Abstract
Our group has previously created a functional neointestine that is capable of restoring absorptive function. However, the endogenous level of vascular endothelial growth factor (VEGF) is markedly reduced in the construct compared to native bowel. Therefore, we wanted to locally deliver VEGF in a sustained fashion to upregulate angiogenesis in the neointestine. Rat recombinant VEGF was encapsulated in poly (lactide-co-glycolide) microspheres by a double emulsion method. Release kinetics and bioactivity were determined in vitro. Tissue-engineered intestine was generated by seeding donor neonatal rat intestinal organoid units onto a biodegradable polyglycolic acid scaffold along with VEGF-containing or empty microspheres, and wrapped in the omentum of recipient rats. After four weeks, the neointestinal cysts were analyzed for morphometry, VEGF levels, epithelial proliferation, and capillary density. Sustained release of biologically active VEGF was confirmed by in vitro studies. Intestinal constructs with VEGF microspheres were significantly larger than those containing empty microspheres. Tissue VEGF levels were significantly higher in neointestine loaded with encapsulated VEGF compared to those without growth factor. Epithelial cellular proliferation and capillary density were significantly increased in the VEGF-containing neointestinal constructs compared to empty constructs. Tissue-engineered intestine responds to sustained delivery of VEGF by upregulating microvasculature and epithelial proliferation.
1. Introduction
Short bowel syndrome is a devastating clinical condition characterized by a deficiency in intestinal absorptive capacity leading to malnutrition, dehydration and electrolyte imbalance in both children and adults. Surgical and pharmacological manipulation of remnant intestine [1,2,3,4] have demonstrated little success in clinical trials while intestinal transplantation has been limited by a high degree of graft failure, a shortage of organ donors and need for immunosuppression [5]. The only proven treatment for this condition is the administration of total parenteral nutrition (TPN) which, although life-sustaining, is associated with significant short- and long-term morbidity, mortality and cost [6,7].
In response to this shortcoming, a prototype tissue-engineered intestine was developed by the Vacanti laboratory [8]. This tissue-engineered intestine exhibits properties of mature intestine such as the maintenance of a membrane potential, expression of brush border enzymes and nutrient transporters, ion transport [9,10], generation of vascular [11] and lymphatic [12] networks and development of a mature mucosal immune system [13].
As with other engineered tissues, one the major challenges has been the need for rapid vascular ingrowth in order to promote engraftment and proper development. This is due in part to the lack of local growth factors that would normally be present in the homeostatic milieu of growing tissues. In order to circumvent this limitatino, our group has examined physiologic regenerative signals for neointestinal maturation such as small bowel resection, partial hepatectomy and portacaval shunt in the host [14]. In addition, we have previously demonstrated that engineered intestine responds to exogenously administered growth factors such as glucagon-like peptide 2 (GLP-2) by exhibiting mucosal hypertrophy and hyperplasia [15]. These studies have clearly established that the neointestine responds to biologic stimulation in a similar fashion to native tissues.
Angiogenesis is the ingrowth of blood vessels into tissues from pre-existing vasculature. It is a critical step for a multitude of important biologic functions such as fetal development, wound healing, and reperfusion after ischemia [16]. It is characterized by a series of steps involving endothelial cells, cytokines, growth factors, and extracellular matrix. One of the key mediators of angiogenesis is the vascular endothelial growth factor (VEGF) whose mitogenic effects include migration and proliferation of endothelial cells, stabilization of neovessels and tissue remodeling [17]. In addition, VEGF has been shown to promote smooth muscle growth and stimulate nerve regeneration after injury [18,19]. VEGF has a limited half-life in circulation and developing tissues require local and persistent exposure to the growth factor to prevent atrophy of neovessels [20] Previously, we have demonstrated that our tissue-engineered intestine exhibits lower levels of VEGF and basic fibroblast growth factor (bFGF) and a fixed capillary density throughout its maturation compared to native juvenile bowel [11].
Therefore, in the present study we applied a polymeric microsphere delivery vehicle to encapsulate VEGF and provide a sustained angiogenic signal to the maturing neointestine.
