Abstract
Considerable evidence indicates early auditory stimulus processing abnormalities in schizophrenia, but the mechanisms are unclear. The present study examined oscillatory phenomena during a paired-click paradigm in the superior temporal gyrus (STG) as a possible core problem. The primary question addressed is whether first click and/or second click group differences in the time-domain evoked response in patients with schizophrenia are due to (1) group differences in the magnitude of poststimulus oscillatory activity, (2) group differences in poststimulus phase-locking, and/or (3) group differences in the magnitude of ongoing background oscillatory activity. Dense-array magnetoencephalography from 45 controls and 45 patients with schizophrenia produced left- and right-hemisphere STG 50- and 100-ms time-frequency evoked, phase-locking, and total power measures. Whereas first click 100-ms evoked theta and alpha abnormalities were observed bilaterally, evoked low beta-band differences were specific to the left hemisphere. Compared to controls, patients with schizophrenia showed more low-frequency phase variability, and the decreased 100-ms S1 evoked response observed in patients was best predicted by the STG phase-locking measure.
Descriptors: Sensory gating, Schizophrenia, M50, M100, Evoked, Phase-locking, Magnetoencephalography
For more than 25 years, an auditory paired-click task has been used to examine auditory processes in individuals with schizophrenia (Adler et al., 1982). Participants are presented with two clicks (S1 and S2) separated by 500 ms, and the 50-ms and sometimes the 100-ms components of the event-related brain potential (ERP) to each click are scored, typically at electrode Cz. An increased ratio score (S2/S1) is frequently observed in patients with schizophrenia, interpreted as a failure to protect processing of the first stimulus by gating out the second stimulus.
According to recent meta-analyses, the exaggerated ratio score is one of the largest and most reliable neuroscience findings in the schizophrenia literature (Bramon, Rabe-Hesketh, Sham, Murray, & Frangou, 2004; Heinrichs, 2004). The clinical significance of this finding is unclear, as few relationships between paired-click ratio scores and symptom or cognitive measures have been noted (e.g., Thoma et al., 2003; but see Potter, Summerfelt, Gold, & Buchanan, 2006). Nevertheless, paired-click abnormalities are considered a key deficit to address via drug development by consortia organized by the National Institute of Mental Health in partnership with the Food and Drug Administration, academia, and the pharmaceutical industry.
Although many studies have focused on the 50-ms response, recent studies have also examined later activity. For example,Boutros, Korzyukov, Jansen, Feingold, and Bell (2004) examined ratio score differences at N100 and P200 to ascertain whether the putative sensory gating deficits in individuals with schizophrenia extend beyond early processes. Patients with schizophrenia had higher S2/S1 ratios (less gating) than controls for N100 and P200, indicating that paired-click group differences continue beyond the preattentive phase. Group differences at 50 and 100 ms may not reflect the same abnormality, however, as recent data suggest that P50 and N100 ratio measures reflect different neural mechanisms, at least in controls (Oranje, Geyer, Bocker, Kenemans, & Verbaten, 2006).
A fundamental question is whether interpretations of ratio score phenomena are correctly focused solely on S2 modulation versus whether at least some of the abnormality in schizophrenia is manifested in other, more or less concurrent features of brain activity. Although the paired-click deficit is often observed in the absence of group differences in S1, several studies have observed a decreased S1 response in patients at 50 ms (e.g., Clementz & Blumenfeld, 2001; Jin et al., 1997; Johannesen et al., 2005; Zouridakis, Boutros, & Jansen, 1997) and 100 ms (e.g., Boutros et al., 2004; Clementz & Blumenfeld, 2001; Clementz, Dzau, Blumenfeld, Matthews, & Kissler, 2003; Jansen, Hegde, & Boutros, 2004). Any systematic difference in the S1 response would affect the S2/S1 ratio and could point to an abnormality other than a failure of S2 gating. Given such confusion in the literature, de Wilde, Bour, Bingemans, Koelman, and Linszen (2007) suggested that “All P50 measures including S1 and the difference between S1–S2 should be reported and considered as possible endophenotypes” (p. 150).
Limited research examining oscillatory activity during the paired-click task supports a primary S1 abnormality. Two studies used electroencephalography (EEG) and magnetoencephalography (MEG) to examine low-frequency (1–20 Hz) and high-frequency (20–40 Hz) activity in patients with schizophrenia during the paired-click paradigm (Blumenfeld & Clementz, 2001; Clementz & Blumenfeld, 2001). Controls had greater low-frequency activity to S1 than did patients. Groups did not differ in S1 or S2 high-frequency activity. Clementz and Blumenfeld suggested that decreased S1 low-frequency activity is inconsistent with a stimulus filtering deficit as a means of explaining normal/schizophrenia group differences on ratio scores. They speculated that patients have difficulty encoding stimuli after long time delays and that attention effects may influence group differences (see also Shelley, Silipo, & Javitt, 1999).
Support for this view was provided by Johannesen et al. (2005), who observed decreased S1 but normal S2 low-frequency activity in schizophrenia. Jansen et al. (2004) found reduced Cz S1 phase synchrony in schizophrenia in the 2- to 12-Hz range (largest deficits observed in the 4- to 8-Hz range 40 to 160 ms after stimulus presentation). In addition, in controls but not patients the amplitude of the 100-ms response was associated with the degree of phase synchrony. This suggests that single-trial theta and alpha phase activity is more variable in patients and that such variability underlies the reduced evoked S1 response. Both Johannesen et al. (2005) and Jansen et al. (2004) concluded that the low S1 amplitude sometimes observed in schizophrenia reflects a diminished capacity to “gate-in” relevant signal.
The few available studies of oscillatory activity during the paired-click paradigm indicate that this is a potentially fruitful approach to understanding brain mechanisms involved in early sensory abnormalities. The primary question addressed in the present study is whether S1 and/or S2 group differences in the time-domain evoked response in patients with schizophrenia are due to (1) group differences in the magnitude of poststimulus oscillatory activity, (2) group differences in poststimulus phaselocking, and/or (3) group differences in the magnitude of ongoing background oscillatory activity.
Multiple brain areas are active during the paired-click task, and abnormalities at one or more brain regions might influence observed S1, S2, and S2/S1 Cz score differences. Lesion (Knight, Staines, Swick, & Chao, 1999), corticography (Grunwald et al., 2003), EEG (Oranje et al., 2006), and animal studies (Miller & Freedman, 1995; Wilson, Babb, Halgren, Wang, & Crandall, 1984) have implicated prefrontal cortex (PFC) and medial temporal areas. A contribution of PFC is further supported by recent catechol-O-methyltransferase work using the paired-click paradigm (Lu, Martin, et al., 2007). Other studies suggest involvement of hippocampus (Adler et al., 1998; Tregellas et al., 2007) and thalamus (Erwin & Buchwald, 1987; Erwin, Mawhinney-Hee, Gur, & Gur, 1991; Tregellas et al., 2007).
The present study examined one node in this putative network, focusing on superior temporal gyrus (STG) activity. A recent series of studies used MEG to localize sources of brain activity during the paired-click paradigm in controls and patients with schizophrenia. These studies examined M50 and M100, the magnetic manifestations of the P50 and N100 neuroelectric components, and established that (1) bilateral STG is the primary area active during the 50-ms period (e.g., Edgar et al., 2003; Huang et al., 2003; Ligeois-Chauvel, Musolino, Badier, Marquis, & Chauvel, 1994); (2) the paired-click 50-ms ratio score deficit is observed in left but not always in right STG in patients with schizophrenia (Thoma et al., 2003); (3) whereas ratio score differences may be specific to the left-hemisphere STG at 50 ms, bilateral STG deficits exist at 100 ms (Hanlon et al., 2005); (4) although Cz P50 and N100 ratio scores and clinical measures were not associated (findings generally consistent with the review of Potter et al., 2006), relationships between left and right STG M50 and M100 ratio scores and cognitive measures were observed (Thoma et al., 2003); and (5) although EEG Cz ratio scores have poor reliability in the classical psychometric sense (Smith, Boutros, & Schwarzkopf, 1994), source-localized M50 STG ratio scores are quite reliable (Lu, Edgar, et al., 2007).
In the present study, source-localized STG activity was examined with the standard paired-click task administered to controls and patients with schizophrenia during 122-channel MEG. Left- and right-hemisphere STG S1 and S2 source strength measures were obtained, and S2/S1 ratio scores calculated. Activity at localized M50 and M100 sources was examined separately. In addition to conventional time-domain analysis, the time-frequency characteristics of source-localized STG activity were examined. For each participant, a complex demodulation transform was applied to obtain localized time-frequency information for each source, and stimulus-evoked, phase-locking, and total power measures were computed.
In a smaller but overlapping sample of subjects included in the present study, Hanlon et al. (2005) observed decreasedM50 S1 in patients with schizophrenia compared to controls. A similar nonsignificant pattern was observed for M100 S1. Analogous M50 and M100 S1 findings were expected in this much larger sample of controls and patients. As such, a primary feature of auditory perception in schizophrenia may be an encoding deficit (at least partially distinct from a gating deficit), manifested as a decreased S1 evoked response and decreased S1 low-frequency activity (Hypotheses 1 and 2). Because decreased phase-locking of S1 Cz low-frequency activity in schizophrenia has been reported (Jansen et al., 2004), it was hypothesized that the decreased S1 STG response is due to more variable single-trial activity rather than to lower-amplitude single-trial activity (Hypothesis 3 above). Although the present literature does not provide support for a specific prediction, STG abnormalities were hypothesized to be specific to poststimulus processes. Thus, no group differences in background activity were expected (Hypothesis 4).
Methods
Participants
Fifty-three participants with chronic schizophrenia (7 female) were recruited from the Albuquerque VA Medical Center and the University of New Mexico Health Sciences Center. Selection criteria were (1) diagnosis of schizophrenia with no other Axis I diagnosis, determined by the Structured Clinical Interview for DSM-IV-Patient Edition (SCID-DSM-IV; American Psychiatric Association, 1994); (2) stable, continuous treatment with one antipsychotic medication for at least 3 months; (3) no history of substance dependence (determined during the SCID-DSM-IV interview); (4) no history of alcohol or other substance abuse in the past 3 months (determined during the SCID-DSM-IV interview); (5) no history of head injury with loss of consciousness for more than 5min; and (6) no psychiatric hospitalization in the last 3 months.
