Abstract
Aims
To determine the effects of digoxin on all-cause mortality and heart failure (HF) hospitalizations, regardless of ejection fraction, accounting for serum digoxin concentration (SDC).
Methods and results
This comprehensive post-hoc analysis of the randomized controlled Digitalis Investigation Group trial (n ¼ 7788) focuses on 5548 patients: 1687 with SDC, drawn randomly at 1 month, and 3861 placebo patients, alive at 1 month. Overall, 33% died and 31% had HF hospitalizations during a 40-month median follow-up. Compared with placebo, SDC 0.5–0.9 ng/mL was associated with lower mortality [29 vs. 33% placebo; adjusted hazard ratio (AHR), 0.77; 95% confidence interval (CI), 0.67–0.89], all-cause hospitalizations (64 vs. 67% placebo; AHR, 0.85; 95% CI, 0.78–0.92) and HF hospitalizations (23 vs. 33% placebo; AHR, 0.62; 95% CI, 0.54–0.72). SDC _ 1.0 ng/mL was associated with lower HF hospitalizations (29 vs. 33% placebo; AHR, 0.68; 95% CI, 0.59–0.79), without any effect on mortality. SDC 0.5–0.9 reduced mortality in a wide spectrum of HF patients and had no interaction with ejection fraction .45% (P ¼ 0.834) or sex (P ¼ 0.917).
Conclusions
Digoxin at SDC 0.5–0.9 ng/mL reduces mortality and hospitalizations in all HF patients, including those with diastolic HF. At higher SDC, digoxin reduces HF hospitalizations but has no effect on mortality or all-cause hospitalizations.
Keywords: Digoxin, heart failure, diastolic heart failure, mortality, hospitalization
Digoxin is the oldest and probably the least expensive drug for heart failure (HF).1 In the Digitalis Investigation Group (DIG) trial, digoxin reduced hospitalizations due to worsening HF without affecting overall mortality in HF patients with ejection fraction (EF) ≤45%.2 For HF patients with EF>45%, digoxin did not affect all-cause mortality or HF-hospitalizations.2 Patients with diastolic HF (clinical HF with normal or near normal EF) constitute over half of all HF patients.3 Yet, little is known about the effect of digoxin in these patients, for whom the benefits of angiotensin-converting enzyme (ACE) inhibitors and beta-blockers are unproven.4 As most diastolic HF patients are elderly and women, in studying the effect of digoxin, it is important to account for SDC, which is an important determinant of therapeutic benefit and harmful adverse effects of digoxin.5-7 However, there are no data on the effect of digoxin at low SDC on outcomes in diastolic HF patients.
Despite U.S. Food and Drug Administration (FDA) approval, and guideline recommendations,1, 4, 8 use of digoxin in HF is in decline.9-11 Recently, ACC/AHA HF guidelines downgraded the role of digoxin in HF.12 This shift in practice and policy is likely to adversely affect HF care and outcomes in the developing nations as local physicians follow guidelines and practice patterns in the U.S., Europe and other developed nations. As most of those patients cannot afford ACE inhibitors or beta blockers, digoxin remains the only affordable therapy for HF. Additionally, as most of these patients also cannot afford evaluation of left ventricular function there is need for a drug that might be beneficial in all HF patients, regardless of EF.
In this context, we conducted the most comprehensive post-hoc re-analysis to date of the DIG dataset to determine the effect of digoxin, accounting for SDC, on outcomes in ambulatory men and women with chronic systolic or diastolic HF. We hypothesized that digoxin at low SDC would reduce all-cause mortality and HF hospitalization in a broad spectrum of ambulatory chronic HF patients with normal sinus rhythm.
METHODS
Study Design
The DIG trial was a randomized controlled trial to evaluate the effects of digoxin on mortality and hospitalization in 7,788 ambulatory adults with chronic HF and normal sinus rhythm.2 Patients received four different daily doses of digoxin or matching placebo (0.125 mg, 0.25 mg, 0.375 mg, and 0.50 mg) based on age, sex, weight, and serum creatinine level.13 Most patients were receiving ACE inhibitors and diuretics. Beta-blockers were not approved for HF at the time of randomization. The design and results of the DIG trial have been previously reported.2
Patients
In the DIG trial, 6,800 HF patients with EF ≤45% (main trial) and 988 patients with EF >45% (ancillary trial) were recruited from the U.S. (186 centers) and Canada (116 centers) between January 1991 and August 1993. As both trials followed very similar protocols we combined the main and ancillary trial datasets to study the effect of digoxin in all HF patients regardless of EF. SDC was measured in a random subset of original DIG participants, and investigators were blinded to the results of the SDC. Investigators were also discouraged from checking SDC for clinical reasons except in life-threatening emergencies.13 As SDC is an important determinant of digoxin effects,5-7 we restricted our analysis to patients with SDC measurements. Of the 3,889 (49.9%) patients randomized to digoxin, data on SDC at 1 month after randomization based on specimens collected at least 6 hours after the last dose of digoxin were available for 1,687 patients. Of these, 982 had low SDC (0.5-0.9 ng/ml) and 705 had high SDC (≥1.0 ng/ml). We chose these cut points of SDC because of their significant association with outcomes.6, 14 Of the 3,899 (50.1%) patients randomized to placebo, 3,861 were alive at 1 month. Our analysis focuses on these patients (N=5,445).
Outcomes
In the DIG trial, the primary outcome was all-cause mortality at a median follow-up of 39.7 months. Vital status of all patients was collected up to December 31, 1995 and was 98.9% complete.15 Pre-specified secondary outcomes included mortality due to cardiovascular causes and HF, and hospitalizations due to all causes, cardiovascular causes, and worsening HF.
Statistical Analysis
We compared baseline characteristics of HF patients receiving placebo and those with low and high SDC using Pearson chi-square tests and one-way analysis of variance tests as appropriate to test for statistical significance. We used Kaplan-Meier analysis and log-rank tests to estimate the effects of low and high SDC on all-cause mortality and HF hospitalizations compared with placebo. We then used bivariate and multivariable Cox proportional hazards regression models to determine the association of low and high SDC with various pre-specified outcomes. In the multivariable model, separate indicators of low and high SDC were entered as independent predictor variables with placebo as the reference category. Covariates used in the multivariable model are displayed in Table 1. Proportional hazards assumptions were checked using log-minus-log scale survival plots for patients receiving placebo and the two SDC groups.
Table 1.
