Abstract
Discontinuation of digoxin is associated with worsening heart failure (HF) symptoms. However, the long-term effects of discontinuation of chronic digoxin therapy on mortality and morbidity in HF have not been well studied. Of the 7788 Digoxin Investigation Group trial participants, 3365 were receiving digoxin before randomization. During the trial, digoxin was continued in 1666 patients and discontinued in 1699 patients. Using multivariable Cox regression analyses, we at first determined the effect of discontinuation of digoxin on mortality and hospitalization during 39.7 months of median follow up. Of the 1666 patients continued on digoxin, 457 had low (0.5–0.9 ng/ml) and 340 had high (≥1.0 ng/ml) serum digoxin concentrations (SDC) after 1 month of therapy and of the 1699 patients whose digoxin was discontinued, 1674 were alive at 1 month. We examined the effects of continuation of digoxin at low or high SDC. Compared with continuation of chronic digoxin therapy, discontinuation of digoxin was associated with significant increase in all-cause hospitalization (adjusted hazard ratio {AHR}=1.18; 95% confidence interval {CI}=1.09–1.28; P<0.0001) and HF hospitalization (AHR=1.35; 95% CI=1.20–1.51; P<0.0001), but had no effect on all cause mortality (AHR=1.06; 95% CI=0.95–1.19; P=0.272). Continuation of digoxin at low SDC, on the other hand, was associated with reduction in all-cause mortality (AHR=0.75; 95% CI=0.63–0.90; P=0.002), all-cause hospitalization (AHR=0.80; 95% CI=0.71–0.91; P=0.001) and HF hospitalization (AHR=0.60; 95% CI=0.50–0.73; P<0.0001). In conclusion, continuation of chronic digoxin therapy at low SDC was associated with reduction in mortality and hospitalization in ambulatory chronic HF patients receiving background therapy with ACE inhibitors and diuretics.
Keywords: Heart failure, digoxin, discontinuation, mortality, hospitalization
The American College of Cardiology / American Heart Association (ACC/AHA) guideline for chronic heart failure (HF) recommends continuation of long-term digoxin therapy in HF patients who are already receiving this drug.1 Discontinuation of digoxin is associated with worsening heart failure (HF) symptoms.2–4 However, little is known about the long-term effect of discontinuation of chronic digoxin therapy on mortality and morbidity in patients with ambulatory chronic heart failure (HF). Even less is known about the effect of continuation of long-term digoxin therapy at low serum digoxin concentration (SDC). In the Digitalis Investigation Group (DIG) trial, 3365 (43%) of the 7788 patients were receiving digoxin at the time of enrollment.5 These patients were randomized to discontinue (placebo arm) or continue (digoxin arm) their chronic digoxin therapy. Data on SDC were collected in a random subset of DIG participants and post hoc analysis using SDC data suggest that the effect of digoxin on outcomes in HF may be dependent on SDC.6,7 These provide a unique opportunity to rapidly and efficiently determine the long-term effect of discontinuation of digoxin as well as the effect of continuation of digoxin therapy at low or high SDC. Because discontinuation of long-term digoxin use is associated with worsening of symptoms,2–4 we hypothesize that discontinuation of digoxin would be associated with increased hospitalization, and because of emerging evidence of the benefit of low SDC,6–8 we hypothesize that continuation of digoxin at low SDC would be associated with reduction in both hospital admissions and mortality.