2. Methods
2.1 Microsphere Preparation
Microspheres were prepared using a double emulsion/solvent extraction technique [21]. Briefly, emulsion #1 was created by sonicating 3 μg of rat recombinant VEGF165 (R&D Systems, Minneapolis, MN) with a poly(lactide-co-glycolide) (PLGA) solution (0.5g of PLGA , 85:15 high viscosity) (Medisorb, Cincinnati, OH) dissolved in 10 ml of ethyl acetate (EtAc) (Sigma, St. Louis, MO). Emulsion #2 was produced by dissolving 0.5g polyvinyl alcohol (PVA) (Sigma) in 9.3 ml double distilled water (ddH20) and 0.7 ml EtAc; 1 ml of emulsion #2 was added to emulsion #1 and the mixture vortexed. The vortexed solution was added to an organic extraction buffer (186 ml ddH20, 14 ml EtAc, 0.2 g PVA) and stirred for three hours to evaporate the EtAc. The solution was filtered through a 0.45 μm membrane pre-conditioned with 70% ethanol (EtOH); the filtrate was centrifuged to pellet the microspheres, and the supernatant was discarded. The microspheres were flash-frozen in liquid nitrogen and lyophilized overnight. For radiolabeled studies, 1μCi of 125I-VEGF was added to the microspheres. (Perkin Elmer, Boston, MA)
2.2 Polymer Fabrication
Polyglycolic acid scaffold tubes were fabricated as previously described [8]. Non-woven 2 mm thick sheets of polyglycolic acid (PGA) (Smith and Nephew, Heslington, NY) were fashioned into 10 mm long cylinders with an outer diameter of 5 mm. The edges were sealed with a 10% PLGA solution. To reinforce the fiber structure, the tubes were sprayed with a 5% poly-L-lactide (1.06 i.v.) solution. (Birmingham Polymers, Pelham, AL) The lyophilized tubes were washed with 500 ml Hank’s balanced salt solution (HBSS) (Gibco, Gaithersburg, MD) The tubes were sterilized by immersion in 100% EtOH for 20 min and coated with 1:100 solution of type I collagen. (Vitrogen, Palo Alto, CA).
2.3 Organoid Separation
Organoid units were prepared according to the method of Evans et al. [22] Briefly, total small intestine from 3–5 day old neonatal Lewis rat pups (Charles River Laboratories, Wilmington MA) was irrigated with ice-cold HBSS before being opened lengthwise along the antimesenteric border and cut into 1–2 mm sections. The tissue was washed three times in HBSS at 4°C with vigorous shaking and enzymatically digested with 0.25 mg/ml of dispase (Boehinger Manheim, Indianapolis, IN) and 800 U/ml of collagenase XI (Sigma) on an orbital shaker at 80 revolutions/min. The digestion was stopped by the addition of 10 ml of high glucose Dulbecco’s Modified Eagle Medium (DMEM) (Gibco) containing 4% fetal bovine serum (FBS) and 4% sorbitol and the mixture centrifuged at 150 g for 5 min. The supernatant was removed and the above procedure repeated three times. Organoid units were reconstituted in high-glucose DMEM with 10% FBS and counted by hemocytometer.
2.4 Construct Implantation
Organoid units (100,000) were loaded into each tubular polymer scaffold, with empty or VEGF-containing microspheres, and allowed to adhere for 1 hr at 4°C. Twelve 200 g adult male Lewis rats were anesthetized with 50mg/kg pentobarbital and a 1.5 cm upper abdominal incision was made in order to exteriorize the greater omentum. Polymer constructs were wrapped in the omentum and secured with 6-O Prolene sutures (Ethicon, Piscataway, NJ) before being returned to the peritoneal cavity and the abdominal wall was closed in two layers. Studies were approved by the Harvard Medical Area Committee on Animals and followed National Institutes of Health guidelines.
2.5 Neointestine Harvest
At four weeks post-implantation, all animals were injected intraperitoneally with 50mg/kg of 5-bromo-2-deoxyuridine (BrDU) (Sigma) one hour before sacrifice. After laparotomy, neointestinal cysts were separated from surrounding tissues and their weight and two radii (long axis a, short axis b) were recorded. Volume of constructs was calculated using a formula for an oblate spheroid (V= (4/3)a2b). They were divided into two pieces along their long axis with one section placed in 10% formalin overnight for paraffin embedding and histology while the other section was snap-frozen in liquid nitrogen for protein analysis.
2.6 In Vitro Characterization of Microspheres
Release kinetics were determined by placing microspheres containing 125I-VEGF in a simulated body fluid consisting of phosphate-buffered saline (PBS) containing calcium and magnesium at 37°C on a shaker table. At pre-determined timepoints, the entire solution was removed and analyzed for radioactivity using a Packard γ counter (GMI Inc, Ramsey, MN). A fresh aliquot of PBS was added and the quantity of 125I-VEGF released was determined by subtracting the radioactivity in each sample from the total radioactivity at the beginning of the experiment. Bioactivity of encapsulated VEGF was determined using a human umbilical vein endothelial cells (HUVEC) (Cell Applications, San Diego, CA) proliferation assay [23]. HUVECs (1 × 104 cells) were cultured and passaged in standard endothelial cell medium conditions with growth supplement onto 6-well plates and allowed to adhere for 24 hr. The plates were then replaced with filtered supplement-free medium conditioned with VEGF released from microspheres or empty microspheres, supplement-free medium alone or supplement-free medium containing 10 ng/ml VEGF; all cells remained in culture for an additional 72 hr. The cells were harvested with a 0.05% trypsin solution and counted. This cycle was continued until the microspheres had undergone 10 successive rounds of HUVEC subculturing and medium conditioning.