Baseline characteristics of patients
| N (%) or mean (±SD) | Placebo (N=3861) | SDC 0.5-0.9 (N=982) | SDC ≥1.0 (N=705) | Overall P |
|---|---|---|---|---|
| Age, years | 63.9 (±10.8) | 62.8 (±10.6) | 65.1 (±10.1) | <0.001 |
| Women | 955 (24.7) | 217 (22.1) | 194 (27.5) | 0.037 |
| Non-whites | 563 (14.6) | 126 (12.8) | 86 (12.2) | 0.129 |
| Body mass index, kg / m2 | 27.4 (±5.35) | 27.2 (±5.23) | 26.8 (±5.38) | 0.016 |
| Heart failure duration, months | 29.7 (±36.4) | 32.2 (±38.7) | 34.7 (±38.6) | 0.002 |
| Primary cause of HF | ||||
| Ischemic | 2654 (68.7) | 672 (68.4) | 507 (71.9) | |
| Hypertensive | 411 (10.6) | 98 (10.0) | 53 (7.5) | 0.119 |
| Idiopathic | 528 (13.7) | 148 (15.1) | 106 (15.0) | |
| Others | 268 (6.9) | 64 (6.5) | 39 (5.5) | |
| Comorbid conditions | ||||
| Prior myocardial infraction | 2445 (63.3) | 624 (63.5) | 461 (65.4) | 0.577 |
| Current angina pectoris | 1030 (26.7) | 262 (26.7) | 195 (27.7) | 0.860 |
| Hypertension | 1826 (47.3) | 433 (44.1) | 334 (47.4) | 0.187 |
| Diabetes mellitus | 1109 (28.7) | 265 (27.0) | 215 (30.5) | 0.284 |
| Chronic kidney disease | 1795 (46.5) | 368 (37.5) | 415 (58.9) | <0.001 |
| Medications | 0.24 (±0.07) | 0.24 (±0.08) | 0.25 (±0.07) | 0.249 |
| Prior digoxin use | 1674 (43.4) | 457 (46.5) | 340 (48.2) | 0.022 |
| ACE inhibitors | 3617 (93.7) | 931 (94.8) | 664 (94.2) | 0.401 |
| Nitrates & hydralazine | 47 (1.2) | 12 (1.2) | 8 (1.1) | 0.982 |
| Potassium-sparing diuretics | 318 (8.2) | 77 (7.8) | 59 (8.4) | 0.905 |
| Other diuretics | 3017 (78.1) | 715 (72.8) | 586 (83.1) | <0.001 |
| Potassium supplement | 1101 (28.5) | 243 (24.7) | 193 (27.4) | 0.061 |
| Dose of study medication | 0.243 (±0.07) | 0.244 (±0.07) | 0.249 (±0.07) | 0.121 |
| Symptoms and signs | ||||
| Dyspnea at rest | 856 (22.2) | 192 (19.6) | 162 (23.0) | 0.150 |
| Dyspnea on exertion | 2926 (75.8) | 721 (73.4) | 532 (75.5) | 0.308 |
| Elevated JVP | 513 (13.3) | 112 (11.4) | 115 (16.3) | 0.014 |
| Third heart sound | 861 (22.3) | 243 (24.7) | 182 (25.8) | 0.056 |
| Pulmonary râles | 632 (16.4) | 134 (13.6) | 144 (20.4) | 0.001 |
| Leg edema | 791 (20.5) | 183 (18.6) | 165 (23.4) | 0.057 |
| NYHA functional class | ||||
| I | 542 (14.0) | 173 (17.6) | 83 (11.8) | |
| II | 2125 (55.0) | 536 (54.6) | 391 (55.5) | 0.002 |
| III | 1125 (29.1) | 259 (26.4) | 208 (29.5) | |
| IV | 69 (1.8) | 14 (1.4) | 23 (3.3) | |
| Heart rate, per minute | 78.6 (±12.6) | 77.2 (±12.7) | 77.6 (±12.7) | 0.004 |
| Blood pressure, mm Hg | ||||
| Systolic | 127.5 (±20.4) | 126.6 (±19.8) | 126.2 (±20.4) | 0.191 |
| Diastolic | 75.2 (±11.1) | 75.6 (±10.8) | 73.9 (±10.9) | 0.005 |
| Chest radiograph findings | ||||
| Pulmonary congestion | 535 (13.9) | 114 (11.6) | 134 (19.0) | <0.001 |
| Cardiothoracic ratio > 0.5 | 2324 (60.2) | 558 (56.84) | 441 (62.6) | 0.048 |
| Laboratory data | ||||
| Serum creatinine, mg/dL | 1.28 (±0.37) | 1.21 (±0.32) | 1.37 (±0.41) | <0.001 |
| Estimated GFR, ml/min/1.73 m2 | 63.4 (±24.5) | 67.1 (±19.41) | 58.0 (±19.1) | <0.001 |
| Serum potassium, mEq/L | 4.33 (±0.43) | 4.33 (±0.46) | 4.35 (±0.44) | 0.626 |
| Ejection fraction, % | 31.9 (±12.5) | 31.9 (±12.1) | 31.0 (±12.3) | 0.176 |
Abbreviation: ACE=angiotensin-converting enzyme, GFR=glomerular filtration rate; JVP=jugular venous pressure, NYHA=New York Heart Association, SDC=serum digoxin concentration
Because patients were not randomized to low or high SDC, there were significant imbalances in baseline covariates between patients receiving placebo, and those with low and high SDC. To account for this selection or predisposition bias, we separately calculated propensity scores or predicted probabilities for the development of low and high SDC for each patient. The propensity score is the conditional probability of receiving an exposure given a vector of measured covariates,16, 17 and is used to adjust for selection bias in causal modeling for observational studies.18
We used non-parsimonious multivariable logistic regression models to estimate propensity scores, using the following baseline characteristics as covariates in the model: age, sex, race, body mass index, duration and etiology of HF, past myocardial infraction, current angina, hypertension, diabetes, use of ACE inhibitors, combined use of nitrates and hydralazine, non-potassium-sparing and potassium-sparing diuretics, potassium supplement, pre-trial use of digoxin, current symptoms of dyspnea at rest and exertion, activity limitation, NYHA class, heart rate, systolic and diastolic blood pressure, elevated jugular venous pressure, pulmonary râles, third heart sound, lower extremity edema, number of symptoms, serum potassium and creatinine, radiological evidence of cardiothoracic ratio greater than 0.5 and pulmonary congestion, and ejection fraction. In addition, the following first-order interaction terms were included in the model: age*creatinine, sex*creatinine, race*creatinine, body mass index*creatinine, diabetes*creatinine, non-potassium-sparing diuretic use*creatinine and pre-trial digoxin use*creatinine.
The models used to estimate propensity scores calibrated and discriminated well. However, because propensity score models are sample-specific adjusters, and are not intended to be used for out-of-sample prediction or estimation of coefficients, measures of fitness and discrimination are not important for the assessment of the model’s effectiveness.19-22 Instead, comparing the balance of baseline covariates between treatment groups before and after adjustment (for instance, matching) is the appropriate way to check the adequacy of the propensity model. The standardized difference is a sensible summary measuring covariate balance between two treatment groups.23, 24 Expressed as a percentage, the standardized difference measures the degree of bias in the means of a covariate, in units of the pooled standard deviation. Our assessment of covariate balance after matching focused on these standardized differences.
Before matching, the mean propensity score for low SDC in the placebo group (n = 3861) was 0.19894 and in the low SDC group (n = 982) was 0.21801, which yielded a standardized difference of 34.6% and t-test p-value <0.0001. Before matching, the mean propensity score for high SDC was 0.15033 in the placebo group (n = 3861) and 0.17694 in the high SDC group (n = 705). This represents a standardized difference of 43.4% and t-test p-value <0.0001.
Using a SPSS macro25 we then matched all 982 patients with low SDC and all 705 high SDC patients separately with 982 and 705 patients (respectively) receiving placebo who had similar propensity scores (within 0.02). After matching, the mean low-SDC propensity score was 0.21803 in the placebo group (n = 982) and 0.21801 in the low SDC group (n = 982) which yields a standardized difference of 0.0%; and t-test p-value = 0.994. After matching, the mean high SDC propensity score was 0.17700 in the placebo group (n = 705) and 0.17694 in the high-SDC group (n = 705) which yields a standardized difference of 0.1%; and t-test p-value = 0.986.
Finally, we re-estimated the associations of digoxin and outcomes in the two matched cohorts using Kaplan-Meier survival and bivariate and multivariable Cox regression analyses, with appropriate adjustments to obtain hypothesis testing results incorporating the matched pairs design using stratification.26 In the multivariable Cox regression model, we also adjusted for propensity score (raw score entered as a linear term) and covariates used for the unmatched cohort, at first separately and then combined together, in both cases incorporating the matched design by embedding it into the model.
To assess potential heterogeneity of effect, we estimated the effects of low SDC (versus placebo) on all-cause mortality in several subgroups of patients: age, gender, race, HF etiology, NYHA class, EF, diabetes, chronic kidney disease (CKD) defined as glomerular filtration rate <60 ml / 1.73 square meter of body surface area, and ACE inhibitor and diuretic use. We tested for first-order interactions in multivariable Cox proportional hazards models entering interaction terms between low SDC and the above covariates.
