Abstract
Background
Organic dust exposure in the agricultural industry results in significant airway disease and lung function decline. Mononuclear phagocytes are key cells that mediate the inflammatory and innate immune response following dust exposure.
Objective
To investigate the effect of organic dust extract (ODE) from modern swine operations on monocyte-derived macrophage (MDM) phenotype and function.
Methods
Peripheral blood monocytes were obtained by elutriation methodology (>99% mCD14+) and differentiated into macrophages in the presence of GM-CSF (1 week) with and without ODE (0.1%). At one week, cells were analyzed by flow cytometry for cell surface marker expression (HLA-DR, CD80, CD86, TLR2, TLR4, mCD14, CD16), phagocytosis (IgG-opsonized zymosan particles), and intracellular killing of Streptococcus pneumoniae. At one week, MDMs were re-challenged with high dose ODE (1%), lipopolysaccharide (LPS), and peptidoglycan (PGN), and cytokines (TNFα, IL-6, IL-10, CXCL8/IL-8) were measured. To elucidate ODE-associated factors, comparisons were made to MDMs conditioned with heat-inactivated dust, endotoxin-depleted dust, LPS, and PGN.
Results
Expression of HLA-DR, CD80, CD86, phagocytosis and intracellular bacterial killing were significantly decreased with ODE- versus control-MDMs. Responses were retained after marked depletion of endotoxin. PGN, LPS and PGN + LPS significantly reduced MDM surface marker expression, and except for LPS alone, also reduced phagocytosis. ODE-MDMs had significantly diminished cytokine responses (TNFα, IL-6, IL-10) following repeat challenge with high dose ODE. Cross-tolerant cytokine responses were also observed.
Conclusion
Repetitive organic dust exposure significantly decreases markers of antigen presentation and host defense function in monocyte-derived macrophages. Bacterial cell components appear to be driving these impaired responses.
Key Messages
Repetitive organic dust exposure impairs monocyte-derived macrophage host defense functions.
Gram positive bacterial cell components may be driving this impaired response.
Clinical implications: Organic dust-induced macrophage dysfunction may be important in respiratory disease development.
Capsule Summary
Repetitive organic dust exposure in vitro impairs host defense function in monocyte-derived macrophages, which appear to be driven by gram positive bacterial cell components. Organic dust-induced macrophage dysfunction may be important in respiratory disease development.
Keywords: Monocyte, Macrophage, Organic dust, Phagocytosis, Intracellular killing, Cell surface molecules, Cytokines, Inflammation, Lipopolysaccharide, Peptidoglycan
INTRODUCTION
In the United States, a variety of farming operations, including modern, largescale concentrated closed animal feeding operations, can generate significant amounts of dust. Chronic organic dust exposure to workers in this agricultural industry, particularly swine production, results in significant airway diseases including rhinitis, bronchitis, and obstructive pulmonary disease. 1 Initial exposure to organic dust induces an intense airways inflammatory response that attenuates over time, however, less well examined are persons repetitively exposed to organic dust environments. These individuals are at an increased risk of lung function decline, persistent inflammation and respiratory disease, but not IgE-mediated disease. 1-4 These observations suggest that chronic organic dust exposure significantly modulates the airways inflammatory response.
Organic dust is a complex mixture containing particulate matter and microbial-associated components that can elicit innate immune responses. Although endotoxin has been well-described for its role as a potent inflammatory stimulus, there has not been a consistent association with endotoxin levels and inflammatory outcomes in swine confinement workers. 5 We and others have also demonstrated that the endotoxin component in swine facility dust does not completely explain the inflammatory response in cultured monocytes, epithelial cells, and whole blood. 6-8 Peptidoglycans (PGNs), cell wall components most commonly found in gram positive bacteria, but also to a lesser degree in gram negative bacteria, are also commonly found in this environment and found to correlate with inflammatory outcomes in exposed workers and mediate inflammation in human alveolar macrophages and epithelial cells. 9, 10 Thus, given the complex nature of organic dust, studies with organic dust samples obtained from these environments are necessary.
Macrophages, which are key innate immune cells that initially respond and are rapidly activated by exposure to inhaled environmental toxins such as organic dust, bacterial endotoxin, particulate air pollution and ozone, have a potential role in the pathogenesis of organic dust-induced respiratory diseases. 11-13 Macrophage derived inflammatory mediators can induce pyrexia, neutrophil recruitment, and activation of airway epithelial cells, 14 Monocytes are precursor cells that can be recruited to sites of inflammation and depending on which maturation and differentiation factors are present in the airways milieu, differentiate into macrophages or dendritic cells. It has now been shown that acute lung inflammation triggers a rapid recruitment of monocytes that replace the resident alveolar macrophage population. 11, 15 It is also recognized that there are different inflammatory cell patterns and macrophage phenotypes present in the airways of subjects with chronic respiratory diseases, which may be driven by various environmental factors. 16-18
Despite the finding that macrophages are increased in swine workers,1 there are few studies investigating the role of repetitive organic dust exposure in monocytes/macrophages. We have previously demonstrated that repeat organic dust exposure from modern swine facilities modulates inflammatory responses in human monocytes as compared to single dust exposure. 6 However, the effect of repetitive organic dust exposure on the differentiation of monocytes to macrophages and the subsequent effect on cell phenotype and function have not been investigated. This information will enhance our understanding of how repetitive organic dust exposure modulates the innate immune response.
In this study, we hypothesized that repetitive organic dust exposure from modern swine facilities would impair human macrophage differentiation and function. To test this hypothesis, macrophages derived from peripheral blood monocytes (MDMs; monocyte-derived macrophages) from healthy individuals were investigated to determine the effect of organic dust exposure on immune cell surface phenotype, phagocytosis, and intracellular bacterial killing. We also evaluated the ability of MDMs cultured in the presence of organic dust, LPS and PGN to secrete pro-inflammatory mediators when subsequently re-challenged with high dose organic dust extract, lipopolysaccharide (LPS), and peptidoglycan (PGN). Moreover, to determine the potential role dust-associated materials play, we compared ODE-treated MDMs with MDMs derived in the presence of LPS, PGN, LPS + PGN, ODE depleted of endotoxin, and heat-inactivated ODE (devoid of organic compounds).
