Abstract
Prospective data relating cardiorespiratory fitness (CRF) with nonfatal cardiovascular disease (CVD) events are limited to studies in men or studies of combined fatal and nonfatal CVD endpoints. The authors examined the association between CRF and nonfatal CVD events in 20,728 men and 5,909 women without CVD at baseline. All participants performed a maximal treadmill exercise test and completed a follow-up health survey in the Aerobics Center Longitudinal Study (Dallas, Texas) between 1971 and 2004. There were 1,512 events in men and 159 events in women during an average follow-up of 10 years. Across incremental CRF groups, age- and examination year-adjusted event rates per 10,000 person-years were 107.9, 75.2, and 50.3 in men (p trend <0.001) and 41.9, 27.7, and 20.8 in women (p trend = 0.002). After further adjustment for smoking, alcohol intake, family history of CVD, and abnormal exercise ECG responses, hazard ratios (95% confidence interval) were 1.00 (referent), 0.82 (0.72, 0.94), and 0.61 (0.53, 0.71) in men, p trend <0.001, and were 1.00 (referent), 0.74 (0.49, 1.13), and 0.63 (0.40, 0.98) in women, p trend = 0.05. After adjustment for other CVD predictors, the association remained significant in men but not in women.
Keywords: exercise, cardiovascular diseases, stroke, women, primary prevention
Cardiovascular disease (CVD) continues to exact a large economic and public health toll in the United States, accounting for nearly 1 million deaths and 6 million hospitalizations in 2003 (1). Physical inactivity is a major modifiable CVD risk factor (2) that is associated with increased risk of fatal and nonfatal CVD events in women and men (3-10). Cardiorespiratory fitness (CRF) is an objective reproducible physiological measure that reflects the functional influences of physical activity habits, genetics, and disease status. Because CRF is less prone to misclassification, it may better reflect the adverse health consequences of a sedentary lifestyle than do self-reported physical activity exposures (11).
CRF is inversely associated with CVD mortality in adults (12-16). Few prospective studies have reported on CRF and nonfatal CVD risk and those that have are limited to studies in men or to combined nonfatal/fatal endpoints (15, 17-21). Although it may be intuitive to expect that CRF would confer protection against nonfatal CVD events in women and men as is seen for fatal CVD, this conclusion can not accurately be drawn from studies of combined nonfatal/fatal events or studies only in men. We examined the prospective association between CRF and nonfatal CVD in women and men in the Aerobics Center Longitudinal Study (ACLS).
MATERIALS AND METHODS
Study population
Participants were 20,728 men and 5,909 women ages 18 to 83 years who completed a baseline examination at the Cooper Clinic (Dallas, TX) during 1971 - 2001. At baseline, all participants were free of known CVD, had normal resting electrocardiograms, and were able to complete an exercise stress test to at least 85 percent of their age-predicted maximal heart rate. All participants responded to at least one mail-back health survey during follow-up. Most participants were Caucasian and from middle and upper socioeconomic strata. Participants provided written consent to participate in the follow-up study.
Baseline examination
The physician examination and clinical measurements were completed after an overnight fast of at least 12 hours (12, 13). Body mass index (BMI, kg/m2) was computed from measured height and weight. After a brief period of quiet sitting, blood pressure was recorded as the first and fifth Korotkof sounds using auscultation methods (22). Serum samples were analyzed for lipids and glucose using standardized automated bioassays. The presence of hypertension, diabetes, and dyslipidemia was based on a history of physician diagnosis or measured phenotypes that met clinical thresholds for each condition. Information on smoking habits (current smoker or not), alcohol intake (drinks per week), and physical activity habits (sedentary or active) was obtained from a questionnaire.
CRF was quantified as the duration of a symptom-limited maximal treadmill exercise test using a modified Balke protocol (12, 23). Exercise duration on this protocol is highly correlated with measured maximal oxygen uptake (r > 0.90) (24, 25). The test endpoint was volitional exhaustion or termination by the supervising physician. The mean (SD) percentage of age-predicted maximal heart rate achieved during exercise was 100.3 (7.0) in women and was 101.2 (7.0) in men. Maximal metabolic equivalents (METs, 1 MET = 3.5 ml O2 uptake·kg-1·min-1) were estimated from the final treadmill speed and grade (26). In previous ACLS reports that have shown low CRF is an independent predictor of mortality and nonfatal disease (12, 13, 27), we have defined low, moderate, and high CRF exposures according to the lowest 20 percent, the middle and upper 40 percent, respectively, of the age and sex-specific distribution of treadmill duration in the overall ACLS population (table 1). To maintain consistency in our study methods and because a widely accepted clinical categorization of CRF does not exist, we used the above approach. CRF by this definition was positively associated with reported physical activity status. The percentage of participants classified as being physically active in the low, moderate, and high CRF groups was 28.8, 54.9, and 86.8 in men and was 33.5, 59.5, and 86.7 in women (p trend <0.001, each). Abnormal exercise electrocardiogram (ECG) responses were broadly defined as rhythm and conduction disturbances and ischemic ST-T wave abnormalities as described in detail elsewhere (28). We have found 90 percent agreement between the ECG interpretation recorded in our database and that of a group of three physicians who read a random sample of 357 patient records (28).
TABLE 1.
