Abstract
Advanced glycation end-products (AGEs) have been found to play a role in inflammation and vascular complications. The receptor for AGE (RAGE) is expressed on many cells and is upregulated during inflammation. To date, RAGE expression or its correlation with NF-kβ expression has not been demonstrated in inflammatory periapical lesions. In this study, periapical tissue was obtained from 38 patients and processed for RAGE and NF-kβ expression using RT-PCR. Various patient variables were examined as predictors for these novel proteins expressions. The results demonstrated a statistically significant positive correlation between the expression of RAGE and NF-kβ (p=0.001). When comparing Caucasians and African-Americans, a statistically significant difference was noted with the African-American group showing a higher expression of RAGE (ANOVA F= 12.746, p=0.002). In conclusion, a positive linear correlation of their presence was demonstrated in inflamed periradicular tissues. This study outlines the possible use of these proteins as potential markers for periapical inflammation.
INTRODUCTION
Advanced glycosilated end-products (AGE) and signaling through their receptors (RAGE) have been shown to impair healing and contribute to several pathologic conditions such as diabetes, atherosclerosis, arthritis, cancers, and periodontal disease. (3, 11). AGE's are the result of spontaneous nonenzymatic glycosilation of proteins or lipids (4, 5). Conditions of hyperglycemia, inflammation, and oxidant stress have been shown to promote AGE formation (9).
AGE's are known to accumulate in intravascular and extravascular tissues by irreversibly crosslinking with connective tissue proteins such as collagen and elastin (6). This accumulation of AGE's within connective tissues causes a loss of elasticity along with destabilization of basement membranes (8, 19).
RAGE's are multiligand receptors belonging to the immunoglobin superfamily (15). Thay are expressed on multiple cell types such as endothelial cells, neutrophils, macrophages, monocytes, lymphocytes (T and B cells), fibroblasts, and neurons (5, 7, 9, 14, 10). Apoptotic smooth muscle cells and macrophages associated with atherosclerotic plaques were shown to have increased levels of RAGE; especially in those of diabetic patients (3). In addition, higher levels of RAGE mRNA have been detected in gingival tissue of diabetics (11).
The actions that are stimulated from the AGE/RAGE complexes appear to be most deleterious. Tumor cells that are RAGE activated seem to show increased proliferation, migration, invasion, and matrix metaloprotease (MMP) production (9). Collinson et al (2002), reported that RAGE activated neutrophils in the presence of Staphylococcus aureus showed an increased phagocytic activity but a decreased ability to kill the bacteria. With the introduction of soluble RAGE (es-RAGE), anti-RAGE antibodies, or antibodies to the AGE, Collinson noted better killing ability by the neutrophils.
Husdon et al (2003), showed in streptozotocin induced diabetic mice that received es-RAGE had less vascular leakage as well as lower levels of TNF-α, IL-6, and MMPs 2,3,9. Other researchers looked at the presence of es-RAGE in chronic joint inflammation and found that rheumatoid arthritis (RA) patients had significantly lower levels of es-RAGE than those of non RA patients (14). These results suggest that by binding the free AGE, there is less RAGE activation and thus less inflammatory sequela.
RAGE activation has also been associated with activation of Nuclear Factor kappa Beta (NF-kβ) (16). NF-kβ is activated by cell surface receptors, of which TNF and IL-1 family Toll receptors play a large part (18). NF-kβ is an essential transcription factor of the immune system with many functions such as apoptosis, lymphoid cell development, lymphoid structure, tumor growth, and osteoclastogenesis (18). NF-kβ activation stimulates the transcription of IL-1, IL-6, and TNF-α which are known to be significant cytokines of inflammation (18). These cytokines will also cause up regulation of NF-kβ (18).
Periradicular inflammation is for most part a reactionary response caused primarily by microorganisms and/or their by-products in the root canal system (19). To date, there are no reported data regarding the existence of RAGE in endodontic periradicular lesions. Therefore, the purpose of this study was to investigate the presence or absence of RAGE expression in periradicular inflammatory lesions of endodontic origin and to examine its relationship with NF-kβ expression.
In part I of this study we correlated the histological degree of inflammation with the presence of RAGE mRNA. The second part of this study was conducted to evaluate the correlation between the expression of RAGE mRNA with NF-kβ expression, a known marker of inflammation.
Material and Methods
Sampling of human tissue was approved by the University of Florida (UFL) Health Science Center Internal Review Board (IRB) and informed consent was obtained from each patient.