To identify baseline predictors of high SDC, we developed a multivariable logistic regression model based on patients receiving digoxin. We used initial bivariate analyses to identify potential predictors. We used chi-square tests and Wilcoxon rank sum tests as appropriate to assess associations of various covariates with high SDC. We then used logistic regression to develop a multivariable model. Age, sex, and race were forced in that model. Covariates significantly associated in the bivariate analysis (p<0.05) were then entered (forward selection: likelihood ratio) into the model. These covariates included digoxin dose, body mass index, serum creatinine, non-potassium-sparing diuretic use, NYHA class, elevated jugular venous pressure, pulmonary râles, lower extremity edema, and radiological evidence of cardiothoracic ratio greater than 0.5 and pulmonary congestion. The following first-order interaction terms were also entered into the model: age*creatinine, sex*creatinine, race*creatinine, and body mass index*creatinine. Digoxin dose 0.25 mg/day and >0.25 mg/day were used as dummy variables, using ≤0.125 mg/day as the reference. This model was first applied in a derivation cohort based on random 50% of patients receiving digoxin (N= 844). Then, we used the predictor variables in a validation cohort consisting of the remaining patients (N= 843). Both models were fit to data during all steps of the regression analyses and had comparable c-statistics (0.71 and 0.69 respectively for the derivation and validation models). Finally, the same model was applied to all 1,687 patients receiving digoxin (Hosmer and Lemeshow goodness of fit test Chi-square=13.38; p=0.10 and c-statistic 0.70). All statistical tests were evaluated using a two-tailed 95% confidence level. All data analyses were performed using SPSS for Windows version 13.0.2.27
RESULTS
Patient Characteristics
Patients (N=5548; low SDC=982, high SDC=705 and placebo=3,861) had a median age of 65 years, 24% were women, 14% were non-white, and 12% had EF >45%. Among the 1,687 randomly-selected patients with SDC, 260 (15%) were receiving digoxin ≤0.125 mg/day, 1237 (73%) were receiving 0.25 mg/day, and 190 (11%) were receiving >0.25 mg/day, with a median dose of 0.25 mg/day.
Baseline patient characteristics are displayed in Table 1. Compared to patients receiving placebo, those with low SDC were younger, had lower mean serum creatinine, and were less likely to have pulmonary congestion and higher NYHA class symptoms, and also less likely to receive diuretics (Table 1). In contrast, compared to placebo patients, those with high SDC were older, had higher mean serum creatinine, and were more likely to have pulmonary congestion and higher NYHA class symptoms, and also more likely to receive diuretics (Table 1).
Matching on propensity scores adequately balanced all 32 covariates included in the propensity model, as well as the eight included interaction terms. All 982 low SDC patients were successfully matched to a placebo patient to within 0.02, and 979 pairs matched to within 0.01 point of propensity scores. All 705 high SDC patients, likewise, were matched to a placebo patient whose propensity score was within 0.02 of the high SDC patient, with 700 pairs actually matched to within 0.01 point. After matching, none of the 32 covariates or 8 interaction terms had large or statistically significant differences between placebo and SDC patients. The standardized difference was 8.2% or lower in absolute value for all covariates and interactions in both the 982 low SDC matched pairs and the 705 high SDC matched pairs, where standardized differences below 10% in absolute value are usually taken to indicate adequate balance.23, 24
Mortality and Hospitalizations
During 40 months of median follow-up, 1854 (33%) patients died from all causes, including 1,456 (26%) from cardiovascular causes, and 650 (12%) from worsening HF. Of the 3,717 (67%) participants hospitalized for all causes, 2,920 (53%) were due to cardiovascular causes and 1,712 (31%) due to worsening HF. No patients in the digoxin group died during the first month and only two patients receiving placebo were lost to follow-up during that period.
Digoxin and Mortality
Compared with 33% deaths among patients receiving placebo, 29% of those with low SDC and 42% of those with high SDC died from all causes during the follow up. In the Kaplan-Meier analysis, compared with placebo, low and high SDC were respectively associated with unadjusted reduction and increase in all-cause mortality (Figure 1). Absolute risk (AR), absolute risk reduction (ARR), unadjusted and adjusted hazard ratios (HR), 95% confidence intervals (CI), and p values for mortality due to all-causes, cardiovascular causes, and worsening HF for patients with low and high SDC compared to placebo are displayed in Table 2. ARR was calculated separately for the two SDC groups relative to placebo patients. Low SDC was associated with a 22% reduction in unadjusted total mortality, which was essentially unchanged after multivariable adjustment for other covariates (Table 2). High SDC was associated with a 23% relative increase in unadjusted mortality, which did not persist after multivariable adjustment (Table 2).
Figure 1.
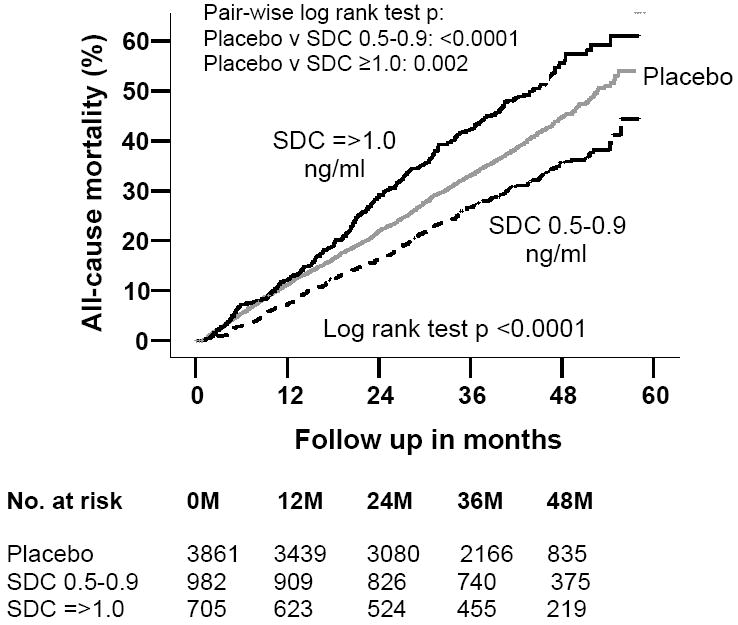
Kaplan-Meier plots for cumulative risk of death due to all causes by serum digoxin concentration (SDC)
Table 2.
Effects of digoxin on mortality (AR =absolute risk, ARR =absolute risk reduction, CV =cardiovascular, HF=heart failure, HR =hazard ratio, CI =confidence interval, SDC =serum digoxin concentration)
| Events / Total | AR (%) | ARR | Crude HR (95% CI) | P value | Adjusted* HR (95% CI) | P value | ||
|---|---|---|---|---|---|---|---|---|
| All-cause mortality | Placebo | 1272 / 3861 | 32.9 | Reference | 1 | Reference | 1 | Reference |
| SDC 0.5-0.9 ng/ml | 288 / 982 | 29.3 | 3.6 | 0.78 (0.69 – 0.89) | <0.0001 | 0.77 (0.67 – 0.89) | <0.0001 | |
| SDC ≥1.0 ng/ml | 294 / 705 | 41.7 | -8.8 | 1.23 (1.08 – 1.39) | 0.002 | 1.06 (0.93 – 1.20) | 0.406 | |
| Cardiovascular mortality | Placebo | 985 / 3861 | 25.5 | Reference | 1 | Reference | 1 | Reference |
| SDC 0.5-0.9 ng/ml | 237 / 982 | 24.1 | 1.4 | 0.84 (0.73 – 0.96) | 0.013 | 0.83 (0.71 – 0.97) | 0.019 | |
| SDC ≥1.0 ng/ml | 234 / 705 | 33.2 | -7.7 | 1.26 (1.10 – 1.46) | 0.001 | 1.07 (0.93 – 1.24) | 0.339 | |
| Heart failure mortality | Placebo | 468 / 3861 | 12.1 | Reference | 1 | Reference | 1 | Reference |
| SDC 0.5-0.9 ng/ml | 86 / 982 | 8.8 | 3.3 | 0.63 (0.50 – 0.80) | <0.0001 | 0.63 (0.49 – 0.82) | <0.0001 | |
| SDC ≥ 1.0 ng/ml | 96 / 705 | 13.6 | -1.5 | 1.09 (0.87 – 1.36) | 0.449 | 0.87 (0.70 – 1.09) | 0.236 |
Adjusted for age, sex, race, body mass index, duration of heart failure, etiology of heart failure, prior myocardial infarction, current angina, hypertension, diabetes, pre-trial use of digoxin, use of angiotensin-converting enzyme inhibitors, diuretics, and combination of hydralazine and nitrates, current dyspnea at rest and dyspnea on exertion, heart rate, systolic and diastolic blood pressure, current jugular venous distension, third heart sound, pulmonary râles, and lower extremity edema, NYHA functional class, pulmonary congestion by chest x-ray, cardiothoracic ratio >0.5, estimated glomerular filtration rate, ejection fraction, and the interaction term for SDC 0.5-0.9 ng/ml and race.