Fifty-two control participants (10 female) were recruited from the local community through newspaper advertisements. Selection criteria were (1) no history of Axis I psychiatric dysfunction as determined by DSM-IV SCID-NP, (2) no history of substance dependence (determined during the SCID-DSM-IV interview), (3) no history of alcohol or other substance abuse in the past 3 months (determined during the SCID-DSM-IV interview), (4) no family history of a psychotic disorder in first-degree relatives by self-report, and (5) no history of head injury with loss of consciousness for more than 5 min or other neurological disease.
Participants signed consent forms approved by the Human Research Review Committee at the University of New Mexico and the Albuquerque VA Medical Center. Participants were asked to refrain from smoking for at least 1 h before examination, because nicotine can affect P50 gating for up to 20 min after ingestion (Adler, Hoffer, Wiser, & Freedman, 1993). To ensure compliance, participants were asked to report to the facility an hour before recordings commenced. During that time, participants were familiarized with the equipment and procedures and were prepared for recording.
For M50, data from 18 patients and 17 controls were removed because goodness-of-fit values from source localization were less than 65%. Thus, M50 data from 35 patients (4 female) and 37 control participants (5 female) were analyzed. For M100, data from 8 patients and 7 controls were removed because goodness-of-fit values were less than 65%. Thus, M100 data from 45 patients (5 female) and 45 control participants (8 female) were analyzed. Conventional M50 ratio score results for a subset of present subjects were presented by Thoma et al. (2003), and conventional M100 ratio score results for a subset of present subjects were presented by Hanlon et al. (2005).
Table 1 presents group demographics on the 45 patients and 45 controls. Six patients and 2 controls were left-handed as assessed by the Waterloo Handedness Questionnaire (Bryden, 1977). In the patient group, 33 were receiving clinical doses of atypical antipsychotics (7 on clozapine, 7 on olanzapine, 6 on aripiprazole, 5 on risperidone, 7 on quetiapine, 1 on ziprasidone), and 12 were receiving clinical doses of typical antipsychotics (4 on fluphenazine, 8 on haloperidol).1 Thirty patients were diagnosed as paranoid subtype and 15 as undifferentiated subtype. Of the controls, 44 identified themselves as White (28 European Americans, 16 Hispanics) and 1 as African American. Of the patients, 43 identified themselves as White (28 European American, 15 as Hispanic) and 2 as American Indian.
Table 1.
Demographic Information: Controls and Patients
| Controls (n = 545) |
Patients (n = 545) |
|||
|---|---|---|---|---|
| Groups | Mean | SD | Mean | SD |
| Age (years)a | 43.40 | 11.06 | 41.53 | 10.48 |
| Education (years)b | 14.40 | 1.77 | 13.36 | 2.36 |
| Patient SESb | 39.14 | 13.78 | 57.66 | 14.92 |
| Parental SESa | 36.52 | 17.88 | 38.23 | 21.10 |
| PANSS total | 61.93 | 20.05 | ||
| SANS total | 31.95 | 13.71 | ||
Group differences in age, t(88) =.80, and parental SES, t(88) = −0.40, were not significant.
Controls were more educated, t(88) = 2.38, p =.02, and had higher SES, t(88) = 5.91, p<.001.
Symptom Assessment
To characterize our patient sample, the Positive and Negative Symptom Scale (PANSS; Kay, Fiszbein, & Opler, 1987) and the Schedule for the Assessment of Negative Symptoms (SANS; Andreasen, 1982) were administered to assess positive and negative symptoms. Symptom scales were administered by trained clinicians or proctored graduate students within 48 h of MEG testing.
Auditory Stimuli
The paired-click paradigm followed the protocol of Adler et al. (1993), where clicks were presented in pairs (S1 and S2) with a 500-ms stimulus onset asynchrony and a variable intertrial interval (ITI) averaging 10 s. The clicks were 3 ms in duration (determined by audiometric calibration) delivered bilaterally through Etymotic earphones placed in each ear canal. To minimize noise produced by movement of the plastic tubes connected to the Etymotic earphones, the tubes were taped to the participant’s ear. Each participant’s hearing threshold was determined, and the peak intensity of the click was presented 35 dB SPL above threshold. If necessary, intensity was decreased to avoid elicitation of a startle reflex. Over approximately 25 min of recording time, click pairs were presented until approximately 150 artifact-free trials were obtained (see below). Groups did not differ in the number of paired-click trials administered, t(88) = 1.51, n.s.
MEG Recordings
MEG data were collected in a magnetically and electrically shielded room (IMEDCO-AG, Switzerland) using a whole-cortex biomagnetometer system manufactured by Neuromag Ltd. of Helsinki, Finland (Ahonen, Hämäläinen, Kajola, Knuutila,& Laine, 1992). The unit contains 122 sensors arrayed as 61 orthogonal pairs of planar gradiometers distributed about the top, back, and sides of the head. At the start of the session, participants were fitted with an electrode cap to which three small coils were attached. These coils provided specification of the position and orientation of the MEG sensors relative to the head. Because it was necessary for the participant’s head to remain in the same place in the MEG dewar across the recording session, foam wedges were inserted between the side of the participant’s head and the inside of the dewar, and a Velcro strap running under the participant’s chin was used to ensure immobility.
To aid in the identification of eyeblink activity, the electrooculogram (EOG; bipolar oblique, upper right, and lower left sites) was collected. Electrode impedances were maintained below 10 kΩ. After a bandpass filter (0.03–150 Hz) and a 60-Hz notch filter, EOG and MEG signals were digitized at 300, 467, or 500 Hz.2
MEG Data Analysis
Source analysis
Source localization and scoring were done blind to participant group. For source localization analyses, epochs 800 ms pre-S1 to 1000 ms post-S1 were defined from the continuous recording. Artifact-contaminated epochs were rejected by amplitude and gradient criteria (amplitude >1200 fT/cm, gradients >800 fT/cm/sample). Noncontaminated epochs were averaged, and a 2-Hz (12 dB/octave, forward) to 40-Hz (12 dB/octave, zero-phase) bandpass filter was applied. To correct for eyeblinks, a typical eyeblink was manually identified in the raw data for each participant. The pattern search function in BESA 5.1 (MEGIS Software GmbH, Gräfelfing, Germany) scanned the raw data to identify other blinks and computed an eyeblink average. An eyeblink was modeled by its first PCA component topography, typically accounting for more than 99% of the variance in the eyeblink average (although the EOG channel was used to help identify eyeblinks in the raw data, the eyeblink source was modeled using only the MEG data). The eyeblink source vector derived for each participant was added to each participant’s source model (see below).
Using all 122 channels of MEG data, determination of the strength, location, and peak latency of M50 sources (40–80 ms poststimulus) and M100 sources (80–125 ms poststimulus) in the left and right hemispheres was accomplished by fitting a dipole source in each hemisphere. A spherical MEG head model was used. A spherical head model was deemed appropriate, as spherical and boundary-element models yield very similar results for STG regions because of the high spherical symmetry of the skull in this region (Leahy, Mosher, Spencer, Huang, & Lewine, 1998).
For modeling S1 M50 and S1 M100, approximately 10 ms of data surrounding the M50 and M100 peak were selected. The eyeblink source vector derived for each participant was included in each participant’s source model to remove eyeblink activity (Berg & Scherg, 1994; Lins, Picton, Berg, & Scherg, 1993), and only dipole sources with goodness-of-fit values exceeding 65% at the maximum of M50/M100 were accepted.3 M50 goodness-of-fit values for controls (mean = 86%, SD = 7.8) and patients (mean = 85%, SD = 6.8) did not differ, t(70) = 0.38, n.s. M100 goodness-of-fit values for controls (mean = 84%, SD = 6.7) and patients (mean = 81%, SD = 6.4) did not differ, t(88) = 1.67, n.s.4
Once the sources were localized, prestimulus (−800 to −300 ms) baseline activity was subtracted from the source waveform, and left and right M50/M100 peak latency and peak source strength were calculated from the largest point in the M50 and M100 scoring windows. The left and right STG locations of the S2 sources were assumed to be the same as those of the S1 sources, and peak latency and strength measures were obtained for S2 from the same sources. The S2 peak was scored within 10 ms of S1 to assure that the same component was chosen for S2 and S1. In the event that no identifiable peak was available, S2 amplitude was scored at the same latency as S1. Scores for each hemisphere and component were expressed as a ratio: S2 dipole peak source strength divided by S1 dipole peak source strength (measured in nano-Ampere-meters, nAm).
Source time-frequency analysis
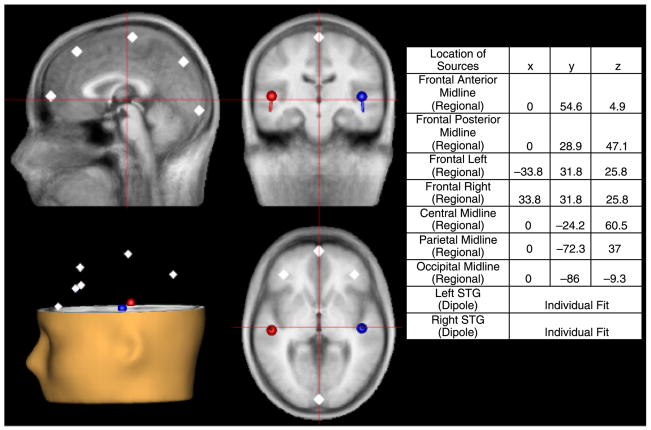
The calculation of single-trial phase and magnitude for the left and right STG sources used a modification of Hoechstetter et al.’s (2004) procedures, where the raw MEG surface activity was transformed into brain space with a model of multiple sources (Scherg, 1990; Scherg & Berg, 1996; Scherg & von Cramon, 1985). As illustrated in Figure 1, the source model was constructed by including (1) the left and right STG dipole sources (localized in each subject), (2) the eyeblink source, and (3) seven fixed regional sources that model brain background activity and serve as probe sources for additional oscillatory activity (Talairach coordinates of probe sources provided in Figure 1).5 The final source model serves as a source montage for the raw MEG(Scherg & Ebersole 1994; Scherg, Ille, Bornfleth, & Berg, 2002). As a result, single-trial data can be transformed from channel space (femto-Tesla, fT) into brain source space where the waveforms are the modeled source activities (current dipole moment, nAm). This spatial filter disentangles the source activities of the different brain regions that overlap at the sensor level.