Methods
DIG was a randomized trial of digoxin in HF patients in normal sinus rhythm receiving diuretics and angiotensin-converting enzyme (ACE) inhibitors (beta-blockers were not approved for HF at the time of randomization).5 DIG was conducted between 1991 and 1993 in 302 centers in the United States (186 centers) and Canada (116 centers). Of the 7788 HF patients (6,800 with ejection fraction ≤45%) 3365 were receiving chronic digoxin therapy before randomization. During the trial, 1666 patients were randomized to the digoxin arm, and thus continued to receive their chronic digoxin therapy. However, 1699 patients were randomized to the placebo arm, and thus their chronic digoxin therapy was discontinued during the trial. Serum digoxin concentration (SDC) is an important predictor of therapeutic and adverse effects of digoxin, and was measured in a random subset of DIG participants.5–7,9 Of the 1666 patients continued on digoxin, 457 had low (0.5–0.9 ng/ml) and 340 had high (≥1.0 ng/ml) SDC after 1 month of therapy, and of the 1699 patients in the placebo group, whose digoxin was discontinued, 1674 were alive at 1 month. Two patients randomized to placebo were censored during the first month after randomization and none had any hospitalizations during that period. Outcomes of interest for this analysis are all-cause mortality, all-cause hospitalizations, and HF hospitalizations during 39.7 months of median follow up. Data on outcomes were 99% complete by December 31, 1995.10
At first we compared the effect of discontinuation of digoxin on outcomes using multivariable Cox proportional hazards regression analysis. Then, we compared baseline characteristics for patients whose digoxin was discontinued (randomized to receive placebo during the trial) and who continued to receive digoxin (randomized to receive digoxin during the trial) at low (0.5–0.9 ng/ml) and high (=>1 ng/ml) SDC. Next, we used Kaplan-Meier survival analysis and log-rank tests to estimate the effects of continuation of digoxin therapy at low and high SDC compared with placebo on all-cause mortality and HF hospitalizations. Finally, Cox proportional hazards regression analyses were used to estimate the effect of continuation of digoxin at low and high SDC on outcomes. In the multivariable models, low and high SDC were used as dummy variables (using placebo as the reference). The covariates used in the multivariable models are age, sex, race, body mass index, duration and etiology of HF, prior myocardial infarction, current angina, hypertension, diabetes, use of ACE inhibitors, diuretics, potassium supplement, and combination of hydralazine and nitrates, current dyspnea at rest and dyspnea on exertion, NYHA class, heart rate, systolic and diastolic blood pressure, elevated jugular venous pressure, third heart sound, pulmonary râles, and lower extremity edema, serum potassium and creatinine, pulmonary congestion and cardiothoracic ratio >0.5 by chest x-ray, and ejection fraction. All statistical tests were evaluated using a 2-tailed 95% confidence level. All data analyses were performed using SPSS for Windows version 14.11
Results
Patients had a mean age of 63 years, 25% were women, 15% non-white, and 10% had ejection fraction >45%. Baseline patient characteristics of the 3 groups are displayed in Table 1. All-cause mortality occurred in 38.5% patients whose chronic digoxin therapy was continued and 39.1% patients whose chronic digoxin therapy was discontinued during the trial (hazard ratio {HR} for discontinuation when compared with continuation=1.03; 95% confidence interval {CI}=0.93–1.15; P=0.574). This association remained essentially unchanged after multivariable risk adjustment (adjusted HR=1.06; 95% CI=0.95–1.19; P=0.272). HF hospitalization occurred in 32.3% of patients in the continuation arm, and in 39.6% of patients in the discontinuation arm (HR=1.35; 95% CI=1.20–1.51; P<0.00010). This association also remained essentially unchanged after multivariable risk adjustment (adjusted HR=1.43; 95% CI=1.28–1.60; P<0.0001). Discontinuation of chronic digoxin therapy was associated with increased risk of hospitalizations due to all causes (HR=1.14; 95% CI=1.05–1.24; P=0.002 and adjusted HR=1.18; 95% CI=1.09–1.28; P<0.0001).
Table 1.