METHODS
Preparation of Organic Dust Extract
Organic dust was obtained from settled surface dust from modern swine confinement animal feeding operation facilities, housing ∼ 500-700 animals. For all experimental studies, the organic dust was placed into solution and sterile filtered (organic dust extract; ODE) by a standard published procedure. 6, 7 To assess the response of endotoxin-depleted dust extract, the ODE was applied to polymixin B (binds and inactivates endotoxin) columns (Pierce, Rockford). The mean endotoxin concentration present after depletion was <0.2 ng/mL. In other experiments, the organic dust was heat-inactivated by heating the dust for 24 hours at 120°C before placing into solution, referred to as heat-inactivated ODE.
Complete analysis of the organic dust can be found on the Online Repository Methods at www.jacionline.org. Briefly, analysis of organic dust prior to placing into extract form revealed trace metals, predominance of Gram-positive bacteria (98%), and high muramic acid (marker of PGN/bacterial biomass). The endotoxin equivalent concentration in ODE 0.1% is 4.8 ng/mL (range: 2.6-7.0 ng/mL) as determined by the Limulus amebocyte lysate gel clot assay (Cambrex, Walkersville, MD).
Monocyte-Derived Macrophage (MDM) Preparation
Populations of monocytes were obtained from the institution’s Elutriation Core Facility. In brief, monocytes were isolated by countercurrent centrifugal elutriation of mononuclear leukocyte-rich fractions of blood cells from healthy donors undergoing leukopheresis. 19 Elutriated monocytes were >99% pure as determined by surface antigen expression of mCD14 measured by flow cytometry. Peripheral blood was taken with written informed consent and studies were approved by the Institutional Review Board.
To induce the in vitro differentiation of monocytes to macrophages (monocyte-derived macrophages; MDM), monocytes were cultured with and without ODE (0.1%) in complete RPMI in the presence of 1000 U/mL of recombinant human granulocyte-macrophage colony stimulating factor (GM-CSF; R&D Systems, Minneapolis, MN). Growth factor supplemented media was changed every 48 hours for 6 days. Successful macrophage differentiation was confirmed by microscopic identification as well as flow cytometric identification. MDMs were incubated without GM-CSF and ODE for 18 hours prior to use in experimental assays to eliminate their direct effect. At the end of 1 week, MDMs cultured with GM-CSF alone and MDMs cultured with GM-CSF and ODE (0.1%) are referred to as control-MDMs and ODE-MDMs, respectively. As others have found that the first 48 hours may be the critical window in monocyte differentiation, 20 monocytes were cultured with ODE (0.1%) for the initial 48 hours only, and then maintained in culture with GM-CSF alone for the remainder of the week, referred to as “MDMs/early ODE exposed.” Schematic of experimental design is depicted in Fig E1A (ODE-MDMs) and Fig E1B (MDMs/early ODE exposed) in the Online Repository at www.jacionline.org. Macrophages were also derived in the presence of LPS (10 ng/mL; 2X equivalent concentration in ODE 0.1%), PGN (4 μg/mL; approximating protein concentration in ODE 0.1%), combination LPS and PGN, heat-inactivated ODE (0.1%), heat-inactivated LPS + PGN, endotoxin-depleted dust extract (0.1%), and iron (ferric ammonium citrate, 40 ng/mL, equivalent concentration in ODE 0.1%). Details of all reagents used can be found in the Online Repository at www.jacionline.org.
Flow cytometry
After 1 week, all MDMs were evaluated for cell surface marker expression by flow cytometry for major histocompatibility class (MHC) II (human leukocyte antigen [HLA]-DR), B7 costimulatory molecules (CD80, CD86), and pathogen-associated receptors, Toll-like receptor (TLR)-2 and TLR4. Cells were also stained for mCD14 (monocyte/macrophage marker) and CD16/FcγRIII (macrophage marker) to ensure appropriate cell differentiation. MDMs (5 X 105 cells) were stained in a standard procedure with antibodies against mCD14, CD16, CD80, CD86, HLA-DR, TLR2, and TLR4 in PBS containing 0.1% bovine albumin. Cells were also incubated with irrelevant isotype control antibodies to account for non-specific binding. Flow-cytometric analyses were performed with the FACSCalibur dual-laser cytometers (Becton-Dickinson Lincoln Park, NJ). Cell surface marker expression was reported as mean fluorescence intensity (MFI) minus isotype background MFI. In all experiments, control cells from the same donor (cells incubated with GM-CSF alone) were run and compared in side-by-side experiments with conditioned cells.
Phagocytosis Assay
Phagocytic ability of MDMs was assessed by flow cytometry utilizing previously published methods. 13 Details can be found online Repository at www.jacionline.org. Phagocytosis of the FITC-labeled IgG-zymosan particles was determined by assessing the proportion of cells in the zymosan-exposed population at 60 minutes compared to cells exposed for 0 minutes (expressed as fold change in MFI). Cells were also quenched with trypan blue and acid wash to eliminate cell surface adhesion versus internalization-associated fluorescence.
Intracellular Bacterial Killing Assay
Killing of Streptococcus pneumoniae by control-MDMs and ODE-MDMs was determined as previously described with some modification. 21 Details can be found online Repository at www.jacionline.org. Percentage of killing was calculated as [(cfu at time 0 - cfu at 60 min)/(cfu at time 0)] X 100.
Cytokine/chemokine assays
To determine cytokine responsiveness of MDMs, after 1 week, control-MDMs and all conditioned-MDMs (5 X 105 cells/mL) in duplicate were subsequently challenged with a high concentration of ODE (1%) and media (control) for five hours. Cell-free supernatant was subsequently harvested and stored at -20°C until assayed for cytokine secretion. To investigate further if cross-tolerance occurs, at 1 week, ODE-MDMs, LPS-MDMs, PGN-MDMs and control-MDMs (5 X 105 cells/mL) in duplicate were restimulated with high concentration ODE (1%), LPS (100 ng/mL), PGN (40 μg/mL), and media alone (control) for five hours. In all experiments, cell counts and cell viability after the 5 hour culture condition was assessed by trypan blue exclusion method.