Age- and sex-specific maximal treadmill exercise duration and estimated MET levels of cardiorespiratory fitness, Aerobics Center Longitudinal Study, Dallas, Texas, 1971-2004
| Men | Women | ||||
|---|---|---|---|---|---|
| Age (years) | Quintile | Duration (min) | METs† | Duration (min) | METs† |
| 20-39 | 1 | <15.0 | <10.4 | <10.3 | <8.2 |
| 2 | 15.0≤min<18.0 | 10.4≤METs<11.7 | 10.3≤min<13.0 | 8.2≤METs<9.4 | |
| 3 | 18.0≤min<20.3 | 11.7≤METs<13.1 | 13.0≤min<15.0 | 9.4≤METs<10.4 | |
| 4 | 20.3≤min≤23.6 | 13.1≤METs≤14.4 | 15.0≤min≤18.0 | 10.4≤METs≤11.7 | |
| 5 | >23.6 | >14.4 | >18.0 | >11.7 | |
| 40-49 | 1 | <13.5 | <9.9 | <8.9 | <7.6 |
| 2 | 13.5≤min<16.1 | 9.9≤METs<10.8 | 8.9≤min<11.0 | 7.6≤METs<8.5 | |
| 3 | 16.1≤min<19.0 | 10.8≤METs<12.2 | 11.0≤min<13.0 | 8.5≤METs<9.4 | |
| 4 | 19.0≤min≤22.0 | 12.2≤METs≤13.5 | 13.0≤min≤16.0 | 9.4≤METs≤10.8 | |
| 5 | >22.0 | >13.5 | >16.0 | >10.8 | |
| 50-59 | 1 | <11.0 | <8.5 | <7.0 | <6.7 |
| 2 | 11.0≤min<13.3 | 8.5≤METs<9.9 | 7.0≤min<9.0 | 6.7≤METs<7.6 | |
| 3 | 13.3≤min<16.0 | 9.9≤METs<10.8 | 9.0≤min<10.7 | 7.6≤METs<8.5 | |
| 4 | 16.0≤min≤19.2 | 10.8≤METs≤12.3 | 10.7≤min≤13.2 | 8.5≤METs≤9.6 | |
| 5 | >19.2 | >12.3 | >13.2 | >9.6 | |
| ≥60 | 1 | <7.8 | <7.2 | <5.5 | <5.8 |
| 2 | 7.8≤min<10.5 | 7.2≤METs<8.5 | 5.5≤min<7.0 | 5.8≤METs<6.7 | |
| 3 | 10.5≤min<13.1 | 8.5≤METs<9.5 | 7.0≤min<9.0 | 6.7≤METs<7.6 | |
| 4 | 13.1≤min≤16.4 | 9.5≤METs≤10.8 | 9.0≤min≤11.3 | 7.6≤METs≤8.6 | |
| 5 | >16.4 | >10.8 | >11.3 | >8.6 | |
Treadmill exercise testing was performed using a modified Balke-Ware protocol as described in the Methods section. Low fitness: Quintile 1; Moderate fitness: Quintile 2 and 3; High fitness: Quintile 4 and 5. Among participants in the current analysis, the distribution of low, moderate and high fitness by the above definition was 19%, 40% and 41% in men, and was 15%, 35% and 50% in women.
METs, metabolic equivalents; 1 MET = 3.5 ml O2 uptake·kg-1·min-1
Assessment of outcomes
CVD events were ascertained from responses to mail-back health surveys in 1982, 1999, and 2004. The aggregate survey response rate across all survey periods in the ACLS is ≈ 65 percent. Nonresponse bias is a concern in epidemiological surveillance and this issue has been investigated in the ACLS (29). Baseline health histories and clinical measures were similar between responders and nonresponders and between early and late responders (29). Total mortality rates also have been similar between responders and nonresponders (unpublished data). CVD endpoints were defined as a physician diagnosis of myocardial infarction (MI) or stroke or a coronary revascularization procedure (coronary artery bypass graft or percutaneous coronary intervention). In participants reporting multiple events, the first event was used for analysis. The primary outcome was all CVD events. Secondary outcomes were coronary heart disease (CHD) events (MI, coronary revascularization) and MI and stroke as separate endpoints. In a random sample of these endpoints (n = 50 each), we applied a standard definition for defining and adjudicating MI, revascularization, and stroke (30, 31). The percentage of agreement between reported events and participant medical records was 88 percent, 100 percent and 89 percent for MI, revascularization, and stroke, respectively.
Statistical analysis
Follow-up time among noncases was computed as the difference between the date of the baseline examination and the date of the last returned survey where the participant reported being free of CVD. Follow-up time among cases was computed as the difference between the baseline examination date and the reported date of the CVD event. If a diagnosis date was not provided, we used the midpoint between the date of the case-finding survey and either the baseline examination date or the date of the last returned survey where the participant reported being free of CVD. The mean (SD) follow-up interval in years was 10.4 (8.1) for men and 10.2 (7.8) for women. Cox proportional hazards regression analysis was used to estimate hazard ratios (HRs) and 95 percent confidence intervals of CVD events according to exposure categories. Multivariable analyses included 6 covariables: age (years), examination year, current smoker (yes/no), alcohol intake (≥5 drinks/wk or not), abnormal exercise ECG responses (present or not), and family history of CVD (present or not). We conducted additional analyses that further adjusted for baseline differences in the following 4 factors that may be intermediate in the causal pathway between CRF and CVD: BMI (<25 vs. ≥25 kg/m2), hypertension, diabetes, and dyslipidemia (present or not for each); although authors debate whether or not an exposure-outcome relationship should be adjusted for biological intermediates (32). To reduce the influence of ascertainment bias due to variable survey response patterns, analyses were stratified on survey year using the STRATA statement in Proc PHREG (SAS v9.1, SAS Institute, Cary, NC). Tests of linear trends across exposure categories were computed using ordinal scoring. The proportional hazards assumption was examined by comparing the cumulative hazard plots grouped on exposure; no appreciable violations were noted. The potential influence of undetected subclinical disease at baseline was evaluated by excluding events that occurred during the first year of follow-up; little change was noted. All p values are 2-sided and p < 0.05 was regarded as statistically significant.
RESULTS
There were 1,512 CVD events (489 MI, 290 strokes, 733 revascularizations) during 215,984 man-years of exposure and 159 CVD events (53 MI, 62 strokes, 44 revascularizations) during 60,158 woman-years of exposure. Compared with noncases, individuals who developed CVD were older, had lower CRF, and had higher prevalence of sedentary habits and other major CVD risk factors (table 2).
TABLE 2.