PART I
Part one of this study was conducted to examine the presence of RAGE and NF-kβ mRNA in apical tissue lesions of previous endodontically treated teeth. Fifteen patients with previous root canal treatment (RCT) and persistent periradicular lesions were identified and treatment planned for a root end resection in accordance with standard care procedures. Patients consisted of seven males and eight females, with an age range from 18−79 (mean age= 58). Immediately following the curettage of the periradicular lesion, half of the sample was submitted to the UF Oral Pathology department for histological analysis and grading of inflammation. The other half was immersed in RNAlater and stored at −80°C.
Histological Examination and Inflammation Grading
Specimens were fixed in 10% neutral buffered formalin, dehydrated in a series of ethanol washes, embedded in paraffin, sectioned, and stained with Hematoxylin and Eosin according to standard protocol. Histological examination of four sections per specimens were conducted by two calibrated oral pathologists and the degree of inflammation (mild, moderate, or severe) was recorded. The control group was classified as non-inflamed. Criteria for each classification of inflammation were as follows:
1 – Mild: Patchy areas of inflammation consisting of a infiltrate (primarily of lymphocytes and plasma cells) interspersed by uniflamed areas of dense fibrous tissue. Very few areas of edema and hyperemia (1−33% of field).
2 –Moderate: Equal areas of inflammation consisting of a mixed infiltrate and uninflammed fibrous tissue. Evidence of edema and hyperemia (35−65% of field)
3 – Severe: Entire tissue section exhibits intense inflammation consisting of a mixed infiltrate (primarily of neutrophils) and heavy zones of edema and hyperemia.(67−100% of field)
PART II
The presence of mRNA RAGE and NF-kβ in periradicular lesions from teeth without endodontic treatment. Periradicular tissue was obtained at the time of extraction from 32 patients that presented with periradicular lesions. Patients consisted of 22 females and 10 males with an age range from 18−75 (mean age= 40). A control group was represented by periodontal ligament samples obtained from the roots of two teeth extracted for orthodontic reasons and five follicles of unerupted 3rd molars. Immediately following extraction the attached tissues were curetted off the root and immersed in RNAlater and stored at −80°C.
RT-PCR
All tissue specimens were thawed and incubated on ice during the RNA extraction process. Specimens were placed into individual micro-centrifuge tubes and total RNA was extracted using the RNeasy Mini Kit (Qiagen) according to manufacturers instructions. Tissue was disrupted by rotor-stator homogenization DNA digestion was performed utilizing the RNase-Free DNase kit (Qiagen). RNA concentration for each sample was estimated using the Biophotometer 6131 (V1.32) (absorbance at 260nm). Concentrations less than 34ng/μl were discarded. Commercially available primers for RAGE II and NF-kβ were used (Geno-Mechanix). Primers for 18s RNA (Sigma-Genosys) were used as control (Table 1). RT-PCR reactions consisted of the following: 5mM of AMV/Tfl, 10mM dNTP, 25mM MgSO4, 50pMol forward and reverse primer, 2.5u AMVRT, 2.5u Tfl DNA, and 350ng isolated RNA for a total volume of 25ul. A negative control containing RNA-free distilled water instead of RNA was included. Reverse transcription was conducted for 45 minutes at 48°C. AMV RT inactivation and denaturation for 2 minutes at 94°C. Reactions then underwent 35 cycles at 94°C for 30 minutes, 63.5°C for 1 minute, and 72°C for 2 minutes. The solutions then underwent final extension at 72°C for 7 minutes. RT-PCR products were separated by 1.2% agarose gel electrophoresis in TBE buffer for 55 minutes at 100V. RT-PCR products were imaged under UV light and densitometrically analyzed using Scion Image for Windows Beta 4.0.2 computer software.
Table 1.