Figure 1.
Source model containing left- and right-hemisphereM50/M100 STG dipole sources and seven regional sources (with two orthogonal components per regional source) that model brain background activity and serve as probe sources for additional oscillatory activity. The accompanying table provides the type of sources (regional or dipole) as well as the fixed location of the regional probe sources in Talairach coordinates.
In each participant, the derived source model was applied to the raw, unfiltered data. Transformation from the time domain to the time-frequency domain used complex demodulation6 procedures (Papp & Ktonas, 1977) implemented in BESA 5.1, using frequencies between 4 and 50 Hz, in steps of 1 Hz. Continuous data were analyzed relative to S1 onset every 50 ms, utilizing ± 78.8 ms and ± 1.42 Hz (50% power) of contiguous data at each 50-ms step. The complex demodulation procedure applied to a given signal returns a complex time-frequency matrix from which power and phase can be extracted.
Evoked time-frequency activity was obtained from each subject’s source waveform. The evoked time-frequency measure assesses oscillatory activity that is strictly time- and phase-locked to the onset of an experimental condition across trials (i.e., has the same phase in every stimulus repetition). Total power and phase-locking measures were extracted from the single-trial complex time-frequency matrix. Total power is a composite measure of phase- and non-phase-locked activity (some authors refer to “total power” as “whole power”). Total power is calculated by averaging the time-frequency spectra of each MEG epoch. When baseline power is subtracted, poststimulus total power assesses the poststimulus increase in the magnitude/power of oscillatory activity (temporal spectral evolution, TSE).
A single-channel phase-locking measure was obtained via a slight modification of the phase-locking procedures applied in BESA (see tutorial on Source Coherence; www.besa.de), replacing the phase difference between two signals with the absolute phase of one signal. Specifically, for each time-frequency bin a single-channel phase-locking index (SC-PLI) was computed as abs , where i= √ −1, the sum is over all N trials, and φ(k) is the phase of the signal in the kth trial. As such, SC-PLI is a normalized measure with SC-PLI = 0 reflecting maximal and SC-PLI = 1 reflecting no phase variability across trials.
For evoked and total power time-frequency measures, percent change from background activity at each frequency (defined as average power in the time interval of −800 to −300 ms) was calculated and removed as a function of frequency and latency (TSE). Given the temporal smear inherent in the complex demodulation time-frequency transforms, the −800 to −300 ms period provided an epoch uncontaminated by poststimulus activity “bleeding” into the prestimulus baseline.
If single-trial activity is of low power (i.e., poor signal-to-noise ratio), the phase measure may be inaccurate. In the present study, if lower amplitude poststimulus activity was observed in patients with schizophrenia, a decreased SC-PLI could simply reflect less accurate single-trial phase estimates. To assess the specificity of phase findings, a SC-PLI score was also obtained from nonartifact trials where single-trial signal strength exceeded a threshold value. Two single-trial magnitude thresholds were chosen such that approximately 10% and 20% of the lowest theta- and alpha-band magnitude trials were removed: 5.0 nAm and 7.5 nAm.
Group Comparisons
For all analyses, subjects more than 3 standard deviations from the group mean were excluded (typically 2 to 3 subjects per variable). For the time-domain analyses, a Group × Component (S1, S2) × Hemisphere analysis of variance (ANOVA) examined differences in source strength separately for M50 and M100. A Group × Hemisphere ANOVA was also run for M50 and M100 source strength ratio scores. For the time-frequency analyses, to limit the number of analyses, time-frequency analyses were computed for M50 and/or M100 only if group source strength differences were observed. Primary time-frequency analyses used t tests to examine activity at each whole-number frequency between 4 and 50Hz in 50-ms bins and from0 to 1000ms following S1 onset, resulting in 920 t tests (46 frequencies × 20 time bins) for each hemisphere. To control family-wise error, a clustering method was used to obtain a corrected p value. The cluster size needed to obtain the desired family-wise correction was determined using a standard fMRI package (AFNI AlphaSim; Ward, 2000), and clustering was performed with custom MATLAB software. An initial p value threshold of.05 for each time-frequency value and a cluster size threshold of nine values (adjacent in time and/or frequency) provided a family-wise corrected p=.05. Where M50 or M100 group differences were observed, a Group × Hemisphere ANOVA examined hemisphere differences. Where M50 or M100 source strength group differences were observed (S1 and/or S2), hierarchal regression examined the contribution of background, phase-locking, and total power to the amplitude measure.
Given possible differences between left- and right-handed subjects in structural/functional brain organization, all analyses were conducted with and without left-handed subjects. Results reported below remained the same after exclusion of left-handed subjects and are thus not separately reported.
Results
Left- and Right-Hemisphere Dipole Source Strength and Ratio Scores
M50
S1 M50 single dipole sources localized bilaterally to Heschl’s gyrus/planum temporale in all participants. Group × Hemisphere ANOVAs on location indicated that groups did not differ in medial-lateral, anterior-posterior, or inferior-superior source location (all p values >.16). Groups also did not differ in M50 S1 latency values in the left hemisphere, t(70) = − 0.61, n.s., or right hemisphere, t(70) = − 1.11, n.s. M50 latency values are reported in Table 2.
Table 2.
M50/M100 Latency, Source Strength, and Background Mean and Standard Deviation (SD) Values
| S1 latency (ms) and SD | S2 latency (ms) and SD | S1 source strength (nAm) and SD | S2 source strength (nAm) and SD | Background Theta | Background Alpha | Background Low beta | |
|---|---|---|---|---|---|---|---|
| M50 | |||||||
| Controls | |||||||
| Left STG | 50.98 (6.7) | 53.75 (7.7) | 14.55 (6.16) | 9.60 (5.26) | 13.18 (5.05) | 15.16 (6.73) | 9.80 (3.89) |
| Right STG | 48.14 (6.6) | 51.13 (7.6) | 14.32 (7.79) | 10.47 (6.97) | 12.95 (6.06) | 15.06 (7.77) | 10.17 (8.57) |
| Patients | |||||||
| Left STG | 51.75 (7.4) | 54.90 (7.7) | 13.57 (8.60) | 10.37 (7.82) | 13.10 (6.02) | 13.18 (5.32) | 8.47 (3.27) |
| Right STG | 49.73 (6.9) | 53.46 (6.5) | 14.24 (9.77) | 10.22 (6.64) | 12.56 (4.88) | 13.23 (6.91) | 8.57 (3.32) |
| M100 | |||||||
| Controls | |||||||
| Left STG | 100.04 (8.3) | 102.85 (11.03) | 24.39a (14.77) | 10.16 (6.86) | 12.97 (5.49) | 14.40 (6.92) | 9.59 (4.77) |
| Right STG | 99.81 (8.9) | 103.46 (11.6) | 24.10a (12.08) | 10.11 (6.38) | 12.25 (5.39) | 14.00 (7.75) | 9.66 (4.77) |
| Patients | |||||||
| Left STG | 99.99 (9.4) | 102.68 (11.9) | 18.26 (9.35) | 8.94 (5.62) | 14.93 (8.29) | 15.36 (8.15) | 10.02 (4.62) |
| Right STG | 98.78 (8.3) | 103.46 (10.29) | 19.86 (7.75) | 10.23 (5.34) | 14.74 (6.94) | 15.66 (8.66) | 9.88 (3.73) |
Note: Background activity is expressed as total power (activity from 4500 to 6500 ms averaged).
Controls versus patients p ≤.05
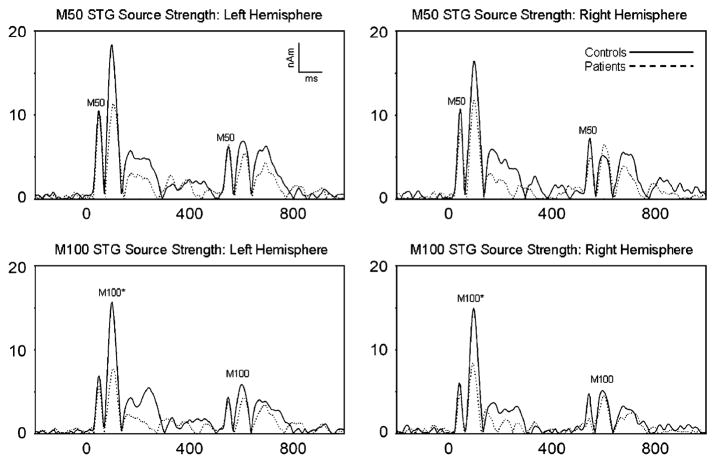
Left and right M50 STG single dipole source waveforms for the patient and control groups are shown in the upper panels in Figure 2 (absolute values plotted). Non-log-transformed peak M50 S1 and S2 source strength values are reported in Table 2. The distribution was skewed, so to obtain a normal distribution M50 source strength values were log transformed. A Group × Hemisphere × Component (S1, S2) ANOVA on M50 source strength produced a main effect of component (S1>S2), F(1,70) = 109.63, p<.001, and there was a trend for a smaller difference between S1 and S2 M50 source strength in patients than controls, Group × Component F(1,70) = 3.09, p=.08. A Group × Hemisphere ANOVA on M50 ratio scores produced no effects, though means indicated less gating in patients.
Figure 2.
M50 (upper panel) and M100 (lower panel) left and right single dipole STG source strength time courses (absolute values plotted) for the patient and control group from −200 to 1000 ms relative to S1 onset. *S1 M100 larger in controls than patients.
