluff, IL). The specimen blocks were mounted on AFM steel stubs (Fig. 1c) using cyanoacrylate adhesive and were ultrasectioned.
All specimens were ultrasectioned with an ultramicrotome (Ultracut E, Reichert-Jung, Vienna, Austria) using a diamond knife (Micro Star Technologies, Huntsville, TX), as previously described [16]. The specimens were ultrasectioned until the cementum in the apical third (Fig. 1a) of the rat molar (Fig. 1c) was exposed. Ultrasectioning was used because it has been shown to be an effective method [16] for creating the relatively flat surface necessary for determining structure, chemical composition and mechanical properties using an AFM, Raman microspectroscopy and AFM-based nanoindentation.
2.2. Specimen preparation for histology
The transversely cut human mandibular molars and the other halves of the rat mandibles were stored in 10% neutral buffered formalin for 2 weeks followed by end-stage decalcification [17] using Cal-EX II decalcifying solution (Fisher Scientific, Fair Lafwn, NJ). The specimens were dehydrated with 80, 95 and 100% Flex alcohol (Richard-Allan Scientific, Kalamazoo, MI) before being embedded in paraffin (Tissue Prep-II, Fisher Scientific, Fair Lawn, NJ) and sectioned on a rotary microtome (Reichert-Jung Biocut, Vienna, Austria) using disposable steel blades (TBF™ Inc., Shur/Sharp™, Fisher Scientific, Fair Lawn, NJ). The paraffin serial sections were mounted on Superfrost Plus microscope slides (Fisher Scientific, Fair Lawn, NJ) deparaffinized with xylene followed by staining with Sirius red F3B (C.I. 35782) and picric acid (American MasterTech Scientific Co., Lodi, CA). The stained tissues were characterized using a light microscope (BX 51, Olympus America Inc., San Diego, CA) and analyzed using Image Pro Plus v6.0 software (Media Cybernetics, Inc., Silver Spring, MD). Polarized light was used to enhance birefringence of collagen stained with picrosirius red [18], thus illustrating collagen fiber orientation. A similar staining procedure was implemented for sections taken from human molars.
2.3. Specimen preparation for micro X-ray tomography
Ground sections, 200 μm thick, were cut longitudinally from hemisected rat mandibles using a low-speed saw (Isomet, Buehler, Lake Bluff, IL). The sections were thinned to ~150 μm by sequential polishing using silicon carbide grit of sizes 1200 and 2400 (Buehler) followed by fine polishing using a diamond suspension slurry of grades 6, 3, 1 and 0.25 μm (Buehler). The specimens were ultrasonicated for 10 s between polishing steps to remove any abrasives. An identical procedure was used to make 150-μm-thick longitudinal specimens from human teeth.
The microscale morphologies of the rat and human ground sections were observed using three-dimensional (3-D) MicroXCT (Xradia, Inc. Concord, CA) at ×20. Computed tomography (CT) facilitates viewing an object in 3-D and allows selection of virtual slices spaced by 1 μm, thus illustrating bulk structure and mineral density variations of inhomogeneous tissues. The transmission X-ray imaging of the specimens was performed using an X-ray tube with a tungsten anode setting of 40 kV at 4 W. The 3-D images were constructed using 421 images taken at 12 0s integration time per image.
2.4. Atomic force microscopy of ultrasectioned surface-blocks for structure analysis
Qualitative and quantitative analyses of the topography were performed using a contact mode AFM under both dry and wet conditions (Nanoscope III, Multimode; DI-Veeco Instruments Inc., Santa Barbara, CA). The ultrasectioned surface was scanned using a Si3N4 tip attached to a “V-shaped” cantilever with a nominal normal spring constant of 0.03 N m−1 (DI-Veeco Instruments Inc., Santa Barbara, CA) at a scanning frequency of 1 Hz. The nominal radius of curvature of the tip was less than 50 nm. Scanning under wet conditions was performed with the specimen and probe immersed in deionized water. The various topographical features were analyzed using Nanoscope III version 4.43r8 software (Nanoscope III, Multimode; DI-Veeco Instruments, Inc., Santa Barbara, CA).
2.5. Raman microspectroscopy for chemical composition
The localization of the relative intensities of organic and inorganic components was determined using Raman microspectroscopy (HR800, Jobin Yvon Inc., HORIBA Group, Edison, NJ) as described previously [14]. An excitation laser with an operating wavelength of 623.8 nm was used. The relative inorganic ( ν1 mode at 960 cm−1) and organic (C-H stretch at 2940 cm−1) contents from cementum to dentin of rat and human molars under dry conditions were recorded by taking the maximum relative intensities at the respective inverse wave-numbers after subtracting the luminescence background. Raman spectra for rat specimens were collected in a grid pattern of 12 points in X and 300 points in Y directions, with a step of 11.6 μm in X and 1.3 μm in Y directions. A lower step size in the Y direction and a magnification of ×10 was chosen to accommodate the relatively smaller transverse sections of rat molars. The data were averaged from two collection periods of 30 s each for each spectrum. The ratio of the relative intensities of organic to inorganic was taken to remove the probable influence of bulk structure on the acquired relative intensities of the organic and inorganic Raman signals. All other experimental parameters remaining the same for human specimens; Raman spectra were collected in a grid pattern of 25 points with a step of 5 μm in the X direction and 100 points with a step of 1 μm in the Y direction at ×50 magnification.