Cytokine/chemokines were assayed by sandwich ELISA as previously published. 6 Cytokine secretion is reported as concentration (pg/mL) per 5 X 105 viable cells as determined upon completion of the experimental protocol by trypan blue exclusion method. In cross-tolerant studies, mean results presented as the percentage of ODE-induced cytokine secretion (ODE-induced cytokine secretion in conditioned-MDMs divided by ODE-induced cytokine secretion in control-MDMs multiplied by 100).
Statistical Analysis
Data are presented as the mean ± standard error of mean (SEM). Statistics were performed using two-tailed non-paired or paired t tests (as appropriate) to determine significant changes among treatment groups.
RESULTS
Organic dust and microbial components modify macrophage cell surface marker expression
To examine the effect of ODE on differentiation and cell surface phenotype expression of macrophages derived from monocytes, human monocytes were incubated with and without ODE (0.1%) in the presence of GM-CSF for 1 week as detailed in the methods section. After 1 week, MDMs demonstrated surface marker expression of mCD14, CD16, HLA-DR, CD80, CD86, consistent with a macrophage phenotype. Compared to control-MDMs, MHC class II molecule (HLA-DR) and B7 costimulatory molecules (CD80 and CD86) were significantly down-regulated with ODE-versus control- treated MDMs (Figure 1A-B). The pathogen-associated receptors, TLR2 and TLR4, expression were not down-regulated. We found that the initial 48 hour exposure to ODE (MDMs/early ODE exposed) was the critical time point to observe the down-regulation of the innate immune cell surface marker expression (Fig 2A).
Figure 1 (A-B).
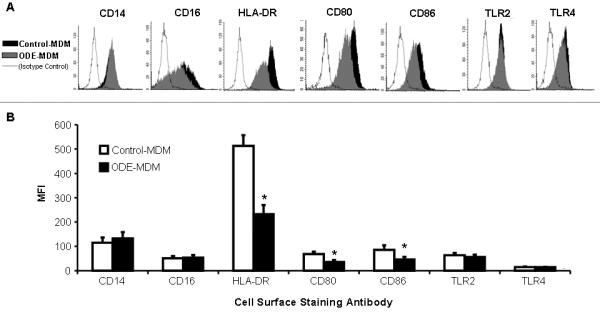
Cell surface marker expression in monocyte-derived macrophages (MDM). After 1 week, control-MDMs and ODE-MDMs demonstrated mCD14 and CD16 expression by flow cytometry. HLA-DR, CD80, and CD86 were diminished with ODE- versus control-MDMs. A, Representative histogram of 4 separate studies; B, Mean ± SEM of the mean fluorescence intensity (MFI) (N=4). *Statistically significant (p<0.05).
Figure 2 (A-F).
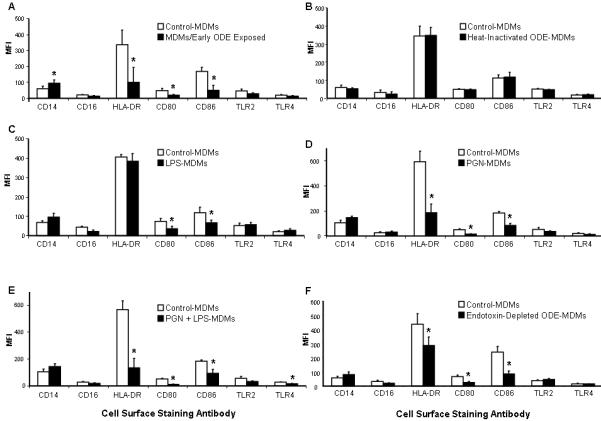
Cell surface marker expression in monocyte-derived macrophages (MDM). After 1 week, control-MDMs were compared to MDMs/early exposed (A; N=3), MDMs conditioned with heat-inactivated dust (B; N=3), LPS 10 ng/mL (C; N=4), PGN 4 μg/mL (D; N=4), and combination LPS + PGN (E; N=4), and endotoxin-depleted ODE (F; N=3). Mean ± SEM of the mean fluorescence intensity (MFI). *Statistically significant (p<0.05).
To determine possible components driving these observations with ODE, macrophages were also derived from monocytes in the presence of LPS, PGN, LPS + PGN, endotoxin-depleted, and heat-inactivated dust extract and evaluated for immune cell surface marker expression. Results of all conditions are shown in Figure 2B-F. LPS-conditioning significantly reduced CD80 and CD86 expression, but did not modify HLA-DR expression as compared to control. Compared to control, PGN-conditioning, combination of LPS + PGN, and endotoxin-depleted ODE significantly diminished HLA-DR, CD80, and CD86 expression. Heat-inactivated dust, iron (data not shown), and heat-inactivated LPS + PGN (data not shown) did not significantly effect the expression of any of the innate immune cell surface markers tested. These results suggest that heat-sensitive and non-endotoxin components in ODE are the dominant factors modulating ODE-induced monocyte-derived macrophage innate immune cell surface marker expression.
Organic dust and PGN exposure diminishes phagocytic ability of macrophages
After 1 week, phagocytosis of IgG-opsonized zymosan particles was measured at 0 and 60 minutes in MDMs treated with and without ODE (0.1%) by flow cytometry. Phagocytosis was demonstrated by a rightward shift in fluorescence (Figure 3) and reported as the proportion of cells in the zymosan-exposed population at 60 minutes compared to cells exposed for 0 minutes (expressed as fold change in MFI). The 0 minute time point represented particle adhesion, while the rightward shift in fluorescence at 60 minutes represented particle internalization as evidenced by shifts in both side (cell granularity) and forward (cell size) scatter, as well as the lack of fluorescence quenching by trypan blue stain or acid washing. Phagocytosis was significantly diminished in ODE-MDMs (Figure 3A-B) and MDMs/early exposed compared to control-MDMs (Fig 4A).