M100
S1 M100 single dipole sources localized bilaterally to Heschl’s gyrus/planum temporale in all participants. Although groups did not differ in medial-lateral or inferior-superior source location (p values >.12), there was less anterior-posterior hemispheric asymmetry in patients than in controls, Group × Hemisphere, F(1,88) = 4.29, p =.04. Simple-effects analyses indicated that patients’ right-hemisphere M100 sources localized approximately 5 mm more posterior than did controls, t(88) = 3.0, p<.005. Groups did not differ in M100 S1 latency values in the left hemisphere, t(88) = 0.31, n.s., or right hemisphere, t(88) = 1.00, n.s. M100 latency values are reported in Table 2.
Left and right M100 STG single dipole source waveforms for the patient and control groups are shown in the lower panels of Figure 2 (absolute values plotted). Peak M100 S1 and S2 source strength values are reported in Table 2. A Group × Hemisphere × Component (S1, S2) ANOVA on M100 source strength produced a main effect of component (S1>S2), F(1,88) = 235.38, p<.001. Supporting Hypothesis 1, patients had weaker S1 responses, Group × Component F(1,87) = 9.09, p<.005, and Tukey HSD tests. A Group × Hemisphere ANOVA on M100 ratio scores produced no effects.
Time-Frequency Measures
As the only significant evoked source strength finding was greater left- and right-hemisphere M100 S1 source strength in controls than patients, time-frequency analyses focused on M100 S1 activity. As the orientation of left/right M100 dipole sources was optimized for M100, only measures obtained from the 100-ms time-frequency bin were used in poststimulus ANOVA and regression analyses (identical findings, however, were obtained examining activity at 150 and 200 ms, as well as when activity from 100 to 200 ms was averaged).
M100 background
Activity across the −800 to −300 ms interval was averaged to obtain a single measure of background activity at each 1-Hz frequency bin. At several frequencies the distribution was skewed, so to obtain a normal distribution background absolute power values were transformed to a logarithmic scale. M100 STG theta-, alpha-, and low beta-band background non-log-transformed power values are reported in Table 2. Patients had marginally greater right-hemisphere M100 STG theta background activity (p =.06).
M100 evoked oscillatory activity
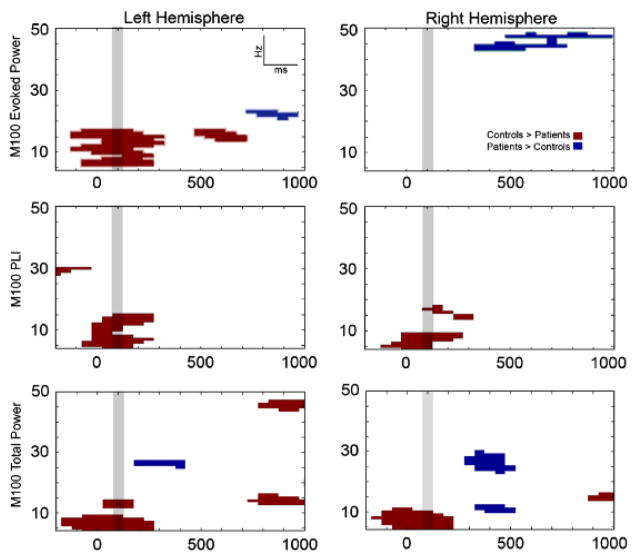
The upper panels of Figure 3 plot family-wise-corrected t tests comparing control and patient M100 evoked percent change measures at each hemisphere and time-frequency bin. Supporting Hypothesis 2, corrected clusters indicated that controls had a greater poststimulus increase in left-hemisphere S1 evoked theta- to low beta-band activity than did patients from 0 to ~300 ms. Controls also had a greater increase in left-hemisphere low beta-band activity from 500 to 600 ms. The group effect was reversed at higher frequencies. In the left hemisphere, greater high beta-band activity was observed between 800 and 1000 ms in patients than in controls. In the right hemisphere, greater gamma-band activity was observed between 900 and 1000 ms in patients than in controls.
Figure 3.
Family-wise-corrected M100 p value plots comparing control and patient STG evoked activity (top panels), single-channel phase-locking values (middle panels), and total power (lower panels), at each time-frequency value from −200 to 1000 ms. Control>patient differences are shown in black, patient>control differences in light gray. The 100-ms bin used for ANOVA and regression analyses is highlighted in dark gray.
To explore the conventional M100 S1 source strength effect reported above, Group × Hemisphere ANOVAs were conducted where significant group differences were observed for 100-ms time-frequency activity. Mean 100 ms STG theta-band (4–7 Hz), alpha-band (8–12 Hz), and low beta-band (13–20 Hz) percent change values for each group are reported in Table 4, below. Although Figure 3 shows S1 theta- to low beta-band group differences primarily in the left hemisphere, ANOVAs generally did not indicate a lateralized abnormality. Thus, as reported in Table 3, controls had a greater evoked theta- and alpha-band 100-ms activity bilaterally (equivalent to the M100 evoked findings). A Group × Hemisphere low beta-band interaction indicated greater evoked left-hemisphere low beta-band activity in controls than patients at 100 ms.
Table 4.
The 100-ms Evoked, Single-Channel Phase-Locking Index (SC-PLI) and Total Power Mean and Standard Deviation (SD) Values
| Evoked 100 ms (% change) |
SC-PLI 100 ms |
Total power 100 ms (% change) |
||||||
|---|---|---|---|---|---|---|---|---|
| Theta | Alpha | Low beta | Theta | Alpha | Low beta | Theta | Alpha | |
| Controls | ||||||||
| Left STG | 832.60 (574.80)a | 710.13 (468.29)a | 325.37 (196.78)b | 0.46 (0.20)a | 0.37 (0.15)a | 0.21 (0.09) | 32.37 (24.72)b | 13.05 (12.61) |
| Right STG | 1024.72 (716.11)a | 813.84 (618.44)a | 317.54 (285.03) | 0.50 (0.19)a | 0.38 (0.15) | 0.22 (0.10) | 31.19 (22.70)b | 14.95 (11.45)a |
| Patients | ||||||||
| Left STG | 566.11 (370.41) | 508.59 (356.43) | 165.85 (103.49) | 0.37 (0.16) | 0.31 (0.11) | 0.18 (0.09) | 17.30 (16.64) | 8.65 (9.56) |
| Right STG | 720.91 (422.22) | 553.84 (346.63) | 297.11 (198.01) | 0.40 (0.16) | 0.33 (0.14) | 0.19 (0.07) | 19.31 (18.02) | 10.46 (9.58) |
Note: Evoked and total power are non-log-transformed values.
Controls versus patients p ≤ 05.
Controls versus patients p ≤.01.
Table 3.
Group × Hemisphere ANOVA on 100-ms Evoked, SC-PLI, and Total Power
| Group |
Hemisphere |
Interaction |
|||||
|---|---|---|---|---|---|---|---|
| F | P | Effect size | F | p | F | p | |
| Evoked (100 ms) | |||||||
| Theta 4 to 7 Hz | 4.63 | .03 | 0.48 (control>patient) | 1.73 | .19 | 0.74 | .39 |
| Alpha 8 to 12 Hz | 5.49 | .02 | 0.49 (control>patient) | 0.68 | .41 | 0.85 | .36 |
| Low beta 13 to 20 Hz | 3.49 | .07 | NA | 0.85 | .36 | 8.38 | .01a |
| Phase-locking index (100 ms) | |||||||
| Theta 4 to 7 Hz | 8.11 | .01 | 0.74 (control>patient) | 4.00 | .05 | 0.07 | .79 |
| Alpha 8 to 12 Hz | 5.04 | .03 | 0.59 (control>patient) | 1.31 | .26 | 0.00 | .95 |
| Low beta 13 to 20 HZ | 3.79 | .06 | 0.41 (control>patient) | 1.84 | .18 | 0.04 | .85 |
| Theta 4 to 7 Hz 5.0 nAm | 7.40 | .01 | 0.72 (control>patient) | 5.26 | .02 | 0.01 | .92 |
| Alpha 4 to 7 Hz 5.0 nAm | 6.28 | .01 | 0.56 (control>patient) | 0.57 | .45 | 0.06 | .80 |
| Low beta 13 to 30 5.0 nAm | 4.72 | .03 | 0.45 (control>patient) | 0.47 | .50 | 0.02 | .90 |
| Theta 4 to 7 Hz 7.5 nAm | 4.47 | .04 | 0.44 (control>patient) | 4.57 | .04 | 0.04 | .85 |
| Alpha 4 to 7 Hz 7.5 nAm | 4.11 | .05 | 0.46 (control>patient) | 1.74 | .19 | 0.00 | .95 |
| Low beta 13 to 30 7.5 nAm | 2.60 | .11 | NA | 1.00 | .32 | 0.06 | .81 |
| Total power (100 ms) | |||||||
| Theta 4 to 7 Hz | 15.84 | .001 | 0.85 (control>patient) | 0.14 | .71 | 0.03 | .87 |
| Alpha 8 to 12 Hz | 6.95 | .01 | 0.57 (control>patient) | 0.21 | .65 | 0.41 | .53 |
Note: NA: not applicable. p<.05 shown in bold.
Evoked S1 low beta left-hemisphere effect size= 0.66.
M100 SC-PLI
The middle panels of Figure 3 plot family-wise- corrected t tests comparing control and patient single-channel phase-locking values for each hemisphere and time-frequency bin. Supporting Hypothesis 3, corrected clusters indicated greater 100-ms single-channel phase-locking in controls than patients bilaterally. SC-PLI ANOVA results in Table 3 show a larger 100-ms theta- to low beta-band SC-PLI in controls than patients bilaterally (trend for low beta-band activity). Mean 100- ms STG theta-, alpha-, and low beta-band single-channel phase-locking values for each group are reported in Table 4. Except for low-beta band findings at the 7.5-nAm threshold, group SC-PLI differences remained significant when computed at the two magnitude thresholds (5.0 nAm and 7.5 nAm). Table 5 shows the average percentage of trials removed from each group at each magnitude threshold.
Table 5.
