Summary
Purpose
The development of nonradioactive and targeted magnetonanoparticles (MNP) capable of crossing the blood–brain barrier (BBB) and of concentrating in the epileptogenic tissues of acute and chronic animal models of temporal lobe epilepsy to render these tissues visible on magnetic resonance imaging (MRI).
Methods
Nonradioactive alpha methyl tryptophan (AMT) was covalently attached to MNP composed of iron oxide and dextran. A rodent model of temporal lobe epilepsy was prepared by injecting kainic acid into the right hippocampus. AMT-MNP or plain MNP was injected in the tail-vein of two animals during the acute stage 3 days after status epilepticus, and AMT-MNP in five animals during the chronic stage. MRIs were obtained before and after particle injection in all animals. Intracranial EEGs were obtained in all chronic animals after completion of MRI studies.
Results
AMT-MNP crossed the BBB and intra-parenchymal uptake was visible on MRI. In the acute condition, AMT-MNP appeared to localize to both hippocampi, whereas plain MNP only identified unilateral, presumably inflammatory, changes. In the chronic condition, AMT-MNP uptake correlated with the occurrence of spontaneous seizures, and the location of uptake appeared to agree with bilateral or unilateral epileptogenicity confirmed by subsequent intracranial EEG.
Discussion
Nonradioactive AMT-MNP can cross the BBB and may accurately localize epileptogenic cerebral regions. The MNP-MRI approach is potentially applicable to the use of any bioactive molecules as ligands for imaging normal and abnormal localized cerebral functions, accurately, safely, and inexpensively.
Keywords: Magnetonanoparticles, Targeted, Epilepsy, Localization, Alpha methyl tryptophan, MRI, Contrast
The American Epilepsy Society and the National Institute of Neurological Disorders and Stroke have coauthored a list of benchmarks to be attained in the search for a cure for epilepsy (Jacobs et al., 2001), the first of which is the identification of a reliable surrogate marker of epileptogenesis and epileptogenicity. A reliable surrogate marker that can be noninvasively measured could (1) provide an inexpensive means to localize the epileptogenic region for surgical resection in patients with pharmacoresistant epilepsy who are candidates for surgery; (2) predict which patients are likely to develop epilepsy following a potentially epileptogenic brain injury, in order to institute antiepileptogenic treatment; and (3) determine which of the many therapeutic approaches, including a large number of antiepileptic drugs, vagus nerve stimulation, and deep brain stimulation, are likely to be effective in individual patients without the need to wait for another seizure to occur. One putative surrogate marker that has received recent attention is alpha methyl tryptophan (AMT), which has been used as a positron emission tomography (PET) ligand to identify epileptogenic tissues in several epilepsy conditions (Fedi et al., 2001; Duchowny, 2003; Juhasz & Chugani, 2003; Juhasz et al., 2003; Natsume et al., 2003; Juhasz et al., 2004; Kagawa et al., 2005).
This proof of principle study demonstrates the use of nanotechnology to covalently attach AMT to magneto-nanoparticles (MNP) visible on magnetic resonance imaging (MRI). These particles cross the blood–brain barrier (BBB) and concentrate in epileptogenic tissues during the acute and chronic stages of an animal model of temporal lobe epilepsy, permitting their localization with standard MRI. Beyond its immediate application in epilepsy, this represents the first demonstration of a novel approach to utilize standard MRI for ligand-based functional neuroimaging, which would have the following advantages: (1) there would be no radiation risk, and thus no limit on the number of times the study could be repeated; (2) the technique could be employed in any MRI facility without need for modification; (3) tracers are relatively easy to synthesize in a short period of time; (4) they could be purchased relatively inexpensively and could be kept for prolonged periods of time without special storage requirements. This MNP-MRI approach is potentially applicable to the use of any bioactive molecules as ligands for imaging normal and abnormal cerebral function, accurately, safely, and inexpensively.
Methods
Particles
The plain MNP were prepared by coprecipitation of dextran with maghemite (γ -Fe2O3) following a procedure similar to that described by Molday (1984). This procedure forms a paramagnetic iron oxide core surrounded by dextran coating. Maghemite is a naturally occurring paramagnetic material found in living organisms such as prokaryotes. Dextran is an α-D-1,6-glucose-linked glucan with side-chains 1–3 linked to the backbone units of the Dextran biopolymer. The degree of branching is approximately 5%. The branches are mostly 1–2 glucose units long. Dextran can be obtained from fermentation of sucrose-containing media by Leuconostoc mesenteroides B512F. The synthesized particles were purified by magnetic separation, which were then washed and redispersed. AMT was then conjugated to functionalized MNP (Akhtari & Engel, 2006). We procured AMT-conjugated MNP’s (AMT-MNP) from Corpuscular Inc. (Cold Springs, NY, U.S.A.) under a fee-for-service agreement and characterized them using atomic force microscopy, magnetic force microscopy, section analysis, and transmission electron microscopy, with and without staining.