Primer Sequences
| Assay | Base pairs | Primer Sequences |
|---|---|---|
| 81sRNA | 63 | Forward: TCTTGGCAAATGCTTTCGCT Reverse: CGCCGCTAGAGGTGAAATTC |
| RAGE II | 323 | Forward: GACTCTTAGCTGGCACTTGGAT Reverse: GGACTTCACAGGTCAGGGTTAC |
| NF-κβ | 484 | Forward: GATCAATGGCTACACAGGACCA Reverse: GTCCTCTTTCTGCACCTTGTCA |
Statistical Analysis
Statistical analysis was conducted utilizing Statistical Package for the Social Sciences (SPSS). For part I of this study, multiple linear regression (MLR) analysis was used to determine if there were any correlations with the outcomes and the following patient variables: age, gender, smoking status, daily alcohol consumption, arthritis, high blood pressure, seasonal allergies, diabetes type II, pain, swelling, sinus tract, degree of inflammation, and size of lesion. Backwards entry selection was used to find association between groups of predictors and outcomes. Additionally, levels of RAGE, NF-kβ expression, and their ratios with 18s RNA control were examined for correlations. Data was reported as Correlation Coefficient (CC) and Significance (2-tailed). Part II of this study utilized MLR in addition to running an ANOVA to compare control vs. diseased and Caucasian vs. African-American groups (p< 0.05)
Results
Four outcomes of interest were obtained via the densitometric analysis: RAGE, NF-kβ, RAGE/18s RNA, NF-kβ /18s RNA.
Part I
4 samples were lost due to low concentrations of RNA. Agarose gels revealed bands for RAGE, NF-kβ, and 18s RNA. The negative control showed no appreciable band. Statistical analysis revealed a statistically significant positive correlation between banding intensity for RAGE and NF-kβ (Pearson Correlation= .691, p= .013). Individually, high blood pressure, presence of swelling, size of preoperative lesion, sinus tract, and degree of inflammation were observed to be the most positively correlated with RAGE expression, but none of statistical significance. The statistically significant models using Backwards Entry Selection are listed in Table 1. The correlation of inflammation and RAGE are illustrated on Table 2.
Table 2.
Backward Entry Selection Data
| |
NF-kB/18s RNA |
RAGE/18s RNA |
NF-kB |
RAGE |
|---|---|---|---|---|
| Diabetic type II β= −.562 |
HBP β= .536 |
Allergies β= −.956 |
HBP β= .614 |
|
| Predictor | Sinus Tract β= −.763 |
Size of Lesion β= .654 |
Diabetic type II β= −.384 |
Arthritis β= .503 |
| |
|
|
Degree of Inflammation β= .597 |
Degree of Inflammation β= .591 |
| Sig. | 0.025 | 0.007 | 0.003 | 0.1 |
The Pearson correlation for the degree of inflammation and RAGE mRNA was 0.351 with a significance of 0.29. The Pearson correlation for NF-kβ was 0.311 with a significance of 0.351. Therefore, there was a moderate positively correlation with the amount of mRNA for RAGE and NF-kβ , but not statistically significant.
Part II: Five samples had inadequate concentrations of RNA and were discarded. Statistical analysis of the remaining samples revealed the same statistical positive correlation between RAGE and NF-kβ (Table 3). All independent factors showed no strong linear correlations. The diseased tissue group showed statistically significant higher densitometric values compared to control for RAGE (F=8.443, p= .008) and NF-kβ (F= 10.568, p= .003); see Chart 1.
Table 3.
Part II: Outcome comparison
| NF-kB / 18s RNA | RAGE / 18s RNA | NF-kB Level | ||
|---|---|---|---|---|
| RAGE Level | Pearson Correlation | −0.283 | −0.102 | 0.657 |
| Sig. (2-tailed) | 0.215 | 0.658 | 0.001 | |
| NF-kB Level | Pearson Correlation | −0.170 | −0.374 | |
| Sig. (2-tailed) | 0.461 | 0.095 | ||
| RAGE / 18s RNA | Pearson Correlation | 0.774 | ||
| Sig. (2-tailed) | 0.000 |
Chart 1. RAGE: control vs diseased.
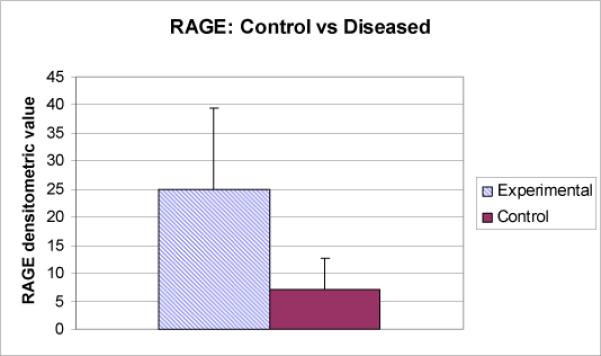
Diseased: Mean 24.955, SD 14.48
Control: Mean 7.228, SD 5.427
F= 8.443
p= 0.008
The “healthy densitometric reading for the Hispanic samples were 9.15 and 14.42; the Caucasians were 0.78,0.86 and 7.88. The reading for the African-American was 10.28.