Figure 3.
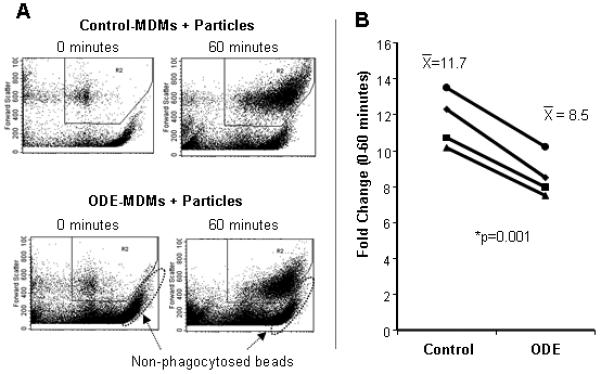
Phagocytosis of IgG-opsonized Saccharomyces cerevisiae zymosan bioparticles. Phagocytosis was diminished in ODE-treated MDMs compared to controls (N=4; p=0.001). A, Representative dot plot of rightward shift in fluorescence from 4 separate studies; B, Fold change in MFI (proportion of cells in the zymosan-exposed population at 60 minutes compared to cells exposed for 0 minutes). *Statistically significant (p<0.05).
Figure 4.
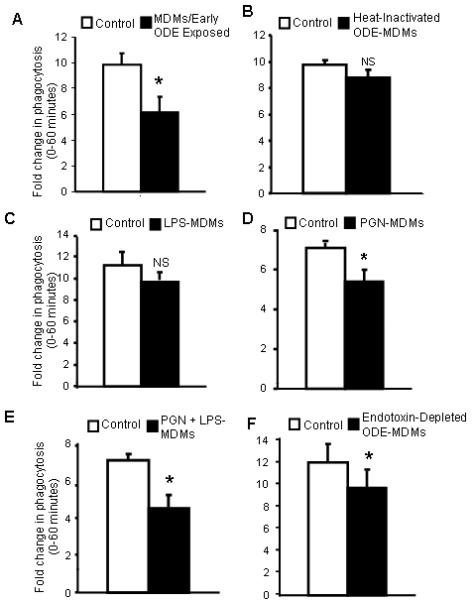
Phagocytosis of IgG-opsonized zymosan bioparticles in monocyte-derived macrophages (MDM). Fold change in MFI on y-axis (proportion of cells in the zymosan-exposed population at 60 minutes compared to cells exposed for 0 minutes) with MDMs/early ODE exposed (A; N=3), MDMs conditioned with heat-inactivated dust (B; N=3), LPS (C; N=3), PGN (D; N=3), and LPS + PGN (E; N=3), endotoxin-depleted ODE (F; N=3). *Statistically significant (p<0.05). NS; non-significant (p>0.05).
We next investigated the effect of LPS, PGN, LPS + PGN, endotoxin-depleted ODE, and heat-inactivation of the dust on the phagocytic ability of MDMs with all results shown in Figure 4B-F. Compared to control-MDMs, there was a slight, but non-significant decrease in phagocytic ability with the heat-inactivated dust extract conditioning. There was no effect on phagocytic ability with iron and heat-inactivated LPS + PGN conditioning (data not shown). LPS-conditioning did not significantly diminish phagocytic ability. However, PGN-conditioning, PGN + LPS, and endotoxin-depleted ODE-MDMs significantly reduced phagocytic ability. These studies suggest that non-endotoxin components in ODE are responsible for impaired phagocytic ability of MDMs.
Organic dust exposure significantly impairs intracellular bacterial killing
To extend the findings regarding phagocytosis in ODE conditioned MDMs, the ability of control-MDMs versus ODE-MDMs to phagocytoze and kill S. pneumoniae was investigated as detailed in the methods section. In these studies, we verified that phagocytosis was significantly impaired (70.6% ± 10.5% reduction in phagocytosis of S. pneumoniae in ODE-MDMs compared to control; N=3) and further demonstrated that intracellular killing of S. pneumoniae was also consistently significantly impaired (72.0±19.8% reduction in killing in ODE-MDMs compared to control; N=3). These studies suggest that ODE-conditioning significantly impairs host defense function marked by both a reduction in phagocytosis and intracellular bacterial killing.
Organic dust exposure effects cytokine responsiveness of MDMs
In these studies, the effect of ODE on innate immune inflammatory cytokine responsiveness of MDMs was investigated. As described in the methods section, after one week in culture, control-MDMs and ODE-MDMs were subsequently re-challenged with ODE (1%) for 5 hours. Control-MDMs and ODE-MDMs secreted TNFα, IL-6, IL-10, and CXCL8 (IL-8) when re-stimulated with ODE (1%) as compared to no restimulation (media control). However, we found that ODE-treated MDMs had significantly reduced secretion of TNFα, IL-6, and IL-10 upon re-stimulation compared to control MDMs (Figure 5 A-C). There was no significant difference in CXCL8 secretion (Figure 5D). There was no difference in cell count or cell viability between treatment conditions to explain these results.
Figure 5.