2.7 Histology and Immunohistochemistry
Deparaffinized and rehydrated 10 μm thick sections were unmasked using microwave antigen retrieval with 0.1M citrate buffer, pH 6.0 for 10 min. Sections were rinsed and then blocked with 10% normal goat serum in PBS for 30 min at room temperature. Sections were incubated with primary antibody (goat anti-CD31 for vessel staining, diluted 1:1000) (Santa Cruz Biotechnology, Santa Cruz, CA) or (mouse anti-BrDU for epithelial proliferation, diluted 1:50) (Sigma) with 0.1M PBS containing 5% normal horse serum and 0.3% Triton X-100, for 1 hr at 25°C. After washing in PBS, sections were incubated with the secondary antibody (biotinylated horse anti-goat antiserum for CD-31 or goat anti-mouse for BrDU) diluted 1:200 in PBS for 1 hr at room temperature. After a brief rinse with PBS, the sections were incubated with 0.3% hydrogen peroxide (H2O2) in methanol for 30 min to quench endogenous peroxidase activity. After rinsing, the sections were reacted with avidin-biotin-horseradish peroxidase complex for 1 hr. After washing in 0.1M Tris HCL-buffered saline, the sections were incubated in 0.05% 3,3′-diaminobenzidine solution containing nickel ammonium sulfate (0.2%) for 5–10 min and counterstained with Mayer’s hematoxylin and mounted. CD31 positive capillaries in 10 random high power fields were counted and normalized to the slide viewing area. BrDU positively-stained cells were calculated as a fraction of the total number of cells per crypt and expressed as a percentage (10 crypts/slide were counted)
2.8 Terminal Deoxynucleotide Transferase Mediated dUTP Nick-End Labeling (TUNEL)
Deparaffinized and rehydrated 10 μm thick sections were exposed to TUNEL mixture containing equilibration buffer, biotinylated nucleotide mix and terminal deoxynucleotidyl transferase for one hour in a humidified chamber at room temperature. (Roche, Indianapolis, IN) Endogenous peroxidase activity was blocked by immersing the sections in 0.3% H2O2 for 3 min at room temperature. Sections were then incubated with anti-digoxigenin and diaminobenzidine chromogen substrate kits according to the manufacturer’s instructions for 30 min. The number of cells with TUNEL positivity and morphological features of apoptosis were counted in five fields and averaged.
2.9 Enzyme-linked Immunosorbent Assay (ELISA)
Tissue VEGF was extracted as previously described [24]. Frozen samples were minced and homogenized in 5 ml of extraction buffer consisting of PBS, 0.05% Triton X-100 and 1mM protease inhibitor 4,2-aminoethylbenzenesulfonylfluoride (Sigma). The mix was sonicated and centrifuged at 1500 g for 10 min at 4°C. The total protein concentration was determined by the bichinchoninic acid method (BCA)(Sigma). Quantikine ELISA kit for rat VEGF was purchased (R&D Systems) and utilized according to the manufacturer’s instructions with standards of known concentration and positive and negative controls. The concentration of VEGF in each sample was calculated as picograms per milligram total protein.
2.10 Statistical Analysis
All continuous data were expressed as the mean ± SEM and p < 0.05 was taken as significant. P values were estimated using Student t-test and when applicable, analysis of variance (ANOVA) with post-hoc Tukey intergroup comparisons. All computations were performed using a commercially available statistical package (Statistica, version 4.3, StatSoft, Tulsa, OK).
3. Results
3.1 In Vitro Microsphere Validation Studies
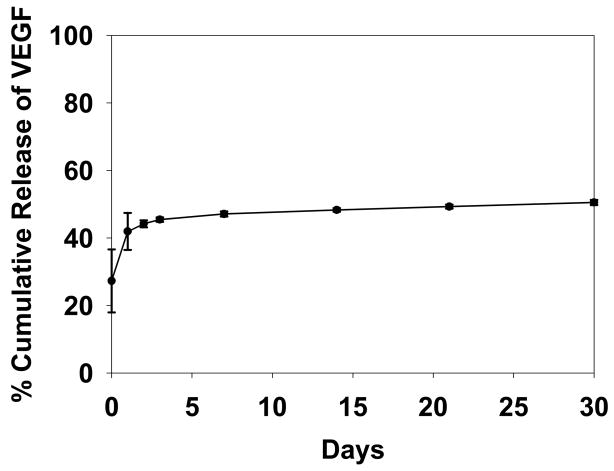
The loading efficiency of VEGF into PLGA microspheres was determined to be 28.7% + 9.5% which was consistent with previous batches fabricated in the Mooney laboratory (personal communication). The release kinetics profile in vitro for the VEGF-loaded microspheres is illustrated in Figure 1. VEGF was released in two phases; an initial burst phase within the first day followed by a steady state release over 14 days with very little release at 30 days.
Figure 1.
Release kinetics of 125I-VEGF microspheres. After an initial burst release, microspheres released a steady state level of VEGF for up to 30 days (n=3)
The bioactivity of encapsulated VEGF released from the microspheres over time was examined using an in vitro HUVEC proliferation assay. The results shown in Figure 2 demonstrate an increased rate of proliferation of HUVECs exposed to exogenous VEGF and to the supernatant of VEGF-loaded microspheres. Empty microspheres did not provide a proliferative stimulus nor a toxic effect on the HUVECs. VEGF released from microspheres demonstrated peak biologic activity after immediate release but remained significant up to 14 d of incubation. After this time, released VEGF from microspheres had a reduced trophic effect compared to fresh VEGF administered to culture media.
