Abstract
The majority of heart failure patients are older adults and most heart failure-related adverse events occur in these patients. However, the independent association of age and outcomes in chronic heart failure is not clearly determined. We categorized 7788 ambulatory chronic heart failure patients who participated in the Digitalis Investigation Group trial as younger and older using the cutoff of 65 years. Propensity scores for age were calculated for each patient and used to match 2381 older patients with 2381 younger patients. The impact of age on mortality and hospitalization during a median 40 months of follow-up was assessed using matched Cox regression methods. All-cause mortality occurred in 877 older patients versus 688 younger patients (hazard ratio when older age was compared with younger age (HR) = 1.26, 95% confidence interval (CI) = 1.12–1.41, p <0.0001). Older patients, when compared with propensity-matched younger patients, also had significantly higher mortality rates due to cardiovascular causes (HR = 1.14; 95% CI = 1.00–1.30, p = 0.044) and worsening heart failure causes (HR = 1.32; 95% CI = 1.07–1.62, p = 0.009). No significant association was found between age and hospitalization due to all causes (HR = 1.08; 95% CI = 0.99–1.18, p = 0.084) and cardiovascular causes (HR = 1.03; 95% CI = 0.93–1.13, p = 0.622). However, hospitalization due to heart failure was significantly increased in older patients (HR = 1.14; 95% CI = 1.01–1.28, p = 0.041). In ambulatory chronic heart failure patients, older age although associated with increased mortality was not associated with increased hospitalizations except for those due to worsening heart failure.
Keywords: Heart failure, mortality, hospitalization, propensity score, older adults
1. Introduction
Aging is associated with multiple morbidities and functional decline that may explain the increased mortality and hospitalization observed in older adults (Sager and Rudberg, 1998; Wolinsky et al., 1993; Yancik et al., 2007). Most heart failure (HF) patients are older adults and most of the HF-related hospitalization and mortality occurs in these patients (Rosamond et al., 2007). Previous studies based on multivariable regression-based risk adjustment models have described age as a predictor for mortality (Cowie et al., 2002; Pocock et al., 2006). However, the extent to which aging is independently associated with mortality and morbidity in HF patients is not well known. The purpose of this study was to examine the effect of age on all-cause and cause-specific mortalities and hospitalizations in a propensity-matched population of chronic HF patients.
2. Subjects and methods
2.1. Data Source and Patients
This study was conducted using retrospective analysis of public-use data sets from the Digitalis Investigation Group (DIG) trial, a randomized clinical trial studying the effects of digoxin in HF patients. Between January 1991 and August 1993 the DIG trial enrolled 7788 ambulatory chronic HF patients from 302 clinical centers across the United States (186 centers) and Canada (116 centers). Of these participants, 4036 (52%) were age 65 or older, and overall ages ranged from 21 to 94. Study design and results from the DIG trial have been previously published (The Digitalis Investigation Group, 1996, 1997). For the purpose of this analysis, we categorized patients into younger and older adults based on an age cutoff of 65 years.
2.2. Outcomes
Mortality and hospitalizations attributed to all causes were the outcome measures used to assess the associations of age and outcomes in HF patients. We also examined associations of age with mortality and hospitalization due to cardiovascular causes and worsening HF. End-of-trial vital status was known for 99% (7691) of DIG participants (Collins et al., 2003).
2.3. Estimation of propensity scores
To determine an independence of association between age and outcomes, we used propensity score matching to assemble a cohort of patients who were well balanced in all measured baseline covariates. First, we used a non-parsimonious multivariable logistic regression model to calculate, for each patient, a propensity score for having age ≥65 years (Ahmed, 2008; Ahmed et al., 2006a,b; Ahmed et al., 2007a,b; Rosenbaum and Rubin, 1983; Rosenbaum and Rubin, 1984; Rubin, 1997; Rubin, 2001; Rubin, 2004). In the model, age ≥65 years was used as the dependent variable, and all covariates shown in Table 1, with the exception of estimated glomerular filtration rate (Levey et al., 1999) and chronic kidney disease (derived values), were entered as covariates into the model.
Table 1.