Among the subset of 982 propensity score pairs matched for low SDC, in 204 of the low SDC patients definitely died before their matched placebo patients. On the other hand, in 252 of those pairs, placebo patients definitely died before the matched low SDC patients. The order of death could not be determined in the remaining 526 pairs. The appropriate matched-samples comparison of hazard rates gave a Z statistic of -2.248 (p = .0246) for the low-SDC (Figure 2, upper panel). Compared with matched placebo, low SDC was associated with reduction in all-cause mortality (unadjusted HR, 0.81; 95% CI, 0.67 to 0.97; p=0.025), which remained essentially unchanged after additional multivariable adjustment (adjusted HR, 0.81; 95% CI, 0.67 to 0.99; p=0.042).
Figure 2.
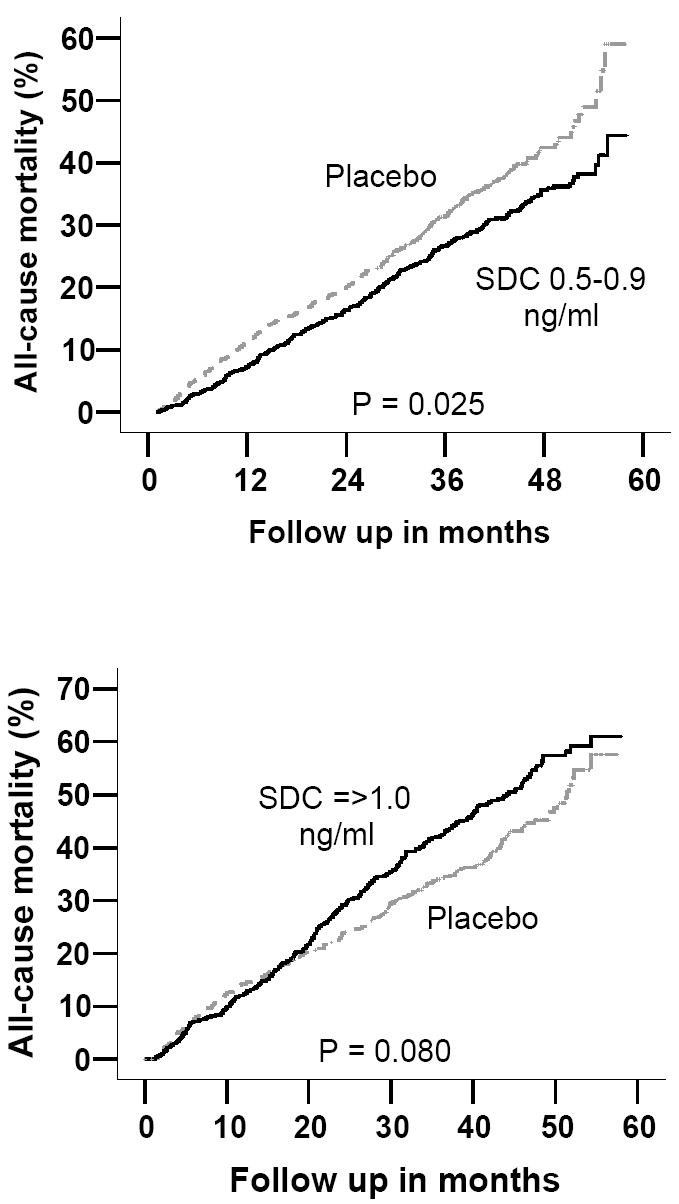
Kaplan-Meier plots for cumulative risk of death due to all causes among patients matched by propensity to develop serum digoxin concentrations (SDC) 0.5-0.9 ng/ml and ≥1.0 ng/ml
In the cohort of 705 propensity-matched pairs for high SDC, among 217 of those pairs, high SDC patients definitely died before the matched placebo. In contrast, in 182 of those pairs, placebo patients definitely died before the matched high SDC patients. In 306 pairs, we could not determine the order of death. The appropriate matched-samples comparison of hazard rates gave a Z statistic of 1.752 (two-tailed p value = 0.0797) for the high-SDC versus placebo comparison (Figure 2, lower panel). Compared to placebo, high SDC was associated with increased mortality that bordered on significance (unadjusted HR, 1.19; 95% CI, 0.98 to 1.45; p=0.080). Even though, this association remained essentially unchanged after multivariable adjustment, it lost its statistical significance (adjusted HR, 1.19; 95% CI, 0.95 to 1.48; p=0.130).
Digoxin and Hospitalization
Compared with 33% placebo patients, 23% of those with low SDC and 29% of those with high SDC were hospitalized due to HF. Kaplan-Meier plots for first hospitalization due to worsening HF are displayed in Figure 3. Low SDC was associated with reduction in the risk for all-cause, cardiovascular, and HF hospitalizations (Table 3). High SDC was associated with a reduction in HF hospitalization, but not with all-cause hospitalization (Table 3).
Figure 3.
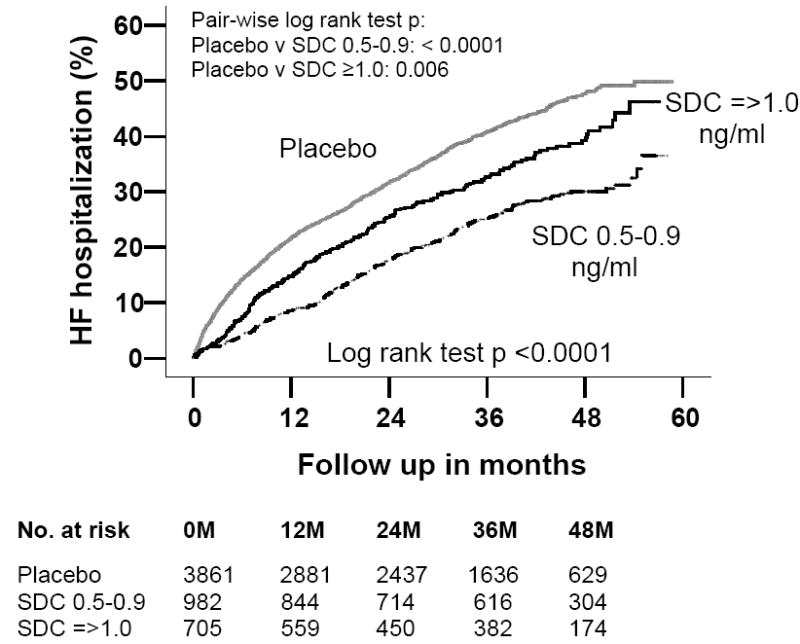
Kaplan-Meier plots for cumulative risk of hospitalization due to worsening heart failure (HF) by serum digoxin concentration (SDC)
Table 3.