Figure 2.

In vitro bioactivity of VEGF as a function of HUVEC proliferation over 72 hrs. Untreated cells did not demonstrate proliferation in serum-free media. HUVECs cultured in serum-free medium supplemented with 10 ng/ml of exogenous VEGF demonstrated an increase in proliferation at all timepoints. HUVECs exposed to serum-free media conditioned with VEGF-loaded microspheres (VEGFMS) had a statistically significant increase in proliferation up to 14 days after conditioning. This was not observed in HUVECs exposed to serum-free media conditioned with empty microspheres (Empty MS) (n=6/group, *p<0.05 compared to untreated group)
3.2 Neointestinal Growth and Development
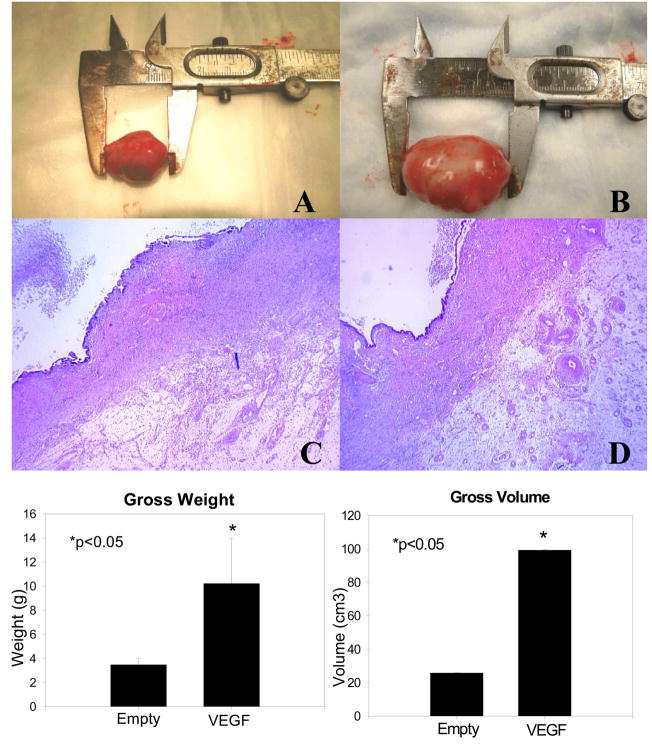
All but two animals (one in each group) produced viable neointestinal cysts for analysis (83% engraftment rate). Microspheres did not have any appreciable effect on the morphologic or histological structure of the neointestine, although it was possible to note the increased vascularity in the outer connective tissue of the VEGF-containing constructs (See Figure 3) Intestinal constructs implanted with VEGF-loaded microspheres were significantly larger (99.3 ± 0.4 cm3 vs. 25.8 ± 0.2 cm3) and heavier (10.2 ± 3.8 g vs. 3.5 ± 0.5 g) (p<0.05) than those implanted with empty microspheres.
Figure 3.
Morphological parameters of Neointestinal Cysts. (A) Gross appearance of neointestinal cyst containing empty microspheres. (B) Gross appearance of neointestinal cyst containing VEGF microspheres. (C) Histological appearance of neointestinal cyst containing empty microspheres (40X magnification). (D) Histological appearance of neointestinal cyst containing VEGF microspheres (40X magnification). Both the volume and the weight were significantly increased in neointestinal cysts containing VEGF microspheres. (n=5/group, *p<0.05)
3.3 In Vivo Angiogenic Response to Released VEGF
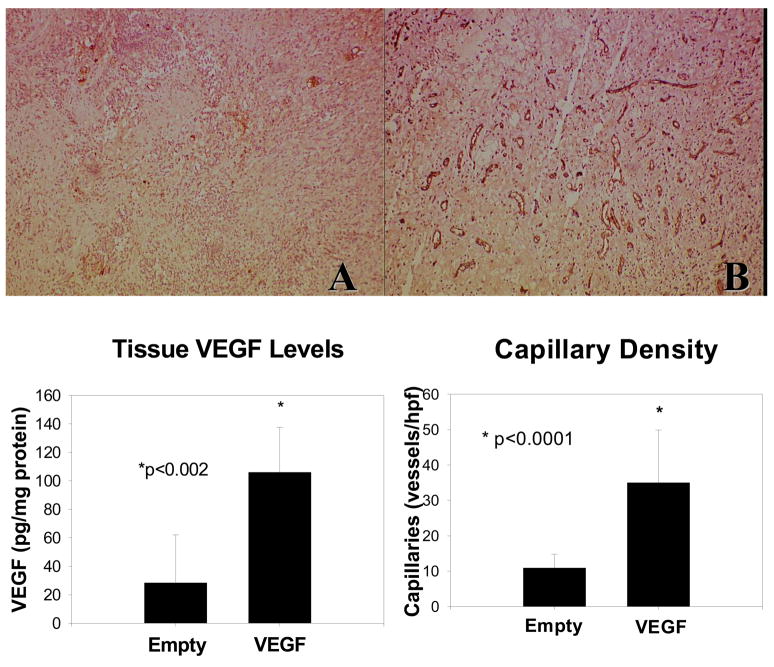
VEGF delivery was confirmed by measuring levels of VEGF in neointestinal constructs. Figure 4 demonstrates that tissue VEGF levels were significantly higher in neointestine loaded with encapsulated VEGF compared to those without growth factor (106 ± 33 pg/mg vs. 28 ± 34 pg/mg) (p<0.05). Neointestinal capillaries were identified by CD31 (PECAM-1) immunopositivity, an endothelial cell marker (Figure 4). In response to the higher tissue VEGF levels, capillary density in the muscular and connective tissue layers of VEGF-containing neointestinal constructs was significantly increased compared to constructs containing microspheres without growth factor (29.8 ± 4.5 vs. 10.3 ± 1.1 capillaries/hpf) (p<0.05)
Figure 4.