Baseline patient characteristics, by age, before and after propensity score matching
| Variable | Before matching
|
After matching
|
||||
|---|---|---|---|---|---|---|
| Age <65 (n = 3752) | Age =65 (n = 4036) | p Value | Age <65 (n = 2381) | Age =65 (n = 2381) | p Value | |
| N (%) or mean (±S.D.) | ||||||
| Female | 800 (21%) | 1126 (28%) | <0.0001 | 556 (23%) | 536 (23%) | 0.507 |
| Non-white | 679 (18%) | 449 (11%) | <0.0001 | 325 (14%) | 331 (14%) | 0.828 |
| Body mass index, (kg/m2) | 26 ± 6 | 26 ± 5 | <0.0001 | 27 ± 5 | 27 ± 5 | 0.742 |
| Duration of HF (months) | 30 ± 35.7 | 29.5 ± 37.1 | 0.025 | 30 ± 36 | 30 ± 38 | 0.724 |
| Primary cause of HF | ||||||
| Ischemic | 2439 (65%) | 2921 (72%) | 1683 (71%) | 1665 (70%) | ||
| Hypertensive | 343 (9%) | 462 (11%) | 231 (9%) | 258 (11%) | ||
| Idiopathic | 662 (18%) | 449 (11%) | <0.0001 | 333 (14%) | 321 (14%) | 0.102 |
| Others | 308 (8%) | 204 (5%) | 152 (6%) | 137 (6%) | ||
| Prior myocardial infarction | 2283 (61%) | 2625 (65%) | <0.0001 | 1541 (65%) | 1568 (66%) | 0.430 |
| Current angina pectoris | 968 (26%) | 1147 (28%) | 0.010 | 649 (27%) | 657 (28%) | 0.820 |
| Hypertension | 1689 (45%) | 1985 (49%) | <0.0001 | 1068 (45%) | 1088 (46%) | 0.575 |
| Diabetes mellitus | 1046 (28%) | 1172 (29%) | 0.258 | 678 (29%) | 695 (29%) | 0.606 |
| Medications | ||||||
| Pre-trial digoxin use | 1662 (44%) | 1703 (42%) | 0.064 | 1014 (43%) | 994 (42%) | 0.581 |
| Trial use of digoxin | 1885 (50%) | 2004 (50%) | 0.618 | 1174 (49%) | 1198 (50%) | 0.506 |
| Angiotensin converting enzyme inhibitors | 3539 (94%) | 3735 (93%) | 0.002 | 2229 (94%) | 2228 (94%) | 1.000 |
| Hydralazine and nitrates | 53 (1%) | 58 (1%) | 1.000 | 30 (1%) | 33 (1%) | 0.798 |
| Diuretics | 2805 (75%) | 3271 (81%) | <0.0001 | 1857 (78%) | 1830 (77%) | 0.359 |
| Potassium-sparing diuretics | 297 (8%) | 299 (7%) | 0.418 | 174 (7%) | 188 (8%) | 0.472 |
| Potassium supplement | 1019 (27%) | 1180 (29%) | 0.044 | 685 (29%) | 664 (28%) | 0.513 |
| Symptoms and signs of HF | ||||||
| Dyspnea at rest | 832 (22%) | 873 (22%) | .565 | 515 (22%) | 514 (22%) | 1.000 |
| Dyspnea on exertion | 2763 (74%) | 3099 (77%) | 0.001 | 1785 (75%) | 1797 (76%) | 0.710 |
| Jugular venous distension | 459 (12%) | 561 (14%) | 0.031 | 293 (12%) | 305 (13%) | 0.631 |
| Third heart sound | 920 (25%) | 926 (23%) | 0.104 | 573 (24%) | 544 (23%) | 0.342 |
| Pulmonary râles | 489 (13%) | 812 (20%) | <0.0001 | 372 (16%) | 384 (16%) | 0.650 |
| Lower extremity edema | 740 (20%) | 893 (22%) | 0.010 | 479 (20%) | 495 (21%) | 0.586 |
| Number of symptom/signs | 5.4 ± 2.1 | 5.5 ± 2.0 | 0.060 | 5.4 ± 2.1 | 5.4 ± 2.0 | 0.714 |
| New York Heart Association functional class | ||||||
| Class I | 587 (16%) | 516 (13%) | 343 (14%) | 328 (14%) | ||
| Class II | 2093 (56%) | 2151 (53%) | 1309 (55%) | 1348 (57%) | ||
| Class III | 1011 (27%) | 1276 (32%) | <0.0001 | 681 (29%) | 665 (28%) | 0.489 |
| Class IV | 61 (2%) | 93 (2%) | 48 (2%) | 40 (2%) | ||
| Heart rate (/minute), | 79 ± 13 | 77 ± 12 | <0.0001 | 78 ± 12 | 78 ± 12 | 0.338 |
| Blood pressure (mm Hg) | ||||||
| Systolic | 125 ± 20 | 130 ± 21 | <0.0001 | 127 ± 21 | 127 ± 19 | 0.889 |
| Diastolic | 76 ± 11 | 74 ± 11 | <0.0001 | 75 ± 11 | 75 ± 11 | 0.611 |
| Chest radiograph findings | ||||||
| Pulmonary congestion | 492 (13%) | 617 (15%) | 0.006 | 324 (14%) | 329 (14%) | 0.866 |
| Cardiothoracic ratio >0.5 | 2154 (57%) | 2536 (63%) | <0.0001 | 1408 (59%) | 1392 (59%) | 0.661 |
| Serum creatinine (mg/dL) | 1.20 ± 0.32 | 1.36 ± 0.40 | <0.0001 | 1.25 ± 0.36 | 1.26 ± 0.32 | 0.277 |
| Serum potassium (mEq/L) | 4.3 ± 0.4 | 4.4 ± 0.4 | <0.0001 | 4.3 ± 0.4 | 4.3 ± 0.4 | 0.265 |
| Ejection fraction (%) | 31 ± 12 | 33 ± 13 | <0.0001 | 31 ± 12 | 32 ± 13 | 0.370 |
2.4. Propensity score matching
A propensity score matching technique was utilized to balance available baseline covariates. Although propensity scores are often used to match baseline characteristics between two treatment groups in an observational study, the method can also be used to match baseline characteristics between two groups of patients based on comorbidities or other characteristics such as age or race (Ahmed, 2008; Ahmed et al., 2006a,b; Ahmed et al., 2007a,b). Using an SPSS macro and a greedy matching protocol, each patient ≥65 years of age was matched with a younger patient based on propensity score (Levesque, 2005). Overall we matched 2381 (59%) patients ≥65 years with 2381 younger patients who had similar propensity scores. The unique aspect of assembling a risk-adjusted well-balanced study cohort using propensity matching is that it can be done without access to outcomes data, thus simulating one key feature of a randomized clinical trial, and therefore adding transparency to study design. However, unlike a randomized clinical trial, this process may or may not balance unmeasured covariates.