Average Percentage of Trials Removed from Each Group at Each Magnitude Threshold for SC-PLI Analyses
| Percentage of Controls (SD) |
Percentage of Patients (SD) |
|||
|---|---|---|---|---|
| M100 Threshold | Left | Right | Left | Right |
| Theta 5.0 nAm | 11.05 (9.05) | 11.95 (9.81) | 13.6 (13.79) | 10.87 (9.39) |
| Alpha 5.0 nAm | 14.32 (11.42) | 15.32 (11.66) | 16.33 (16.64) | 12.98 (10.91) |
| Low beta 5.0 nAm | 30.07 (21.51) | 28.83 (19.51) | 27.92 (22.64) | 24.37(15.29) |
| Theta 7.5 nAm | 22.04 (15.93) | 23.85 (16.30) | 25.70 (21.10) | 21.61 (15.33) |
| Alpha 7.5 nAm | 27.06 (18.49) | 29.15 (18.29) | 28.89 (22.91) | 24.65 (16.79) |
| Low beta 7.5 nAm | 48.51 (25.16) | 48.09 (24.35) | 44.97 (26.81) | 42.89 (21.68) |
M100 total power
The lower panels of Figure 3 plot family-wise- corrected t tests comparing groups on the change in 100-ms total power in each hemisphere and time-frequency bin. Corrected clusters indicated a greater poststimulus increase in left and right-hemisphere S1 theta- to alpha-band activity in controls than in patients from 0 to ~ 300 ms. Between 400 and ~ 500 ms a greater theta- and high beta-band poststimulus decrease in controls than patients was observed (right>left). Finally, a greater increase in low beta- and gamma-band poststimulus activity in controls than patients was observed from 800 to 1000 ms. As shown in Table 3, ANOVAs indicated theta- and alphaband 100-ms poststimulus differences bilaterally. Mean 100-ms STG theta- and alpha-band total power percent change values for each group are reported in Table 4.
Regression Models
As M100 S1 group differences primarily reflected theta- to low beta-band activity, STG time-frequency regression analyses included only those frequencies. To assess the degree to which background and 100-ms time-frequency measures predicted M100 amplitude, hierarchical multiple regression was conducted with group entered in the first block, theta- to low beta-band background activity in the second block, 100-ms theta- to betaband total power percent change values in the third block, and 100-ms theta- to beta-band SC-PLI values in the fourth block. Analyses were rerun leaving the first two blocks unchanged and switching the order in which phase and total power were added. To investigate group differences in the association between M100 S1 amplitude scores and 100-ms total power and phase, all two way interaction terms were entered in the fifth block. Three-way and higher interaction terms were not examined. Outliers previously identified for each of the above variables were excluded, removing 16 subjects. In all regressions, results were unchanged when outliers were included.
The first four blocks (i.e., not including the interaction terms) explained 76% of the variance in M100 S1 source strength in the left hemisphere, F(10,74) = 24.14, p<.001, and 75% of the variance in the right hemisphere, F(10,74) = 22.75, p<.001. As shown in Table 6, whereas in both hemispheres single-channel phase-locking accounted for additional variance in M100 source strength when entered before or after total power, the total power measure did not account for additional variance when entered after phase-locking.
Table 6.
Hierarchical Regressions with Time-Frequency Measures Predicting M100 Source Strength
| M100 source strength | Block 1 R2 change | Block 2 R2 change | Block 3 R2 change |
|---|---|---|---|
| Left | Background | Total power | Phase-locking |
| 0.44 | 0.22 | 0.12 | |
| Background | Phase-locking | Total power | |
| 0.44 | 0.34 | 0.01 | |
| Right | Background | Total power | Phase-locking |
| 0.48 | 0.22 | 0.07 | |
| Background | Phase-locking | Total power | |
| 0.48 | 0.28 | 0.01 |
p<.01 shown in bold.
Discussion
The present study sought to determine whether abnormal STG oscillatory activity during the paired-click paradigm characterizes schizophrenia and how abnormal STG oscillatory activity might contribute to the typically reported ratio score abnormality. Given evidence of decreased low-frequency S1 activity in patients with schizophrenia in paired-click as well as other auditory tasks (e.g., Gilmore, Clementz, Buckley, 2004; Winterer et al., 2000), the first two hypotheses were that patients would show a smaller STG S1-evoked response as well as decreased evoked low-frequency STG S1 activity, supporting an encoding deficit. Given decreased phase-locking of Cz S1 activity in schizophrenia during the paired-click task (Jansen et al., 2004), the third hypothesis was that patients would show decreased STG S1 single-channel phase-locking and that this would account for patients’ decreased STG S1-evoked response. The fourth hypothesis was the group differences would reflect poststimulus processes, indicated by no group differences in background oscillatory activity.
Supporting Hypothesis 1, patients had smaller M100 S1 amplitudes bilaterally. Supporting Hypothesis 2, patients had less 100- ms evoked theta- to low beta-band activity (theta and alpha bilaterally, low-beta left-hemisphere). Evoked time-frequency results could indicate that in schizophrenia 100-ms low frequency STG poststimulus activity is less phase-locked, is of lower amplitude, or both. Supporting Hypothesis 3, patients had less left/right 100-ms theta- to low beta-band phase-locking. Although total power analyses unexpectedly also revealed a greater increase in poststimulus theta- to alpha-band activity in controls than in patients, regression analyses indicated that phase-locking was a better predictor ofM100 S1 source strength than 100-ms total power in both groups. Supporting Hypothesis 4, group differences in background activity were generally not observed.
Present results replicated findings of decreased evoked low frequency activity following S1 in patients with schizophrenia (e.g., Boutros et al., 2004; Clementz & Blumenfeld, 2001; Clementz et al., 2003; Johannesen et al., 2005). Results also replicated the decreased low-frequency Cz S1 phase-locking in schizophrenia (Jansen et al., 2004). Whereas Jansen et al. observed an association between phase-locking and Cz S1 amplitude only in controls, in the present study 100-ms phase-locking predicted significant variance in M100 S1 amplitude in controls and patients. Present results indicate a clear association between phase-locking and evoked S1 amplitude in both groups when STG source activity is examined.
There are indications that early event-related fields (ERFs) such as M50 and M100 may partly result from the phase-locking of ongoing oscillations (Makeig, Debener, Onton, & Delorme, 2004). To the extent that M50 and M100 are generated by a reorganization of ongoing oscillations in the MEG (e.g., Basar 1980; Brandt, 1997; David, Harrison, & Friston, 2005; Sayers, Beagley, & Hensall, 1974), present findings indicate a deficit in patients with schizophrenia to synchronize the phase of ongoing oscillatory activity. Thus, as Jansen et al. (2004) noted, a deficit in the ability of the brain to respond to novel stimuli by synchronizing the phase of ongoing oscillatory activity may be an important mechanism underlying the reported evoked potential abnormalities in schizophrenia.
Some findings in other studies, however, support the classical additive power model that ERPs/ERFs reflect transient, fixed latency, and fixed polarity (evoked) responses to a stimuli superimposed on the “background EEG/MEG” (Hillyard, 1985; Mäkinen, Tiitinen, & May, 2005). Other studies observe a combination of phase-locking and evoked alpha activity (Fuentemilla, Marco-Pallares, & Grau, 2006; Min et al., 2007). In the present study, theta- to beta-range background activity and 100- ms single-channel phase-locking predicted ~ 75% of the variance in M100 source strength, suggesting a phase-locking phenomena. Although STG phase-locking measure predicted unique variance in M100 source strength in the present study, it has been observed that an evoked component superimposed on random, ongoing oscillationsmimics phase reset, and that a large phase-locking value does not provide clear evidence against the evoked model (Hanslmayr et al., 2007; Mäkinen et al., 2005; Yeung, Bogacz, Holroyd, & Cohen, 2004). Given the above, alternative interpretations of the present findings are that (1) patients with schizophrenia produce fewer transient, fixed latency, and fixed polarity responses on top of background MEG (a strictly evoked interpretation) or (2) patients with schizophrenia produce few evoked responses and less phase-locking (a combined evoked and phase-locking interpretation).
Single-trial, low-amplitude evoked responses in patients would manifest as increased phase variability (phase is less accurately assessed in low-amplitude signals) and also produce a positive correlation between phase-locking and S1 source strength. Although a decreased evoked response could account for the present findings, an evoked interpretation is unlikely, as phase findings were unchanged when 20% of the lowest theta and alpha-band magnitude trials were removed. To what extent phase reset and evoked responses contribute to the generation of M100, however, remains a question to be addressed in future studies (although difficult if not impossible to determine if both spectral power and phase coherence contributions are simultaneously present). Present analyses underscore the need to examine all aspects of event-related brain dynamic state space (Makeig et al., 2004). Regardless of the mechanism underlying the M100 S1 group differences, present results indicate an inability in patients with schizophrenia to properly modulate first-click low frequency activity.
S1 evoked and total power findings are consistent with the hypothesis that low-frequency group differences reflect a deficit related to encoding and further processing of stimuli (Boutros et al., 2004; Clementz & Blumenfeld, 2001). Subcortical structures such as the thalamus appear to be involved in generation of theta and alpha oscillatory activity (e.g., Llinás & Steriade, 2006). In addition, it is hypothesized that hippocampal theta synchronization is related to the encoding of new information through long-term potentiation (Klimesch, 1999). Although oscillatory activity was examined at localized STG sources, STG theta-band activity may reflect theta activity that is induced in the cortex via cortico-hippocampal feedback loops (Miller, 1991). Thus, thalamacortical dysfunction (thalamus to STG) and/or cortico-hippocampal dysfunction may contribute to the observed STG theta and alpha abnormalities. As noted in the introduction, to fully understand paired-click processes (and thus control/patient differences), more work is needed to identify the neural substrates involved in paired-click processing and to assess connectivity between these areas in controls and patients.