Baseline characteristics of study participants by sex and cardiovascular disease event status, Aerobics Center Longitudinal Study, 1971-2004
| Men | Women | |||
|---|---|---|---|---|
| Characteristic | Noncase (n = 19,216) (mean (SD) or %) | Case (n = 1,512) (mean (SD) or %) | Noncase (n = 5,750) (mean (SD) or %) | Case (n = 159) (mean (SD) or %) |
| Age (years) | 43.9 (9.6) | 50.3 (8.7) | 44.4 (10.2) | 52.3 (10.0) |
| Body mass index (kg/m2) | 26.1 (3.6) | 26.4 (3.3) | 23.0 (3.8) | 23.6 (3.5) |
| Treadmill time (minutes) | 17.6 (5.0) | 15.4 (4.8) | 13.1 (4.6) | 10.5 (4.1) |
| Maximal METs | 11.5 (2.5) | 10.5 (2.3) | 9.4 (2.1) | 8.2 (1.9) |
| Lipids (mmol/L) | ||||
| Total cholesterol | 5.4 (1.0) | 5.9 (1.0) | 5.2 (1.0) | 5.7 (1.0) |
| HDL cholesterol | 1.2 (0.3) | 1.1 (0.3) | 1.6 (0.4) | 1.6 (0.4) |
| Triglycerides | 1.5 (1.2) | 1.8 (1.3) | 1.1 (0.8) | 1.3 (0.8) |
| Fasting blood glucose (mmol/L) | 5.6 (0.9) | 5.8 (1.4) | 5.2 (0.7) | 5.5 (1.2) |
| Blood pressure (mmHg) | ||||
| Systolic | 121.7 (13.7) | 126.5 (14.9) | 113.4 (14.5) | 121.7 (15.6) |
| Diastolic | 81.0 (9.7) | 83.4 (9.7) | 75.7 (9.5) | 80.2 (9.5) |
| Sedentary (%) | 36.7 | 42.1 | 30.9 | 34.1 |
| Current smoker (%) | 18.3 | 19.4 | 9.2 | 14.5 |
| Alcohol intake (≥5 drinks/wk) † (%) | 40.6 | 39.3 | 18.9 | 24.5 |
| Abnormal exercise ECG (%) | 4.4 | 14.6 | 4.9 | 13.2 |
| Hypertension ‡ (%) | 30.7 | 45.0 | 17.4 | 40.9 |
| Diabetes mellitus§ (%) | 4.9 | 9.2 | 3.0 | 4.4 |
| Hypercholesterolemia # (%) | 18.5 | 34.1 | 13.5 | 25.2 |
| Hypercholesterolemia # (%) | 14.7 | 21.5 | 4.5 | 11.3 |
| Low HDL cholesterol # (%) | 54.5 | 68.1 | 25.9 | 40.3 |
| Dyslipidemia # (%) | 82.7 | 91.9 | 52.6 | 70.4 |
| Family history of CVD (%) | 15.8 | 18.5 | 18.5 | 17.0 |
SD, standard deviation; METs, maximal metabolic equivalents; HDL cholesterol, high density lipoprotein cholesterol; ECG, electrocardiogram; CVD, cardiovascular disease
One unit of alcohol is defined as 12 ounces (3.41 dL) of beer, 5 ounces (1.421 dL) of wine, or 1.5 ounces (0.4262 dL) of hard liquor.
Hypertension is defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or a history of physician diagnosis
Diabetes mellitus is defined as a fasting plasma glucose concentration ≥7.0 mmol/L (126 mg/dL), a history of physician diagnosis, or insulin use.
Hypercholesterolemia is defined as total cholesterol ≥6.20 mmol/L (240 mg/dL) or a history of physician diagnosis.
Hypertriglyceridemia is defined as triglyceride ≥2.26 mmol/L (200 mg/dL).
Low HDL cholesterol is defined as <1.03 mmol/L (40 mg/dL).
Dyslipidemia is defined as the presence of one or more of the above lipid abnormalities.
An inverse gradient (p trend < 0.001) of total CVD event rates was observed across CRF groups in men (table 3). After adjusting for covariables, men with moderate and high CRF had an 18 percent and 39 percent lower CVD risk than men with low CRF (p trend < 0.001). The inverse association remained significant after additional adjustment for BMI, hypertension, diabetes, and dyslipidemia (p trend < 0.001). Similar inverse patterns of association were observed between CRF and each secondary outcome.
TABLE 3.
Rates and hazard ratios for cardiovascular disease events by CRF groups in men, Aerobics Center Longitudinal Study, Dallas, Texas, 1971-2004
| Events | Rate† | HR‡ | 95% CI‡ | HR§ | 95% CI§ | |
|---|---|---|---|---|---|---|
| Total CVD | ||||||
| Low CRF | 345 | 107.9 | 1.00 | Referent | 1.00 | Referent |
| Moderate CRF | 664 | 75.2 | 0.82 | 0.72, 0.94 | 0.89 | 0.78, 1.02 |
| High CRF | 503 | 50.3 | 0.61 | 0.53, 0.71 | 0.75 | 0.64, 0.87 |
| p linear trend | <0.001 | <0.001 | <0.001 | |||
| Coronary heart disease | ||||||
| Low CRF | 289 | 88.9 | 1.00 | Referent | 1.00 | Referent |
| Moderate CRF | 533 | 60.5 | 0.81 | 0.70, 0.94 | 0.89 | 0.77, 1.03 |
| High CRF | 400 | 40.3 | 0.61 | 0.52, 0.71 | 0.76 | 0.64, 0.90 |
| p linear trend | <0.001 | <0.001 | 0.001 | |||
| Myocardial infarction | ||||||
| Low CRF | 123 | 35.6 | 1.00 | Referent | 1.00 | Referent |
| Moderate CRF | 212 | 24.1 | 0.80 | 0.64, 1.01 | 0.87 | 0.69, 1.09 |
| High CRF | 154 | 16.2 | 0.60 | 0.46, 0.77 | 0.73 | 0.56, 0.96 |
| p linear trend | <0.001 | <0.001 | 0.02 | |||
| Stroke | ||||||
| Low CRF | 56 | 19.0 | 1.00 | Referent | 1.00 | Referent |
| Moderate CRF | 131 | 14.8 | 0.86 | 0.63, 1.18 | 0.90 | 0.65, 1.24 |
| High CRF | 103 | 10.0 | 0.63 | 0.45, 0.89 | 0.71 | 0.49, 1.01 |
| p linear trend | <0.001 | 0.005 | 0.04 | |||
HR, Hazard ratio; CI, Confidence interval; CVD, cardiovascular disease; CRF, cardiorespiratory fitness.
There were 66,887, 70,222, and 78,872 man-years of follow-up in the low, moderate, and high fitness groups, respectively.
Rate per 10,000 person-years adjusted for age and examination year.
Adjusted for the above plus current smoking (yes or not), alcohol intake (≥5 drinks/wk, or not), family history of CVD (present or not), and abnormal exercise electrocardiogram responses (present or not).
Adjusted for the above plus body mass index (<25 or ≥25 kg/m2), hypertension, diabetes, or dyslipidemia (present or not for each).
In women (table 4), total CVD event rates were inversely associated with CRF (p trend = 0.002). After adjustment for covariables, women with moderate and high CRF had a 26 percent and 37 percent lower risk of CVD events than women with low CRF (p trend = 0.05). CRF remained inversely associated with CVD risk after additional adjustment for intermediate risk factors, although the trend was not significant (p trend =0.30). CRF was inversely associated with CHD event rates (p trend = 0.004); however, significance was attenuated by adjustment for covariables (p trend =0.09) and intermediate risk factors (p trend =0.49). Lower MI and stroke rates also were observed in women with moderate and high CRF, but these associations were not statistically significant.
TABLE 4.