2.5. Quantification of bias reduction: standardized differences
To assess the balance between groups achieved through propensity score matching, absolute standardized differences were calculated for each variable. The acceptable level of balance is taken to be a standardized difference under 10% (Ahmed et al., 2006a,b; Normand et al., 2001).
2.6. Statistical Analysis
To compare baseline characteristics between the older and younger age groups we used Pearson Chi square and Wilcoxon rank-sum tests for the pre-matched population and McNemar’s test and paired sample t-test for the matched population as appropriate. Kaplan-Meier survival analysis and matched Cox proportional hazard analysis were used to determine the association between age and various outcomes. Log-minus-log scale survival plots were used to check proportional hazards assumptions. We conducted subgroup analyses and tested for interactions to examine if there was any heterogeneity in the association between age and mortality. All statistical tests were evaluated using two-tailed 95% confidence levels, and a p <0.05 was required for significance. SPSS for Windows (Version 14) was used for all data analysis (SPSS, 2006).
3. Results
3.1. Patient characteristics
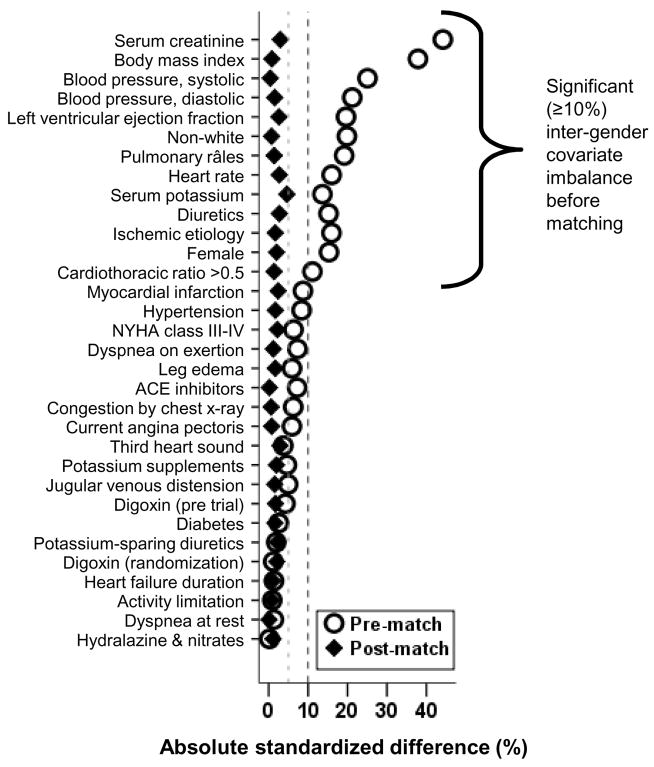
Overall, 23% of patients in the matched study population were female, 14% were non-white, and the mean ages of the two groups were 71 (±5.0) years and 56 (±7.0) years. Baseline characteristics for both age groups before and after matching for are displayed in Table 1. Before matching, patients age ≥65 years (n=4036) were more likely than younger patients (n=3752) to be female, white and have ischemic heart disease, hypertension, cardiomegaly, pulmonary râles, higher serum creatinine and be receiving diuretics. The mean ages (±SD) of the older and younger groups were 72 (±5.4) years and 55 (±7.9) years, respectively. Interestingly, the duration of HF was similar in young and older patients. After matching, patients were balanced on all measured covariates (Table 1). Absolute standard differences between age groups were <5% for all measured covariates after propensity score matching (Figure 1), indicating a substantial reduction of bias (Ahmed et al., 2006a,b; Normand et al., 2001).
Figure 1.