Tissue VEGF levels and capillary density. Tissue VEGF levels were significantly increased in neointestinal cysts containing VEGF-loaded microspheres compared to those with empty microspheres. (n=5) (*p<0.002) Immunohistochemical staining of PECAM-1 (CD31) in submucosal and muscular layers of neointestinal cysts containing (A) empty microspheres and (B) VEGF-loaded microspheres (100X magnification, representative images). Quantification of capillary density demonstrated a significantly increased capillary density in B than A. (n=5/group, *p<0.0001)
3.4 In Vivo Epithelial Response to Released VEGF
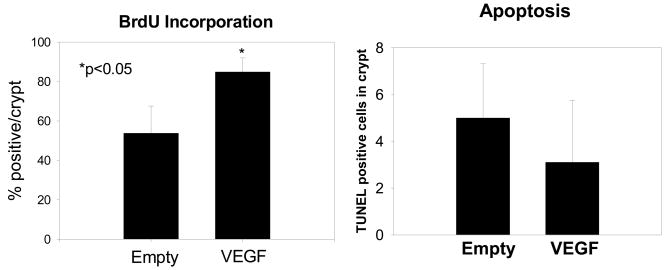
Neointestinal epithelial proliferation was quantified by measuring BrDU incorporation in crypts while apoptosis was measured by TUNEL staining (Figure 5). Constructs implanted with VEGF-releasing microspheres had increased rates of epithelial proliferation in neoinstestinal crypts compared to those implanted with empty microspheres, 84.9 ± 7.1% vs. 53.8 ± 13.8% BrDU staining cells per crypt, respectively. However, apoptosis remained unchanged between groups (5.0 ± 2.3 vs. 3.1 ± 2.6 TUNEL staining cells per crypt).
Figure 5.
Epithelial proliferation and apoptosis. BrDU incorporation in crypts was significantly increased in neointestinal crypts containing VEGF-loaded microspheres demonstrating increased proliferation. (n=5/group) (*p<0.05) Apoptosis measured by TUNEL staining showed no difference in programmed cell death between neointestinal cysts containing empty or VEGF-loaded microspheres. (n=5/group)
4. Discussion
One of the greatest challenges in engineering organs is the adequate delivery and maintenance of oxygen and nutrients to the developing tissue. The biologic phenomenon of angiogenesis is particularly critical in the early stages of engraftment and development when cells transplanted onto scaffolds must maintain metabolic demands of cell division, tissue differentiation and physiologic function. One of the current challenges of the tissue-engineered intestine is the difficulty in forming constructs longer than a few centimeters that would be essential in order to expand absorptive surface area. Previous studies have confirmed that implantation of neointestine in rats undergoing massive small bowel resection reverses weight loss in these animals indirectly suggesting some recovery of absorption [25]. However, in order for tissue-engineered intestine to be a viable clinical option, the fluid and nutrition balances must be achieved on a much larger scale for higher order organisms. We hypothesized that a deficiency in angiogenic growth factors normally present in rapidly developing tissues contribute to this growth inhibition since the measured levels in our constructs fell below those of native juvenile bowel [11].
The present study addresses this issue by encapsulating VEGF in PLGA microspheres for localized and sustained release of VEGF to the maturing neointestine. PLGA microspheres have been utilized to encapsulate several growth factors in different systems [26]. Although VEGF-containing PLGA microspheres have been employed in other applications to stimulate angiogenesis [27], this is to our knowledge the first report of using this method to deliver growth factor to a three-dimensional, multi-cell lineage, physiologically complex engineered tissue such as intestine.