Rates and hazard ratios for cardiovascular disease events by CRF groups in women, Aerobics Center Longitudinal Study, Dallas, Texas, 1971-2004
| Events | Rate† | HR‡ | 95% CI‡ | HR§ | 95% CI§ | |
|---|---|---|---|---|---|---|
| Total CVD | ||||||
| Low CRF | 35 | 41.9 | 1.00 | Referent | 1.00 | Referent |
| Moderate CRF | 63 | 27.7 | 0.74 | 0.49, 1.13 | 0.83 | 0.54, 1.28 |
| High CRF | 61 | 20.8 | 0.63 | 0.40, 0.98 | 0.78 | 0.49, 1.23 |
| p linear trend | 0.002 | 0.05 | 0.30 | |||
| Coronary heart disease | ||||||
| Low CRF | 22 | 26.6 | 1.00 | Referent | 1.00 | Referent |
| Moderate CRF | 40 | 17.7 | 0.79 | 0.47, 1.35 | 0.93 | 0.54, 1.60 |
| High CRF | 35 | 11.8 | 0.61 | 0.35, 1.09 | 0.82 | 0.45, 1.48 |
| p linear trend | 0.004 | 0.09 | 0.49 | |||
| Myocardial infarction | ||||||
| Low CRF | 12 | 13.7 | 1.00 | Referent | 1.00 | Referent |
| Moderate CRF | 24 | 10.5 | 0.92 | 0.45, 1.88 | 1.08 | 0.53, 2.22 |
| High CRF | 17 | 6.1 | 0.62 | 0.28, 1.36 | 0.81 | 0.36, 1.82 |
| p linear trend | 0.03 | 0.19 | 0.55 | |||
| Stroke | ||||||
| Low CRF | 13 | 15.3 | 1.00 | Referent | 1.00 | Referent |
| Moderate CRF | 23 | 10.0 | 0.65 | 0.33, 1.31 | 0.68 | 0.34, 1.38 |
| High CRF | 26 | 9.1 | 0.64 | 0.31, 1.30 | 0.69 | 0.33, 1.44 |
| p linear trend | 0.18 | 0.28 | 0.40 | |||
Abbreviations, †, ‡, and § are the same as in Table 3.
There were 19,808, 19,504, and 20,853 woman-years of follow-up in the low, moderate, and high fitness groups, respectively.
We also examined whether CRF predicted CVD events independent of reported physical activity status. Age and examination year adjusted rates of total CVD events (per 10,000 person-years) were inversely associated with physical activity status in men (sedentary = 78.4 vs. active = 64.8, p < 0.001) but not in women (sedentary = 22.3 vs. active =28.5, p = 0.20). After adjusting for age, examination year, and physical activity status, hazard ratios (95 percent confidence intervals) in the low, moderate, and high CRF groups were 1.00 (referent), 0.77 (0.68, 0.89), and 0.55 (0.47, 0.64), p trend < 0.001, in men; and were 1.00 (referent), 0.67 (0.44, 1.01), and 0.57 (0.33, 0.81), p trend = 0.005, in women. Results were similar for secondary outcomes.
We next examined whether other risk predictors modified the association between CRF and total CVD events (tables 5 and 6). In men, after adjusting for age and examination year, each 1-minute increment of maximal exercise was, on average, associated with a 3 to 9 percent (p < 0.05) lower CVD risk in each risk factor group, adverse or not. The consistency in the direction and magnitude of association between CRF and CVD suggested that there was little effect modification across risk factor categories. Further adjustment for the other risk factors eliminated some but not all of the associations. Results were similar for CHD events and for MI (data not shown). In women, the pattern of association between CRF and CVD risk was variable across risk factor groups and statistical power often was limited by a small number of events.
TABLE 5.
Hazard ratios for total CVD events per 1-minute increment in maximal exercise duration according to CVD risk factor categories in men, Aerobics Center Longitudinal Study, Dallas, Texas, 1971-2004
| Risk Factor | N | Events | HR‡ | 95% CI‡ | p value | HR§ | 95% CI§ | p value |
|---|---|---|---|---|---|---|---|---|
| Age, years | ||||||||
| <55 | 17,532 | 1,025 | 0.94 | 0.92, 0.95 | <0.001 | 0.95 | 0.94, 0.97 | <0.001 |
| ≥55 | 3,196 | 487 | 0.96 | 0.94, 0.98 | <0.001 | 0.98 | 0.96, 1.00 | 0.11 |
| Current smoker | ||||||||
| No | 16,922 | 1,218 | 0.95 | 0.94, 0.96 | <0.001 | 0.97 | 0.96, 0.99 | <0.001 |
| Yes | 3,806 | 294 | 0.93 | 0.90, 0.96 | <0.001 | 0.94 | 0.91, 0.96 | <0.001 |
| Family history of CVD | ||||||||
| No | 17,411 | 1,233 | 0.95 | 0.94, 0.96 | <0.001 | 0.97 | 0.96, 0.99 | <0.001 |
| Yes | 3,317 | 279 | 0.91 | 0.88, 0.94 | <0.001 | 0.93 | 0.90, 0.95 | <0.001 |
| Exercise ECG responses | ||||||||
| Normal | 19,667 | 1,292 | 0.95 | 0.94, 0.96 | <0.001 | 0.96 | 0.95, 0.98 | <0.001 |
| Abnormal | 1,061 | 220 | 0.96 | 0.93, 0.99 | 0.007 | 0.97 | 0.94, 1.01 | 0.12 |
| Body mass index, kg/m2 | ||||||||
| 18.5-24.9 | 8,701 | 573 | 0.94 | 0.92, 0.96 | <0.001 | 0.96 | 0.94, 0.98 | <0.001 |
| ≥25 | 12,027 | 939 | 0.95 | 0.94, 0.97 | <0.001 | 0.97 | 0.96, 0.99 | 0.001 |
| Hypertension | ||||||||
| No | 14,143 | 832 | 0.95 | 0.93, 0.96 | <0.001 | 0.96 | 0.95, 0.98 | <0.001 |
| Yes | 6,585 | 680 | 0.95 | 0.94, 0.97 | <0.001 | 0.97 | 0.95, 0.99 | <0.001 |
| Diabetes | ||||||||
| No | 19,653 | 1,373 | 0.95 | 0.93, 0.96 | <0.001 | 0.97 | 0.95, 0.98 | <0.001 |
| Yes | 1,075 | 139 | 0.96 | 0.93, 1.00 | 0.048 | 0.98 | 0.93, 1.02 | 0.27 |
| Total cholesterol | ||||||||
| <6.20 mmol/L (240 mg/dl) | 16,668 | 997 | 0.94 | 0.93, 0.95 | <0.001 | 0.96 | 0.94, 0.97 | <0.001 |
| ≥6.20 mmol/L (240 mg/dl) | 4,060 | 515 | 0.97 | 0.95, 0.99 | 0.003 | 0.98 | 0.96, 1.00 | 0.08 |
HR, hazard ratio; CI, confidence interval; CVD, cardiovascular disease; ECG, electrocardiogram.
The point and interval estimates are the risk of CVD events that are associated, on average, with each 1-minute increment in treadmill exercise duration.
Adjusted for age and examination year.
Adjusted for the above plus each of the other risk factors in the table.
TABLE 6.