Love plots for absolute standardized differences before and after propensity score matching comparing covariate values for patients age <65 years and age ≥65 years.
3.2. Association of age and mortality
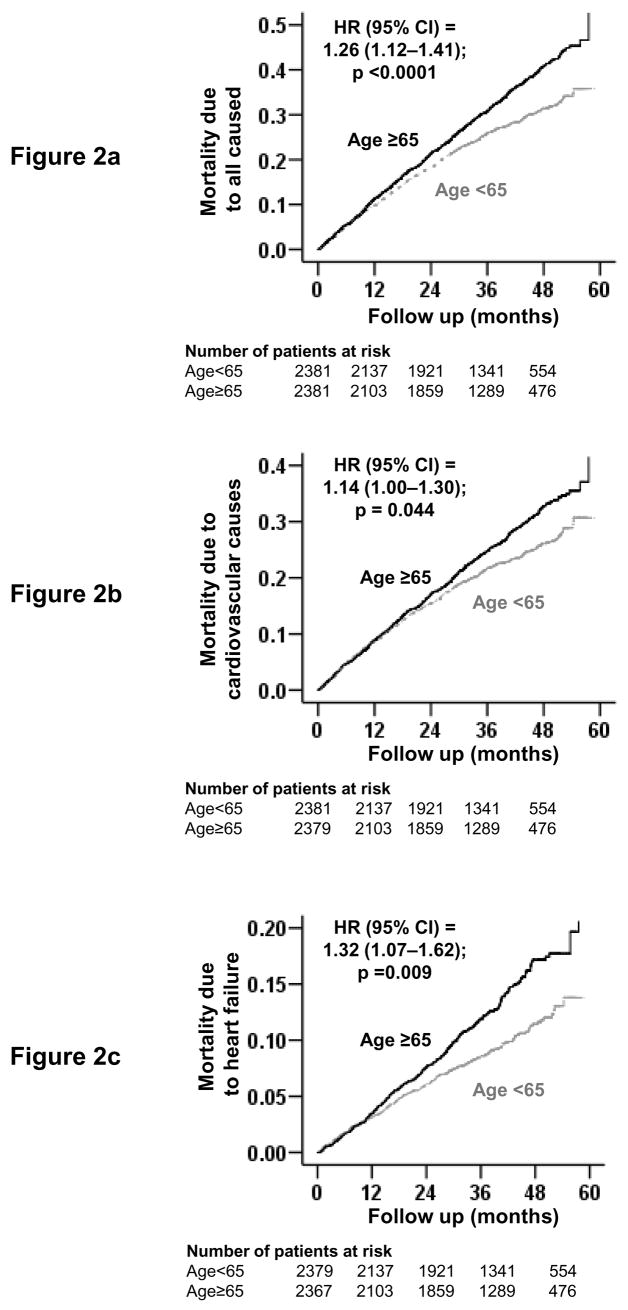
Overall, 1565 (33%) of the 4762 propensity-matched patients died during a median follow-up of 38 months; 1223 of these mortalities were attributed to cardiovascular causes and 516 to worsening HF. All-cause mortality occurred in 877 patients age ≥65 years versus 688 younger patients during 6904 and 7125 total years of follow-up, respectively. Mortality rates for older and younger patients were, respectively, 1270 and 966 per 10,000 person-years of follow-up (hazard ratio (HR) = 1.26, 95% confidence interval (CI) = 1.12–1.41, p <0.0001; Table 2). Older patients, when compared with propensity-matched younger patients, also had significantly increased mortality rates due to cardiovascular causes (HR = 1.14; 95% CI =1.00–1.30; p = 0.044; Table 2) and worsening HF causes (HR = 1.32; 95% CI =1.07–1.62; p = 0.009; Table 2). Kaplan-Meier survival curves for all-cause, cardiovascular, and HF mortality are displayed in Figure 2.
Table 2.
Mortality by causes in heart failure patients before and after matching by propensity scores for age ≥65 years.
| Rate, per 10,000 person-years (Events/total follow-up years)
|
Absolute rate difference* (per 10,000 person-years) | Hazard ratio† (95% confidence interval) | P value | ||
|---|---|---|---|---|---|
| Age <65 | Age ≥65 | ||||
| Pre-match | (N=3752) | (N=4036) | |||
| All-cause | 901 (1030/11430) | 1377 (1576/11443) | + 476 | 1.53 (1.41–1.66) | <0.0001 |
| Cardiovascular | 741 (847/11430) | 1053 (1205/11443) | + 312 | 1.42 (1.30–1.55) | <0.0001 |
| Progressive heart failure | 293 (335/11430) | 500 (572/11443) | + 207 | 1.71 (1.50–1.96) | <0.0001 |
| Post-match | (N=2381) | (N=2381) | |||
| All-cause | 966 (688/7125) | 1270 (877/6904) | + 305 | 1.26 (1.12–1.41) | <0.0001 |
| Cardiovascular | 786 (560/7125) | 960 (663/6904) | + 174 | 1.14 (1.00–1.30) | 0.044 |
| Progressive heart failure | 305 (217/7125) | 433 (299/6904) | + 129 | 1.32 (1.07–1.62) | 0.009 |
Absolute differences in rates of events per 10,000 person-year of follow-up were calculated by subtracting the event rates in the age <65 years group from the event rates in the age ≥65 group (before values were rounded).