Baseline characteristics of heart failure (HF) patients with prior digoxin use (n=2471)
| N (%) or mean (±SD) | Digoxin discontinued, on placebo (N=1674) |
Digoxin continued at SDC 0.5–0.9 (N=457) |
Digoxin continued at SDC ≥1.0 (N=340) |
Overall P |
|---|---|---|---|---|
| Age, years | 64 (±11) | 62 (±11) | 65 (±10) | 0.002 |
| Women | 409 (24%) | 98 (21%) | 104 (31%) | 0.011 |
| Non-whites | 266 (16%) | 63 (14%) | 35 (10%) | 0.024 |
| BMI, kg/sq.m | 27 (±5) | 27(±5) | 26 (±5) | 0.058 |
| Heart rate, beats per minute | 79 (±12) | 77 (±12) | 76 (±13) | <0.0001 |
| HF duration, months | 36 (±39) | 37 (±42) | 41 (±40) | 0.074 |
| NYHA class III-IV | 541 (32%) | 137 (30%) | 114 (34%) | 0.523 |
| Ischemic etiology | 1119 (67%) | 314 (69%) | 245 (74%) | 0.158 |
| Ejection fraction, % | 31 (±12) | 31 (±12) | 31 (±11) | 0.963 |
| Ejection fraction >45% | 179 (11%) | 39 (9%) | 30 (9%) | 0.287 |
| Hypertension | 750 (45%) | 198 (43%) | 145 (43%) | 0.698 |
| Diabetes | 447 (27%) | 142 (31%) | 104 (31%) | 0.097 |
| Chronic kidney disease | 775 (46%) | 165 (36%) | 168 (55%) | <0.0001 |
| ACE inhibitor use | 1581 (94%) | 438 (96%) | 323 (95%) | 0.483 |
| Diuretic use | 1397 (84%) | 369 (81%) | 299 (88%) | 0.0251 |
| Dose of study medication | 0.24 (±0.1) | 0.24 (±0.1) | 0.24 (±0.1) | 0.119 |
| Pulmonary congestion | 220 (13%) | 58 (13%) | 70 (21%) | 0.001 |
| Cardiothoracic ratio >0.5 | 1013 (61%) | 273 (60%) | 223 (66%) | 0.176 |
| Serum creatinine, mg/dL | 1.3 (±0.4) | 1.2 (±0.3) | 1.4 (±0.4) | <0.0001 |
| eGFR, ml/min/1.73 sq.m | 64 (±29) | 69 (±21) | 58 (±18) | <0.0001 |
| Serum potassium, mEq/L | 4.3 (±0.5) | 4.3 (±0.5) | 4.3 (±0.4) | 0.478 |
ACE=angiotensin-converting enzyme; BMI=body mass index; CKD=chronic kidney disease, defined as estimated baseline glomerular filtration rate (GFR) <60 ml/1.73 m2 body surface area; eGFR=estimate glomerular filtration rate; NYHA=New York Heart Association; SDC=serum digoxin concentration
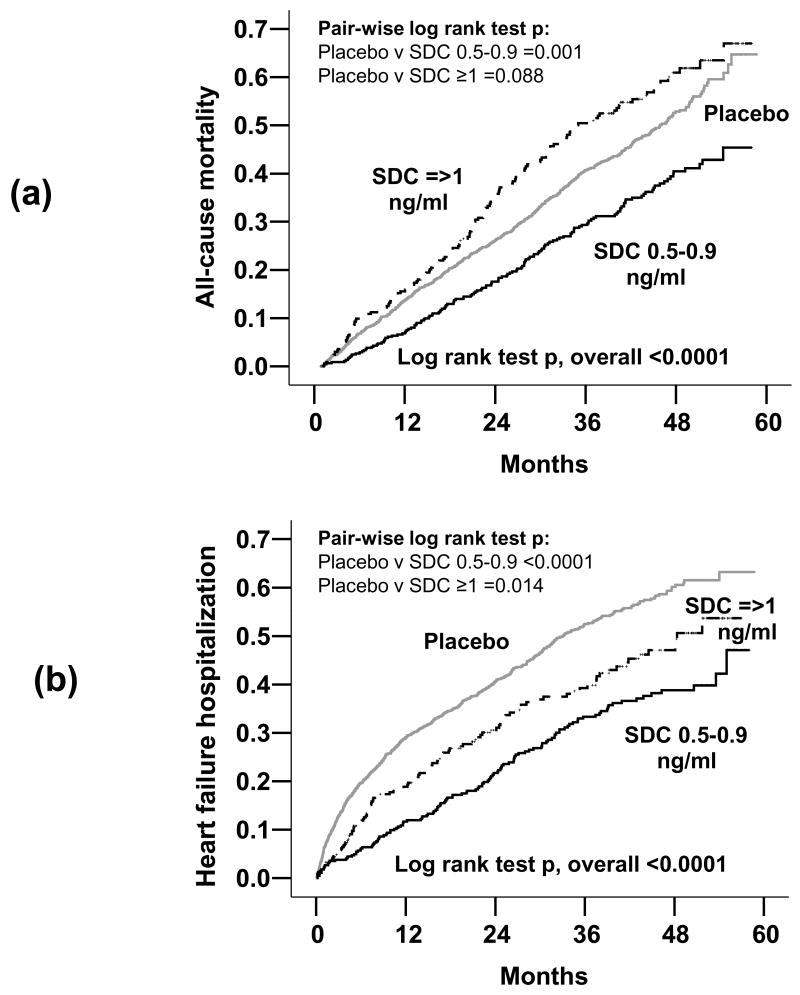
All-cause mortality occurred in 38% of patients whose chronic digoxin therapy was discontinued, in 32% of the patients in the digoxin continuation arm with low SDC (HR=0.73; 95% CI=0.61–0.87; P=0.001) and in 45% of those in the continuation arm with high SDC (HR=1.17; 95% CI=0.98–1.39; P=0.088) died from all causes (Table 2). These associations remained essentially unaltered after multivariable adjustment for other covariates. Kaplan-Meier plots for unadjusted mortality and HF hospitalization for patients with continued digoxin therapy at low and high SDC are displayed in Figure 1.