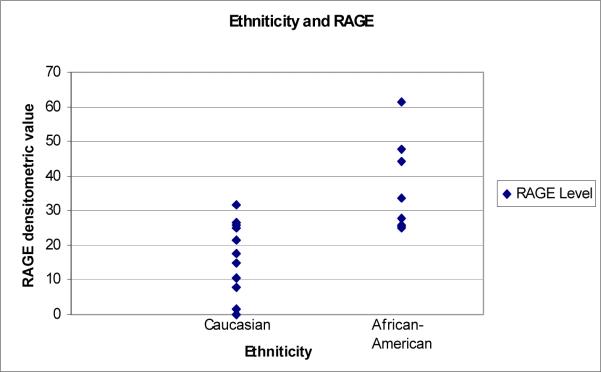
The “diseased” group consisted of eight African-Americans and the reminder thirteen were Caucasians. The densitometric readings for the Caucasians were 0.00−31.57 with a mean value of 17.93. The readings for the African –American group was 25−61.53 with a mean value of 36.37. Comparing Caucasians and African-Americans using ANOVA, a statistically significant difference was noted with the African-American group showing a higher expression of RAGE mRNA (F= 12.746, p=0.002); see Image 1 and Chart 2.
Image 1.

RAGE
Chart 2.
Caucasian: Mean 17.930, SD 10.173
African-American: Mean 36.371, SD 13.463
F= 12.746
p= 0.002
Discussion
In part I of this study the relationship between the degree of inflammation with the outcomes was evaluated. The sample size utilized was too small for any significant linear relationships to be revealed between the patient variables and outcomes. We had selected general guidelines for gauging inflammation instead of counting specific cell types, when counting may had been more meaningful. Size of lesion and grading of inflammation were not accomplished in part II due to the facts that panoramic radiographs were used and no biopsies were taken for extractions.
The results of this experiment qualitatively show the presence of RAGE mRNA in periapical tissues and a marked higher expression in inflamed lesions of endodontic origin as compared to healthy periradicular tissues. In addition, the expression of NF-kβ was shown to be positively correlated with the expression of RAGE.
Bergstrom et al (2004) found a positive association between chronic smoking and the amount of periodontal pocketing and alveolar bone loss. The same researchers found no associations between chronic smoking and periradicular health or RCT teeth. Additionally, Marending et al (2005) did not find any association for smoking and failure to heal after RCT. In this study, we did not find any association with chronic smokers and the expression of RAGE or NF-kβ.
NF-kβ expression is essential for RANK expressing osteoclast precursors to differentiate (18). Sabeti et al (2005), has shown the presence of the receptor activator for NF-kβ (RANKL) in periapical lesions. Zhang and Peng (2005) were able to show in a rat model that the levels of RANKL positive cells in periapical lesions were highest at 14 days after the pulpal insult (21). Lee et al (2005) found that the major surface proteins of Treponema maltophilum and Treponema lecithinolyticum increased NF-kβ activation which thus increased the expression of intercellular adhesion molecule 1 (ICAM-1) along with IL-1B, IL-6, and IL-8. RAGE activation has also been shown to activate NF-kβ (20, 16).
The expression of RANKL has also shown an active role in osteoclast formation in compressed synovial cells,while continuous compressive force induced osteoclastic bone destruction in the TMJ (22).
Estrogen deficiency–induced severe periapical bone resorption which might be mediated by overexpression of RANKL in the lesion. Also, an early stage of estrogen deficiency induced an accelerated osteoblastogenesis/antiosteoclastogenesis probably associated with a resistant response to bone resorption (23).
Increased RAGE levels have been detected in the synovial tissues of patients with inflammatory joint diseases (7). AGE/RAGE interactions stimulate the release of inflammatory cytokines such as IL-1, IL-2, and TNF-α (6, 15). These interactions also stimulate increased levels of growth factors (TGF-B, CTGF, VEGF) (6) and modulate the expression of adhesive and prothrombotic molecules of fibroblasts (15).
In addition, RAGE and its role in diabetes has aggressively studied. Poor healing of the dental pulp and periradicular tissues in diabetic patients is potentially caused by diminished circulation and impaired neutrophils ( 1 ). AGEs not only cause pathologic changes to the vascular system, but also cause inhibition of killing by neutrophils (5).