Hazard ratios for total CVD events per 1-minute increment of maximal exercise duration according to cardiovascular disease risk factor categories in women, Aerobics Center Longitudinal Study, Dallas, Texas, 1971-2004
| Risk Factor | N | Events | HR‡ | 95% CI‡ | p value | HR§ | 95% CI§ | p value |
|---|---|---|---|---|---|---|---|---|
| Age, years | ||||||||
| <55 | 4,864 | 93 | 0.94 | 0.89, 0.99 | 0.03 | 0.96 | 0.91, 1.02 | 0.23 |
| ≥55 | 1,045 | 66 | 0.96 | 0.89, 1.04 | 0.30 | 0.98 | 0.90, 1.06 | 0.61 |
| Current smoker | ||||||||
| No | 5,358 | 136 | 0.96 | 0.92, 1.01 | 0.14 | 0.98 | 0.93, 1.03 | 0.49 |
| Yes | 551 | 23 | 0.89 | 0.77, 1.01 | 0.07 | 0.87 | 0.75, 1.00 | 0.05 |
| Family history of CVD | ||||||||
| No | 4,820 | 132 | 0.95 | 0.91, 1.00 | 0.05 | 0.97 | 0.92, 1.02 | 0.23 |
| Yes | 1,089 | 27 | 0.93 | 0.83, 1.04 | 0.22 | 0.97 | 0.85, 1.10 | 0.61 |
| Exercise ECG responses | ||||||||
| Normal | 5,604 | 138 | 0.95 | 0.90, 0.99 | 0.03 | 0.96 | 0.91, 1.01 | 0.11 |
| Abnormal | 305 | 21 | 1.00 | 0.85, 1.17 | 0.97 | 1.12 | 0.93, 1.34 | 0.24 |
| Body mass index, kg/m2 | ||||||||
| 18.5-24.9 | 4,644 | 119 | 0.94 | 0.89, 0.94 | 0.01 | 0.95 | 0.90, 1.01 | 0.07 |
| ≥25 | 1,265 | 40 | 1.02 | 0.91, 1.14 | 0.80 | 1.03 | 0.92, 1.16 | 0.60 |
| Hypertension | ||||||||
| No | 4,846 | 94 | 0.97 | 0.92, 1.03 | 0.33 | 1.00 | 0.94, 1.06 | 0.93 |
| Yes | 1,063 | 65 | 0.94 | 0.87, 1.01 | 0.07 | 0.92 | 0.85, 0.99 | 0.04 |
| Diabetes | ||||||||
| No | 5,732 | 152 | 0.94 | 0.90, 0.99 | 0.01 | 0.96 | 0.91, 1.01 | 0.08 |
| Yes | 177 | 7 | 1.09 | 0.82, 1.44 | 0.57 | 1.15 | 0.73, 1.79 | 0.55 |
| Total cholesterol | ||||||||
| <6.20 mmol/L (240 mg/dl) | 5,091 | 119 | 0.93 | 0.89, 0.98 | 0.009 | 0.95 | 0.90, 1.01 | 0.07 |
| ≥6.20 mmol/L (240 mg/dl) | 818 | 40 | 0.99 | 0.90, 1.09 | 0.86 | 1.03 | 0.93, 1.13 | 0.63 |
Abbreviations, †, ‡ and § are the same as in Table 5.
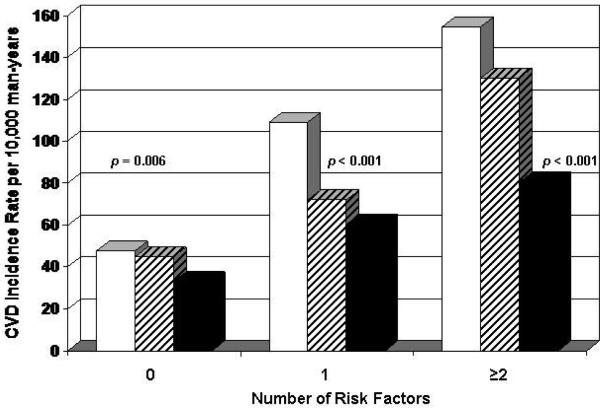
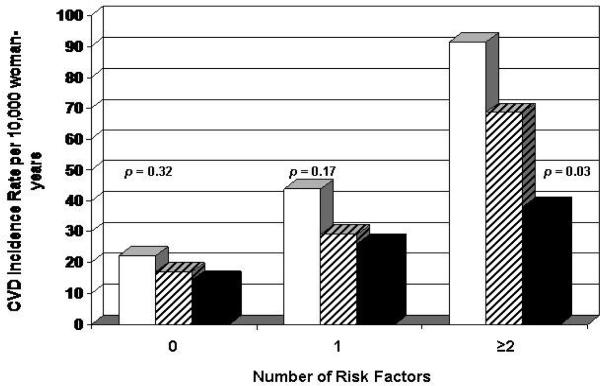
To examine whether CRF had prognostic value beyond an individual's pretest probability of having a CVD event, we computed CVD rates by CRF levels grouped on the number of major CVD risk factors at baseline (figures 1 and 2). By convention (33), individuals with 0 risk factors would be classified as low risk (e.g., expected 10-year probability <10 percent), whereas those with 1 or more risk factors would have an intermediate to high CVD risk (e.g., 10-year probability ≥10 percent). In men, we observed inverse gradients of CVD rates across CRF categories within each risk factor stratum (p < 0.01 each). Similar inverse patterns of association were seen in women, but the rate differences were not statistically significant.
Figure 1.
Age and examination year adjusted rates of total CVD events (per 10,000 person-years) by levels of CRF and number of major CVD risk factors (current smoking, hypertension, hypercholesterolemia, diabetes, and family history of CVD) in 20,728 men, Aerobics Center Longitudinal Study, Dallas, Texas, 1971-2004. White bars represent low fitness, striped bars moderate fitness, and black bars high fitness. The p values are for a test of linear trend across CRF groups. The number of men (and cases) in the low, moderate, and high CRF groups were 878 (42), 2,886 (143), and 4,099 (154) in those with 0 risk factors; 1,548 (120), 3,422 (266), and 3,131 (226) in those with 1 risk factor; and 1,541 (183), 1,987 (255), and 1,236 (123) in those with ≥2 risk factors. CRF, cardiorespiratory fitness; CVD, cardiovascular disease.
Figure 2.