Whereas MEG recordings are very sensitive to tangentially oriented sources such as STG sources, MEG recordings are less sensitive to pure radially oriented sources as well as deep sources such as hippocampus or thalamus. In contrast, EEG is often well suited for examining such sources. Several of our studies strongly support the view that 50-ms Cz activity primarily reflects STG activity. Predicting P50 Cz scalp-recorded activity from bilateral STG sources derived from whole-cortex MEG, Huang et al. (2003) showed that virtually all of the variance in P50 (87% in Sz patients, 96% in controls) is accounted for by STG sources. Although Edgar et al. (2003) found relatively weak associations between P50 and M50 suppression ratios, the weak correlations may simply reflect the fact that, because P50 gating does not correlate with itself in terms of psychometric reliability, P50 ratio scores cannot correlate well with anything else, although in contrast the M50 ratio is quite reliable (Lu, Edgar, et al., 2007). In contrast to 50-ms Cz activity, multiple brain regions clearly contribute to the 100-ms Cz response (see introduction). As some of these brain areas are not well modeled with MEG, and given the need to examine STG and non-STG areas to fully understand paired-click activity, studies simultaneously examining whole cortex MEG and EEG are needed.
In general, significant Group × Hemisphere interactions were not obtained. One of the few exceptions to bilateral findings was 100-ms evoked low beta-band differences in the left hemisphere. Decreased STG gray matter in patients with schizophrenia is observed in most published studies (see McCarley et al., 1999; Shenton, Dickey, Frumin, & McCarley, 2001), and MRI studies examining STG structural abnormalities frequently report left>right hemisphere group differences (e.g., Hirayasu et al., 1998; Kasai et al., 2003). Given that present evoked beta-band findings were specific to the left hemisphere, an examination of the relationship between low beta-range activity and STG structural measures is of interest. It is hypothesized that decreased left-hemisphere STG gray matter would be associated specifically with beta-band abnormalities. Corticography studies examining the 100-ms STG response indicate that it is made up of at least three distinct responses (N1a ~ 75 ms, N1b ~ 100 ms, N1c ~ 120 ms), not all of which are consistently observed in scalp recordings (e.g., Godey, Schwartz, de Graff, Chauvel, & Liégois-Chauvel, 2001). Present results likely reflect N1b, as the mean peak of the localized 100-ms activity was approximately 99 ms in both groups (SD ~ 11 ms in each group). Godey et al. noted that N1b likely reflects activity from lateral portions of Heschl’s gyrus. Although N1b activity was localized, given the spatial resolution inherent in MEG (perhaps 3–5 mm; Miller, Elbert, Sutton, & Heller, 2007), M100 STG sources most likely reflect activity from multiple auditory areas in or near Heschl’s gyrus.
For M50, patients showed smaller S1–S2 differences, a finding consistent with the literature, as the obtained Group × Condition trend (p=.08) can be regarded as significant given that a one-tailed test could easily be justified. The group difference in the conventional ratio score, although in the expected direction, did not approach significance. There is some evidence to suggest that atypical antipsychotics improve P50 ratio score abnormalities (Becker et al., 2004; Light, Geyer, Clementz, Cadenhead, & Braff, 2000; Nagamoto et al., 1996;Yee, Nuechterlein, Morris,& White, 1998), and most patients in the present study were taking atypical antipsychotics. In a recent meta-analysis, de Wilde et al. (2007) reported significantly larger ratio score effects in paired click studies conducted at the University of Colorado versus in laboratories elsewhere. De Wilde et al. suggested that between laboratory findings may reflect differences in data acquisition (differences in sound intensity, subjects run supine versus upright). Thus, present findings are broadly in line with our prior findings as well as those of other laboratories. Although in this and a prior study using a small subset of the present sample (Hanlon et al., 2005) no S2 group differences were observed, in Hanlon et al. gating ratios correlated with S2 and not S1 source strength. In addition, in two other studies using a subset of the present sample, ratio score and not the individual components were associated with symptom (Thoma et al., 2003) and genetic measures (Lu, Martin, et al., 2007). At present, continued focus on all paired-click measures—S1, S2, and the ratio score—is warranted.
Similar to Jansen et al. (2004), no group differences in the ability to phase synchronize the ongoing EEG in response to S2 were found. Although manifested in a marginal Group × Component interaction, S2 differences were not observed in either the time-domain or time-frequency analyses. Differences in the location and/or orientation of 50 and 100 S1 and S2 could account for the present failure to observe stronger S2 group differences. This is unlikely, as analyses examining 50- and 100- ms time-domain-evoked and time-frequency-evoked and total power using regional sources rather than dipolar sources also revealed no S2 STG group differences (regional source results can be obtained from the first author; see also Edgar et al., 2006).7
Additional points in support of the present method can be made, regarding source localization in general and STG localization in particular: (a) A spherical head model is particularly good for localization in STG, because of the spherical shape of the head there. (b)MEG is particularly well positioned to localize activity already known generally to be in dorsal STG, because of the orientation of the dipole and the position and orientation of the MEG sensors (see Edgar et al., 2003, for an exploration of variance in STG P50/M50 dipole orientation and the consistent success of MEG in localizing the dipole in the face of that variance). (c) More generally, MEG is much less dependent on head model than is EEG, for example in its conductivity assumptions. For that and other reasons, MEG localization can be quite effective, matching or exceeding fMRI for superficial sources such as STG (see Miller et al., 2007). (d) Whereas a beamformer approach has the advantage of creating an automated image, beamformers are based on the assumption that brain processes are temporally uncorrelated. Because left/right STG activity is highly correlated, standard beamformer approaches would be especially challenged to separate them, which is centrally important for the present project (Huang et al., 2004). Similar concerns apply to an ICA/PCA approach. In addition, even if left/right STG sources could be individually identified via ICA/PCA, it is not clear that the ICA/PCA components reflecting STG activity could be easily identified in each subject. Given these concerns and the success our and other laboratories have had assessing STG activity via source localization strategies, as well as the fact that under proper circumstances source localization strategies can handle highly correlated sources, source localization procedures were preferred over beamformer and PCA/ICA.
Finally, although the time-frequency family-wise corrected plots in Figure 3 show group differences during the ITI, these results must be taken as tentative, as dipole orientation was optimized to examine ~ 100-ms activity. In addition to ITI activity, activity at higher frequencies may not have been optimally assessed, given the epoch length used for the time-frequency transforms. To the extent that early high-frequency activity is of short duration (i.e., nonstationary across the transformed epoch), group differences at higher frequencies would not have been optimally assessed. Clementz and Blumenfeld (2001) and Blumenfeld and Clementz (2001) also did not observe group differences in gamma-band activity at S1 or S2. As in the present study, however, the epoch they used to examine high-frequency activity was not optimal.
Present findings replicate previous studies reporting decreased evoked 1 to 20 Hz activity following S1 in individuals with schizophrenia during the paired-click task, providing support for a primary encoding deficit. Compared to controls, patients with schizophrenia showed more low-frequency phase variability, and the decreased M100 S1 evoked response observed in patients was best predicted by the STG phase-locking measure. Results suggest that a focus of future research should be the identification of neural network processes that generate STG low-frequency and beta-band activity and the identification of the specific neural network processes that are abnormal in schizophrenia.
Acknowledgments
This research was supported by a National Alliance for Research on Schizophrenia and Depression Young Investigator Award and grants from the National Institute of Mental Health (R01 MH61358, R01 MH65304, T32MH19554), the National Institute of Drug Abuse (R21 DA1411), the Biomedical Research Institute of New Mexico, the University of Illinois Beckman Institute and Intercampus Research Initiative in Biotechnology, and the Mental Illness and Neuroscience Discovery Institute.
The authors thank Fernando Torres, Robin Douglas, and Jeanne Schneider for help with this project. The authors also thank Dr. Patrick Berg for analysis advice.
Footnotes
Because the number of patients receiving a particular antipsychotic was small, examining the effect of specific medications on time-frequency measures was not possible. As sample size in this ongoing project increases, medication effects will be examined. Studies examining first episode and/or medication naïve subjects would also be of interest.
Data acquisition software and hard drive were upgraded during the course of data collection, and digitization rate was increased.
Although a 65% goodness-of-fit value is lower than in some other studies (including our own previous studies), most studies localized STG sources by using a subset of the total MEG channels. Goodness-of-fit values would generally be larger, simply because there is less total residual activity in the modeled sites after STG sources have been modeled.
Although the control and patient groups’ 50- and 100-ms goodness-of-fit (GoF) values did not differ, removing subjects with low GoF values may produce a potential confound. An alternative strategy would be to include all subjects. It is not clear, however, how to include subjects with low GoF values. Specifically, in most subjects with low GoF values, the 50- and 100-ms sources localize deep within or even outside the head. To include these subjects, one option would be to place a dipole (or regional source) at a standard STG location in all subjects or in those subjects. Some thought would need to be given as to whether sources should be placed at a standard Talairach coordinate or instead placed with reference to a subject-specific brain structure (e.g., Heschl’s gyrus; or maybe do both). In addition, whereas such studies would seem to require the use of a regional source because it would not be possible to determine the optimal dipole orientation for each subject, one runs into the problem of computing a phase-locking measure from regional sources. The development of methods to perform such analyses would be valuable. In the present study, localizing 50- and 100-ms activity for each subject, excluding subjects with a low GoF, seemed appropriate and still provided a sizable N in each group.
The source model applied in the present study was derived from a standard BESA source model (starting with the BESA source model BR_Brain_Regions_LR.BSA), removing sources near the STG.
Complex demodulation uses a finite impulse response (FIR) low-pass filter with a Gaussian wave shape in the time domain. Complex demodulation is equivalent to a wavelet transformation using Morlet-type wavelets with a fixed time-frequency resolution across frequencies.
Whereas using the STG S1 dipole to score the STG S2 response is problematic if the true STG S1 and STG S2 dipoles differ somewhat in location or orientation, using regional sources avoids that problem.