VEGF delivery from microspheres was confirmed in vitro by radioactive tracer and bioassay studies. Encapsulated VEGF had its most profound trophic effect on HUVECs within three days of conditioning mirroring its burst release profile and exceeding that of 10 ng/ml of exogenous VEGF. This would suggest much higher concentrations of active VEGF were released from the microspheres. In addition, a significant proliferative response was still elicited after 14 days of media conditioning. However this was reduced and less than that achieved by the exogenous VEGF presumably due to the decreased presence of released growth factor. Previous studies have examined the role of varying the ratio of lactide to glycolide in the polymer in order to tailor degradation rates to achieve desired release kinetics [28]. We chose to use 85:15 PLGA as our delivery vehicle given its favorable low-burst and sustained release profile over several weeks for our application. Some authors have expressed concern with the organic solvent in the double emulsion/solvent extraction technique denaturing the protein payload [23]. In addition, the acidic microenvironment generated by the hydrolysis of the polymer into its lactic and glycolic acid components has been associated with protein breakdown [29]. However, the bioactivity of the growth factor was preserved both during processing and release as evidenced by the robust proliferative response of HUVECs to encapsulated VEGF released in tissue culture media. This observation is critical since the growing constructs would have outstripped its oxygen supply unless neovascularization was stimulated in a sustained fashion over the course of engraftment and development into neointestine. Incorporation of VEGF into the polymer scaffold itself has been achieved [30], but these models are associated with a rapid, burst release of the growth factor which may not be applicable to our construct.
During neointestinal engraftment, the transplanted organoid units must obtain their sustenance from passive diffusion of oxygen and nutrients from the vascularized omentum of the host through the entire thickness of the polymer scaffold. This distance can approach several millimeters to a centimeter depending on the dimensions of the cylindrical scaffold dimensions and polymer spraying time of the PLLA coating. Since new blood vessel formation occurs at a rate of less than 1 mm per day, local application of an endothelial cell mitogen such as VEGF is required to accelerate neovessel growth and facilitate nutrient transport [31] Increased blood vessel formation was noted in the outer layers of the neointestine including in the host omentum suggesting trans-polymer diffusion of released VEGF, although this was not directly measured in this study. It has been demonstrated that pre-encapsulated VEGF in microspheres incorporated into the polymer scaffold yielded VEGF concentrations of 10 ng/ml up to 2 cm away from the scaffold [32]. This approach may be able to support a larger intestinal segment by providing a gradient of growth factor concentration over a wider area thereby supporting neovessels at progressive stages of development.
Microsphere incubation with organoid units did not have any detrimental effect on the morphology or ultrastructure of the resulting neointestinal construct. Spatial orientation of mucosa to serosa as well as villus-crypt architecture was preserved. This suggests the presence of degrading PLGA does not alter the attachment of the organoids to the PGA scaffold, nor the migration of cells to their appropriate topographic sites (inner mucosa for epithelium and outer serosa for mesenchymal elements). Although the intercellular signaling for cell migration in organoid units transplanted into scaffolds remains elusive, it seems that angiogenic stimuli are not involved.
A significant increase in the size and weight of the constructs exposed to VEGF microspheres was noted signifying a trophic effect on neointestinal tissues. This was confirmed by the presence of tissue VEGF levels that were significantly higher than controls and comparable to those of juvenile native jejunum [11]. In addition, a higher density of capillary networks in both the muscular and serosal layers of the neointestine was achieved by the application of VEGF-releasing microspheres. In rodents, as the juvenile intestine grows rapidly during weaning, metabolic demands are met by augmenting capillary networks in the bowel wall in order to support the lengthening tissue [33]. Therefore, VEGF-containing neointestinal constructs are able to mimic the in vivo angiogenic maturation of growing intestinal tissue. However, the functionality of the engineered tissue when anastomosed to native gut and actual bowel perfusion will require further investigation.
In response to increased vascularity and therefore greater access to nutrients and oxygen, the neointestinal epithelium responded by upregulating cellular proliferation as evidenced by BrDU incorporation into rapidly dividing progenitor cells in the crypt compartment. No effect was noted in cellular apoptotic rates suggesting a lack of neoplastic transformation. This implies that the enhanced neointestine has the capability to regenerate its mucosa through repopulation from crypt cells. Under normal conditions, the gut is constantly exposed to a harsh environment consisting of osmotic loads, digestive enzymes, and bacterial toxins, but it is able to survive despite these challenges. This is achieved by a constant turnover of villus tip cells and by migration of mucosal cells adjacent to denuded areas. Further investigation will be needed to determine if the angiogenic and regenerative properties of growth factor enriched neointestine will translate into improved absorptive capacity and resistance to injury.
5. Conclusion
In summary, sustained polymeric delivery of VEGF with polymer microspheres to the tissue-engineered intestine is feasible. The proliferative effects on the capillary network and epithelial crypts of the tissue-engineered intestine may serve to enhance its resilience and functional capacity. The ability to deliver other critical growth factors such as bFGF and GLP-2 is likely to further optimize the neointestine and may ultimately help to create an engineered organ of vital biologic function in desperate demand.