Effects of digoxin on hospitalizations (AR =absolute risk, ARR =absolute risk reduction, CV =cardiovascular, HF=heart failure, HR =hazard ratio, CI =confidence interval, SDC =serum digoxin concentration)
| Events / Total | AR (%) | ARR | Crude HR (97.5% CI) | P value | Adjusted* HR (97.5% CI) | P value | ||
|---|---|---|---|---|---|---|---|---|
| All-cause hospitalization | Placebo | 2594 / 3861 | 67.2 | Reference | 1 | Reference | 1 | Reference |
| SDC 0.5-0.9 ng/ml | 625 / 982 | 63.6 | 3.6 | 0.82 (0.75 – 0.89) | <0.0001 | 0.85 (0.78 – 0.92) | <0.0001 | |
| SDC ≥1.0 ng/ml | 498 / 705 | 70.6 | -3.4 | 1.05 (0.95 – 1.15) | 0.333 | 0.95 (0.87 – 1.05) | 0.331 | |
| Cardiovascular hospitalization | Placebo | 2059 / 3861 | 53.3 | Reference | 1 | Reference | 1 | Reference |
| SDC 0.5-0.9 ng/ml | 471 / 982 | 48.0 | 4.5 | 0.77 (0.70 – 0.86) | <0.0001 | 0.79 (0.72 – 0.88) | <0.0001 | |
| SDC ≥1.0 ng/ml | 390 / 705 | 55.3 | -2.0 | 1.01 (0.90 – 1.12) | 0.891 | 0.91 (0.82 – 1.01) | 0.086 | |
| Heart failure hospitalization | Placebo | 1279 / 3861 | 33.1 | Reference | 1 | Reference | 1 | Reference |
| SDC 0.5-0.9 ng/ml | 229 / 982 | 23.3 | 9.8 | 0.60 (0.52 – 0.69) | <0.0001 | 0.62 (0.54 – 0.72) | <0.0001 | |
| SDC ≥1.0 ng/ml | 204 / 705 | 28.9 | 4.2 | 0.81 (0.70 – 0.94) | 0.005 | 0.68 (0.59 – 0.79) | <0.0001 |
Adjusted for the same covariates as in Table 2.
In the propensity-matched cohort, compared to patients receiving placebo, low SDC was associated with 38% unadjusted (HR, 0.62; 95% CI, 0.51 to 0.76; p<0.0001) and multivariable adjusted (HR, 0.62; 95% CI, 0.50 to 0.79; p<0.0001) reduction in HF hospitalization. Compared to placebo, matched high SDC patients also had significant reduction in HF hospitalization: unadjusted HR, 0.61 (95% CI, 0.49 to 0.76; p<0.0001) and adjusted HR, 0.53 (95% CI, 0.40 to 0.69; p<0.001).
Subgroup Analysis
Associations between low SDC and all-cause mortality in various subgroups of patients are displayed in Figure 4. Low SDC was associated with reduced mortality in most subgroups, with the exception of nonwhites (adjusted p for interaction =0.006). There was no significant interaction of low SDC with ejection fraction >45% (p=0.834) or gender (p=0.917).
Figure 4.
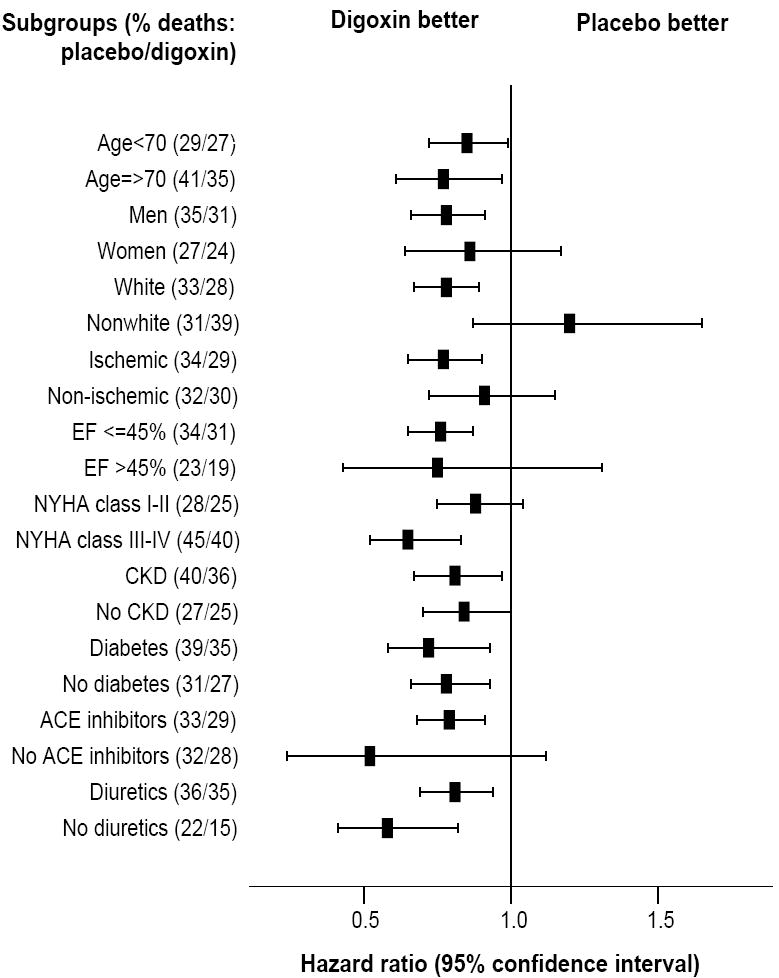
Hazard ratio (95% confidence interval) for all-cause mortality in subgroups of patients with heart failure with serum digoxin concentration 0.5-0.9 ng/dL and placebo (ACE= angiotensin-converting enzyme, CKD= chronic kidney disease, EF= ejection fraction, NYHA= New York Heart Association)
Predictors of high SDC
Among patients receiving digoxin (N=1,687), 41.8% had high SDC. In the derivation sample (N=844), 42.5% patients had high SDC and in the validation sample (N=843), 41.0% patients had high SDC. Age, gender, dose of digoxin, serum creatinine, use of diuretics, and pulmonary congestion were predictors of high SDC in both derivation and validation cohorts. Median predicted probability for high SDC was 0.40 (range 0.05 to 0.96). Unadjusted and multivariable covariate adjusted odds ratios (OR) and 95% confidence intervals (CI) for high SDC based on all 1,687 patients are presented in Table 5. Compared to patients receiving a daily digoxin dose of ≤0.125 mg, those receiving 0.25 mg (adjusted OR, 3.17, 95% CI, 2.25 to 4.47; p<0.0001) and >0.25 mg (adjusted OR, 8.70, 95% CI, 5.04 to 15.00; p<0.0001) had greater odds for high SDC.
DISCUSSION
The results of our study demonstrate that digoxin reduces HF hospitalization in a wide spectrum of ambulatory chronic HF patients in normal sinus rhythm receiving diuretics and ACE inhibitors. In addition, digoxin reduces all-cause mortality and all-cause hospitalization at low SDC. Our data suggest that digoxin can play an important role in HF care, regardless of ejection fraction or gender. In the developed nations, where diuretics and ACE inhibitors are the most commonly used regimen for HF therapy, digoxin can play an important role in reducing morbidity and mortality, and decreasing health care costs. In the developing nations, where most HF patients do not have a left ventricular function evaluation and cannot afford ACE inhibitors and beta-blockers, digoxin is the only available drug that can play a critical role in reducing mortality and morbidity.
The beneficial effects of digoxin observed by us are likely due to hemodynamic and neurohormonal properties of digoxin. Digitalis is the only inotropic agent that does not increase mortality and improves neurohormonal profile in HF.28, 29 The effects of digoxin have long been known to be dependent on SDC.1, 5, 30, 31 However, ours is the first study to demonstrate a beneficial effect of low SDC in all HF patients, including those with diastolic HF. As risk of hospitalization is relatively high in diastolic HF patients, digoxin can play an important role in reducing morbidity and health care costs. Furthermore, digoxin reduces mortality and all-cause hospitalization if care is taken to achieve a SDC between 0.5 and 0.9 ng/ml.