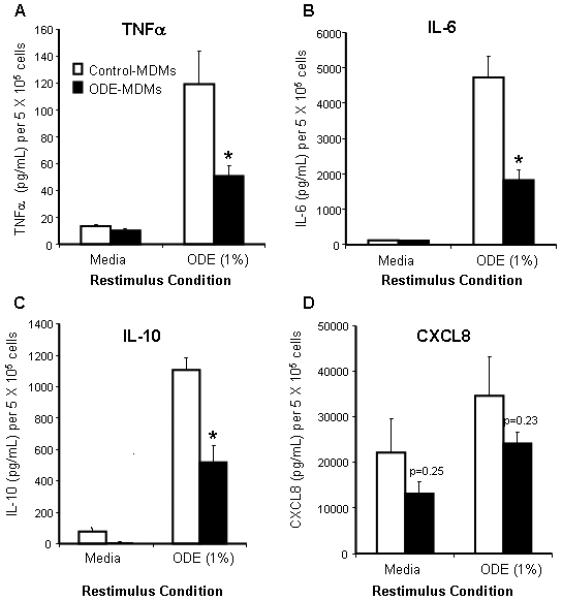
Secretion of cytokines/chemokines in monocyte-derived macrophages (MDMs) at 1 week after restimulation with ODE 1% for 5 hours. TNFα (A), IL-6 (B), IL-10 (C) secretion was dampened in ODE-MDMs compared to control-MDMs (N=5; p<0.05). CXCL8 (D) was not significantly altered (N=5). Mean results are presented per 5 X 105 cells ± SEM. *Statistically significant (p<0.05).
We next investigated the cytokine responsiveness of all conditioned MDMs to restimulation with 1% ODE (cross-tolerant studies). MDMs conditioned with LPS, PGN, LPS + PGN, endotoxin-depleted ODE, and early ODE exposure (initial 48 hours only), but not heat-inactivated ODE and heat-inactivated LPS + PGN (data not shown), demonstrated significant impairment in TNFα and IL-6 secretion (Fig E2A-B). Restimulation with relatively high concentration LPS of MDMs conditioned with ODE, LPS and PGN also demonstrated impairment of TNFα and IL-6 secretion (FigE3A-B). This cross-tolerant pattern of impaired TNFα and IL-6 was also observed when conditioned MDMs were re-stimulated with relatively high concentration of PGN (Fig E3C-D). Figures E2A-B and Figures E3A-D can be found on the Online Repository at www.jacionline.org.
DISCUSSION
Organic dust exposure is an important environmental factor that has been implicated in increased morbidity among repetitively exposed subjects. 1 Macrophages are key innate immune cells derived from monocytes that have a potential role in the pathogenesis of organic-dust induced respiratory disease; however, little is known about the effect of repeated organic dust exposure on macrophage phenotype and function. In this study, repetitive in vitro organic dust exposure resulted in an overall impairment in macrophage host defense capability. This was demonstrated by a marked reduction in expression of cell surface molecules associated with antigen presentation, phagocytic and bactericidal ability, and cytokine responsiveness.
Macrophages can orchestrate inflammation and activate T cell responses through antigen presenting ability. The first signal in the antigen presentation-T cell activation process is regulated by MHC Class II molecules, and the classic second signal is regulated by the B7 co-stimulatory molecules, CD80 and CD86. Our data demonstrate that repetitive swine facility dust exposure significantly down-regulates MHC Class II and co-stimulatory molecule (CD80 and CD86) expression. The reduction in the expression of HLA-DR and co-stimulatory molecules on macrophages would be consistent with an impaired host defense state.22 We speculate that this state may favor a decreased ability to induce an appropriate acquired immune response to microbial stimuli, which may ultimately perpetuate or promote bacterial colonization within the airways milieu.
Interestingly, our findings with repetitive organic dust exposure are consistent with observations made in subjects with chronic obstructive pulmonary disease (COPD). Alveolar macrophages from subjects with COPD and/or chronic smokers have lower expression of co-stimulatory molecules and MHC Class II compounds. 16 17 In contrast, macrophages from subjects with allergic asthma appear to over express CD80 and CD86 and also MHC Class I and II molecules compared to normal controls. 18 23 It is well-recognized that workers who are routinely exposed to swine barns have chronic bronchitis and progressive lung function decline as features of their disease, and this is particularly true in young workers aged 25-35 years.3 24 Thus, our in vitro model is consistent with the phenotype of macrophages from subjects with chronic bronchitis who have as a central feature of their disease, elevated neutrophilic inflammation and bacterial infection.
Another key macrophage function is phagocytosis, a process by which cellular debris and pathogens are removed. In this study, we directly measured macrophage phagocytosis by examining ingestion of fluorescently labeled-zymosan particles by flow cytometry. 13 25 We found that phagocytic ability was significantly impaired in organic dust-treated macrophages as compared to controls and verified this by demonstrating significantly impaired phagocytosis of S. pneumoniae by plate counting. We then found that intracellular killing of S. pneumoniae was significantly impaired in organic dust conditioned macrophages. We interpret these findings to also be consistent with a COPD/chronic bronchitis-macrophage phenotype. Others have suggested that alveolar macrophage phagocytic ability might be diminished in subjects with COPD. 16, 26
Macrophages also elicit inflammatory responses through production and secretion of cytokines/chemokines. We found that at 1 week, macrophages differentiated in the presence of organic dust can still release TNFα, IL-6, IL-10, CXL8 when restimulated with high dose ODE, but when compared to non-dust treated cells (control), the secretion of TNFα, IL-6, IL-10 was significantly decreased. The organic dust conditioned MDMs were also cross-tolerant to other microbial stimuli (LPS and PGN). These observations are also consistent with a COPD/chronic bronchitis phenotype whereby macrophages from subjects with COPD/chronic bronchitis secrete lower amounts of inflammatory cytokines compared to normal controls. 27
An interesting finding in this study is that the impairment in organic dust induced monocyte-derived macrophage host defense functions occur during the initial culture exposure time interval. Exposure of fresh monocytes to organic dust for 48 hours followed by washing and maintaining the cells in culture with GM-CSF for the remaining week demonstrated similar findings to exposing cells to organic dust for the entire week. Consistent with this observation, others have found that signaling necessary for monocyte survival and development into macrophages may end during the first 48 hour of culture in GM-CSF.20
The organic dust utilized was obtained from modern swine confinement facilities that are becoming increasingly common, replacing the smaller farming operations. In this study, we sought to better characterize the organic dust obtained from these modern facilities in our region (Midwest United States). We found that gram positive bacteria predominated in the dust, and in particular, muramic acid, a gram positive bacterial component of peptidoglycan. Finally, several elemental compounds such as Fe, which could elicit inflammatory responses and effect phagocytosis, were found to exist in the dust, but at relatively low levels.