Age and examination year adjusted rates of total CVD events (per 10,000 person-years) by levels of CRF and number of major CVD risk factors (current smoking, hypertension, hypercholesterolemia, diabetes, and family history of CVD) in 5,909 women, Aerobics Center Longitudinal Study, Dallas, Texas, 1971-2004. White bars represent low fitness, striped bars moderate fitness, and black bars high fitness. The p values are for a test of linear trend across CRF groups. The number of women (and cases) in the low, moderate, and high CRF groups were 355 (8), 1,068 (21), and 1,690 (25) in those with 0 risk factors; 349 (14), 725 (22), and 963 (24) in those with 1 risk factor; and 178 (13), 303 (20), and 278 (12) in those with ≥2 risk factors. CRF, cardiorespiratory fitness; CVD, cardiovascular disease.
DISCUSSION
Several prospective studies have shown that CRF is inversely associated with CVD mortality in asymptomatic women and men (12-16). Only a few studies in men have reported on CRF and risk of nonfatal CVD events (17, 18). To evaluate the true role of CRF in primary CVD prevention, it is important to determine whether CRF is related to incident events that are survived and not merely to mortality, and whether protection is conferred both in women and men. The present study demonstrated that higher CRF was associated with significantly lower rates of nonfatal CVD events. The inverse pattern of association was present in women and men, and in those with a low or a moderate/high pretest probability of CVD. Significant associations generally persisted after considering the potential confounding or modifying effects of physical activity status and other risk factors, although some associations were attenuated in women due to low statistical power. Inverse patterns of association also were seen between CRF and nonfatal CHD events and when MI and stroke were considered separately. This investigation is one of the largest prospective studies, and to our knowledge the first in women to relate an objectively measured CRF exposure with the incidence of several nonfatal CVD endpoints in initially asymptomatic adults.
Three of the study findings deserve further comment. First, CRF predicted primary CVD events independent of reported physical activity status. Because physical activity assessment was crude in the present study caution must be taken when considering the implications of this finding. Accurate questionnaire-based assessment of physical activity habits is difficult, particularly in women (11). This partly may explain the lack of association between physical activity and CVD in the present women. Our findings suggest that assessment of CRF in asymptomatic women and men may provide important prognostic information above that obtained from self-reported physical activity habits. Clinicians should, therefore, consider the benefits and feasibility of more routine exercise testing.
In men, the inverse gradient of CVD risk across CRF groups remained significant after adjustment for confounding by age, smoking, family history of CVD, abnormal exercise ECG responses, and factors that may be intermediate in the causal pathway between CRF and CVD (BMI, dyslipidemia, hypertension, and diabetes). The present findings of a strong independent association between CRF and nonfatal CVD in men are consistent with previous ACLS findings on CRF and CVD mortality (12, 13), with findings in Finnish men on CRF and nonfatal CVD (17), and with findings from studies that have related CRF (15, 20, 21, 34) or reported physical activity (5, 6, 8) with combined fatal/nonfatal CVD in men. Similar patterns of association generally were seen in women. Lack of a significant association in the fully adjusted model that included biological intermediates may be due to the small number of cases and is consistent with some (5, 8, 20) but not all (7, 9, 10, 16, 34) studies on PA or CRF and CVD risk in women. For example, CRF predicted CVD mortality risk in women and men in the Lipid Research Clinics study (16), whereas it was significantly associated with combined fatal/nonfatal CHD events in men but not women in the Framingham Heart Study (20). Additional prospective data on CRF exposures and nonfatal CVD events are needed in women to expand on the findings reported here and elsewhere.
A second major finding was that the inverse association between CRF and CVD generally was consistent in strata of other CVD predictors. The prognostic value of CRF is particularly noteworthy in men who were older and who had diabetes, exercise ECG abnormalities, or co-existing risk factors at baseline. A sharp rise in the risk of a first CVD event occurs in adults ages 45 to 60 (1). We observed that men ages 55 years or older had a 3-fold higher risk of CVD events than their younger counterparts. Diabetes and multiple co-existing risk factors now are seen as coronary risk equivalents in asymptomatic adults (33). In our study, 10-year CVD risk was 50 percent greater in men with diabetes and was 3-fold greater in men with ≥2 risk factors than in men without either condition. Abnormal exercise ECG responses also are predictive of CVD events (20, 21, 28) and were associated with a 2-fold higher risk of CVD events among men in our study. Even in these high-risk subgroups of men, higher functional capacity was associated with significantly lower CVD event rates. Stratified analyses were more variable in women, however greater functional capacity tended to be associated with lower CVD risk across risk factor stratum. CVD rates also were lower across incremental CRF groups in women with ≥2 risk factors. Statistical significance of these cross-tabulations in women was limited by the small number of events.
Collectively, the present results suggest that CRF is an important prognostic factor for nonfatal CVD in asymptomatic men beyond information obtained from the exercise electrocardiogram and traditional risk factors. Higher CRF is protective against CVD events in those with a moderate/high or a low pretest probability of CVD. Assessing functional capacity in asymptomatic women likely is of similar benefit to CVD risk assessment as in men (8); however, additional data are needed to confirm the suggestive findings reported here.
A third noteworthy issue is the variety of CVD endpoints that were related to baseline CRF levels. A recent review of published prospective data on physical activity, CRF, and CVD outcomes indicated that the strongest inverse associations were for CVD mortality in men, and that additional data are needed in women and on nonfatal endpoints such as MI and stroke (35). In the present women and men, CRF not only was inversely related with total CVD events, but also with MI, and with MI and coronary revascularization combined. MI or sudden death is the first clinical manifestation in many adults, among whom risk factors often are normal or only slightly elevated (33). The findings reported herein and elsewhere (13, 16, 17, 20) suggest that low CRF is a significant predictor of atherothrombotic CVD events independent of the presence or absence of traditional risk factors. Assessment of CRF in clinical settings could, therefore be an important tool to facilitate more effective primary CVD prevention. Effective strategies are needed to better integrate exercise testing into CVD risk assessment (36).
CRF also was inversely associated with stroke incidence in men, which is consistent with findings on CRF and stroke mortality in the ACLS (37) and in Finnish studies (19). Others have reported inverse associations between physical activity and stroke in women (4, 5). The inverse trend in stroke events across CRF groups was not significant in the present women, which may partly be due to the small number of stroke events. We were not able to differentiate between hemorrhagic and ischemic strokes, and stroke subtype modifies the association between physical activity and stroke risk (4, 38). Additional studies on activity, fitness, and stroke are needed to expand on our suggestive findings of an inverse association.
Strengths of the current study include the extensive baseline examination to detect subclinical disease, the use of measured risk factors and of maximal exercise testing to quantify CRF, the large person-years of follow-up, and the variety of CVD endpoints. We also accounted for variable patterns of survey responses in our analyses, an approach not typically used in cohort studies such as ours (4, 9, 38). The inverse associations generally were graded and independent of traditional risk factors, which strengthens causal inferences. Biological plausibility for these associations may, for example, be through enhanced endothelial cell function and coronary flow reserve, reduced myocardial oxygen demand under a variety of circumstances, higher myocardial arrhythmia threshold, improved endogenous thrombolytic activity, and lower levels of circulating atherothrombotic cytokines which may promote coronary plaque stabilization (11).