Table 2.
Effects of continuation of digoxin at low and high serum digoxin concentrations (SDC)
| Events / total follow-up years (Rate per 10,000 person-years of follow up) |
Crude hazard ratio (95% confidence interval) |
P value | Adjustd* hazard ratio (95% confidence interval) |
P value | ||
|---|---|---|---|---|---|---|
| All-cause mortality | Digoxin discontinued, on placebo (N=1674) | 636 / 4823 (1319) |
1 | Reference | 1 | Reference |
| Digoxin continued at SDC 0.5–0.9 (N=457) | 148 / 1530 (967) |
0.73 (0.61 – 0.87) |
0.001 | 0.75 (0.63 – 0.90) |
0.002 | |
| Digoxin continued at SDC ≥1.0 (N=340) | 153 / 994 (1539) |
1.17 (0.98 – 1.39) |
0.088 | 1.03 (0.86 – 1.12) |
0.580 | |
| All-cause hospitalization | Digoxin discontinued, on placebo (N=1674) | 1167 / 2653 (4399) |
1 | Reference | 1 | Reference |
| Digoxin continued at SDC 0.5–0.9 (N=457) | 302 / 918 (3290) |
0.79 (0.70 – 0.90) |
<0.0001 | 0.80 (0.70 – 0.91) |
0.001 | |
| Digoxin continued at SDC ≥1.0 (N=340) | 236 / 572 (4126) |
0.95 (0.83 – 1.10) |
0.504 | 0.94 (0.88 – 1.01) |
0.102 | |
| HF hospitalization | Digoxin discontinued, on placebo (N=1674) | 665 / 3730 (1783) |
1 | Reference | 1 | Reference |
| Digoxin continued at SDC 0.5–0.9 (N=457) | 133 / 1321 (1007) |
0.61 (0.50 – 0.73) |
<0.0001 | 0.60 (0.50 – 0.73) |
<0.0001 | |
| Digoxin continued at SDC ≥1.0 (N=340) | 114 / 850 (1341) |
0.78 (0.64 – 0.95) |
0.014 | 0.83 (0.76 – 0.92) |
<0.0001 | |
Adjusted for age, sex, race, body mass index, duration of heart failure, etiology of heart failure, prior myocardial infarction, current angina, hypertension, diabetes, use of angiotensin-converting enzyme inhibitors, diuretics, potassium supplement, and combination of hydralazine and nitrates, current dyspnea at rest and dyspnea on exertion, activity limitation, NYHA functional class, heart rate, systolic and diastolic blood pressure, elevated jugular venous pressure, third heart sound, pulmonary râles, and lower extremity edema, serum potassium and creatinine, pulmonary congestion and cardiothoracic ratio >0.5 by chest x-ray, and ejection fraction.
Figure 1.
Kaplan-Meier plots for unadjusted cumulative risk of (a) all-cause mortality, and (b) heart failure (HF) hospitalizations
All-cause hospitalizations occurred in 70% patients in the digoxin discontinuation group, in 66% of patients in the digoxin continuation group with low SDC (HR=0.79; 95% CI=0.70–0.90; P<0.0001) and in 69% of those in the continuation group with high SDC (HR=0.95; 95% CI=0.83–1.10; P=0.496; Table 2). There were no significant changes in these associations after multivariable adjustment for other covariates. HF hospitalizations occurred in 40% of patients in the discontinuation group, 29% of those in the low SDC group (HR=0.61; 95% CI=0.54–0.73; P<0.0001) and 34% of those in the high SDC group (HR=0.78; 95% CI=0.64–0.95; P=0.014) (Table 3). These associations also remained essentially unchanged after multivariable adjustment for other covariates.
Table 3.