2.6. AFM-based nanoindentation for site-specific determination of reduced elastic modulus
Nanoindentation was performed using an AFM coupled with a load–displacement transducer (Triboscope Micromechanical Test Instrument, Hysitron Inc., Minneapolis, MN). The coupling facilitates structure examination followed by site-specific indentation. The probe was a diamond Berkovich indenter with radius of curvature less than 100 nm. Indentation was performed under wet conditions by immersing the respective specimen and the probe in water. The maximum normal load was 1000 μN, with load, hold and unload for 3 s each. The distance between any two indents varied between 5 and 7 μm for both rat and human specimens to avoid indentation of empty physical entities, such as lacunae within cementum. Calibration was done using a fused silica standard and the reduced elastic modulus was evaluated using the Oliver–Pharr method [19].
3. Results
In this study the collagen fiber orientations defining the structure of cementum and CDJ are described relative to the longitudinal axis (Z) of a tooth. AFM, histology and MicroXCT results offer structure variations within transverse planes (2-D XY plane) assumed to be orthogonal to the longitudinal axis of a tooth.
3.1. Structure of cementum and CDJ using histology
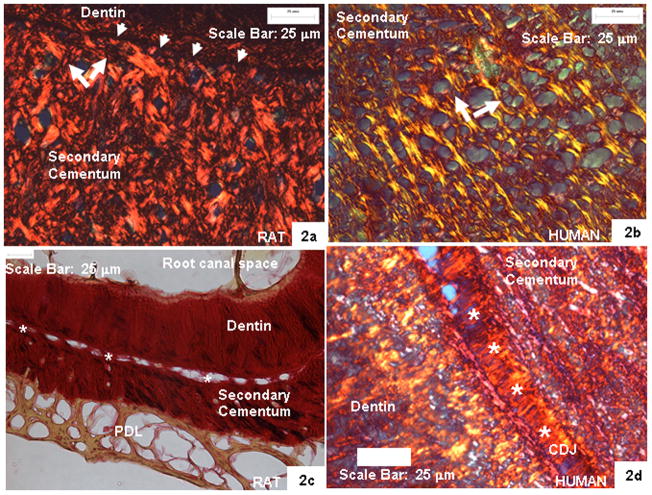
Microscale observations along the longitudinal axis (Z) of a tooth illustrated spheroidal secondary cementum in rats, giving a bulbous appearance, compared to the elliptical–paraboloid deposition in humans. Polarized microscopy of bulk cementum across species illustrated radial and circumferential collagen fibers relative to the longitudinal axis of a tooth (white arrows in bulk cementum (Fig. 2a and b)). However, a dark band between cementum and root dentin was observed in rats (white arrow heads in Fig. 2a). Under normal light microscopy the dark band manifested into an interface containing 0.7- to 2- μm-wide sparsely located ligament-like bridges (white asterisks in Fig. 2c) defining secondary cementum attachment to root dentin in rats. This mode of attachment was also observed in human molars; however, the interface was 15–30 μm (white asterisks in Fig. 2d) wide. At the root apex, cementum appeared to be attached directly to root mantle dentin with no discernible interface in both humans and rats.
Fig. 2.
Polarized micrographs illustrating secondary cementum and CDJ (white asterisks) in rat (a, c) and human (b, d) molars. Notice the woven fabric-like cementum structure (white arrows) and collagen-fiber bridges (white asterisks) defining CDJ in both species.
3.2. Micro- and nanoscale structural analysis of cementum and CDJ using an AFM
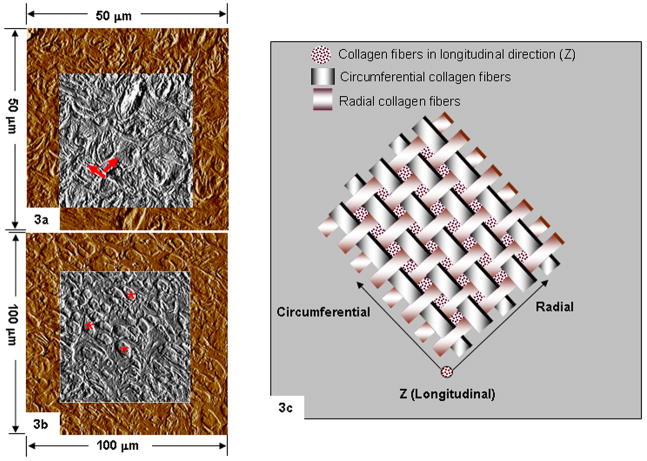
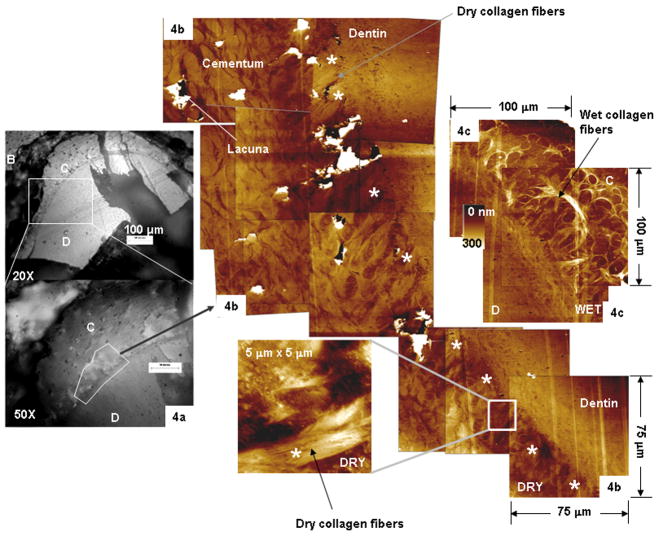
Collagen fibers in radial and circumferential directions relative to longitudinal axis of a tooth were observed (Figs. 3a and 3b) in human specimens. Scanning ultrasectioned surface-blocks of rat specimens (Fig. 4) with an AFM probe indicated: 1) distinctly visible 1.5 to 2.5 μm thick radial collagen fiber bundles bridging cementum with dentin (Fig. 4b) 2) partial swelling of the collagen fiber bundles (indicated by a slight increase in height compared to dry conditions) within cementum under wet conditions (Fig. 4c). The pores seen in histology sections of a rat-CDJ were also seen in ultrasectioned surface blocks under dry and wet conditions (Fig. 4b).