Our interpretation is that metals likely play a minor role in the responses observed here because dust that was heat-inactivated at 120°C, a process that inactivates the biologics and leaves the metals intact, did not significantly modify our markers of immune cell function and phenotype or cytokine secretion. Phagocytosis was slightly decreased, but this was not statistically significant. In addition, we found that soluble iron at the comparable low concentrations found in the organic dust extract did not affect macrophage host defense (data not shown). It appears our observations are more in line with studies using ambient air pollution particles and/or diesel exhaust particles. 13 28 In these studies, a slight decrease in phagocytic ability was observed in macrophages, but the major immune effects were found to be secondary to the organic or biologic components in the particles.
Given the inherent complexity with the organic dust samples, we chose to investigate the effects of organic dust depleted of endotoxin, LPS and PGN individually and LPS and PGN combined in order to further understand the relative contribution of gram positive and gram negative bacterial components in our in vitro macrophage model. We previously reported that bacterial components such as LPS on PM2.5-10, play an important role in driving innate immune responses (phagocytosis, mCD14, HLA-DR, mTNFa) on airway macrophages following a single (acute) in vivo exposure of healthy volunteers to coarse PM. 13 In this current study, we found that repetitive exposure to LPS during macrophage differentiation resulted in diminished costimulatory molecule expression (CD80 and CD86), but exerted no change in MHC Class II expression. PGN-conditioning and organic dust depleted of endotoxin demonstrated similar findings to the ODE-conditioning, namely marked reduction in MHC class II, CD80, and CD86 expression. Combination of LPS + PGN-conditioning further reduced innate immune cell surface marker expression. As anticipated, LPS did not significantly affect phagocytic ability, but PGN-conditioning, combination LPS + PGN, and organic dust depleted of endotoxin significantly reduced phagocytosis. The observation that PGN inhibits phagocytic ability has been supported by others. 29 Our findings in this study are also consistent with other studies and our previous studies demonstrating that the endotoxin component in swine barn dust does not completely explain the immune inflammatory response in cultured airway epithelial cells and monocytes. 6-8
It appears then that gram positive bacterial components may be the driving component of the organic dust-induced responses observed in our study, but LPS + PGN together most closely mimicked the observations we observed with ODE, suggesting that combinations of biological agents are important in real life exposures. However, it would be an oversimplification to state that a TLR2 and/or TLR4 agonist completely explains the effect observed with organic dust as we did not observe any modulation in TLR2 and TLR4 cell surface expression with organic dust conditioning. Studies in our laboratory also have failed to show that blocking TLR2 by commercially available antibodies reverse the impaired host defense response in organic dust conditioned MDMs (data not shown). However, due to the complexity of blocking TLR2 in one week cell culture systems of human cells, we plan to study the role of TLR2 in a murine model of organic dust exposure before making definitive conclusions. Besides TLR2, other potential receptor targets include several of the PGN recognition molecules, including mCD14, Nod1, Nod2, and a family of PGN recognition proteins (PGRPs).30 Our study does add to the growing body of literature that various combinations of bacterial cell surface components might exert more powerful activities on host defense cells than that of individual components. 31 Organic dust is one such environmental factor whereby the study of various combinations of bacterial cell components will be critical for future therapeutic options.
In conclusion, macrophages differentiated in culture from peripheral blood monocytes from healthy volunteers showed impaired host defense function following exposure to organic dust. Various bacterial cell wall components, particularly gram positive peptidoglycan, appeared to drive these responses. The in vitro model utilized in this study can next be applied to subjects with chronic airways disease and will also be important when investigating possible therapeutic interventions aimed at preventing impaired host defense function with organic dust exposure.
Supplementary Material
Schematic representation of experimental protocol showing elutriated monocytes cultured with GM-CSF (1000 IU/mL) with and without low concentration organic dust extract (ODE; 0.1%) for six days (E1). Monocytes cultured with GM-CSF and ODE (0.1%) for 48 hours, and washed and maintained in culture for the remainder 4 days (E2; MDMs/early ODE exposed). Macrophages rested for 18 hours prior to experimental assays.
Cytokine secretion in monocyte-derived macrophages (MDMs) at 1 week after restimulation with ODE (1%) for 5 hours. TNFα (A) and IL-6 (B) secretion was dampened in all conditioned-MDMs compared to control-MDMs except for heat-inactivated ODE-MDMs (N=3). Mean results are presented as the percentage of ODE-induced cytokine secretion in control MDMs ± SEM. *Statistically significant (p<0.05).
Cytokine secretion in monocyte-derived macrophages (MDMs) at 1 week after restimulation with LPS (100 ng/mL; E2A-B) and PGN (40 μg/mL; E2C-D). TNFα (E2A and E2C) and IL-6 (E2B and E2D) secretion was dampened in all conditioned-MDMs compared to control-MDMs (N=3). Mean results are presented as the percentage of LPS or PGN -induced cytokine secretion in control MDMs ± SEM. *Statistically significant (p<0.05).
Acknowledgements
The authors wish to thank Art Heires and Mary U. Snitily for assisting in the experiments, and Linda Wilkie, Victoria Smith and Charles Kuzinski, PhD, in the UNMC Cell Analysis Facility for assistance with flow cytometry measurements. The authors also wish to thank Myhahn Che and Howard E. Gendelman, MD, in the UNMC Elutriation Facility for isolating monocytes by centrifugal elutriation. The authors also thank Christina Pehrson, MLT, at Lund University, Sweden, for assistance with chemical marker analysis, and Lisa Chudomelka for manuscript preparation assistance.
Study supported by grants from the National Institute of Environmental Health Sciences (K08: ES015522-01; JAP), American Academy of Allergy, Asthma Immunology Interest Section Grant Award (JAP), and National Institute of Occupational Safety Health (1R01OH008539-01; DJR).