Digitalis is the oldest and probably the least expensive drug for the management of HF. Yet, despite FDA approval and guideline recommendations, there has been a recent decline in the use of digoxin in HF patients.9-11, 32 The recent ACC/AHA HF guidelines have also downgraded the role of digoxin in HF care, while promoting expensive device-based therapies with selective indications.12 This change in policy and practice regarding digoxin use appears to be due in part to lack of mortality benefit,31, 33 in particular in those with diastolic HF.4 Despite recent evidence to the contrary,14, 34 a widely publicized earlier study questioning the safety of digoxin in women35 also likely played a role. Finally, availability of newer neurohormonal antagonists such as beta-blockers and aldosterone antagonists,4 lack of industry sponsorship for digoxin, and aggressive promotion of expensive devices with selective indications10, 11 have further slighted the role of digoxin in HF care. However, our data demonstrate that in ambulatory men and women with chronic stable systolic or diastolic HF, use of digoxin was associated with over 30% reduction in HF hospitalization regardless of SDC, and significant reduction in mortality and hospitalizations from all causes at low SDC. This is important as HF is one of the major causes of hospitalization, especially among older adults, and HF hospitalizations are expensive and associated with poor patient outcomes.
Our findings support a more expanded role of digoxin in HF. Digoxin should be used in systolic HF patients with or without atrial fibrillation who continue to remain symptomatic despite therapy with ACE inhibitors or angiotensin receptor blockers (ARB), and beta-blockers, or for those who cannot afford or tolerate these drugs. Specifically, digoxin should be used for relief of HF symptoms before considering aggressive therapy with high-dose non-potassium-sparing diuretics, as these drugs may increase mortality and hospitalizations.36, 37 Second, digoxin should be considered before initiating an aldosterone antagonist as these drugs often cause hyperkalemia.38 Third, digoxin should be prescribed before adding an ARB to an ACE inhibitor. In the Val-HeFT and CHARM-added trials, addition of ARB’s, which are expensive, did not result in reduction in mortality and was associated with increased adverse effects.32, 39 Finally, digoxin should be tried before considering cardiac resynchronization therapy (CRT), which is invasive and costly.10, 11, 40 In addition, CRT is reserved for systolic HF patients with wide QRS complex and response to CRT is often unpredictable.
The role of digoxin in the management of HF in developing nations deserves special consideration. Digoxin, along with diuretics, forms the basis of HF therapy in most developing countries. However, clinicians in those countries often follow guidelines and practice patterns in the U.S. and Europe. As the use of digitalis glycosides is being discouraged in developed nations,41 clinicians in the developing countries are also likely to decrease use of digitalis. This could have the untoward consequence of increasing mortality and morbidity in millions of HF patients worldwide. This is important as most HF patients in developing countries cannot afford expensive therapy with ACE inhibitors and beta-blockers. In addition, our data suggest that digoxin is beneficial regardless of EF. Thus, digoxin can play a very special role in HF care in developing nations as most HF patients there cannot afford an echocardiographic evaluation of left ventricular function.
Correlates of high SDC identified in our study can be used to reduce the risk of high SDC in situations where SDC cannot be measured. Based on our observations, we suggest that for young men with clinically stable HF (no pulmonary congestion by chest x-ray and not receiving diuretics) and normal renal function, a daily digoxin dose of 0.25 mg or lower would be therapeutic. In contrast, for HF patients who are elderly, women, have pulmonary congestion or receiving diuretics, or have renal impairment, a daily dose of 0.125 mg would probably be more appropriate. For patients with multiple risk factors for high SDC, such as elderly women with CKD, or elderly women with pulmonary congestion or receiving diuretics, digoxin should be started at a daily dose of 0.0625 mg. When possible, SDC should be monitored to guide therapy for these patients.
To the best of our knowledge, this is the most comprehensive and in-depth analysis of the DIG dataset based on pre-defined outcomes. DIG was the largest multicenter randomized controlled trial of digoxin in patients with HF with the longest follow up. Because most of the studies in the literature either were based on subgroups of DIG patients,14, 35, 42 did not account for SDC,35 were of shorter duration or used intermediate outcomes,6, 43-46 our findings provide the strongest evidence of a significant benefit of digoxin in a wide spectrum of HF patients.
HF patients in the DIG trial were not receiving beta-blockers or aldosterone antagonists. A post-hoc analysis of the U.S. Carvedilol Trials data demonstrated that digoxin use was associated with a reduction in the combined endpoint of death or hospitalization from all causes in patients both receiving (relative risk, 0.64; 95% CI, 0.52 to 0.79) and not receiving (relative risk, 0.82; 95% CI, 0.71 to 0.99) carvedilol.47 In the RALES trial, the survival benefit of spironolactone, an aldosterone antagonist, was only significant in patients receiving digoxin.48 Therefore, there is no reason to withhold digoxin from systolic HF patients receiving beta-blockers or aldosterone antagonists. As there is currently no evidence of survival benefit from beta-blockers or aldosterone antagonists in patients with diastolic HF, digoxin could be considered in preference to those drugs in this population.
Several limitations of our study must be acknowledged. The results of this study are based on post-hoc analysis and patients were not randomized to a certain SDC. Despite our efforts to balance baseline covariate distribution by propensity score matching, bias due to unmeasured covariates cannot be ruled out as a possible explanation of our results. Although we can never know if such a hidden bias exists, careful sensitivity analysis permits us to quantify the size of hidden bias that would be required to invalidate our conclusions. Our sensitivity analysis demonstrates that for an unmeasured binary covariate (unrelated to covariates in our propensity model) to explain away our results, that unmeasured covariate would need to increase the odds of a patient receiving digoxin by at least 48% and would also need to be a near-perfect predictor of all-cause mortality, suggesting that these results are at least somewhat resistant to hidden bias.
About 22% of DIG participants randomized to placebo were receiving open labeled digoxin, and about 20% of those randomized to digoxin were not receiving the drug.2 In our study, all patients in the treatment group were receiving digoxin at 1 month follow-up, as indicated by the presence of a detectable serum digoxin level. However, digoxin may have been subsequently discontinued in some patients, and some of the placebo patients may have been started on digoxin during follow-up. However, as our analysis starts at 30 days after randomization, we were not able to estimate an early effect of digoxin.
Results of our study are based on predominantly white, male, and relatively younger patients with mild to moderate HF and normal sinus rhythm. Our finding of a significant interaction between low SDC and race might be due to chance and lacks biological basis. Subgroup analyses without biological basis can be misleading. For example, in the MERIT-HF trial, survival benefit of extended release metoprolol was only observed in HF patients in Europe, and not in the U.S.49
In summary, our comprehensive post-hoc analysis of data from the DIG trial indicates that at low doses, which is likely to achieve low SDC (<0.9 ng/ml), digoxin reduces mortality and hospitalization in a broad spectrum of ambulatory men and women with chronic systolic or diastolic HF. In systolic HF, digoxin should be used in patients who are symptomatic despite ACE inhibitors or ARB and beta-blocker therapy. Use of digoxin should be considered before CRT, high-dose diuretics, aldosterone antagonists, or adding an ARB to an ACE inhibitor.
Table 4.
Predictors of serum digoxin concentration ≥1.0 ng/ml among patients receiving digoxin (N=1687)
| Unadjusted odds ratio | P value | Adjusted odds ratio | P value | |
|---|---|---|---|---|
| Age, years | 1.02 (1.01 – 1.03) | <0.0001 | 1.02 (1.01 – 1.03) | 0.002 |
| Female sex | 1.34 (1.07 – 1.67) | 0.011 | 2.21 (1.70 – 2.88) | <0.0001 |
| Nonwhite race | 0.94 (0.70 – 1.27) | 0.699 | 0.84 (0.61 – 1.16) | 0.286 |
| Body mass index | 0.99 (0.97 – 1.01) | 0.149 | 0.95 (0.93 – 0.98) | <0.0001 |
| Serum creatinine | 3.45 (2.59 – 4.60) | <0.0001 | 5.98 (4.21 – 8.50) | <0.0001 |
| Use of diuretic | 1.84 (1.44 – 2.34) | <0.0001 | 1.56 (1.21 – 2.02) | 0.001 |
| Pulmonary congestion by chest x-ray | 1.79 (1.36 – 2.34) | 0.001 | 1.66 (1.25 – 2.22) | 0.001 |
| Digoxin 0.25 mg/ day | 1.09 (0.88 – 1.36) | 0.431 | 3.17 (2.25 – 4.47) | <0.0001 |
| Digoxin >0.25 mg/ day | 1.12 (0.83 – 1.52) | 0.473 | 8.70 (5.04 – 15.00) | <0.0001 |
Adjusted for the same covariates as in Table 2
Acknowledgments
The Digitalis Investigation Group (DIG) study was conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with the Digitalis Investigation Group (DIG) Investigators. This manuscript has been reviewed by NHLBI for scientific content and consistency of data interpretation with previous The Digitalis Investigation Group (DIG) publications and significant comments have been incorporated prior to submission for publication.