References
- Adler LE, Hoffer LD, Wiser A, Freedman R. Normalization of auditory physiology by cigarette smoking in schizophrenia patients. American Journal of Psychiatry. 1993;150:1856–1861. doi: 10.1176/ajp.150.12.1856. [DOI] [PubMed] [Google Scholar]
- Adler LE, Olincy A, Waldo M, Harris JG, Griffith J, Stevens K, et al. Schizophrenia, sensory gating, and nicotinic receptors. Schizophrenia Bulletin. 1998;24:189–202. doi: 10.1093/oxfordjournals.schbul.a033320. [DOI] [PubMed] [Google Scholar]
- Adler LE, Pachtman E, Franks R, Pecevich M, Waldo MC, Freedman R. Neurophysiological evidence for a deficit in inhibitor mechanisms involved in sensory gating in schizophrenia. Biological Psychiatry. 1982;17:639–654. [PubMed] [Google Scholar]
- Ahonen AI, Hämäläinen MS, Kajola MJ, Knuutila JET, Laine PP. A 122-channel magnetometer covering the whole head. In: Dittmar A, Froment AC, editors. Proceedings of the Satellite Symposium on Neuroscience and Technology, 14th Annual Conference of IEEE Engineering in Medicine and Biology Society; Lyon: IEEE Engineering and Medicine and Biology Society; 1992. pp. 16–20. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 1994. [Google Scholar]
- Andreasen NC. Negative symptoms in schizophrenia: definition and reliability. Archives of General Psychiatry. 1982;39:784–788. doi: 10.1001/archpsyc.1982.04290070020005. [DOI] [PubMed] [Google Scholar]
- Basar E. EEG-brain dynamics: Relation between EEG and brain evoked potentials. New York: Elsevier; 1980. pp. 375–391. [Google Scholar]
- Becker J, Gomes I, Ghisol ES, Schuch A, Ramos FL, Ehlers JA, et al. Clozapine, but not typical antipsychotics, correct P50 suppression deficit in patients with schizophrenia. Clinical Neurophysiology. 2004;115:396–401. doi: 10.1016/j.clinph.2003.09.018. [DOI] [PubMed] [Google Scholar]
- Berg P, Scherg M. A multiple source approach to the correction of eye artifacts. Electroencephalography and Clinical Neurophysiology. 1994;90:229–241. doi: 10.1016/0013-4694(94)90094-9. [DOI] [PubMed] [Google Scholar]
- Blumenfeld LA, Clementz BA. Response to the first stimulus determines reduced auditory evoked response suppression in schizophrenia: Single trials analysis using MEG. Clinical Neurophysiology. 2001;112:1650–1659. doi: 10.1016/s1388-2457(01)00604-6. [DOI] [PubMed] [Google Scholar]
- Boutros NN, Korzyukov O, Jansen B, Feingold A, Bell M. Sensory gating deficits during the mid-latency phase of information processing in medicated schizophrenia patients. Psychiatry Research. 2004;126:203–215. doi: 10.1016/j.psychres.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Bramon E, Rabe-Hesketh S, Sham P, Murray RM, Frangou S. Meta-analysis of the P300 and P50 waveforms in schizophrenia. Schizophrenia Research. 2004;70:315–329. doi: 10.1016/j.schres.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Brandt ME. Visual and auditory evoked phase resetting of the alpha EEG. International Journal of Psychophysiology. 1997;26:285–298. doi: 10.1016/s0167-8760(97)00771-x. [DOI] [PubMed] [Google Scholar]
- Bryden MP. Measuring handedness with questionnaires. Neuropsychologia. 1977;15:617–624. doi: 10.1016/0028-3932(77)90067-7. [DOI] [PubMed] [Google Scholar]
- Clementz BA, Blumenfeld LD. Multichannel electroencephalographic assessment of auditory evoked response suppression in schizophrenia. Experimental Brain Research. 2001;139:377–390. doi: 10.1007/s002210100744. [DOI] [PubMed] [Google Scholar]
- Clementz BA, Dzau JR, Blumenfeld LD, Matthews S, Kissler J. Ear of stimulation determines schizophrenia-normal brain activity differences in an auditory paired-stimuli paradigm. European Journal of Neuroscience. 2003;18:2853–2858. doi: 10.1111/j.1460-9568.2003.03027.x. [DOI] [PubMed] [Google Scholar]
- David O, Harrison L, Friston KJ. Modelling event-related responses in the brain. NeuroImage. 2005;25:756–770. doi: 10.1016/j.neuroimage.2004.12.030. [DOI] [PubMed] [Google Scholar]
- de Wilde OM, Bour LJ, Bingemans PM, Koelman JHTM, Linszen DH. Ameta-analysis of P50 studies in patients with schizophrenia and relatives: Differences in methodology between research groups. Schizophrenia Research. 2007;97:137–151. doi: 10.1016/j.schres.2007.04.028. [DOI] [PubMed] [Google Scholar]
- Edgar JC, Huang M, Miller GA, Sherwood A, Adler LE, Canive JM. Interpreting abnormality: An EEG and MEG study of P50 and the auditory paired-stimulus paradigm. Biological Psychology. 2003;65:1–20. doi: 10.1016/s0301-0511(03)00094-2. [DOI] [PubMed] [Google Scholar]
- Edgar JC, Matos-Lamourt A, Weisend MP, Thoma RJ, Hanlon FM, Adler LE, et al. Evoked and induced low frequency superior temporal gyrus abnormalities in schizophrenia. In: Cheyne D, Ross B, Stroink G, Weinberg H, editors. 15th International Conference on Biomagnetism, Final Program and Book of Abstracts, 84; Vancouver. 2006. [Google Scholar]
- Erwin RJ, Buchwald JS. Midlatency auditory evoked responses in the human and the cat model. Electroencephalography and Clinical Neurophysiology. 1987;40(Supppl):461–467. [PubMed] [Google Scholar]
- Erwin RJ, Mawhinney-Hee M, Gur RC, Gur RE. Midlatency auditory evoked responses in schizophrenia. Biological Psychiatry. 1991;30:430–442. doi: 10.1016/0006-3223(91)90304-5. [DOI] [PubMed] [Google Scholar]
- Fuentemilla L, Marco-Pallares J, Grau C. Modulation of spectral power and of phase resetting of EEG contributes differentially to the generation of auditory event-related potentials. NeruoImage. 2006;30:909–916. doi: 10.1016/j.neuroimage.2005.10.036. [DOI] [PubMed] [Google Scholar]
- Gilmore CS, Clementz BA, Buckley PF. Rate of stimulation affects schizophrenia-normal differences on the N1 auditoryevoked potential. Brain Imaging. 2004;15:2713–2717. [PubMed] [Google Scholar]
- Godey B, Schwartz D, de Graff JB, Chauvel P, Liégeois-Chauvel C. Neuromagnetic source localization of auditory evoked fields and intracerebral evoked potentials: A comparison of data in the same patients. Clinical Neurophysiology. 2001;112:1850–1859. doi: 10.1016/s1388-2457(01)00636-8. [DOI] [PubMed] [Google Scholar]
- Grunwald T, Pezer N, von Oertzen J, Fernandez G, Schaller C, Elger CE. Neuronal substrates of sensory gating within the human brain. Biological Psychiatry. 2003;53:511–519. doi: 10.1016/s0006-3223(02)01673-6. [DOI] [PubMed] [Google Scholar]
- Hanlon FM, Miller GA, Thoma RJ, Irwin J, Jones A, Moses SN, et al. DistinctM50 andM100 auditory gating deficits in schizophrenia. Psychophysiology. 2005;42:417–427. doi: 10.1111/j.1469-8986.2005.00299.x. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S, Klimesch W, Sauseng P, Gruber W, Doppelmayr M, Freunberger R, et al. Alpha phase reset contributes to the generation of ERPs. Cerebral Cortex. 2007;17:1–8. doi: 10.1093/cercor/bhj129. [DOI] [PubMed] [Google Scholar]
- Heinrichs RW. Meta-analysis and the science of schizophrenia: Variant evidence or evidence of variants? Neuroscience Biobehavioral Review. 2004;28:379–394. doi: 10.1016/j.neubiorev.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Hillyard SA. Electrophysiology of human selective attention. Trends in Neuroscience. 1985;8:400–405. [Google Scholar]
- Hirayasu Y, Shenton ME, Salisbury DF, Dickey CC, Fisher IA, Mazzoni P, et al. Lower left temporal lobe MRI volumes in patients with first-episode schizophrenia compared to psychotic patients with first-episode affective disorder and normal subjects. American Journal of Psychiatry. 1998;155:1384–1391. doi: 10.1176/ajp.155.10.1384. [DOI] [PubMed] [Google Scholar]
- Hoechstetter K, Bornfleth H, Weckesser D, Ille N, Berg P, Scherg M. BESA source coherence: A new method to study cortical oscillatory coupling. Brain Topography. 2004;16:233–238. doi: 10.1023/b:brat.0000032857.55223.5d. [DOI] [PubMed] [Google Scholar]
- Huang MX, Edgar JC, Thoma RJ, Hanlon FM, Moses SN, Lee RR, et al. Predicting EEG responses using MEG sources in superior temporal gyrus reveals source asynchrony in patients with schizophrenia. Clinical Neurophysiology. 2003;114:835–850. doi: 10.1016/s1388-2457(03)00041-5. [DOI] [PubMed] [Google Scholar]
- Huang MX, Shih J, Lee RR, Harrington DL, Thoma RJ, Weisend MP, et al. Commonalities and differences among vectorized beamformers in electromagnetic source imaging. Brain Topography. 2004;16:139–158. doi: 10.1023/b:brat.0000019183.92439.51. [DOI] [PubMed] [Google Scholar]
- Jansen BH, Hegde A, Boutros NN. Contribution of different EEG frequencies to auditory evoked potential abnormalities in schizophrenia. Clinical Neurophysiology. 2004;115:523–533. doi: 10.1016/j.clinph.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Jin Y, Potkin SG, Patterson JV, Sandman CA, Hetrick WP, Bunney WE. Effects of P50 temporal variability on sensory gating in schizophrenia. Psychiatry Research. 1997;70:71–81. doi: 10.1016/s0165-1781(97)03091-6. [DOI] [PubMed] [Google Scholar]
