Abstract
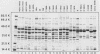
Haemophilus aegyptius and Haemophilus influenzae biotype III are morphologically and biochemically similar; however, their outer membrane protein (Sarkosyl insoluble) profiles are distinct. Of 18 strains of H. aegyptius examined, 15 had a type 1 protein profile, and 3 had a type 2 profile, whereas the 5 strains of H. influenzae biotype III examined had three other protein profile types. All Haemophilus strains examined had 31- and 76-kilodalton (kDa) proteins and minor proteins with molecular masses between 20 and 100 kDa. H. aegyptius, with a type 1 protein profile, had major outer membrane proteins with apparent molecular masses of 27, 35.5, and 41.5 kDa, and H. aegyptius, with a type 2 protein profile, had 26-, 29-, 39.5-, and 41-kDa proteins. The type strain of H. influenzae biotype III had three major outer membrane proteins with apparent molecular masses of 29, 38.5 and 40 kDa. Four other strains designated as H. influenzae biotype III had major outer membrane proteins between 27 and 41.5 kDa representing two additional protein profiles.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albritton W. L., Setlow J. K., Thomas M., Sottnek F., Steigerwalt A. G. Heterospecific transformation in the genus Haemophilus. Mol Gen Genet. 1984;193(2):358–363. doi: 10.1007/BF00330693. [DOI] [PubMed] [Google Scholar]
- Barenkamp S. J., Munson R. S., Jr, Granoff D. M. Outer membrane protein and biotype analysis of pathogenic nontypable Haemophilus influenzae. Infect Immun. 1982 May;36(2):535–540. doi: 10.1128/iai.36.2.535-540.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. J. Modification of the rapid fermentation test for Neisseria gonorrhoeae. Appl Microbiol. 1974 Jun;27(6):1027–1030. doi: 10.1128/am.27.6.1027-1030.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS D. J., PITTMAN M., GRIFFITTS J. J. Hemagglutination by the Koch-Weeks bacillus (Hemophilus aegyptius). J Bacteriol. 1950 Mar;59(3):427–431. doi: 10.1128/jb.59.3.427-431.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulley J. R., Grieve P. A. A simple technique for eliminating interference by detergents in the Lowry method of protein determination. Anal Biochem. 1975 Mar;64(1):136–141. doi: 10.1016/0003-2697(75)90415-7. [DOI] [PubMed] [Google Scholar]
- Kersters K., De Ley J. Identification and grouping of bacteria by numerical analysis of their electrophoretic protein patterns. J Gen Microbiol. 1975 Apr;87(2):333–342. doi: 10.1099/00221287-87-2-333. [DOI] [PubMed] [Google Scholar]
- Kilian M. A taxonomic study of the genus Haemophilus, with the proposal of a new species. J Gen Microbiol. 1976 Mar;93(1):9–62. doi: 10.1099/00221287-93-1-9. [DOI] [PubMed] [Google Scholar]
- Kilian M., Mordhorst C. H., Dawson C. R., Lautrop H. The taxonomy of haemophili isolated from conjunctivae. Acta Pathol Microbiol Scand B. 1976 Jun;84(3):132–138. doi: 10.1111/j.1699-0463.1976.tb01915.x. [DOI] [PubMed] [Google Scholar]
- LEIDY G., HAHN E., ALEXANDER H. E. Interspecific transformation in Hemophilus: a possible index of relationship between H. influenzae and H. aegyptius. Proc Soc Exp Biol Med. 1959 Oct;102:86–88. doi: 10.3181/00379727-102-25151. [DOI] [PubMed] [Google Scholar]
- LEIDY G., JAFFEE I., ALEXANDER H. E. FURTHER EVIDENCE OF A HIGH DEGREE OF GENETIC HOMOLOGY BETWEEN H. INFLUENZAE AND H. AEGYPTIUS. Proc Soc Exp Biol Med. 1965 Mar;118:671–679. doi: 10.3181/00379727-118-29935. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loeb M. R., Smith D. H. Outer membrane protein composition in disease isolates of Haemophilus influenzae: pathogenic and epidemiological implications. Infect Immun. 1980 Dec;30(3):709–717. doi: 10.1128/iai.30.3.709-717.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazloum H. A., Kilian M., Mohamed Z. M., Said M. D. Differentiation of Haemophilus aegyptius and Haemophilus influenzae. Acta Pathol Microbiol Immunol Scand B. 1982 Apr;90(2):109–112. doi: 10.1111/j.1699-0463.1982.tb00090.x. [DOI] [PubMed] [Google Scholar]
- Murphy T. F., Dudas K. C., Mylotte J. M., Apicella M. A. A subtyping system for nontypable Haemophilus influenzae based on outer-membrane proteins. J Infect Dis. 1983 May;147(5):838–846. doi: 10.1093/infdis/147.5.838. [DOI] [PubMed] [Google Scholar]
- PITTMAN M., DAVIS D. J. Identification of the Koch-Weeks bacillus (Hemophilus aegyptius). J Bacteriol. 1950 Mar;59(3):413–426. doi: 10.1128/jb.59.3.413-426.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sottnek F. O., Biddle J. W., Kraus S. J., Weaver R. E., Stewart J. A. Isolation and identification of Haemophilus ducreyi in a clinical study. J Clin Microbiol. 1980 Aug;12(2):170–174. doi: 10.1128/jcm.12.2.170-174.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiede J. J., Pagano M. The application of robust calibration to radioimmunoassay. Biometrics. 1979 Sep;35(3):567–574. [PubMed] [Google Scholar]