Fig. 3.
AFM micrographs of secondary human cementum illustrating radial and circumferential collagen fibers (red arrows) (a) and collagen fibers (red asterisks) in the longitudinal (Z) direction (b). A schematic representing secondary cementum as a woven fabric-like structure due to collagen fibers in the radial, circumferential and longitudinal directions (c).
Fig. 4.
(a) Reflectance light micrographs of an ultrasectioned surface block illustrating the mineralized tissues of interest. (b) Composite AFM micrographs of cementum (C) and root dentin (D) under dry conditions illustrating the porous interface, including the collagen fibers: a 5 μm × 5 μm region illustrating collagen fiber bundle at the cementum–dentin junction (CDJ). (c) 100 μm × 100 μm AFM micrographs of cementum (C) and root dentin (D) under wet conditions illustrating the partially swollen collagen fibers and interface.
3.3. MicroXCT™ of bulk cementum including various interfaces
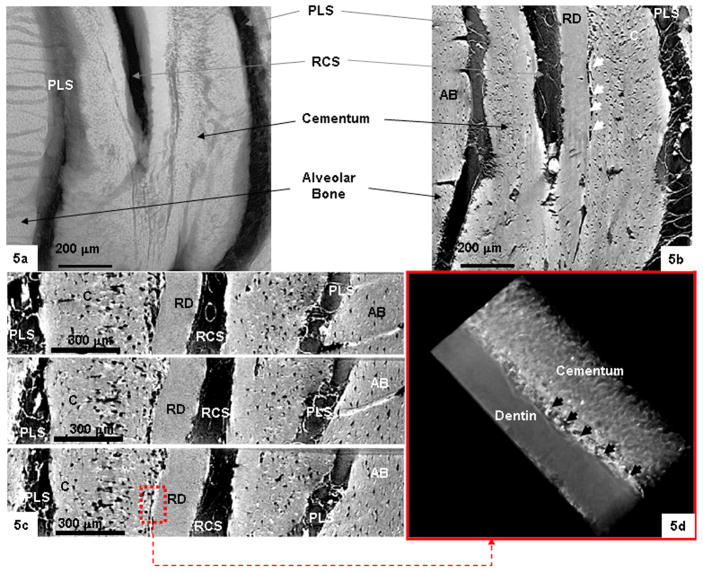
Microradiography (Fig. 5a) and tomography slices in the longitudinal (Fig. 5b) and transverse (Figs. 5c and 6a and b) directions of the ground sections from both species demonstrated a similar cementum structure and interface defining its attachment to root dentin. In particular, ligament-like connections were observed between cementum and root dentin over a width of 4–7 μm in rats (white arrows in Fig. 5b and black arrows in Figs. 5d) compared to well-organized radially oriented collagen fiber bridges within a 15–30 μm interface in humans (Fig. 6). Pores, noticed as black voids, between ligament-like connections were observed in both species: however, more were found in rat CDJ (Fig. 5). Similarly several dark regions representative of lacunae within cementum were observed in both species (Figs. 5 and 6). Additionally, the alveolar bone, root canal and periodontal ligament spaces can be observed in transverse (Fig. 5c) and longitudinal (Fig. 5b) sections in rat specimens. Contrary to rat cementum, human cementum was lamellar (Fig. 6) in appearance.
Fig. 5.
(a) A radiograph of the ground section taken at ×20 using MicroXCT. (b, c) Transverse and longitudinal slices taken from different areas of tomograph illustrating the bulk structure of CDJ (white arrows in (b)), including other interfaces such as the periodontal ligament space (PLS) and periodontal ligament (PL) between alveolar bone (AB) and cementum (C). The noticeable black regions are considered to be voids either representative of lacunae or pores due material/region architecture. (d) A tomograph of the red region marked in (c) illustrating the CDJ (black arrows). RCS: root canal space; RD: root dentin.