Acknowledgments
The authors wish to acknowledge the technical assistance of Jan Rounds. Portions of this manuscript were presented at the American Gastroenterological Association/Digestive Disease Week Annual Meeting, May 23, 2006. This work was funded by a National Institutes of Health F-32 Grant DK65483 (FGR) and Association for Academic Surgery/Karl Storz Endoscopy Research Fellowship (FGR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Warner BW, Chaet MS. Nontransplant surgical options for management of the short bowel syndrome. J Ped Gastroenterol and Nutrition. 1993;17:1–12. doi: 10.1097/00005176-199307000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Thompson JS. Surgical considerations in the short bowel syndrome. Surg Gyn Obstet. 1993;176:89–101. [PubMed] [Google Scholar]
- 3.Byrne TA, Persinger RL, Young LS, Ziegler TR, Wilmore DW. A new treatment for patients with short-bowel syndrome: growth hormone, glutamine, and a modified diet. Ann Surg. 1995;222:243–255. doi: 10.1097/00000658-199509000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scolapio JS, Camilleri M, Fleming CR, Oenning LV, Burton DD, Sebo TJ, et al. Effect of growth hormone, glutamine, and diet on adaptation in short bowel syndrome: a randomized, controlled study. Gastroenterol. 1997;113:1074–81. doi: 10.1053/gast.1997.v113.pm9322500. [DOI] [PubMed] [Google Scholar]
- 5.Todo S, Reyes J, Furukawa H, Abu-Elmagd K, Lee RG, Tzakis A, et al. Outcome analysis of 71 clinical intestinal transplantations. Ann Surg. 1995;222:270–9. doi: 10.1097/00000658-199509000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leaseburge LA, Winn NJ, Schloerb PR. Liver test alterations with total parenteral nutrition and nutritional status. JPEN J Parenter Enteral Nutr. 1992;26:348–352. doi: 10.1177/0148607192016004348. [DOI] [PubMed] [Google Scholar]
- 7.Howard L, Hassan N. Home parenteral nutrition: 25 years later. Gastroenterol Clin N. 1998;27:481–512. doi: 10.1016/s0889-8553(05)70015-7. [DOI] [PubMed] [Google Scholar]
- 8.Organ GM, Mooney DJ, Hansen LK, Schloo B, Vacanti JP. Transplantation of enterocytes utilizing polymer-cell constructs to produce a neointestine. Transplant Proc. 1992;24(6):3009–11. [PubMed] [Google Scholar]
- 9.Choi RS, Riegler M, Pothoulakis C, Kim BS, Mooney D, Vacanti M, et al. Studies of brush border enzymes, basement membrane components, and electrophysiology of tissue-engineered neointestine. J Ped Surg. 1998;33(7):991–7. doi: 10.1016/s0022-3468(98)90520-6. [DOI] [PubMed] [Google Scholar]
- 10.Tavakkolizadeh A, Berger UV, Stephen AE, Kim BS, Mooney D, Hediger MA, et al. Tissue-engineered neomucosa: morphology, enterocyte dynamics, and SGLT1 expression topography. Transplantation. 2003;75:181–5. doi: 10.1097/01.TP.0000044101.03656.9F. [DOI] [PubMed] [Google Scholar]
- 11.Gardner-Thorpe J, Grikscheit TC, Ito H, Perez A, Ashley SW, Vacanti JP, et al. Angiogenesis in tissue-engineered small intestine. Tissue Eng. 2003;9:1255–61. doi: 10.1089/10763270360728161. [DOI] [PubMed] [Google Scholar]
- 12.Duxbury MS, Grikscheit TC, Gardner-Thorpe J, Rocha FG, Ito H, Perez A, Ashley SW, et al. Transplantation. 2004;77(8):1162–6. doi: 10.1097/01.tp.0000121506.34924.3c. [DOI] [PubMed] [Google Scholar]
- 13.Perez A, Grikscheit TC, Blumberg RS, Ashley SW, Vacanti JP, Whang EE. Tissue- engineered small intestine: ontogeny of the immune system. Transplantation. 2002;74:619–23. doi: 10.1097/00007890-200209150-00006. [DOI] [PubMed] [Google Scholar]
- 14.Kim SS, Kaihara S, Benvenuto M, Choi RS, Kim BS, Mooney DJ, et al. Regenerative signals for tissue-engineered small intestine. Transplant Proc. 1999;31:657–60. doi: 10.1016/s0041-1345(98)01737-0. [DOI] [PubMed] [Google Scholar]
- 15.Ramsanahie A, Duxbury MS, Grikscheit TC, Perez A, Rhoads DB, Gardner-Thorpe J, et al. Effect of GLP-2 on mucosal morphology and SGLT1 expression in tissue-engineered neointestine. Am J Physiol Gastrointest Liver Physiol. 2003;285(6):G1345–52. doi: 10.1152/ajpgi.00374.2002. [DOI] [PubMed] [Google Scholar]
- 16.Folkman J, Shing Y. Angiogenesis. J Biol Chem. 1992;267:10931–10934. [PubMed] [Google Scholar]
- 17.Banai S, Jaklitsch MT, Shou M, Lazarous DF, Scheinowitz M, Biro S, et al. Angiogenic-induced enhancement of collateral blood flow to ischemic myocardium by vascular endothelial growth factor in dogs. Circulation. 1994;89:2183–9. doi: 10.1161/01.cir.89.5.2183. [DOI] [PubMed] [Google Scholar]