The homogeneity of our population sample in sociodemographic factors enhances the internal validity of our findings by reducing confounding by these factors. Although the self-referred origin and homogeneity of our cohort also may be seen as a weakness, we believe that our data are no less meaningful than those from population samples of adults referred to exercise testing for clinical reasons (39), or data from other selected cohorts that have been influential in preventive cardiology (6, 14, 20). Our findings should be generalized carefully to other adult populations. We did not have sufficient information on medication usage, menopausal status, or dietary habits to include in our analysis. It is possible that residual confounding by these factors may exist, although it seems unlikely that it would account for all of the observed association between CRF and CVD. Future studies should include such information to expand on the findings reported here. Women tend to manifest CVD events 10 years later than men. In the present study the age distribution in women was insufficient for grouped analysis beyond 55 years of age. Genetics clearly contribute to maximal CRF (40, 41). Nonetheless, CRF can be enhanced in most individuals through participation in moderate and vigorous physical activities, such as brisk walking, bicycling, and jogging, for 30 minutes or more on most days of the week (2).
We conclude that CRF is a significant determinant of nonfatal primary CVD events in women and men. Assessment of CRF provides important prognostic information independent of exercise ECG responses and traditional risk factors, and in those with high and low pretest probabilities of CVD. Exercise testing to assess functional capacity may enhance CVD risk stratification beyond conventional office-based methods in asymptomatic adults. We believe that clinicians should consider the benefits of assessing CRF, and should vigilantly counsel their sedentary patients to become more physically active and improve their CRF as a cornerstone of primary CVD prevention.
Acknowledgments
ACKNOWLEDGEMENTS
Supported by NIH grants AG06945 and HL62508, and by the Communities Foundation of Texas on recommendation of Nancy Ann and Ray L. Hunt.
The authors thank Dr. Kenneth H. Cooper for establishing the Aerobics Center Longitudinal Study, the Cooper Clinic physicians and technicians for collecting the baseline data, and Gaye Christmas for editorial assistance.
Abbreviations
- BMI
body mass index
- CHD
coronary heart disease
- CI
confidence interval
- CRF
cardiorespiratory fitness
- CVD
cardiovascular disease
- ECG
electrocardiogram
- HR
hazard ratio
- MET
metabolic equivalent
- MI
myocardial infarction
- SD
standard deviation
Footnotes
Conflict of interest: none declared.
Reference List
- 1.Thom T, Haase N, Rosamond W, et al. Heart disease and stroke statistics--2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:e85–151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- 2.Thompson PD, Buchner D, Pina IL, et al. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the council on clinical cardiology (subcommittee on exercise, rehabilitation, and prevention) and the council on nutrition, physical activity, and metabolism (subcommittee on physical activity) Arterioscler Thromb Vasc Biol. 2003;23:E42–E49. doi: 10.1161/01.ATV.0000087143.33998.F2. [DOI] [PubMed] [Google Scholar]
- 3.Leon AS, Connett J, Jacobs DR, Jr., et al. Leisure-time physical activity levels and risk of coronary heart disease and death. The Multiple Risk Factor Intervention Trial. JAMA. 1987;258:2388–95. [PubMed] [Google Scholar]
- 4.Hu G, Sarti C, Jousilahti P, et al. Leisure time, occupational, and commuting physical activity and the risk of stroke. Stroke. 2005;36:1994–9. doi: 10.1161/01.STR.0000177868.89946.0c. [DOI] [PubMed] [Google Scholar]
- 5.Salonen JT, Puska P, Tuomilehto J. Physical activity and risk of myocardial infarction, cerebral stroke and death: a longitudinal study in Eastern Finland. Am J Epidemiol. 1982;115:526–37. doi: 10.1093/oxfordjournals.aje.a113334. [DOI] [PubMed] [Google Scholar]
- 6.Paffenbarger RS, Jr., Hyde RT, Wing AL, et al. A natural history of athleticism and cardiovascular health. JAMA. 1984;252:491–5. [PubMed] [Google Scholar]
- 7.Rockhill B, Willett WC, Manson JE, et al. Physical activity and mortality: a prospective study among women. Am J Public Health. 2001;91:578–83. doi: 10.2105/ajph.91.4.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Connor GT, Hennekens CH, Willett WC, et al. Physical exercise and reduced risk of nonfatal myocardial infarction. Am J Epidemiol. 1995;142:1147–56. doi: 10.1093/oxfordjournals.aje.a117573. [DOI] [PubMed] [Google Scholar]
- 9.Lee I-M, Rexrode KM, Cook NR, et al. Physical activity and coronary heart disease in women: is “no pain, no gain” passe? JAMA. 2001;285:1447–54. doi: 10.1001/jama.285.11.1447. [DOI] [PubMed] [Google Scholar]
- 10.Manson JE, Greenland P, LaCroix AZ, et al. Walking compared with vigorous exercise for the prevention of cardiovascular events in women. N Engl J Med. 2002;347:716–25. doi: 10.1056/NEJMoa021067. [DOI] [PubMed] [Google Scholar]
- 11.Haskell WL, Leon AS, Caspersen CJ, et al. Cardiovascular benefits and assessment of physical activity and physical fitness in adults. Med Sci Sports Exerc. 1992;24(suppl 6):S201–S220. [PubMed] [Google Scholar]
- 12.Blair SN, Kohl HW, III, Paffenbarger RS, Jr., et al. Physical fitness and all-cause mortality: a prospective study of healthy men and women. JAMA. 1989;262:2395–401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 13.Blair SN, Kampert JB, Kohl HW, et al. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA. 1996;276:205–10. [PubMed] [Google Scholar]
- 14.Ekelund LG, Haskell WL, Johnson JL, et al. Physical fitness as a predictor of cardiovascular mortality in asymptomatic North American men: The Lipid Research Clinic's mortality follow-up study. N Engl J Med. 1988;319:1379–84. doi: 10.1056/NEJM198811243192104. [DOI] [PubMed] [Google Scholar]