- Johannesen JK, Kieffaber PD, O’Donnell BF, Shekhar A, Evans JD, Hetrick WP. Contributions of subtype and spectral frequency analyses to the study of P50 ERP amplitude and suppression in schizophrenia. Schizophrenia Research. 2005;78:269–284. doi: 10.1016/j.schres.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale for schizophrenia. Schizophrenia Bulletin. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kasai K, Shenton ME, Salisbury DF, Hirayasu Y, Onitsuka T, Spencer MH, et al. Progressive decrease of left Heschl gyrus and planum temporale gray matter volume in first-episode schizophrenia: A longitudinal magnetic resonance imaging study. Archives of General Psychiatry. 2003;60:766–775. doi: 10.1001/archpsyc.60.8.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Research Reviews. 1999;29:169–195. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Knight RT, Staines WR, Swick D, Chao LL. Prefrontal cortex regulates inhibition and excitation in distributed neural networks. Acta Psychologica. 1999;101:159–178. doi: 10.1016/s0001-6918(99)00004-9. [DOI] [PubMed] [Google Scholar]
- Leahy RM, Mosher JC, Spencer ME, Huang MX, Lewine JD. A study of dipole localization accuracy for MEG and EEG using a human skull phantom. Electroencephalography and Clinical Neurophysiology. 1998;107:159–173. doi: 10.1016/s0013-4694(98)00057-1. [DOI] [PubMed] [Google Scholar]
- Ligeois-Chauvel C, Musolino A, Badier JM, Marquis P, Chauvel P. Evoked potentials recorded from the auditory cortex in man: Evaluation and topography of the middle latency components. Electroencephalography and Clinical Neurophysiology. 1994;92:414–421. doi: 10.1016/0168-5597(94)90064-7. [DOI] [PubMed] [Google Scholar]
- Light GA, Geyer MA, Clementz BA, Cadenhead KS, Braff DL. Normal P50 suppression in schizophrenia patients treated with atypical antipsychotic medications. American Journal of Psychiatry. 2000;157:767–771. doi: 10.1176/appi.ajp.157.5.767. [DOI] [PubMed] [Google Scholar]
- Lins OG, Picton TW, Berg P, Scherg M. Ocular artifacts in recording EEGs and event-related potentials. II: Source dipoles and source components. Brain Topography. 1993;6:65–78. doi: 10.1007/BF01234128. [DOI] [PubMed] [Google Scholar]
- Llinás RR, Steriade M. Bursting of thalamic neurons and states of vigilance. Journal of Neurophysiology. 2006;95:3297–3308. doi: 10.1152/jn.00166.2006. [DOI] [PubMed] [Google Scholar]
- Lu B, Edgar JC, Smith AK, Jones AJ, Lewis SF, Miller GA, et al. Improved test-retest reliability of 50-ms paired-click auditory gating using magnetoencephalography source modeling. Psychophysiology. 2007;44:86–90. doi: 10.1111/j.1469-8986.2006.00478.x. [DOI] [PubMed] [Google Scholar]
- Lu BL, Martin K, Edgar JC, Smith AK, Lewis SF, Escamilla MA, et al. Effect of catechol O-methyltransferase Val158-Met polymorphism on the P50 sensory gating endophenotype in schizophrenia. Biological Psychiatry. 2007;62:822–825. doi: 10.1016/j.biopsych.2006.11.030. [DOI] [PubMed] [Google Scholar]
- Makeig S, Debener S, Onton J, Delorme A. Mining event-related brain dynamics. Trends in Cognitive Sciences. 2004;8:204–210. doi: 10.1016/j.tics.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Mäkinen V, Tiitinen H, May P. Auditory event-related responses are generated independently of ongoing brain activity. NeuroImage. 2005;24:961–968. doi: 10.1016/j.neuroimage.2004.10.020. [DOI] [PubMed] [Google Scholar]
- McCarley RW, Wible CG, Frumin M, Hirayasu Y, Levitt JJ, Fischer IA, et al. MRI anatomy of schizophrenia. Biological Psychiatry. 1999;45:1099–1119. doi: 10.1016/s0006-3223(99)00018-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CL, Freedman R. The activity of hippocampal interneurons and pyramidal cells during the response of the hippocampus to repeated auditory stimuli. Neuroscience. 1995;69:371–381. doi: 10.1016/0306-4522(95)00249-i. [DOI] [PubMed] [Google Scholar]
- Miller GA, Elbert T, Sutton BP, Heller W. Innovative clinical assessment technologies: Challenges and opportunities in neuroimaging. Psychological Assessment. 2007;19:58–73. doi: 10.1037/1040-3590.19.1.58. [DOI] [PubMed] [Google Scholar]
- Miller R. Cortico-hippocampal interplay and the representation of contexts in the brain. Berlin: Springer; 1991. [Google Scholar]
- Min BK, Busch NA, Debener S, Kranczioch C, Hanslmayr S, Engel AK, et al. The best of both worlds: Phase-reset of human EEG alpha activity and additive power contribute to ERP generation. International Journal of Psychophysiology. 2007;65:58–68. doi: 10.1016/j.ijpsycho.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Nagamoto HT, Adler LE, Hea RA, Griffith JM, McRae KA, Freedman R. Gating of auditory P50 in schizophrenics: Unique effects of clozapine. Bioogical Psychiatry. 1996;40:181–188. doi: 10.1016/0006-3223(95)00371-1. [DOI] [PubMed] [Google Scholar]
- Oranje B, Geyer MA, Bocker KBE, Kenemans JL, Verbaten MN. Prepulse inhibition and P50 suppression: Commonalities and dissociations. Psychiatry Research. 2006;143:147–158. doi: 10.1016/j.psychres.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Papp N, Ktonas P. Critical evaluation of complex demodulation techniques for the quantification of bioelectrical activity. Journal of Biomedical Science. 1977;13:135–143. [PubMed] [Google Scholar]
- Potter D, Summerfelt A, Gold J, Buchanan RW. Review of clinical correlates of P50 sensory gating abnormalities in patients with schizophrenia. Schizophrenia Bulletin. 2006;32:692–700. doi: 10.1093/schbul/sbj050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayers BM, Beagley HA, Hensall WR. The mechanism of auditory evoked EEG responses. Nature. 1974;247:481–483. doi: 10.1038/247481a0. [DOI] [PubMed] [Google Scholar]
- Scherg M. Fundamentals of dipole source potential analysis. Auditory evoked magnetic fields and electric potentials. In: Gandori M, Hoke M, Romani GL, editors. Advances in audiology. Vol. 6. Basel: Karger; 1990. pp. 40–69. [Google Scholar]
- Scherg M, Berg P. New concepts of brain source imaging and localization. Electroencephalography and Clinical Neurophysiology. 1996;46(Suppl):127–137. [PubMed] [Google Scholar]
- Scherg M, Ebersole JS. Brain source imaging of focal and multifocal epileptiform EEG activity. Clinical Neurophysiology. 1994;24:51–60. doi: 10.1016/s0987-7053(05)80405-8. [DOI] [PubMed] [Google Scholar]
- Scherg M, Ille N, Bornfleth H, Berg P. Advanced tools for digital EEG review: Virtual source montages, whole-head mapping, correlation, and phase analysis. Journal of Clinical Neurophysiology. 2002;19:91–112. doi: 10.1097/00004691-200203000-00001. [DOI] [PubMed] [Google Scholar]
- Scherg M, von Cramon D. A new interpretation of the generators of BAEP waves I–V: Results spatio-temporal dipole model. Electroencephalography and Clinical Neurophysiology. 1985;62:290–299. doi: 10.1016/0168-5597(85)90006-1. [DOI] [PubMed] [Google Scholar]
- Shelley AM, Silipo G, Javitt DC. Diminished responsiveness of ERPs in schizophrenic subjects to changes in auditory stimulation parameters: Implications for theories of cortical dysfunction. Schizophrenia Research. 1999;37:65–79. doi: 10.1016/s0920-9964(98)00138-8. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophrenia Research. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DA, Boutros NN, Schwarzkopf SB. Reliability of P50 event-related potential indices of sensory gating. Psychophysiology. 1994;31:495–502. doi: 10.1111/j.1469-8986.1994.tb01053.x. [DOI] [PubMed] [Google Scholar]
- Thoma RJ, Hanlon FM, Moses SN, Edgar JC, Huang MX, Weisend MP, et al. Lateralization of auditory sensory gating and neuropsychological dysfunction in schizophrenia. American Journal of Psychiatry. 2003;160:1595–1605. doi: 10.1176/appi.ajp.160.9.1595. [DOI] [PubMed] [Google Scholar]
- Tregellas JR, Davalos DB, Rojas DC, Waldo MC, Gibson L, Wylie K, et al. Increased hemodynamic response in the hippocampus, thalamus and prefrontal cortex during abnormal sensory gating in schizophrenia. Schizophrenia Research. 2007;92:262–272. doi: 10.1016/j.schres.2006.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward DB. Simultaneous inference for FMRI data. [accessed July 27, 2006];2000 http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf.
- Wilson CL, Babb TL, Halgren E, Wang ML, Crandall PH. Habituation of human limbic neuronal response to sensory stimulation. Experimental Neurology. 1984;84:74–97. doi: 10.1016/0014-4886(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Winterer G, Ziller M, Dorn H, Frick K, Mulert C, Wuebben Y, et al. Schizophrenia: Reduced signal-to-noise ratio and impaired phase-locking during information processing. Clinical Neurophysiology. 2000;111:837–849. doi: 10.1016/s1388-2457(99)00322-3. [DOI] [PubMed] [Google Scholar]
- Yee CM, Nuechterlein KH, Morris SE, White PM. P50 suppression in recent-onset schizophrenia: Clinical correlates and risperidone effects. Journal of Abnormal Psychology. 1998;107:691–698. doi: 10.1037//0021-843x.107.4.691. [DOI] [PubMed] [Google Scholar]
- Yeung N, Bogacz R, Holroyd CB, Cohen JD. Detection of synchronized oscillations in the electroencephalogram: An evaluation of methods. Psychophysiology. 2004;41:822–832. doi: 10.1111/j.1469-8986.2004.00239.x. [DOI] [PubMed] [Google Scholar]
- Zouridakis G, Boutros NN, Jansen BH. A fuzzy clustering approach to study the auditory P50 component in schizophrenia. Psychiatry Research. 1997;69:169–181. doi: 10.1016/s0165-1781(96)02979-4. [DOI] [PubMed] [Google Scholar]