Hazard ratios and confidence intervals (CI) were estimated from matched Cox proportional hazards models
Figure 2.
Kaplan-Meier plots for mortality due to (a) all-causes, (b) cardiovascular causes, and (c) worsening heart failure
3.3. Age and hospitalization
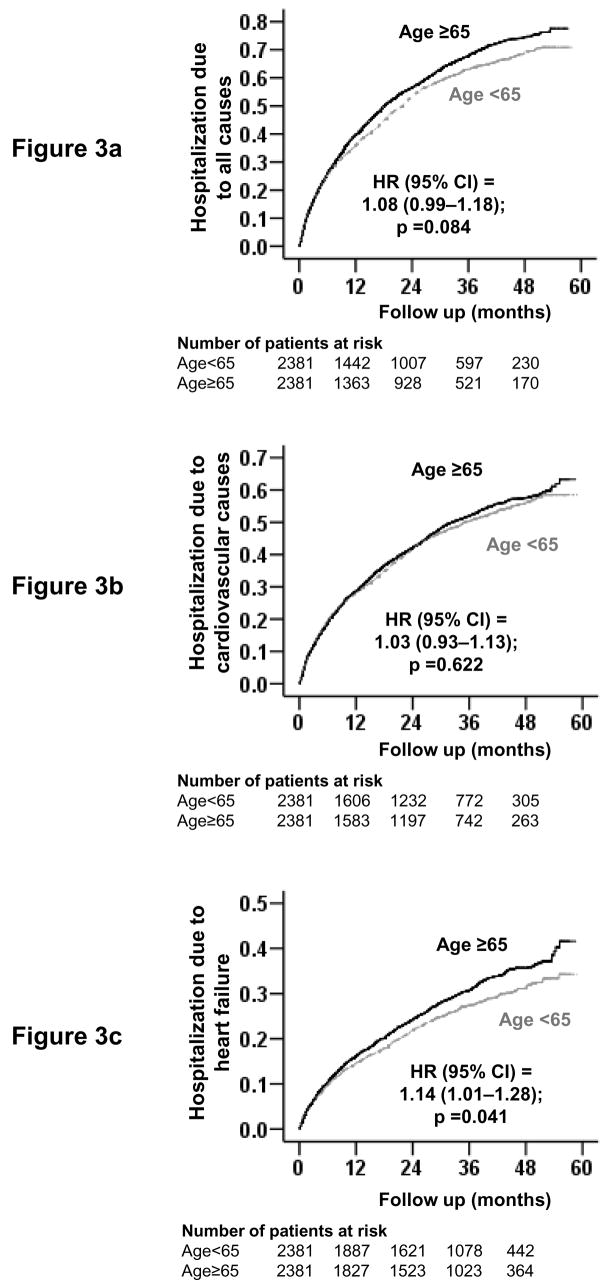
Overall, 3106 patients had hospitalizations due to all causes, including 2421 due to cardiovascular causes and 1379 due to worsening HF. All-cause hospitalization occurred in 1611 patients age ≥65 years compared with 1495 younger patients during 3991 and 4307 years of follow-up, respectively. Rates for all-cause hospitalization per 10,000 person-years were 3471 for younger patients and 4036 for older patients (HR = 1.08; 95% CI = 0.99–1.18; p = 0.084; Table 3). There was no significant association between age and hospitalization due to cardiovascular causes. Rates of HF hospitalization per 10,000 person-years were 1058 and 1248 respectively for younger and older patients (HR = 1.14; 95% CI = 1.01–1.28; p = 0.041; Table 3). Kaplan-Meier survival curves for all-cause, cardiovascular, and HF hospitalizations are shown in Figure 3.
Table 3.
Hospitalizations† by causes in heart failure patients before and after matching by propensity scores for age ≥65 years.
| Rate, per 10,000 person-years (Events/total follow-up years)
|
Absolute rate difference (per 10,000 person-years) | Hazard ratio† (95% confidence interval) | P value | ||
|---|---|---|---|---|---|
| Age <65 | Age ≥65 | ||||
| Pre-match | (N=3752) | (N=4036) | |||
| All-cause | 3378 (2333/6906) | 4293 (2795/6510) | + 915 | 1.23 (1.17–1.30) | <0.0001 |
| Cardiovascular | 2346 (1873/7983) | 2705 (2137/7899) | + 359 | 1.13 (1.06–1.20) | <0.0001 |
| Worsening heart failure | 1033 (1014/9819) | 1321 (1273/9634) | + 289 | 1.25 (1.15–1.30) | <0.0001 |
| Post-match | (N=2381) | (N=2381) | |||
| All-cause | 3471 (1495/4307) | 4036 (1611/3991) | + 565 | 1.08 (0.99–1.18) | 0.084 |
| Cardiovascular | 2399 (1197/4989) | 2531 (1224/4836) | + 132 | 1.03 (0.93-1.13) | 0.622 |
| Worsening heart failure | 1058 (650/6146) | 1248(729/5842) | + 190 | 1.14 (1.01–1.28) | 0.041 |
| Number of total hospitalizations | 4388 | 4763 | + 375 | ||
Data shown include the first hospitalization of each patient for each cause.