Our study is of interest as it may shed light on mechanisms related to healing of endodontic lesions, currently we went a step further and with a larger sample size are investigating potential differences between diabetic and non-diabetic patients.
Conclusion
Within the parameters of this study there is a higher level of RAGE mRNA expression in periapical lesions of endodontic origin as compared to healthy periradicular tissues.
Expression of NF-kβ mRNA was shown to be positively correlated with expression of RAGE mRNA.
African-Americans have higher levels of expression of RAGE mRNA in periradicular lesions of endodontic origin than Caucasians.
RAGE mRNA levels may be a potential marker for periapical inflammation
References
- 1.Bender IB, Bender AB. Diabetes Mellitus and the Dental Pulp. J Endod. 2003;29:383–388. doi: 10.1097/00004770-200306000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Bergstrom J, Babcan J, Eliasson S. Tobacco smoking and dental periapical condition. Eur J Oral Sci. 2004;112:115–120. doi: 10.1111/j.1600-0722.2004.00112.x. [DOI] [PubMed] [Google Scholar]
- 3.Burke A, Kolodgie F, Zieske A, Fowler D, Weber D, Varghese J, Farb A, Virmani R. Morphologic Findings of Coronary Atherosclerotic Plaques in Diabetics: A Postmortem Study. Arterioscler Thromb Vasc Biol. 2004;24:1266–1271. doi: 10.1161/01.ATV.0000131783.74034.97. [DOI] [PubMed] [Google Scholar]
- 4.Cerami C, Founds H, Nicholl I, Mitsuhashi T, Giordano D, Vanpatten S, Lee A, Al-Abeb Y, Vlassara H, Bucala R, Cerami A. Tobacco smoke is a source of toxic reactive glycation products. Proc. Natl. Acad. Sci. USA. 1997;94:13915–13920. doi: 10.1073/pnas.94.25.13915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collinson K, Parhar R, Saleh S, Meyer B, Kwaasi A, Hammami M, Schmidt A, Stern D, Al-Mohanna F. RAGE-mediated neutrophil dysfunction is evoked by advanced glycation end products (AGEs). J of Leukocyte Biol. 2002;71:433–444. [PubMed] [Google Scholar]
- 6.Cooper M. Importance of Advanced Glycation End Products in Diabetes-Associated Cardiovascular and Renal Disease. Am J of Hypert. 2004;17:31S–38S. doi: 10.1016/j.amjhyper.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 7.Drinda S, Franke S, Ruster M, Petrow P, Pulling O, Stein G, Hein G. Identification of the receptor for advanced glycation end products in synovial tissue of patients with rheumatoid arthritis. Rheumatol Int. 2005;25:411–413. doi: 10.1007/s00296-004-0456-y. [DOI] [PubMed] [Google Scholar]
- 8.Feng L, Matsumoto C, Schwartz A, Schmidt A, Stern D, Pile-Spellman J. Chronic Vascular Inflammation in Patients With Type 2 Diabetes. Diabetes Care. 2005;28:379–384. doi: 10.2337/diacare.28.2.379. [DOI] [PubMed] [Google Scholar]
- 9.Hudson B, Bucciarelli L, Wendt T, Sakaguchi T, Lalla E, Qu W, Lu Y, Lee L, Stern D, Naka Y, Ramasamy R, Yan S, Yan S, D'Agati V, Schmidt A. Blockade of receptor for advanced glycation endproducts: a new targer for therapeutic intervention in diabetic complications and inflammatory disorders. Arch of Biochem and Biophysics. 2003;419:80–88. doi: 10.1016/j.abb.2003.08.030. [DOI] [PubMed] [Google Scholar]
- 10.Ichikawa K, Yoshinari M, Iwase M, Wakisaka M, Doi Y, Iino K, Yamamoto M, Fujishima M. vanced glycosylation end products induced tissue factor expression in human monocyte-like U937 cells and increased tissue factor expression in monocytes from diabetic patients. Atherosclerosis. 1998;136:281–287. doi: 10.1016/s0021-9150(97)00221-9. [DOI] [PubMed] [Google Scholar]