Abbreviations
- COPD
Chronic obstructive pulmonary disease
- HLA-DR
Human Leukocyte Antigen-DR
- IL-6
Interleukin-6
- IL-10
Interleukin-10
- CXCL8
Interleukin-8
- LPS
Lipopolysaccharide
- MHC
Major histocompatibility complex
- MFI
Mean fluorescence intensity
- MDM
Monocyte-derived macrophage
- ODE
Organic Dust Extract
- PGN
Peptidoglycan
- PBS
Phosphate buffered saline
- TLR
Toll-like receptor
- TNF-α
Tumor necrosis factor-alpha
Footnotes
Disclosure: Some of the results of these studies have been presented at AAAAI National Meeting (2008, oral abstract #257).
References
- 1.Von Essen S, Romberger D. The respiratory inflammatory response to the swine confinement building environment: the adaptation to respiratory exposures in the chronically exposed worker. J Agric Saf Health. 2003;9:185–96. doi: 10.13031/2013.13684. [DOI] [PubMed] [Google Scholar]
- 2.Palmberg L, Larssson BM, Malmberg P, Larsson K. Airway responses of healthy farmers and nonfarmers to exposure in a swine confinement building. Scand J Work Environ Health. 2002;28:256–63. doi: 10.5271/sjweh.673. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz DA, Donham KJ, Olenchock SA, Popendorf WJ, Van Fossen DS, Burmeister LF, et al. Determinants of longitudinal changes in spirometric function among swine confinement operators and farmers. Am J Respir Crit Care Med. 1995;151:47–53. doi: 10.1164/ajrccm.151.1.7812571. [DOI] [PubMed] [Google Scholar]
- 4.Portengen L, Preller L, Tielen M, Doekes G, Heederik D. Endotoxin exposure and atopic sensitization in adult pig farmers. J Allergy Clin Immunol. 2005;115:797–802. doi: 10.1016/j.jaci.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 5.Donham KJ, Reynolds SJ, Whitten P, Merchant JA, Burmeister L, Popendorf WJ. Respiratory dysfunction in swine production facility workers: dose-response relationships of environmental exposures and pulmonary function. Am J Ind Med. 1995;27:405–18. doi: 10.1002/ajim.4700270309. [DOI] [PubMed] [Google Scholar]
- 6.Poole JA, Wyatt TA, Von Essen SG, Hervert J, Parks C, Mathisen T, et al. Repeat organic dust exposure-induced monocyte inflammation is associated with protein kinase C activity. J Allergy Clin Immunol. 2007;120:366–73. doi: 10.1016/j.jaci.2007.04.033. [DOI] [PubMed] [Google Scholar]
- 7.Romberger DJ, Bodlak V, Von Essen SG, Mathisen T, Wyatt TA. Hog barn dust extract stimulates IL-8 and IL-6 release in human bronchial epithelial cells via PKC activation. J Appl Physiol. 2002;93:289–96. doi: 10.1152/japplphysiol.00815.2001. [DOI] [PubMed] [Google Scholar]
- 8.Muller-Suur C, Larsson K, Grunewald J. Organic dust-induced interleukin-12 production activates T- and natural killer cells. Eur Respir J. 2002;20:686–90. doi: 10.1183/09031936.02.02002002. [DOI] [PubMed] [Google Scholar]
- 9.Zhiping W, Malmberg P, Larsson BM, Larsson K, Larsson L, Saraf A. Exposure to bacteria in swine-house dust and acute inflammatory reactions in humans. Am J Respir Crit Care Med. 1996;154:1261–6. doi: 10.1164/ajrccm.154.5.8912733. [DOI] [PubMed] [Google Scholar]
- 10.Larsson BM, Larsson K, Malmberg P, Palmberg L. Gram positive bacteria induce IL-6 and IL-8 production in human alveolar macrophages and epithelial cells. Inflammation. 1999;23:217–30. doi: 10.1023/a:1020269802315. [DOI] [PubMed] [Google Scholar]
- 11.Arjomandi M, Witten A, Abbritti E, Reintjes K, Schmidlin I, Zhai W, et al. Repeated exposure to ozone increases alveolar macrophage recruitment into asthmatic airways. Am J Respir Crit Care Med. 2005;172:427–32. doi: 10.1164/rccm.200502-272OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lay JC, Alexis NE, Kleeberger SR, Roubey RA, Harris BD, Bromberg PA, et al. Ozone enhances markers of innate immunity and antigen presentation on airway monocytes in healthy individuals. J Allergy Clin Immunol. 2007;120:719–22. doi: 10.1016/j.jaci.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Alexis NE, Lay JC, Zeman K, Bennett WE, Peden DB, Soukup JM, et al. Biological material on inhaled coarse fraction particulate matter activates airway phagocytes in vivo in healthy volunteers. J Allergy Clin Immunol. 2006;117:1396–403. doi: 10.1016/j.jaci.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 14.Borish LC, Steinke JW. 2. Cytokines and chemokines. J Allergy Clin Immunol. 2003;111:S460–75. doi: 10.1067/mai.2003.108. [DOI] [PubMed] [Google Scholar]
- 15.Maus UA, Janzen S, Wall G, Srivastava M, Blackwell TS, Christman JW, et al. Resident alveolar macrophages are replaced by recruited monocytes in response to endotoxin-induced lung inflammation. Am J Respir Cell Mol Biol. 2006;35:227–35. doi: 10.1165/rcmb.2005-0241OC. [DOI] [PubMed] [Google Scholar]
- 16.Lofdahl JM, Wahlstrom J, Skold CM. Different inflammatory cell pattern and macrophage phenotype in chronic obstructive pulmonary disease patients, smokers and non-smokers. Clin Exp Immunol. 2006;145:428–37. doi: 10.1111/j.1365-2249.2006.03154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pons AR, Noguera A, Blanquer D, Sauleda J, Pons J, Agusti AG. Phenotypic characterisation of alveolar macrophages and peripheral blood monocytes in COPD. Eur Respir J. 2005;25:647–52. doi: 10.1183/09031936.05.00062304. [DOI] [PubMed] [Google Scholar]