MRI phantom studies
A concentric cylindrical plastic phantom was constructed to determine proper imaging sequences and to quantify the effect of MNP on shortening the T2 (spin-spin relaxation time) and T2* relaxation time of protons in solution; T2* measures the effects on tissue protons due to local inhomogeneity caused by their immediate surroundings. The inner cylinder was filled with 1 μl of MNP and 1 ml of tap water. The outer cylinder was filled with tap water only. MR images (Bruker BioSpin, 7T, Ettlingen, Germany) of the phantom were obtained using T2* sequences. Sequential images were obtained with repetition time (TR) held constant at 600 ms while echo acquisition time (TE) was varied from 5.5 ms to 13 ms in increments of 2.5 ms.
Animals
All procedures described in this study were approved by the University of California, Los Angeles, Institutional Animal Care and Use Committee. A total of seven Lewis rats (205–300 g) were used in the experiments.
Kainic acid injection
Eighteen rats were anesthetized by isoflurane gas and, after additional local anesthesia, were fixed in a stereotaxic frame. Kainic acid (KA) (0.4 μg/0.2 μl normal saline) was injected unilaterally in the right posterior hippocampus (6 mm posterior, 4 mm lateral, and 7 mm deep from bregma) through 3.0 mm burr holes with a 1 μl Hamilton micro-syringe over 20 min. Status epilepticus was observed in all rats 10–20 min after KA injection. Two rats were studied during the acute stage, 3 days after status.
Video monitoring of animals
The remaining 16 rats were housed in individual cages and video monitored on a 24-h basis, with 12 h daylight and 12 h infrared illumination for night monitoring. The wavelength of infrared illumination was above 760 nm, which is not visible to rodents. Tapes were reviewed each day using high-speed playback. The rats were monitored for up to 10 months. Rats that showed behavioral seizures were shifted at 2-week intervals into and out of video monitoring, while rats that did not develop behavioral seizures remained under video monitoring continuously. Five rats with frequent spontaneous seizures and one which never exhibited behavioral seizures were chosen for the chronic MRI studies and subsequent electrophysiological recordings. One rat was found to have a right hippocampal abscess on histological evaluation. Because we were unable to determine if this was caused by the cannula prior to MRI, or the recording electrodes after MRI, this animal is not included in the data presented here.
MRI animal studies
MRI scans (7T, 6000TR/50TE, T2 sequences, Spin Echo (SE), 192 × 192, 25 slices, 1–1.6 mm slice thickness, 0.1 mm interslice distance, 3–4 cm FOV, 19 min acquisition time), Bruker Biospin, 7T) were acquired on two rats immediately after initial recovery from status epilepticus (acute state), and the rest of the animals 2–8 months after initial KA injection (chronic state). MR images were acquired before particle injection (AMT-MNP 300 μmol/kg body weight; 15 mg/kg, Dousset et al., 1999a, in six rats, plain MNP, also 15 mg/kg, in one rat) in the tail vein, as well as 2–24 h after tail vein injection to compare the differences between images. Each animal had at least two images taken at different intervals, including an early image at 2–6 h, and a later image up to 24 h after AMT-MNP injection.
Densitometry
Areas of (negative) signal enhancement were identified visually on MR images after injection with AMT-MNP. The signal intensities of these areas were measured using MRIcro software (http://www.sph.sc.edu/comd/rorden/). The signal intensities of the corresponding areas on baseline images were also measured. Reference signal intensity was also measured randomly in areas of hippocampus that did not show signal variation before and after injection with AMT-MNP. The signal intensities of the clusters of signal enhancement and references were normalized to that of the cerebellum in rats 3–7, and nonenhanced brain tissues in rats 1 and 2, in each corresponding image to eliminate possible global signal intensity changes in repeated scans. Hence, four groups of densitometry data were generated, two sets corresponding to areas of signal enhancement and two reference sets. Densitometry values from the edematous right hippocampi in rats 1 and 2 were not included in the final statistical analysis.
Microelectrode implantation
After MRI studies, chronic animals were anesthetized with isofluorane gas and given atropine (0.04 mg I.M). Pairs of tungsten wires (50 μm in diameter) with 0.5 mm vertical tip separation were placed in the right angular bundle to stimulate perforant path afferents to the hippocampus (AP = −7.0 mm from bregma, ML = 3.5 mm and DV = 6.5 mm from the surface of neocortex) (Paxinos & Watson, 1997). Recording microelectrodes consisted of four tungsten wires glued together with 2.0 mm vertical tip separation. They were implanted bilaterally such that the two middle microelectrodes were located in a region of MRI contrast enhancement, the coordinates of which were determined using the Paxinos Atlas (Paxinos & Watson, 1997). The mean impedance of the microelectrodes was 52 ± 2.8 kohms and the mean amplitude of baseline noise was 26 ± 0.14 μV.
Electrophysiological data acquisition and analysis
One week after electrode implantation, continuous (24 h/day) recordings of intracranial electrical activity were performed for 3 weeks. Two 4-channel MOSFET input operational amplifiers were mounted in the cable connector to eliminate cable movement artifacts. Physiological data were recorded wide-band (0.1–3.0 kHz) and sampled at 10 kHz/channel with 12 bit digitizers on a Pentium PC using DataPac (Run Technologies, Torrance, CA, U.S.A.) or RC-Electronics (Santa Barbara, CA, U.S.A.) software.