- 11.Katz J, Bhattacharyya I, Farkhondeh-Kish F, Perez F, Caudle R, Heft M. Expression of the receptor of advanced glycation end products in gingival tissues of type 2 diabetes patients with chronic periodontal disease: a study utilizing immunohistochemistry and RT-PCR. J Clin Periodontol. 2005;32:40–44. doi: 10.1111/j.1600-051X.2004.00623.x. [DOI] [PubMed] [Google Scholar]
- 12.Lee SH, Kim KK, Choi BK. Upregulation of intercellular adhesion molecule 1 and proinflammatory cytokines by the major surface protein of Treponema maltophilum and Treponema lecithinolyticum, the phylogenetic group IV oral spirochetes associated with periodontitis and endodontic infections. Infect Immun. 2005 Jan;73:268–276. doi: 10.1128/IAI.73.1.268-276.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marending M, Peters O, Zehnder M. Factors affecting the outcome of orthograde root canal therapy in a general dentistry hospital practice. Oral Surg Oral Med Oral Pathol and Endodon. 2005;99:119–124. doi: 10.1016/j.tripleo.2004.06.065. [DOI] [PubMed] [Google Scholar]
- 14.Pullerits R, Bokarewa M, Dahlberg L, Tarkowski A. Decreased levels of soluble receptor for advanced glycation end products in patients with rheumatoid arthritis indicating deficient inflammatory control. Arthritis Res Ther. 2005;7:R817–24. doi: 10.1186/ar1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramasamy R, Vannucci S, Yan S, Herold K, Yan S, Schmidt A. Advanced glycation end products and RAGE: a common thread in aging, diabetes, neurodegeneration, and inflammation. Glycobiology. 2005;15:16R–28R. doi: 10.1093/glycob/cwi053. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez-Ayala E, Anderstam B, Suliman M, Seeberger A, Heimburger O, Lindholm B, Stenvinkel P. Enhanced RAGE-mediated NFkB stimulation in inflamed hemodialysis patients. Atherosclerosis. 2005;180:333–340. doi: 10.1016/j.atherosclerosis.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 17.Sabeti M, Kermani S, Valles Y, Rostein I. Detection of Receptor Activator of NF-kB Ligand in Apical Periodontitis. J of Endod. 2002;31:17–18. doi: 10.1097/01.don.0000134210.95473.a7. [DOI] [PubMed] [Google Scholar]
- 18.Xing L, Bushnell TP, Carlson L, Tai Z, Tondravi M, Siebenlist U, Young F, Boyce BF. NF-kappaB p50 and p52 expression is not required for RANK-expressing osteoclast progenitor formation but is essential for RANK- and cytokine-mediated osteoclastogenesis. J Bone Miner Res. 2002;17:1200–10. doi: 10.1359/jbmr.2002.17.7.1200. [DOI] [PubMed] [Google Scholar]
- 19.Möller AJ, Fabricius L, Dahlén G, Ohman AE, Heyden G. Influence on periapical tissues of indigenous oral bacteria and necrotic pulp tissue in monkeys. Scand J Dent Res. 1981;89:475–484. doi: 10.1111/j.1600-0722.1981.tb01711.x. [DOI] [PubMed] [Google Scholar]
- 20.Yan S, Ramasamy R, Naka Y, Schmidt A. Glycation, Inflammation, and RAGE A Scaffold for the Macrovascular Complications of Diabetes and Beyond. Circ Res. 2003;93:1159–1169. doi: 10.1161/01.RES.0000103862.26506.3D. [DOI] [PubMed] [Google Scholar]
- 21.Zhang X, Peng B. Immunolocalization of Receptor Activator of NF Kappa B Ligand in Rat Periapical Lesions. J of Endod. 2005;31:574–577. doi: 10.1097/01.don.0000153839.74546.66. [DOI] [PubMed] [Google Scholar]
- 22.Ichimiya H, Takahashi T, Ariyoshi W, Takano H, Matayoshi T, Nishihara T. Compressive mechanical stress promotes osteoclast formation through RANKL expression on synovial cells. Oral Surg Oral Med Oral Pathol and Endodon. 2007;(103):334–341. doi: 10.1016/j.tripleo.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 23.Zhang X, Peng B, Fan M, Bian Z, Chen Z. The Effect of Estrogen Deficiency on Receptor Activator of Nuclear Factor Kappa B Ligand and Osteoprotegerin Synthesis in Periapical Lesions Induced in Rats. Journal of Endod. 2007;33:1053–1056. doi: 10.1016/j.joen.2007.04.005. [DOI] [PubMed] [Google Scholar]