Absolute differences in rates of events per 10,000 person-year of follow-up were calculated by subtracting the event rates in the age ≥65 years group from the event rates in the age >65 years group (before values were rounded).
Hazard ratios and confidence intervals (CI) were estimated from matched Cox proportional hazards models
Figure 3. Kaplan-Meier plots for hospitalization due to (a) all-causes, (b) cardiovascular causes, and (c) worsening heart failure.
(Kaplan-Meier plots do not account for matching within the data, but HR estimates are calculated based on matched pairs)
3.4. Subgroup analyses
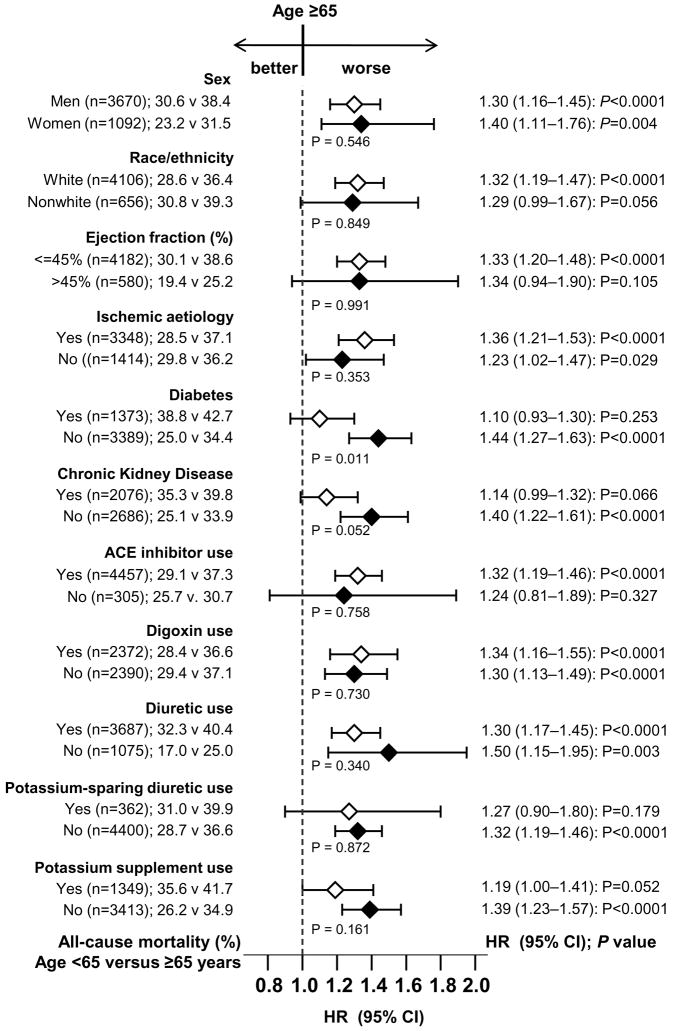
The effects of age on mortality were also evident across various subgroups of patients, as shown in Figure 4. There were no significant interactions between older age and any of the subgroups except for diabetes (P for interaction = 0.011).
Figure 4.
Association of age ≥65 years and all-cause mortality in subgroups of propensity score matched heart failure patients (middle p values are for interaction).
4. Discussion
4.1. Key study findings
Findings from this study indicate that among ambulatory chronic HF patients, compared to patients younger than 65 years, those ≥65 years were more likely to die from all causes, cardiovascular causes and progressive HF, but not more likely to be hospitalized for all causes or cardiovascular causes. Older age was associated with hospitalization due to worsening HF. The findings are interesting as they demonstrate the independent effect of age on the association of older age with mortality and hospitalization. These results are important as most HF patients are ≥65 years and HF is the number one reason for hospital admission for population ≥65 years (Rosamond et al., 2007). Further, it has been projected that the prevalence of HF may double over the next several decades as the baby-boomer generation ages.