- 18.Meirer R, Gurunlouglu R, Siemionow M. Neurogenic perspective on vascular endothelial growth factor: review of the literature. J Reconstr Microsurg. 2001;17(8):625–30. doi: 10.1055/s-2001-18818. [DOI] [PubMed] [Google Scholar]
- 19.Burgess WH, Maciag T. The heparin-binding (fibroblast) growth factor family of proteins. Annu Rev Biochem. 1989;58:575–606. doi: 10.1146/annurev.bi.58.070189.003043. [DOI] [PubMed] [Google Scholar]
- 20.Lazarous DF, Shou M, Scheinowitz M, Hodge E, Thirumurti V, Kitsiou AN, et al. Comparative effects of basic fibroblast growth factor and vascular endothelial growth factor on coronary collateral development and arterial response to injury. Circulation. 1996;94:1074–82. doi: 10.1161/01.cir.94.5.1074. [DOI] [PubMed] [Google Scholar]
- 21.Rosca ID, Watari F, Uo M. Microparticle formation and its mechanism in single and double emulsion fashion. J Control Release. 2004;99:271–80. doi: 10.1016/j.jconrel.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Evans GS, Flint N, Somers AS, Eyden B, Potten CS. The development of a method for the preparation of rat intestinal epithelial cell primary cultures. J Cell Sci. 1992;101:219–31. doi: 10.1242/jcs.101.1.219. [DOI] [PubMed] [Google Scholar]
- 23.King TW, Patrick CW. Development and in vitro characterization of vascular endothelial growth factor (VEGF)-loaded poly(DL-lactic-co-glycolic acid)/poly(ethylene glycol) microspheres using a solid encapsulation/single emulsion/solvent technique. J Biomed Mater Res. 2000;51(3):383–90. doi: 10.1002/1097-4636(20000905)51:3<383::aid-jbm12>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 24.Ishii H, Oota I, Takuma T, Inomata Developmental expression of vascular endothelial growth factor in the masseter muscle of rats. Arch Oral Biol. 2001;46:77– 82. doi: 10.1016/s0003-9969(00)00081-9. [DOI] [PubMed] [Google Scholar]
- 25.Grikscheit TC, Siddique A, Ochoa E, Srinivasan A, Alsberg E, Hodin RA, et al. Tissue-engineered small intestine improves recovery after massive small bowel resection. Ann Surg. 2004;240(5):748–54. doi: 10.1097/01.sla.0000143246.07277.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen S, Yoshioka T, Lucarelli M, Hwang LH, Langer R. Controlled delivery systems for proteins based on poly(lactic/glycolic acid) microspheres. Pharm Res. 1991;8:713–20. doi: 10.1023/a:1015841715384. [DOI] [PubMed] [Google Scholar]
- 27.Cleland JL, Duenas ET, Park A, Daugherty A, Kahn J, Kowalski J, et al. Development of poly-(D,L-lactide-coglycolide) microsphere formulations containing recombinant human vascular endothelial growth factor to promote local angiogenesis. J Control Release. 2001;72:13–24. doi: 10.1016/s0168-3659(01)00258-9. [DOI] [PubMed] [Google Scholar]
- 28.Chung TW, Tsai YL, Hsieh JH, Tsai WJ. Different ratios of lactide and glycolide in PLGA affect the surface property and protein delivery characteristics of the PLGA microspheres with hydrophobic additives. J Microencapsul. 2006;23(1):15–27. doi: 10.1080/02652040500286110. [DOI] [PubMed] [Google Scholar]
- 29.Fu K, Pack DW, Klivanov AM, Langer R. Visual evidence of acidic environment within degrading poly(lactic-co-glycolic acid) (PLGA) microspheres. Pharm Res. 2000;17:100–6. doi: 10.1023/a:1007582911958. [DOI] [PubMed] [Google Scholar]
- 30.Murphy WL, Peters MC, Kohn DH, Mooney DJ. Sustained release of vascular endothelial growth factor from mineralized poly(lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials. 2000;21:2521–7. doi: 10.1016/s0142-9612(00)00120-4. [DOI] [PubMed] [Google Scholar]
- 31.Mikos AG, Sarakinos G, Ingber DE, Vacanti J, Langer R. Prevascularization of porous biodegradable polymer sponges. Biotech Bioeng. 1993;42:716–23. doi: 10.1002/bit.260420606. [DOI] [PubMed] [Google Scholar]
- 32.Ennett AB, Kaigler D, Mooney DJ. Temporally regulated delivery of VEGF in vitro and in vivo. J Biomed Mater Res. 2006;79(1):176–84. doi: 10.1002/jbm.a.30771. [DOI] [PubMed] [Google Scholar]
- 33.Unthank JL, Lash JM, Bohlen HG. Maturation of the rat intestinal microvasculature from juvenile to early adult life. Am J Physiol Gastrointest Liver. 1990;259:G282–9. doi: 10.1152/ajpgi.1990.259.2.G282. [DOI] [PubMed] [Google Scholar]