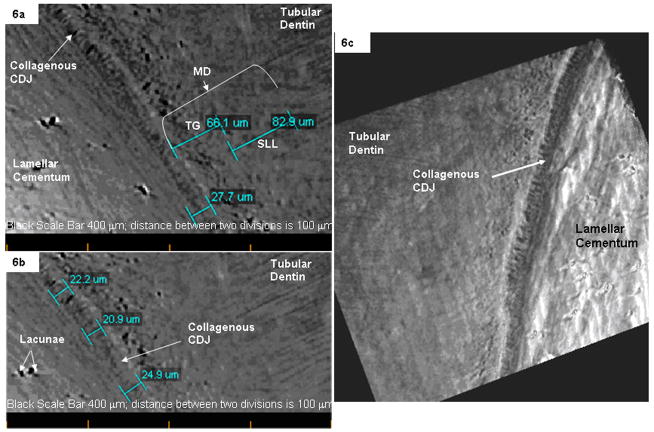
Fig. 6.
(a) Longitudinal slices taken from a tomograph illustrating mantle dentin (MD),which is a combination of a Tomes granular layer (TG, most often observed in ground sections) and a structureless layer (SLL) between human lamellar cementum and tubular root dentin. (b) The width of the CDJ between bulk cementum with lacunae (dark regions) and tubular dentin. (c) A tomograph illustrating radially oriented collagen fibers within the CDJ in between tubular dentin and cementum.
3.4. Chemical composition of cementum, root dentin and the interface
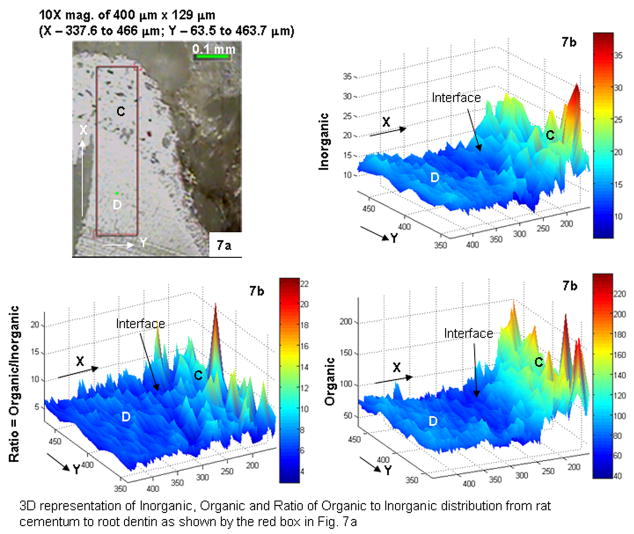
In rats, Raman microspectroscopy provided relative concentration distributions of organic and inorganic materials, and the ratio of relative concentration of organic (C-H stretch at 2940 cm−1) to inorganic materials ( ν1 mode at 960 cm−1) within different areas (Fig. 7a). Cementum was lower in inorganic compared to organic content, resulting in a higher ratio of organic to inorganic concentration compared to root dentin (Fig. 7b). A 10- to 50-μm-wide interface with a lower ratio of organic to inorganic matte was observed between cementum and root dentin (Fig. 7b).
Fig. 7.
(a) Light microscopy area images of a 400 μm × 129 μm region bracketing the CDJ. (b) 3-D surface plots of the region illustrating a variation in inorganic, organic and a ratio of organic to inorganic contents. C: cementum; D: dentin.
In humans, the organic and inorganic contents were higher in root dentin compared to cementum, resulting in a relatively uniform distribution of their ratio between the two materials. However, a 70- to 100-μm-wide interface between cementum and dentin was found, with a higher ratio of organic to inorganic concentrations (these results are not shown because the distribution was similar to results published on 65- to 80-year-old males [14]).
3.5. Elastic modulus values of cementum, root dentin and the interface
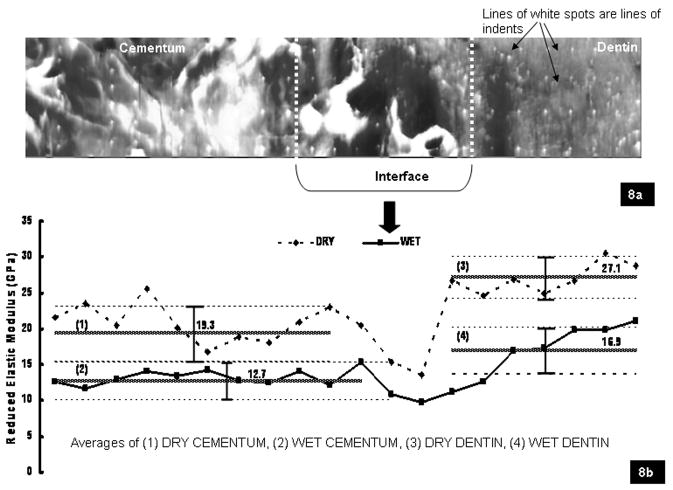
Fig. 8a illustrates lines of nanoindents from cementum to root dentin in a rat molar. Fig. 8b is a representative curve illustrating the variation in reduced elastic modulus values for 9- to 12-month-old rat cementum to root dentin under dry and wet conditions along a line of indents. The horizontal lines (1–4 in Fig. 8b) represent average values of reduced elastic modulus and corresponding standard deviations for dry and wet cementum and root dentin, taking into account all specimens (n=5). Lower values of elastic modulus within a 10–50 μm interface under dry and wet conditions were observed (Fig. 8b). The observed ranges within this region under dry (10–20 GPa) and wet (8–18 GPa) conditions were similar. Under wet conditions a gradual gradient in elastic modulus from cementum to root dentin was observed (Fig. 8b), contrary to the dip under dry conditions (Fig. 8b). Student’s t-test illustrated significant differences (p < 0.05) between dry and wet cementum, root dentin, and CDJ respectively between species (Table 1). Although no significant differences in root dentin between species were observed, rat cementum and CDJ had significantly higher modulus values (p < 0.05) than human cementum and CDJ when compared under wet conditions.