4.2. Explanation of study findings
The association of age with increased mortality is not surprising as aging is associated with increased morbidities and functional decline (Sager and Rudberg, 1998; Wolinsky et al., 1993; Yancik et al., 2007). This is reflected by stronger associations of age with unadjusted mortality (Table 2). However, after matching when all measured baseline characteristics were balanced, these associations became weaker, but remained significant, suggesting an independent association of age with mortality in chronic HF. However, the association of age with mortality was not apparent until after the first 2 years of follow-up (Figure 2). On the other hand, in the case of hospitalization, significant unadjusted associations of age with all-cause and cardiovascular hospitalization became nonsignificant after matching (Table 3). A possible explanation of increased cardiovascular mortality without an associated increase in cardiovascular hospitalization among older adults is that many of these non-HF cardiovascular deaths may be due to lack of symptoms (e.g. silent ischemia) and sudden cardiac deaths (e.g. ventricular arrhythmias), precluding hospital admissions. Older adults are also known to underestimate symptoms, attributing them to aging, and also wishing to avoid emergency room visits and hospital admissions due to long waiting times and prior experience of confusion and delirium (Rothschild et al., 2000). However, that paradigm may not be totally applicable to older adults with symptoms of HF.
In the matched cohort, the association of age with HF hospitalization became weaker but retained a significant independent association. These findings suggest that, although older adults with chronic HF may be less likely to be hospitalized due to other cardiovascular causes, they are more likely to respond to their HF symptoms. This may be due to the fact that unlike other cardiovascular symptoms, such as chest pain, which may be mild or absent in older adults with myocardial ischemia, symptoms of HF, such as shortness of breath or fatigue, are almost never silent.
4.3. Clinical and public health implications
What are the implications for this apparent disconnect between mortality and hospitalizations among elderly HF patients? The results of our study suggest that in older adults with chronic HF, hospitalizations are largely driven by comorbidities and severity of disease and, to a smaller extent, due to aging. Further, our data suggest that older adults may be more likely to ignore non-HF cardiovascular symptoms such as chest pain or palpitation and non-cardiovascular symptoms, but may be more responsive to HF symptoms. HF is the leading cause of hospitalization among older adults (Rosamond et al., 2007) and doubling of the HF population in the coming decades will likely further increase the burden on the health care delivery system. The findings of the current analysis underscore the need for proper outpatient management of older adults with chronic HF in a timely manner. Appropriate patient and family/caregiver education including recognition and treatment of precipitating factors will likely help avoid unnecessary hospitalizations as well as improve quality of life (Sui et al., 2007). An alternate approach might be to improve symptom recognition in outpatient settings and increase appropriate utilization of hospice and palliative care services.
4.4. Literature comparison
Our finding of increased mortality among older HF patients compared with younger patients is consistent with previous studies that show an association between age and HF outcomes (Cowie et al., 2002; Pocock et al., 2006; Roger et al., 2004). These studies have identified age as a predictor for mortality in multivariable regression-based risk adjustment models used for other predictors. However, none of these studies have compared mortality and hospitalization rates between propensity-matched older and younger HF patients.
4.5. Limitations
Although propensity score matching is a powerful technique to provide balance in all measured covariates, it is possible that unmeasured covariates may not be completely balanced. However, for an unmeasured covariate to significantly impact our findings, it must be strongly associated with age and the outcomes, but not be strongly associated with any of the many measured baseline covariates included in the DIG trial.
4.6. Conclusion
In a propensity-matched population of HF patients who were well balanced in all measured covariates, older age was independently associated with increased mortality but not with increased hospitalization except for those due to worsening HF.
Acknowledgments
The DIG study was conducted and supported by the NHLBI in collaboration with the DIG Investigators. This Manuscript was prepared using a limited access dataset obtained from the NHLBI and does not necessarily reflect the opinions or views of the DIG Study or the NHLBI.
Funding/Support
Dr. Ahmed is supported by the National Institutes of Health through grants from the National Heart, Lung, and Blood Institute (R01-HL085561 and P50-HL077100), and a generous gift from Ms. Jean B. Morris of Birmingham, Alabama.