- 18.Lensmar C, Katchar K, Eklund A, Grunewald J, Wahlstrom J. Phenotypic analysis of alveolar macrophages and lymphocytes following allergen inhalation by atopic subjects with mild asthma. Respir Med. 2006;100:918–25. doi: 10.1016/j.rmed.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 19.Wahl SM, Katona IM, Stadler BM, Wilder RL, Helsel WE, Wahl LM. Isolation of human mononuclear cell subsets by counterflow centrifugal elutriation (CCE). II. Functional properties of B-lymphocyte-, T-lymphocyte-, and monocyte-enriched fractions. Cell Immunol. 1984;85:384–95. doi: 10.1016/0008-8749(84)90252-1. [DOI] [PubMed] [Google Scholar]
- 20.Hashimoto SI, Komuro I, Yamada M, Akagawa KS. IL-10 inhibits granulocyte-macrophage colony-stimulating factor-dependent human monocyte survival at the early stage of the culture and inhibits the generation of macrophages. J Immunol. 2001;167:3619–25. doi: 10.4049/jimmunol.167.7.3619. [DOI] [PubMed] [Google Scholar]
- 21.Gentry MJ, Snitily MU, Preheim LC. Phagocytosis of Streptococcus pneumoniae measured in vitro and in vivo in a rat model of carbon tetrachloride-induced liver cirrhosis. J Infect Dis. 1995;171:350–5. doi: 10.1093/infdis/171.2.350. [DOI] [PubMed] [Google Scholar]
- 22.Mokart D, Guery BP, Bouabdallah R, Martin C, Blache JL, Arnoulet C, et al. Deactivation of alveolar macrophages in septic neutropenic ARDS. Chest. 2003;124:644–52. doi: 10.1378/chest.124.2.644. [DOI] [PubMed] [Google Scholar]
- 23.Balbo P, Silvestri M, Rossi GA, Crimi E, Burastero SE. Differential role of CD80 and CD86 on alveolar macrophages in the presentation of allergen to T lymphocytes in asthma. Clin Exp Allergy. 2001;31:625–36. doi: 10.1046/j.1365-2222.2001.01068.x. [DOI] [PubMed] [Google Scholar]
- 24.Zejda JE, Hurst TS, Rhodes CS, Barber EM, McDuffie HH, Dosman JA. Respiratory health of swine producers. Focus on young workers. Chest. 1993;103:702–9. doi: 10.1378/chest.103.3.702. [DOI] [PubMed] [Google Scholar]
- 25.Alexis NE, Lay JC, Zeman KL, Geiser M, Kapp N, Bennett WD. In vivo particle uptake by airway macrophages in healthy volunteers. Am J Respir Cell Mol Biol. 2006;34:305–13. doi: 10.1165/rcmb.2005-0373OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berenson CS, Garlipp MA, Grove LJ, Maloney J, Sethi S. Impaired phagocytosis of nontypeable Haemophilus influenzae by human alveolar macrophages in chronic obstructive pulmonary disease. J Infect Dis. 2006;194:1375–84. doi: 10.1086/508428. [DOI] [PubMed] [Google Scholar]
- 27.Berenson CS, Wrona CT, Grove LJ, Maloney J, Garlipp MA, Wallace PK, et al. Impaired alveolar macrophage response to Haemophilus antigens in chronic obstructive lung disease. Am J Respir Crit Care Med. 2006;174:31–40. doi: 10.1164/rccm.200509-1461OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yin XJ, Dong CC, Ma JY, Roberts JR, Antonini JM, Ma JK. Suppression of phagocytic and bactericidal functions of rat alveolar macrophages by the organic component of diesel exhaust particles. J Toxicol Environ Health A. 2007;70:820–8. doi: 10.1080/15287390701209766. [DOI] [PubMed] [Google Scholar]
- 29.Leong PA, Cohen MS. Group A streptococcal peptidoglycan-polysaccharide inhibits phagocytic activity of human polymorphonuclear leukocytes. Infect Immun. 1984;45:378–83. doi: 10.1128/iai.45.2.378-383.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dziarski R. Peptidoglycan recognition proteins (PGRPs) Mol Immunol. 2004;40:877–86. doi: 10.1016/j.molimm.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 31.Yang S, Tamai R, Akashi S, Takeuchi O, Akira S, Sugawara S, et al. Synergistic effect of muramyldipeptide with lipopolysaccharide or lipoteichoic acid to induce inflammatory cytokines in human monocytic cells in culture. Infect Immun. 2001;69:2045–53. doi: 10.1128/IAI.69.4.2045-2053.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Schematic representation of experimental protocol showing elutriated monocytes cultured with GM-CSF (1000 IU/mL) with and without low concentration organic dust extract (ODE; 0.1%) for six days (E1). Monocytes cultured with GM-CSF and ODE (0.1%) for 48 hours, and washed and maintained in culture for the remainder 4 days (E2; MDMs/early ODE exposed). Macrophages rested for 18 hours prior to experimental assays.
Cytokine secretion in monocyte-derived macrophages (MDMs) at 1 week after restimulation with ODE (1%) for 5 hours. TNFα (A) and IL-6 (B) secretion was dampened in all conditioned-MDMs compared to control-MDMs except for heat-inactivated ODE-MDMs (N=3). Mean results are presented as the percentage of ODE-induced cytokine secretion in control MDMs ± SEM. *Statistically significant (p<0.05).
Cytokine secretion in monocyte-derived macrophages (MDMs) at 1 week after restimulation with LPS (100 ng/mL; E2A-B) and PGN (40 μg/mL; E2C-D). TNFα (E2A and E2C) and IL-6 (E2B and E2D) secretion was dampened in all conditioned-MDMs compared to control-MDMs (N=3). Mean results are presented as the percentage of LPS or PGN -induced cytokine secretion in control MDMs ± SEM. *Statistically significant (p<0.05).