Fig. 8.
(a) Topographical map of an indented area illustrating indents (white spots). (b) Representative graph illustrating variation of reduced elastic modulus from cementum to dentin under dry and wet conditions along a line of indents. The lowest modulus values under dry and wet conditions were observed at the interface. Additionally, under wet conditions a gradual increase in modulus was observed when compared to dry conditions. The horizontal lines represent the average reduced elastic modulus values of dry cementum (1), wet cementum (2), dry dentin (3) and wet dentin (4) from all specimens (n=5), with corresponding standard deviation bars.
Table 1.
Average reduced elastic modulus of dry and wet cementum, CDJ and dentin for humans and rats
| HUMAN (GPa) | RAT (GPa) | |||||
|---|---|---|---|---|---|---|
| CEMENTUM | DENTIN | INTERFACE | CEMEMTUM | DENTIN | INTERFACE | |
| DRY | 150 ± 359 | 23 8 ± 3.68 | 17.7 ± 3.19 | 19.3 ± 3.85 | 27.1 ± 3.02 | 18.9 ± 5.50 |
| WET | 3.73 ± 1.81 | 14.2 ± 5.10 | 1 5 ± 0.71 | 12.7 ± 2.62 | 16.9 ± 3.16 | 11.6 ± 3.20 |
| Fractional Decrease | 0.75 | 0.40 | 0.91 | 0.34* | 0.37 | 0.38* |
Indicates a significant difference between rat and human wet cementum and CDJ
4. Discussion
The results of this study form a basis for future studies of the entire periodontal complex and its degradation due to periodontal disease using an animal model. In this study the structure, site-specific chemical composition and mechanical properties of rat cementum and its interface with root dentin were compared to human specimens taken from a physiologically equivalent age group.
From a biomaterials perspective, a significant challenge in this study was to define equivalent age groups for rats and humans to compare the structures, chemical compositions and mechanical properties of cementum, root dentin and the interface. The mean life span of Sprague-Dawley rats is approximately 2 years [20] and for humans has been reported to be 80–85 years [21]. Based on their reduced breeding capability during middle age, a rat of 9–12 months can be considered physiologically equivalent to a human of 40–55 years. The results in this study were limited to erupted and fully functional molars included comparisons with corresponding human tissues for: (i) the structure of cementum; (ii) the cementum attachment to root dentin; and (iii) variation in chemical composition and its manifestation as a variation in reduced elastic modulus: a descriptor of site-specific properties of cementum, cementum–dentin junction and root dentin.
4.1. Contrary to primary cementum, secondary cementum is a woven fabric-like biomaterial
Primary cementum predominantly consists of radial collagen fibers in humans and rats. The architecture of secondary human cementum has been described as a twisted plywood structure with an alternating lamellar pattern [5]. We found that the architecture of secondary human and rat cementums can be represented as a woven fabric with wide radial and narrower circumferential collagen fibers (Fig. 3c). Fibers in the longitudinal Z direction running through interspaces of circumferential and radial collagen fibers could join the woven-fabric planes (Fig. 3c), providing the necessary 3-D architecture. Apart from sustaining different modes of mechanical loads across species, the woven fabric-like structure provides tissue porosity and permeability.
In our previous and current studies, scanning with an AFM and comparing similar areas of cementum and its interface with root dentin under dry and wet conditions facilitated the understanding of the behavior of site-specific regions under in vivo simulated conditions [8,14,16]. In particular, rat collagen fibers were partially swollen (Fig. 4c), contrary to the consistently observed hydrated collagen fibers in human cementum [8]. The differences in hydration were indicated by small, sometimes significant height variations between dry and wet collagen fibers. Additionally, in human molars we confirmed an inference made by others that the hypomineralized and glycosaminoglycan-rich primary or radial fibers extend through bulk cementum and attach to root dentin [8,22–24]. Based on results from this study, the structure is similar for rats but the chemical composition could be significantly different, resulting in varying packing densities and permeability of cementum across species.
4.2. Both species have cementum attached to root dentin via collagen fiber bridges
Several names were given to the 10- to 50-μm-wide discernible layer that attaches bulk cementum to root dentin [25–28]. From a materials science and mechanical engineering approach, we continue to define the discernible layer as a cementum–dentin junction/interface [16,29].
Structurally, the CDJ in both species can be considered as a region of interspaced collagen fiber bridges formed during development after the breakdown of Hertwig’s epithelial root sheath (HERS) [30,31]. The rat CDJ was compared to human CDJ using histology (Fig. 2), AFM (Figs. 3 and 4) and MicroXCT (Figs. 5 and 6) imaging techniques, and the results were consistent and complementary.