Effect of digoxin on outcomes at the end of 2 years in systolic & diastolic heart failure (HF) patients
| Outcomes | Original data (N=7788) with 6800 SHF and 988 DHF patients |
||||
|---|---|---|---|---|---|
| Systolic HF* | Placebo (n=3403) | Digoxin (n=3397) | Absolute rate difference (%) | Hazard ratio (95% CI) | P value |
|
|
|||||
| HF hospitalization or HF mortality | 999 (29%) | 735 (22%) | − 7.8 | 0.69 (0.63–0.76) | <0.001 |
| HF hospitalization | 920 (27%) | 667 (20%) | − 7.4 | 0.68 (0.62–0.75) | <0.001 |
|
|
|||||
| Diastolic HF† | Placebo (n=496) | Digoxin (n=492) | Absolute rate difference (%) | Hazard ratio (95% CI) | P value |
|
|
|||||
| HF hospitalization or HF mortality | 90 (18%) | 67 (14%) | − 4.5 | 0.71 (0.52–0.98) | 0.034 |
| HF hospitalization | 86 (17%) | 59 (12%) | − 5.3 | 0.66 (0.47–0.91) | 0.012 |
| Matched data (N=1832) with 916 SHF and 916 DHF patients |
|||||
| Systolic HF | Placebo (n=450) | Digoxin (n=466) | Absolute rate difference (%) | Hazard ratio (95% CI) | P value |
|
|
|||||
| HF hospitalization or HF mortality | 114 (25%) | 89 (19%) | − 6.2 | 0.72 (0.55–0.95) | 0.022 |
| HF hospitalization | 102 (23%) | 80 (17%) | − 5.5 | 0.73 (0.54–0.97) | 0.033 |
|
|
|||||
| Diastolic HF | Placebo (n=460) | Digoxin (n=456) | Absolute rate difference (%) | Hazard ratio (95% CI) | P value |
|
|
|||||
| HF hospitalization or HF mortality | 86 (19%) | 62 (14%) | − 5.1 | 0.69 (0.50–0.95) | 0.025 |
| HF hospitalization | 82 (18%) | 55 (12%) | − 5.6 | 0.64 (0.45–0.90) | 0.010 |
Adapted from: GlaxoSmithKline. Lanoxin (digoxin) tablets, USP: Full prescribing information. 2001.
Adapted from: Ahmed A, et al. Circulation 2006; 114(5):397–403.
Discussion
The results of our analysis suggest that discontinuation of chronic digoxin therapy was associated with long-term increase in hospital admission, but no such increase in mortality in ambulatory chronic HF patients receiving ACE inhibitors and diuretics. However, these data also suggest that continuation of chronic digoxin therapy at low SDC was associated not only with reduction in hospital admission, but also with reduction in mortality.
About half of all patients in the DIG trial were receiving chronic digoxin therapy prior to randomization. These patients were probably receiving digoxin because they were more symptomatic or had more advanced HF, and thus were more likely to benefit from the continued use of digoxin. Treatment effects often depend on severity of disease and/or associated comorbidity.12 We speculate that the mortality benefit of digoxin at low SDC was due to the neurohormonal modulating properties of digoxin,13–15 which are mediated via its inhibition of the sodium-potassium adenosine triphosphatase enzyme in non-cardiac tissues.16–18 To what extent reduction in heart rate contributed to the beneficial effect of digoxin is unknown. We had no data on heart rate changes at the end of the study.
Prior studies have documented that discontinuation of digoxin therapy results in worsening symptoms in systolic HF patients.2–4,19 These studies in systolic HF were limited by small sample sizes, short duration of follow up, and lack of mortality or hospitalizations as outcomes. Instead, the results of the current analysis are based on the DIG trial, which is the largest clinical trial of digoxin in systolic and diastolic HF, with long follow up for an array of cause-specific mortalities and hospitalizations as outcomes. The current analysis is also distinguished from prior studies by its adjustment for SDC. In 1 study digoxin in HF patients with increased heart rate, after 72 months of follow-up, compared with patients who were adherent with their digoxin therapy, those who were non-adherent had increased mortality and hospitalizations..20 In that study, SDC was used to determine adherence to digoxin therapy.