The authors wish to thank Jerome L. Fleg, MD, National Heart Lung and Blood Institute for his critical review of the manuscript.
Grant Support: Dr. Ahmed is supported by a National Institute on Aging, National Institutes of Health grant 1-K23-AG19211-03.
Footnotes
Contributors Ali Ahmed conceived the study hypothesis and design, and wrote the paper in collaboration with Michael Rich, Mihai Gheorghiade and Thomas Love. Ali Ahmed did the biostatistical analyses with assistance from Inmaculada Aban, Thomas Love and Donald Lloyd-Jones. All authors analyzed and interpreted the data, participated in critical revision of the paper for important intellectual content, and approved the final version of the article. Ali Ahmed and Inmaculada Aban had full access to the data.
Dedication The authors wish to dedicate this article to the memory of Thomas W. Smith, MD (1936-1997) who played a crucial role in enhancing our understanding of digoxin in heart failure that lead to the DIG trial.
Abstract Presentation An oral abstract based on this manuscript will be presented at the 2005 American Heart Association Scientific Sessions in Dallas, Texas on November 15.
Contributor Information
Ali Ahmed, University of Alabama at Birmingham, and VA Medical Center, Birmingham, AL, USA.
Michael W. Rich, Washington University School of Medicine, St. Louis, MO, USA.
Thomas E. Love, Case Western Reserve University, Cleveland OH, USA.
Donald M. Lloyd-Jones, Northwestern University, Chicago, IL, USA.
Inmaculada B. Aban, University of Alabama at Birmingham, Birmingham, AL, USA.
Wilson S. Colucci, Boston University, Boston, MA, USA.
Kirkwood F. Adams, Jr, University of North Carolina, Chapel Hill, NC, USA.
Mihai Gheorghiade, Northwestern University, Chicago, IL, USA.
References
- 1.Gheorghiade M, Adams KF, Jr, Colucci WS. Digoxin in the management of cardiovascular disorders. Circulation. 2004;109:2959–2964. doi: 10.1161/01.CIR.0000132482.95686.87. [DOI] [PubMed] [Google Scholar]
- 2.The Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997;336:525–533. doi: 10.1056/NEJM199702203360801. [DOI] [PubMed] [Google Scholar]
- 3.Hogg K, Swedberg K, McMurray J. Heart failure with preserved left ventricular systolic function; epidemiology, clinical characteristics, and prognosis. J Am Coll Cardiol. 2004;43:317–327. doi: 10.1016/j.jacc.2003.07.046. [DOI] [PubMed] [Google Scholar]
- 4.Hunt SA, Baker DW, Chin MH, Cinquegrani MP, Feldman AM, Francis GS, Ganiats TG, Goldstein S, Gregoratos G, Jessup ML, Noble RJ, Packer M, Silver MA, Stevenson LW, Gibbons RJ, Antman EM, Alpert JS, Faxon DP, Fuster V, Jacobs AK, Hiratzka LF, Russell RO, Smith SC., Jr ACC/AHA Guidelines for the Evaluation and Management of Chronic Heart Failure in the Adult: Executive Summary A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1995 Guidelines for the Evaluation and Management of Heart Failure): Developed in Collaboration With the International Society for Heart and Lung Transplantation; Endorsed by the Heart Failure Society of America. Circulation. 2001;104:2996–3007. doi: 10.1161/hc4901.102568. [DOI] [PubMed] [Google Scholar]
- 5.Smith TW, Butler VP, Jr, Haber E. Determination of therapeutic and toxic serum digoxin concentrations by radioimmunoassay. N Engl J Med. 1969;281:1212–1216. doi: 10.1056/NEJM196911272812203. [DOI] [PubMed] [Google Scholar]
- 6.Adams KF, Jr, Gheorghiade M, Uretsky BF, Patterson JH, Schwartz TA, Young JB. Clinical benefits of low serum digoxin concentrations in heart failure. J Am Coll Cardiol. 2002;39:946–953. doi: 10.1016/s0735-1097(02)01708-4. [DOI] [PubMed] [Google Scholar]
- 7.Rathore SS, Curtis JP, Wang Y, Bristow MR, Krumholz HM. Association of serum digoxin concentration and outcomes in patients with heart failure. JAMA. 2003;289:871–878. doi: 10.1001/jama.289.7.871. [DOI] [PubMed] [Google Scholar]
- 8.Remme WJ, Swedberg K. Guidelines for the diagnosis and treatment of chronic heart failure. Eur Heart J. 2001;22:1527–1560. doi: 10.1053/euhj.2001.2783. [DOI] [PubMed] [Google Scholar]
- 9.Fonarow GC, Yancy CW, Heywood JT. Adherence to heart failure quality-of-care indicators in US hospitals: analysis of the ADHERE Registry. Arch Intern Med. 2005;165:1469–1477. doi: 10.1001/archinte.165.13.1469. [DOI] [PubMed] [Google Scholar]
- 10.Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, Carson P, DiCarlo L, DeMets D, White BG, DeVries DW, Feldman AM. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–2150. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 11.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 12.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup ML, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure) [August 17, 2005];American College of Cardiology Web Site. 2005 doi: 10.1016/j.jacc.2005.08.022. Available at: http://www.acc.org/clinical/guidelines/failure//index.pdf. [DOI] [PubMed]
- 13.The Digitalis Investigation Group. Protocol: Trial to evaluate the effect of digitalis on mortality n heart failure. NHLBI; 1991. [Google Scholar]
- 14.Adams KF, Jr, Patterson JH, Gattis WA, O’Connor CM, Lee CR, Schwartz TA, Gheorghiade M. Relationship of serum digoxin concentration to mortality and morbidity in women in the digitalis investigation group trial: a retrospective analysis. J Am Coll Cardiol. 2005;46:497–504. doi: 10.1016/j.jacc.2005.02.091. [DOI] [PubMed] [Google Scholar]
- 15.Collins JF, Howell CL, Horney RA. Determination of vital status at the end of the DIG trial. Control Clin Trials. 2003;24:726–730. doi: 10.1016/j.cct.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 16.Rosenbaum PR, Rubin DB. Reducing bias in observational studies using subclassification on the propensity score. J Am Stat Asso. 1984;79:516–524. [Google Scholar]
- 17.Rubin DB. Using propensity score to help design observational studies: Application to the tobacco litigation. Health Services and Outcomes Research Methodology. 2001;2:169–188. [Google Scholar]
- 18.Aronow HD, Topol EJ, Roe MT, Houghtaling PL, Wolski KE, Lincoff AM, Harrington RA, Califf RM, Ohman EM, Kleiman NS, Keltai M, Wilcox RG, Vahanian A, Armstrong PW, Lauer MS. Effect of lipid-lowering therapy on early mortality after acute coronary syndromes: an observational study. Lancet. 2001;357:1063–1068. doi: 10.1016/S0140-6736(00)04257-4. [DOI] [PubMed] [Google Scholar]
- 19.Weitzen S, Lapane KL, Toledano AY, Hume AL, Mor V. Weaknesses of goodness-of-fit tests for evaluating propensity score models: the case of the omitted confounder. Pharmacoepidemiol Drug Saf. 2004;14:227–238. doi: 10.1002/pds.986. [DOI] [PubMed] [Google Scholar]