The histology of human specimens as seen using light microscopy (Fig. 2d) and MicroXCT (Fig. 6) illustrated 15- to 30-μm-wide collagen fiber bridges from cementum to root dentin, with variations in width depending on the location of any given section. However, using an AFM, we were able to differentiate the behavior of 10- to 50-μm-wide human CDJ by comparing its swelling under wet conditions to a valley under dry conditions relative to the adjacent predominantly mineralized tissues: cementum and root dentin [8]. The observed pores in all three imaging techniques across species could be remnants of HERS after its breakdown, also known as epithelial rests of Malassez [30,31], which appear to be pore-like structures found between bulk cementum and root dentin. These were more frequently seen using all three imaging techniques and especially in AFM micrographs of rat specimens (Fig. 4b).
4.3. Rat cementum and CDJ is hypermineralized compared to that in humans
Raman microspectroscopy results on human specimens illustrated similar ratios of organic to inorganic matter for cementum and root dentin. Additionally, the observed trends in ratio of organic to inorganic matter from cementum to root dentin, including the interface, for 40- to 55-year-old humans were similar to those published for an older human age group (65–80 years) [14]. However, similar intensities of organic content in human cementum and root dentin could imply that human cementum has a lower packing density.
Dry hypomineralized tissues result in the extracellular matrix having a collapsed structure and an overestimation in the elastic modulus using nanoindentation. Additionally, the direction of indentation load relative to fiber orientation can influence the indentation modulus. In this study the indentation direction was perpendicular to the radially oriented collagen fibers. However, it should be noted that the values could be significantly different if the indentation direction were perpendicular to the transverse section of collagen fibers.
Under wet conditions, the site-specific load resisting capabilities within individual tissues can be identified using nanoindentation. The basic cementum constituents including collagen and glycosaminoglycans vary with development and age across species [13]. Human cementum was more hygroscopic due to dominance of hydrophilic organic components (collagen and glycosaminoglycans) within the radial collagen fibers. In comparison, rat cementum was less hygroscopic and had a significantly lower decrease in elastic modulus when hydrated (75% in humans compared to 34% in rats). The significant differences in percent reduction in elastic modulus under wet conditions suggest that rat cementum is more mineralized than human cementum. In addition, it is possible that the packing density of wet rat cementum is similar to dry rat cementum. Hence, it would be of interest to map the organic and inorganic intensities of cementum, and its interface with root dentin, across species under wet conditions using Raman microspectroscopy.
The organic to inorganic ratio in 40- to 55-year-old humans increased within a 70-to 100-μm-wide interface, similar to the trend observed in 65- to 80-year-old humans [14]. Additionally, an intermediate and distinct hygroscopic CDJ [8,29] between cementum and root dentin was found that had a significant percent decrease in elastic modulus (91%) when hydrated. This could indicate that in humans the dominant basic constituent within collagen fiber bridges of the CDJ is organic matter. However, the presence of a phosphate peak within the human CDJ could indicate the presence of a mineral, thus ruling out a true soft-tissue-like characteristic [14]. Additionally, the lower measured intensities using Raman microspectroscopy could also imply a lower packing density of material within the interfacial region [14]. Moreover, an accurate width of the interface and intensity mapping can be obtained by tuning the optics of the Raman microspectrometer to match the laser spot size and step size.
In rats, the presence of lower ratios of organic to inorganic matter and absence of a distinct hygroscopic CDJ manifested in a lower percent decrease in elastic modulus (38% in rats compared to 91% in humans) (Table 1). This could indicate that in rats the substantial mineral content reinforces the collagen fiber bridges of the CDJ. It should be noted that although the collagen fibers forming bridges could be more mineralized in rats than in humans, the mode of attachment is similar. Despite the higher modulus and the lack of a distinct hygroscopic CDJ in rats, a gradual increase in modulus from cementum to root dentin (Fig. 8b) similar to that seen in humans [14] was observed under wet conditions. However, the width (rats: 0.7–15 μm; humans: 15–30 μm) and properties of the CDJ between species could vary during development and growth, and could also be influenced by function and the size of the animal. Additionally, macroscale biomechanical testing could highlight the greater load-bearing capacity in the radial direction due to the observed preferred orientation of collagen fibers.
Despite the observation of distinct structures forming cementum, root dentin and the interface between them in both species, no distinct variations within each tissue or region could be recorded using MicroXCT. Owing to the equipment and processing limitations of the MicroXCT instrument, the data presented are valid only for structure and not mineral density between species. However, future calibration of the MicroXCT instrument will provide a quantitative measure of mineral density of respective mineralized tissues and their interfaces.
The observed cementum and CDJ structure and chemical composition variations in this study can be linked to the key developmental events describing cementum attachment to root dentin as follows: (i) after the breakdown of the Hertwig’s epithelial root sheath, the collagen fibrils of cementum and root dentin blend, forming an attachment between the two materials; (ii) following the deposition of non-collagenous proteins, mineralization of root dentin starts and spreads through the CDJ and cementum, resulting in a mineralized matrix [30,32]; and (iii) during the early stages of development, mineralization of the CDJ occurs within a short time (approximately 3 days), causing a decrease in sulphated glycosaminoglycans [13,32]. The first explains the attachment of cementum to root dentin, while the second and third explain the process and fast rate of CDJ mineralization in a rat molar during development.