These findings of our study are consistent with the 2005 ACC/AHA HF guideline recommendation that if a HF patient “is taking digoxin but not an ACEI or a beta-blocker, treatment with digoxin should not be withdrawn, but appropriate therapy with the neurohormonal antagonists should be instituted.”1 These findings provide evidence in support of this recommendation and also suggest that when digoxin is continued, it should be continued at low SDC. HF is the number 1 reason for hospitalizations for older adults in the US and is responsible for about 1 million discharges with a primary discharge diagnosis of HF every year.21 However, when used in low doses to achieve low SDC, digoxin may also reduce mortality, which is an important consideration for HF patients who cannot afford or tolerate ACE inhibitors and beta-blockers.7
Our study has several limitations. Importantly all analyses are based on subgroups of patients. SDC used in this analysis was at 1 month after randomization and may not reflect SDC throughout the trial. DIG participants were relatively young, predominantly male and white, in normal sinus rhythm and were not receiving beta-blockers. However, a post-hoc analysis of the carvedilol trials in HF suggest that the digoxin associated reduction in the combined outcome of all-cause mortality or all-cause hospitalization was observed regardless of beta-blocker use.22 Despite these limitations, the results of our study suggest that discontinuation of chronic digoxin therapy was associated with increased hospitalizations with no effect on mortality, and that continuation of chronic digoxin therapy at low (0.5–0.9 ng/ml) SDC was associated with reduction in mortality and hospitalizations.
Acknowledgments
“The Digitalis Investigation Group (DIG) study was conducted and supported by the NHLBI in collaboration with the DIG Investigators. This Manuscript was prepared using a limited access dataset obtained by the NHLBI and does not necessarily reflect the opinions or views of the DIG Study or the NHLBI.”
Funding/Support: Dr. Ahmed is supported by the National Institutes of Health through grants from the National Institute on Aging (1-K23-AG19211-04) and the National Heart, Lung, and Blood Institute (1-R01-HL085561-01 and P50-HL077100).
Footnotes
Author Contributions
Dr. Ahmed conceived the study hypothesis and design, and prepared the manuscript. All authors interpreted the data, participated in critical revision of the paper for important intellectual content, and approved the final version of the article. Drs. Ahmed and Weaver had full access to data. Dr. Ahmed did statistical analyses in consultation with Drs. Rich and Weaver.
Conflict of Interest Disclosures: None
References
- 1.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 2.Adams KF, Jr, Gheorghiade M, Uretsky BF, Young JB, Ahmed S, Tomasko L, Packer M. Patients with mild heart failure worsen during withdrawal from digoxin therapy. J Am Coll Cardiol. 1997;30:42–48. doi: 10.1016/s0735-1097(97)00133-2. [DOI] [PubMed] [Google Scholar]
- 3.Packer M, Gheorghiade M, Young JB, Costantini PJ, Adams KF, Cody RJ, Smith LK, Van Voorhees L, Gourley LA, Jolly MK. Withdrawal of digoxin from patients with chronic heart failure treated with angiotensin-converting-enzyme inhibitors. RADIANCE Study. N Engl J Med. 1993;329:1–7. doi: 10.1056/NEJM199307013290101. [DOI] [PubMed] [Google Scholar]
- 4.Uretsky BF, Young JB, Shahidi FE, Yellen LG, Harrison MC, Jolly MK. Randomized study assessing the effect of digoxin withdrawal in patients with mild to moderate chronic congestive heart failure: results of the PROVED trial. PROVED Investigative Group. J Am Coll Cardiol. 1993;22:955–962. doi: 10.1016/0735-1097(93)90403-n. [DOI] [PubMed] [Google Scholar]
- 5.The Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997;336:525–533. doi: 10.1056/NEJM199702203360801. [DOI] [PubMed] [Google Scholar]
- 6.Rathore SS, Curtis JP, Wang Y, Bristow MR, Krumholz HM. Association of serum digoxin concentration and outcomes in patients with heart failure. JAMA. 2003;289:871–878. doi: 10.1001/jama.289.7.871. [DOI] [PubMed] [Google Scholar]