Footnotes
Conflict of Interest Disclosures: None
References
- Ahmed A, Aronow WS. A propensity-matched study of the association of physical function and outcomes in geriatric heart failure. Arch Gerontol Geriatr. 2008;46:161–172. doi: 10.1016/j.archger.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed A, Husain A, Love TE, Gambassi G, Dell'Italia LJ, Francis GS, Gheorghiade M, Allman RM, Meleth S, Bourge RC. Heart failure, chronic diuretic use, and increase in mortality and hospitalization: an observational study using propensity score methods. Eur Heart J. 2006a;27:1431–1439. doi: 10.1093/eurheartj/ehi890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed A, Perry GJ, Fleg JL, Love TE, Goff DC, Jr, Kitzman DW. Outcomes in ambulatory chronic systolic and diastolic heart failure: a propensity score analysis. Am Heart J. 2006b;152:956–966. doi: 10.1016/j.ahj.2006.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed A, Aban IB, Vaccarino V, Lloyd-Jones DM, Goff DC, Jr, Zhao J, Love TE, Ritchie C, Ovalle F, Gambassi G, Dell'Italia LJ. A propensity-matched study of the effect of diabetes on the natural history of heart failure: variations by sex and age. Heart. 2007a;93:1584–1590. doi: 10.1136/hrt.2006.113522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed A, Zannad F, Love TE, Tallaj J, Gheorghiade M, Ekundayo OJ, Pitt B. A propensity-matched study of the association of low serum potassium levels and mortality in chronic heart failure. Eur Heart J. 2007b;28:1334–1343. doi: 10.1093/eurheartj/ehm091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins JF, Howell CL, Horney RA. Determination of vital status at the end of the DIG trial. Control Clin Trials. 2003;24:726–730. doi: 10.1016/j.cct.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Cowie MR, Fox KF, Wood DA, Metcalfe C, Thompson SG, Coats AJ, Poole-Wilson PA, Sutton GC. Hospitalization of patients with heart failure: a population-based study. Eur Heart J. 2002;23:877–885. doi: 10.1053/euhj.2001.2973. [DOI] [PubMed] [Google Scholar]
- Levesque R. Macro. In: Levesque R, editor. SPSS® Programming and Data Management, 2nd Edition. A Guide for SPSS® and SAS® Users. 2. SPSS Inc; Chicago, IL: [accessed 04.06.05]. http://www.spss.com/spss/data_management_book.htm. [Google Scholar]
- Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Int Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- Normand ST, Landrum MB, Guadagnoli E, Ayanian JZ, Ryan TJ, Cleary PD, McNeil BJ. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54:387–398. doi: 10.1016/s0895-4356(00)00321-8. [DOI] [PubMed] [Google Scholar]
- Pocock SJ, Wang D, Pfeffer MA, Yusuf S, McMurray JJ, Swedberg KB, Ostergren J, Michelson EL, Pieper KS, Granger CB. Predictors of mortality and morbidity in patients with chronic heart failure. Eur Heart J. 2006;27:65–75. doi: 10.1093/eurheartj/ehi555. [DOI] [PubMed] [Google Scholar]
- Roger VL, Weston SA, Redfield MM, Hellermann-Homan JP, Killian J, Yawn BP, Jacobsen SJ. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292:344–350. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O’Donnell CJ, Roger V, Rumsfeld J, Sorlie P, Steinberger J, Thom T, Wasserthiel-Smoller S, Hong Y for the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics--2007 Update: A Report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- Rosenbaum PR, Rubin DB. The central role of propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- Rosenbaum PR, Rubin DB. Reducing bias in observational studies using subclassification on the propensity score. J Am Stat Assoc. 1984;79:516–524. [Google Scholar]
- Rothschild JM, Bates DW, Leape LL. Preventable medical injuries in older patients. Arch Intern Med. 2000;160:2717–2728. doi: 10.1001/archinte.160.18.2717. [DOI] [PubMed] [Google Scholar]
- Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med. 1997;127:757–763. doi: 10.7326/0003-4819-127-8_part_2-199710151-00064. [DOI] [PubMed] [Google Scholar]
- Rubin DB. Using Propensity Scores to Help Design Observational Studies: Application to the Tobacco Litigation. Health Serv Outcomes Res Methodol. 2001;2:169–188. [Google Scholar]
- Rubin DB. On principles for modeling propensity scores in medical research. Pharmacoepidemiol Drug Saf. 2004;13:855–857. doi: 10.1002/pds.968. [DOI] [PubMed] [Google Scholar]
- Sager MA, Rudberg MA. Functional decline associated with hospitalization for acute illness. Clin Geriatr Med. 1998;14:669–679. [PubMed] [Google Scholar]
- SPSS. SPSS for Windows, Version 14. SPSS Inc; Chicago, IL: 2006. [Google Scholar]
- Sui X, Gheorghiade M, Zannad F, Young JB, Ahmed A. A propensity matched study of the association of education and outcomes in chronic heart failure. Int J Cardiol Epub. 2007 doi: 10.1016/j.ijcard.2007.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Digitalis Investigation Group. Rationale, design, implementation, and baseline characteristics of patients in the DIG trial: a large, simple, long-term trial to evaluate the effect of digitalis on mortality in heart failure. Control Clin Trials. 1996;17:77–97. doi: 10.1016/0197-2456(95)00065-8. [DOI] [PubMed] [Google Scholar]
- The Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. The Digitalis Investigation Group. N Engl J Med. 1997;336:525–533. doi: 10.1056/NEJM199702203360801. [DOI] [PubMed] [Google Scholar]
- Wolinsky FD, Callahan CM, Fitzgerald JF, Johnson RJ. Changes in functional status and the risks of subsequent nursing home placement and death. J Gerontol. 1993;48:S94–101. [PubMed] [Google Scholar]
- Yancik R, Ershler W, Satariano W, Hazzard W, Cohen HJ, Ferrucci L. Report of the national institute on aging task force on comorbidity. J Gerontol A Biol Sci Med Sci. 2007;62:275–280. doi: 10.1093/gerona/62.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]