- 20.Weitzen S, Lapane KL, Toledano AY, Hume AL, Mor V. Principles for modeling propensity scores in medical research: a systematic literature review. Pharmacoepidemiol Drug Saf. 2004;13:841–853. doi: 10.1002/pds.969. [DOI] [PubMed] [Google Scholar]
- 21.Love TE. Using Propensity Scores: Stratification, Adjustment, Sensitivity and Strategies. [May 11. 2004]; Available at http://www.chrp.org/propensity/
- 22.Rubin DB. On principles for modeling propensity scores in medical research. Pharmacoepidemiol Drug Saf. 2004;13:855–857. doi: 10.1002/pds.968. [DOI] [PubMed] [Google Scholar]
- 23.D’Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 24.Normand ST, Landrum MB, Guadagnoli E, Ayanian JZ, Ryan TJ, Cleary PD, McNeil BJ. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54:387–398. doi: 10.1016/s0895-4356(00)00321-8. [DOI] [PubMed] [Google Scholar]
- 25.Macro Levesque R. In: SPSS® Programming and Data Management, 2nd Edition A Guide for SPSS® and SAS® Users. 2. Levesque R, editor. Chicago, IL: SPSS Inc; [June 4, 2005]. 2005. Available online at: http://www.spss.com/spss/data_management_book.htm. [Google Scholar]
- 26.Klein JP, Moschberger ML. Survival analysis: Techniques for censored and truncated data. 2. New York, NY: Springer; 2003. [Google Scholar]
- 27.SPSS for Windows, Rel. 13.0.2. [computer program]. Version. Chicago, IL: SPSS Inc.; 2005. [Google Scholar]
- 28.Antonello A, Cargnielli G, Ferrari M, Melacini P, Montanaro D. Effect of digoxin on plasma-renin-activity in man. Lancet. 1976;2:850. doi: 10.1016/s0140-6736(76)91231-9. [DOI] [PubMed] [Google Scholar]
- 29.Gheorghiade M, Ferguson D. Digoxin. A neurohormonal modulator in heart failure? Circulation. 1991;84:2181–2186. doi: 10.1161/01.cir.84.5.2181. [DOI] [PubMed] [Google Scholar]
- 30.Bertler A, Redfors A. Plasma-digoxin concentration. Lancet. 1971;2:50. doi: 10.1016/s0140-6736(71)90044-4. [DOI] [PubMed] [Google Scholar]
- 31.van Veldhuisen DJ. Low-dose digoxin in patients with heart failure. Less toxic and at least as effective? J Am Coll Cardiol. 2002;39:954–956. doi: 10.1016/s0735-1097(02)01710-2. [DOI] [PubMed] [Google Scholar]
- 32.McMurray JJ, Ostergren J, Swedberg K, Granger CB, Held P, Michelson EL, Olofsson B, Yusuf S, Pfeffer MA. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet. 2003;362:767–771. doi: 10.1016/S0140-6736(03)14283-3. [DOI] [PubMed] [Google Scholar]
- 33.Eichhorn EJ, Gheorghiade M. Digoxin--new perspective on an old drug. N Engl J Med. 2002;347:1394–1395. doi: 10.1056/NEJMp020118. [DOI] [PubMed] [Google Scholar]
- 34.Domanski M, Fleg J, Bristow M, Knox S. The effect of gender on outcome in digitalis-treated heart failure patients. J Card Fail. 2005;11:83–86. doi: 10.1016/j.cardfail.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 35.Rathore SS, Wang Y, Krumholz HM. Sex-based differences in the effect of digoxin for the treatment of heart failure. N Engl J Med. 2002;347:1403–1411. doi: 10.1056/NEJMoa021266. [DOI] [PubMed] [Google Scholar]
- 36.Domanski M, Norman J, Pitt B, Haigney M, Hanlon S, Peyster E. Diuretic use, progressive heart failure, and death in patients in the Studies Of Left Ventricular Dysfunction (SOLVD) J Am Coll Cardiol. 2003;42:705–708. doi: 10.1016/s0735-1097(03)00765-4. [DOI] [PubMed] [Google Scholar]
- 37.Ahmed A, Husain A, Gambassi G, Dell’Italia LJ, Allman RM, Bourge RC. Diuretics increase mortality and hospitalization in ambulatory patients with chronic systolic hart failure: A propensity score analysis. Paper presented at: American Heart Association Scientific Sessions, Dallas, Texas. Circulation. 2005;112:II–674. [Google Scholar]
- 38.Juurlink DN, Mamdani MM, Lee DS, Kopp A, Austin PC, Laupacis A, Redelmeier DA. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Engl J Med. 2004;351:543–551. doi: 10.1056/NEJMoa040135. [DOI] [PubMed] [Google Scholar]
- 39.Cohn JN, Tognoni G. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345:1667–1675. doi: 10.1056/NEJMoa010713. [DOI] [PubMed] [Google Scholar]
- 40.Young JB, Abraham WT, Smith AL, Leon AR, Lieberman R, Wilkoff B, Canby RC, Schroeder JS, Liem LB, Hall S, Wheelan K. Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: the MIRACLE ICD Trial. JAMA. 2003;289:2685–2694. doi: 10.1001/jama.289.20.2685. [DOI] [PubMed] [Google Scholar]
- 41.Packer M. End of the oldest controversy in medicine. Are we ready to conclude the debate on digitalis? N Engl J Med. 1997;336:575–576. doi: 10.1056/NEJM199702203360809. [DOI] [PubMed] [Google Scholar]
- 42.Rathore SS, Krumholz HM. Digoxin therapy for heart failure: safe for women? Ital Heart J. 2003;4:148–151. [PubMed] [Google Scholar]
- 43.Packer M, Gheorghiade M, Young JB, Costantini PJ, Adams KF, Cody RJ, Smith LK, Van Voorhees L, Gourley LA, Jolly MK. Withdrawal of digoxin from patients with chronic heart failure treated with angiotensin-converting-enzyme inhibitors. RADIANCE Study. N Engl J Med. 1993;329:1–7. doi: 10.1056/NEJM199307013290101. [DOI] [PubMed] [Google Scholar]
- 44.Uretsky BF, Young JB, Shahidi FE, Yellen LG, Harrison MC, Jolly MK. Randomized study assessing the effect of digoxin withdrawal in patients with mild to moderate chronic congestive heart failure: results of the PROVED trial. PROVED Investigative Group. J Am Coll Cardiol. 1993;22:955–962. doi: 10.1016/0735-1097(93)90403-n. [DOI] [PubMed] [Google Scholar]
- 45.Gheorghiade M, Hall VB, Jacobsen G, Alam M, Rosman H, Goldstein S. Effects of increasing maintenance dose of digoxin on left ventricular function and neurohormones in patients with chronic heart failure treated with diuretics and angiotensin-converting enzyme inhibitors. Circulation. 1995;92:1801–1807. doi: 10.1161/01.cir.92.7.1801. [DOI] [PubMed] [Google Scholar]
- 46.Slatton ML, Irani WN, Hall SA, Marcoux LG, Page RL, Grayburn PA, Eichhorn EJ. Does digoxin provide additional hemodynamic and autonomic benefit at higher doses in patients with mild to moderate heart failure and normal sinus rhythm? J Am Coll Cardiol. 1997;29:1206–1213. doi: 10.1016/s0735-1097(97)00057-0. [DOI] [PubMed] [Google Scholar]
- 47.Eichhorn EJ, Lukas MA, Wu B, Shusterman N. Effect of concomitant digoxin and carvedilol therapy on mortality and morbidity in patients with chronic heart failure. Am J Cardiol. 2000;86:1032–1035. A1010–1031. doi: 10.1016/s0002-9149(00)01146-2. [DOI] [PubMed] [Google Scholar]
- 48.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 49.AstraZeneca LP. Toprol XL (metoprolol succinate) extended release tablets. Full prescribing information. [June 30, 2005];AstraZeneca LP. http://www.astrazeneca-us.com/pi/toprol-xl.pdf.