- 7.Ahmed A, Rich MW, Love TE, Lloyd-Jones DM, Aban IB, Colucci WS, Adams KF, Gheorghiade M. Digoxin and reduction in mortality and hospitalization in heart failure: a comprehensive post hoc analysis of the DIG trial. Eur Heart J. 2006;27:178–186. doi: 10.1093/eurheartj/ehi687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adams KF, Jr, Gheorghiade M, Uretsky BF, Patterson JH, Schwartz TA, Young JB. Clinical benefits of low serum digoxin concentrations in heart failure. J Am Coll Cardiol. 2002;39:946–953. doi: 10.1016/s0735-1097(02)01708-4. [DOI] [PubMed] [Google Scholar]
- 9.Adams KF, Jr, Patterson JH, Gattis WA, O’Connor CM, Lee CR, Schwartz TA, Gheorghiade M. Relationship of serum digoxin concentration to mortality and morbidity in women in the digitalis investigation group trial: a retrospective analysis. J Am Coll Cardiol. 2005;46:497–504. doi: 10.1016/j.jacc.2005.02.091. [DOI] [PubMed] [Google Scholar]
- 10.Collins JF, Howell CL, Horney RA. Determination of vital status at the end of the DIG trial. Control Clin Trials. 2003;24:726–730. doi: 10.1016/j.cct.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 11.SPSS. SPSS for Windows, Rel. 14. Chicago, IL: SPSS Inc., Chicago, IL; 2006. [Google Scholar]
- 12.Rothwell PM. Treating individuals 2. Subgroup analysis in randomised controlled trials: importance, indications, and interpretation. Lancet. 2005;365:176–186. doi: 10.1016/S0140-6736(05)17709-5. [DOI] [PubMed] [Google Scholar]
- 13.Gheorghiade M, Ferguson D. Digoxin. A neurohormonal modulator in heart failure? Circulation. 1991;84:2181–2186. doi: 10.1161/01.cir.84.5.2181. [DOI] [PubMed] [Google Scholar]
- 14.Rahimtoola SH. Digitalis therapy for patients in clinical heart failure. Circulation. 2004;109:2942–2946. doi: 10.1161/01.CIR.0000132477.32438.03. [DOI] [PubMed] [Google Scholar]
- 15.Gheorghiade M, van Veldhuisen DJ, Colucci WS. Contemporary use of digoxin in the management of cardiovascular disorders. Circulation. 2006;113:2556–2564. doi: 10.1161/CIRCULATIONAHA.105.560110. [DOI] [PubMed] [Google Scholar]
- 16.Ferguson DW, Berg WJ, Sanders JS, Roach PJ, Kempf JS, Kienzle MG. Sympathoinhibitory responses to digitalis glycosides in heart failure patients. Direct evidence from sympathetic neural recordings. Circulation. 1989;80:65–77. doi: 10.1161/01.cir.80.1.65. [DOI] [PubMed] [Google Scholar]
- 17.Covit AB, Schaer GL, Sealey JE, Laragh JH, Cody RJ. Suppression of the renin-angiotensin system by intravenous digoxin in chronic congestive heart failure. Am J Med. 1983;75:445–447. doi: 10.1016/0002-9343(83)90346-7. [DOI] [PubMed] [Google Scholar]
- 18.Torretti J, Hendler E, Weinstein E, Longnecker RE, Epstein FH. Functional significance of Na- K-ATPase in the kidney: effects of ouabain inhibition. Am J Physiol. 1972;222:1398–1405. doi: 10.1152/ajplegacy.1972.222.6.1398. [DOI] [PubMed] [Google Scholar]
- 19.Adams KF, Jr, Gheorghiade M, Uretsky BF, Young JB, Patterson JH, Tomasko L, Packer M. Clinical predictors of worsening heart failure during withdrawal from digoxin therapy. Am Heart J. 1998;135:389–397. doi: 10.1016/s0002-8703(98)70313-8. [DOI] [PubMed] [Google Scholar]
- 20.Miura T, Kojima R, Mizutani M, Shiga Y, Takatsu F, Suzuki Y. Effect of digoxin noncompliance on hospitalization and mortality in patients with heart failure in long-term therapy: a prospective cohort study. Eur J Clin Pharmacol. 2001;57:77–83. doi: 10.1007/s002280100272. [DOI] [PubMed] [Google Scholar]
- 21.Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O’Donnell CJ, Roger V, Rumsfeld J, Sorlie P, Steinberger J, Thom T, Wasserthiel-Smoller S, Hong Y. Heart disease and stroke statistics--2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 22.Eichhorn EJ, Lukas MA, Wu B, Shusterman N. Effect of concomitant digoxin and carvedilol therapy on mortality and morbidity in patients with chronic heart failure. Am J Cardiol. 2000;86:1032–1035. A1010–1031. doi: 10.1016/s0002-9149(00)01146-2. [DOI] [PubMed] [Google Scholar]