Rat molars continue to develop from the time of birth up to 4 months, after which growth slows significantly [33]. The root formation in rats is 17–20 times faster than in humans [7]. This implies that 9- to 12-month-old rat specimens when compared to 40- to 55-year-old humans could be significantly more mineralized, as suggested from this study. The need for this evolutionary variation could be attributed to the fundamental physiological variations across species, including diet. However, in both species, the question that remains to be answered is the need for the observed stiffness-graded interface between cementum and root dentin in both species (Fig. 8). Based on physical property observations of rat and human cementum interface with root dentin, it is hypothesized that the stiffness-graded interface distributes the cyclic functional and occasional impact loads on a tooth.
5. Conclusion
Rat and human cementum can be represented as a woven fabric-like material that provides tissue permeability. In both species the attachment of cementum with root dentin is defined by a stiffness-graded interface. Additionally, macroscale biomechanical testing could highlight the greater load-bearing capacity in the radial direction due to the observed preferred orientation of collagen fibers of the CDJ. However, it is concluded that cementum and CDJ from a 9- to 12-month-old rat is more mineralized, with a higher packing density, and displays noticeably decreased collagen fiber hydration in cementum and the cementum–dentin interface and a significantly higher modulus compared to a physiologically equivalent 40- to 55-year-old human. Furthermore, the results of the study illustrate that the extensions of observations made from animal models to humans should be justified with substantial and equivalent comparisons of data across age ranges (life spans) of different mammalian species.
Acknowledgments
Support was provided by NIH/1K99DE018212-01, NIH/R00DE018212, UCSF-Academic Senate Individual Investigator Grant, and NIH/NIDCR T32DE07306 (SPH). The authors thank Liling Wu, Peking University Health Science Center, and E LeRoy, HORIBA Jobin Yvon Inc., for technical discussions on equivalent age groups for rats and humans, and Raman microspectroscopy. The authors thank Mahesh Mankani, UCSF Department of Surgery, for the generous supply of rats, and Peter Sargent, Department of Cell and Tissue Biology, UCSF, for the use of the ultramicrotome. Additionally, the authors thank the Lawrence Livermore National Laboratory for the use of Raman microspectrometer and Linda Prentice, Department of Orofacial Sciences, UCSF, for histology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bosshardt DD. Are cementoblasts a subpopulation of osteoblasts or a unique phenotype? Critical Reviews in Oral Biology & Medicine, Journal of Dental Research. 2005;84(5):390–406. doi: 10.1177/154405910508400501. [DOI] [PubMed] [Google Scholar]
- 2.Klausen B. Microbiological and immunological aspects of experimental periodontal disease in rats: a review article. Journal of Periodontology. 1991;62:59–73. doi: 10.1902/jop.1991.62.1.59. [DOI] [PubMed] [Google Scholar]
- 3.Page RC, Schroeder E. Periodontitis in Man and Other Animals. Basel: Karger; 1982. [Google Scholar]
- 4.Yamamoto T, Domon T, Takahashi S, Islam MD, Suzuki R, Wakita M. The regulation of fiber arrangement in advanced cellular cementogenesis of human tooth. Journal of Periodontal Research. 1998;33:83–90. doi: 10.1111/j.1600-0765.1998.tb02296.x. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto T, Domon T, Takahashi S, Islam NM, Suzuki R. Wakita M Twisted plywood structure of an alternating lamellar pattern in cellular cementum of human teeth. Anatomical Embryology. 2000;202(1):25–30. doi: 10.1007/pl00008241. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto T, Domon T, Takahashi S, Islam N, Suzuki R. The initial attachment of cemental fibers to the root dentin surface in acellular and cellular cementogenesis in rat molars. Annals of Anatomy. 2001;183:123–128. doi: 10.1016/S0940-9602(01)80030-3. [DOI] [PubMed] [Google Scholar]
- 7.Bosshardt DD, Schroeder H. Cementogenesis reviewed: a comparison between human premolars and rodent molars. The Anatomical Record. 1996;245:267–292. doi: 10.1002/(SICI)1097-0185(199606)245:2<267::AID-AR12>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 8.Ho SP, Marshall SJ, Ryder MI, Marshall GW. The tooth attachment mechanism defined by structure, chemical composition and mechanical properties of collagen fibers in the periodontium. Biomaterials. 2007;28(35):5238–45. doi: 10.1016/j.biomaterials.2007.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamamoto T, Domon T, Takahashi S, Arambawatta AK, Wakita M. Immunolocation of proteoglycans and bone-related noncollagenous glycoproteins in developing acellular cementum of rat molars. Cell and Tissue Research. 2004;317(3):299–312. doi: 10.1007/s00441-004-0896-4. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto T, Domon T, Takahashi S, Islam N, Suzuki R, Wakita M. The structure and function of the cemento-dentinal junction in human teeth. Journal of Periodontal Research. 1999;34:261–268. doi: 10.1111/j.1600-0765.1999.tb02252.x. [DOI] [PubMed] [Google Scholar]