- 15.Lakka TA, Venalainen JM, Rauramaa R, et al. Relation of leisure-time physical activity and cardiorespiratory fitness to the risk of acute myocardial infarction in men. N Engl J Med. 1994;330:1549–54. doi: 10.1056/NEJM199406023302201. [DOI] [PubMed] [Google Scholar]
- 16.Mora S, Redberg RF, Cui Y, et al. Ability of exercise testing to predict cardiovascular and all-cause death in asymptomatic women: a 20-year follow-up of the lipid research clinics prevalence study. JAMA. 2003;290:1600–7. doi: 10.1001/jama.290.12.1600. [DOI] [PubMed] [Google Scholar]
- 17.Laukkanen JA, Kurl S, Salonen R, et al. The predictive value of cardiorespiratory fitness for cardiovascular events in men with various risk profiles: a prospective population-based cohort study. Eur Heart J. 2004;25:1428–37. doi: 10.1016/j.ehj.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 18.Miller GJ, Cooper JA, Beckles GL. Cardiorespiratory fitness, all-cause mortality, and risk of cardiovascular disease in Trinidadian men--the St James survey. Int J Epidemiol. 2005;34:1387–94. doi: 10.1093/ije/dyi193. [DOI] [PubMed] [Google Scholar]
- 19.Kurl S, Laukkanen JA, Rauramaa R, et al. Cardiorespiratory fitness and the risk for stroke in men. Arch Intern Med. 2003;163:1682–8. doi: 10.1001/archinte.163.14.1682. [DOI] [PubMed] [Google Scholar]
- 20.Balady GJ, Larson MG, Vasan RS, et al. Usefulness of exercise testing in the prediction of coronary disease risk among asymptomatic persons as a function of the Framingham risk score. Circulation. 2004;110:1920–5. doi: 10.1161/01.CIR.0000143226.40607.71. [DOI] [PubMed] [Google Scholar]
- 21.Bodegard J, Erikssen G, Bjornholt JV, et al. Reasons for terminating an exercise test provide independent prognostic information: 2014 apparently healthy men followed for 26 years. Eur Heart J. 2005;26:1394–401. doi: 10.1093/eurheartj/ehi278. [DOI] [PubMed] [Google Scholar]
- 22.Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45:142–61. doi: 10.1161/01.HYP.0000150859.47929.8e. [DOI] [PubMed] [Google Scholar]
- 23.Balke B, Ware RW. An experimental study of physical fitness in Air Force personnel. US Armed Forces Med J. 1959;10:675–88. [PubMed] [Google Scholar]
- 24.Pollock ML, Bohannon RL, Cooper KH, et al. A comparative analysis of four protocols for maximal treadmill stress testing. Am Heart J. 1976;92:39–46. doi: 10.1016/s0002-8703(76)80401-2. [DOI] [PubMed] [Google Scholar]
- 25.Pollock ML, Foster C, Schmidt D, et al. Comparative analysis of physiologic responses to three different maximal graded exercise test protocols in healthy women. Am Heart J. 1982;103:363–73. doi: 10.1016/0002-8703(82)90275-7. [DOI] [PubMed] [Google Scholar]
- 26.American College of Sports Medicine . ACSM's guidelines for exercise testing and prescription. 6th ed. Lippincott Williams & Wilkins; Philadelphia, PA: 2000. [Google Scholar]
- 27.Barlow CE, Lamonte MJ, Fitzgerald SJ, et al. Cardiorespiratory fitness is an independent predictor of hypertension incidence among initially normotensive healthy women. Am J Epidemiol. 2006163:142–50. doi: 10.1093/aje/kwj019. [DOI] [PubMed] [Google Scholar]
- 28.Gibbons LW, Mitchell TL, Wei M, et al. Maximal exercise test as a predictor of risk for mortality from coronary heart disease in asymptomatic men. Am J Cardiol. 2000;86:53–8. doi: 10.1016/s0002-9149(00)00827-4. [DOI] [PubMed] [Google Scholar]
- 29.Macera CA, Jackson KL, Davis DR, et al. Patterns of non-response to a mail survey. J Clin Epidemiol. 1990;43:1427–30. doi: 10.1016/0895-4356(90)90112-3. [DOI] [PubMed] [Google Scholar]
- 30.Luepker RV, Apple FS, Christenson RH, et al. Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation. 2003;108:2543–9. doi: 10.1161/01.CIR.0000100560.46946.EA. [DOI] [PubMed] [Google Scholar]
- 31.Kelly-Hayes M, Robertson JT, Broderick JP, et al. The American Heart Association Stroke Outcome Classification. Stroke. 1998;29:1274–80. doi: 10.1161/01.str.29.6.1274. [DOI] [PubMed] [Google Scholar]
- 32.Manson JE, Stampfer MJ, Hennekens CH, et al. Body weight and longevity. A reassessment. JAMA. 1987;257:353–8. [PubMed] [Google Scholar]
- 33.Greenland P, Smith SC, Jr., Grundy SM. Improving coronary heart disease risk assessment in asymptomatic people: role of traditional risk factors and noninvasive cardiovascular tests. Circulation. 2001;104:1863–7. doi: 10.1161/hc4201.097189. [DOI] [PubMed] [Google Scholar]
- 34.Roger VL, Jacobsen SJ, Pellikka PA, et al. Prognostic value of treadmill exercise testing: a population-based study in Olmsted County, Minnesota. Circulation. 1998;22(98):2836–41. doi: 10.1161/01.cir.98.25.2836. [DOI] [PubMed] [Google Scholar]
- 35.Kohl HW., III Physical activity and cardiovascular disease: evidence for a dose response. Med Sci Sports Exerc. 2001;33(suppl 6):S472–S483. doi: 10.1097/00005768-200106001-00017. [DOI] [PubMed] [Google Scholar]
- 36.Lauer M, Froelicher ES, Williams M, et al. Exercise testing in asymptomatic adults: a statement for professionals from the American Heart Association Council on Clinical Cardiology, Subcommittee on Exercise, Cardiac Rehabilitation, and Prevention. Circulation. 2005;112:771–6. doi: 10.1161/CIRCULATIONAHA.105.166543. [DOI] [PubMed] [Google Scholar]
- 37.Lee CD, Blair SN. Cardiorespiratory fitness and stroke mortality in men. Med Sci Sports Exerc. 2002;34:592–5. doi: 10.1097/00005768-200204000-00005. [DOI] [PubMed] [Google Scholar]
- 38.Hu FB, Stampfer MJ, Colditz GA, et al. Physical activity and risk of stroke in women. JAMA. 2000;283:2961–7. doi: 10.1001/jama.283.22.2961. [DOI] [PubMed] [Google Scholar]
- 39.Myers J, Prakash M, Froelicher V, et al. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346:793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- 40.Bouchard C, Daw EW, Rice T, et al. Familial resemblance for VO2max in the sedentary state: The HERITAGE Family Study. Med Sci Sports Exerc. 1998;30:252–8. doi: 10.1097/00005768-199802000-00013. [DOI] [PubMed] [Google Scholar]
- 41.Bouchard C, An P, Rice T, et al. Familial aggregation of VO2max response to exercise training: results from the HERITAGE Family Study. J Appl Physiol. 1999;87:1003–8. doi: 10.1152/jappl.1999.87.3.1003. [DOI] [PubMed] [Google Scholar]