- 11.Matias MA, Li H, Young WG, Bartold PM. Immunohistochemical localization of fibromodulin in the periodontium during cementogenesis and root formation in the rat molar. Journal of Periodontal Research. 2003;38:502–507. doi: 10.1034/j.1600-0765.2003.00682.x. [DOI] [PubMed] [Google Scholar]
- 12.Sato R, Yamamoto H, Kasai K. Yamauchi M Distribution pattern of versican, link protein and hyaluronic acid in the rat periodontal ligament during experimental tooth movement. Journal of Periodontal Research. 2002;37:15–22. doi: 10.1034/j.1600-0765.2002.90770.x. [DOI] [PubMed] [Google Scholar]
- 13.Tomazela-Herndl SA, Arana-Chavez VE. Ultrastructure of early mineral deposition during hyaline layer formation in rat molars. Archives of Oral Biology. 2001;46(4):305–11. doi: 10.1016/s0003-9969(00)00131-x. [DOI] [PubMed] [Google Scholar]
- 14.Ho SP, Balooch M, Marshall SJ, Marshall GW. Local properties of a functionally graded interphase between cementum and dentin. Journal of Biomedical Materials Research Part A. 2004;70(3):480–9. doi: 10.1002/jbm.a.30105. [DOI] [PubMed] [Google Scholar]
- 15.White JM, Goodis HE, Marshall SJ, Marshall GW. Sterilization of teeth by gamma radiation. Journal of Dental Research. 1994;73(9):1560–7. doi: 10.1177/00220345940730091201. [DOI] [PubMed] [Google Scholar]
- 16.Ho SP, Goodis H, Balooch M, Nonomura G, Marshall SJ, Marshall G. The effect of sample preparation technique on determination of structure and nanomechanical properties of human cementum hard tissue. Biomaterials. 2004;25(19):4847–57. doi: 10.1016/j.biomaterials.2003.11.047. [DOI] [PubMed] [Google Scholar]
- 17.Sheehan DC, Hrapchak BB. Theory and Practice of Histotechnology. 2. The C.V. Mosby Company; 1980. [Google Scholar]
- 18.Junqueira LCU, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochemical Journal. 1979;11:447–455. doi: 10.1007/BF01002772. [DOI] [PubMed] [Google Scholar]
- 19.Oliver WC. An improved technique for determining hardness and elastic-modulus using load and displacement sensing indentation experiments. Journal of Materials Research. 1992;7(6):1564–1583. [Google Scholar]
- 20.Sucknow MA, Weisbroth SH, Franklin CL, editors. The laboratory Rat. Elsevier Academic Press; Burlington, MA: 2006. [Google Scholar]
- 21.Perls T, Kunkel LM, Puca AA. The genetics of exceptional human longevity. Journal of Molecular Neuroscience. 2002;19(1–2):233–8. doi: 10.1007/s12031-002-0039-x. [DOI] [PubMed] [Google Scholar]
- 22.Dewey KW. Normal and pathological cementum formation. Dental Cosmos. 1926;68:560–85. [Google Scholar]
- 23.Furseth R. The fine structure of acellular cementum in human young premolars. Scandinavian Journal of Dental Research. 1974;82:437–41. doi: 10.1111/j.1600-0722.1974.tb00398.x. [DOI] [PubMed] [Google Scholar]
- 24.Selvig KA. The fine structure of human cementum. Acta ondontologica Scandinavica. 1965;23:423–41. doi: 10.3109/00016356509007523. [DOI] [PubMed] [Google Scholar]
- 25.Bodecker CFW. The distribution of living matter in human dentine, cement, and enamel. Dental Cosmos. 1957;20:582–590. [Google Scholar]
- 26.Benez L. Befunde an der Dentinzement Grenze. Zeitschrift für Stomatologie. 1927;5:877–896. [Google Scholar]
- 27.el Mostehy MR, Stallard RE. Intermediate cementum. Journal of Periodontal Research. 1968;3:24–29. [PubMed] [Google Scholar]
- 28.Hopewell-Smith A. Concerning human cementum. Journal of Dental Research. 1920;2:59–76. [Google Scholar]
- 29.Ho SP, Sulyanto RM, Marshall SJ, Marshall GW. The cementum–dentin junction also contains glycosaminoglycans and collagen fibrils. Journal of Structural Biology. 2005;151(1):69–78. doi: 10.1016/j.jsb.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 30.Nanci A, Bosshardt DD. Structure of periodontal tissues in health and disease. Periodontology 2000. 2006;40:11–28. doi: 10.1111/j.1600-0757.2005.00141.x. [DOI] [PubMed] [Google Scholar]
- 31.Luan X, Ito Y, Diekwisch TGH. Evolution and development of Hertwig’s epithelial root sheath. Developmental Dynamics. 2006;235:1167–1180. doi: 10.1002/dvdy.20674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tomalez-Herndl SA, Arana-Chevez VE. Localisation of sulphated glycoconjugates during hyaline layer formation in rat molars by ultrastructural cytochemistry. Journal of Molecular Histology. 2004;35:63–68. doi: 10.1023/b:hijo.0000021089.75408.7c. [DOI] [PubMed] [Google Scholar]
- 33.Schour J, Massler M. The teeth. In: Farris EJ, Griffith JQ, editors. The rat in Laboratory Investigation. 2. J. B. Lippincott; Philadelphia, PA: 1949. [Google Scholar]