When present, seizures were detected, counted, and scored (Racine, 1972a, 1972b) by reviewing videotapes. Electrophysiological data were analyzed off-line on a Pentium computer, using DataPac software. Evoked potentials were analyzed after averaging (n = 5).
An electrographic seizure was defined as a period of consistent, repetitive changes in amplitude and frequency of electrical activity that was clearly different from interictal activity (details provided in the Results section) and which persisted for more than 10 s. During transition to ictus, the time of seizure onset was considered to be the time of first appearance of a consistent increase in EEG frequency in one or more wide band recording channels, determined by a moving spectral analysis for epochs of 500 ms with 250 ms overlap.
EEG interictal spikes (IIS) and high frequency oscillations were analyzed after band-pass filtering at 20–500 Hz, and 80–500 Hz, respectively. A digital bandpass filter (FIR, roll-off = −36dB) limited each frequency band. A Hamming window was applied to the filtered signal. Detected events were considered IIS when they met 5:1 signal/background-noise ratio and pathological high frequency oscillations (pHFOs) when they met 3:1 signal/background-noise ratio criteria and persisted for at least 30 ms. IIS and pHFO rates were calculated during three 30-min periods of immobility and slow wave sleep and the ratio of total number of IIS recorded on the injected side to the number recorded on the noninjected side was determined. Power spectrum analysis of pHFO was performed using wavelet function in the Matlab library (Math-Works, Inc., Natick, MA, U.S.A.).
Histological procedures
At the conclusion of experiments, rats 3, 4, and 7 were perfused using 0.9% saline followed by 0.1% sodium sulfide and 4% paraformaldehyde. All solutions were buffered in 0.12 molar Millonings buffer (pH 7.3) containing 0.002% CaCl2. Brains were immediately removed after perfusion and fixed in 4% paraformaldehyde and 1× phosphate buffer overnight.
Glial fibrillary acid protein (GFAP) stain
The brains were embedded in paraffin and cut to 6 μm thickness. A 3% Hydrogen Peroxide bath quenched endogenous peroxidase activity and the deparaffinized sections were incubated for 30 min in Tris-EDTA buffer, pH 8.0, at 120°C for antigen retrieval. Sections were incubated overnight in GFAP (DAKO Corporation, Carpinteria, CA, U.S.A.) at a 1:100 dilution, followed by 1-h incubation at 37°C in ImmPRESS anti-rabbit Ig reagent (Vector Laboratories, Inc., Burlingame, CA, U.S.A.). The sections were developed using DAB peroxidase substrate (Vector Laboratories, Inc.) and counterstained with hematoxylin (Biomeda Corporation, Foster City, CA, U.S.A.).
Perl’s iron stain
Paraffin-embedded brain tissue was cut to 6 μm thickness. Deparaffinized sections were incubated in stock potassium ferrocyanide solution (10%) for 5 min. Immediately prior to use, working potassium ferrocyanide solution was prepared (70 ml stock potassium ferrocyanide, 30 ml 10% HCl) and applied for 20 min. Sections were counterstained with Nuclear Fast Red for 5 min.
Results
Characteristics of nanoparticles
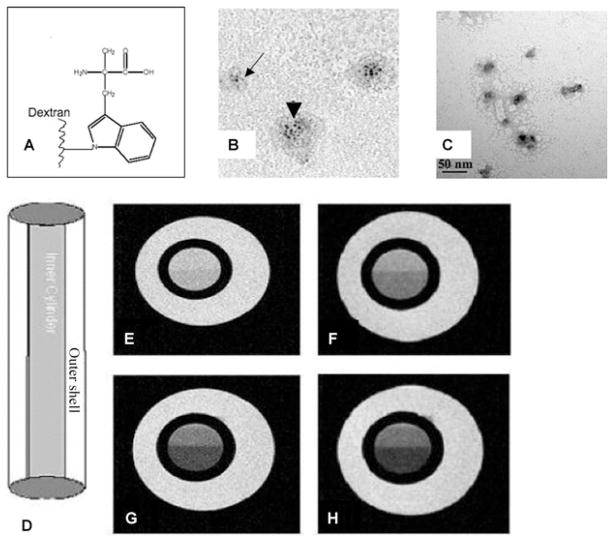
The schematic diagram for particle construct is shown in Fig. 1A. Figs. 1B and C show the unstained and stained (uranyl acetate) transmission electron microscopy of AMT-MNP particles. The particles had an average diameter of 10–20 nm with an average iron-oxide core diameter of 2–3 nm
Figure 1.
Top, characterization of MNP. (A) Schematic diagram of AMT conjugated to dextran coating, (B) Transmission electron microscopy (TEM) (no staining) shows single particle core (arrow) and aggregates (arrowhead) which occur during drying of the sample on the grid, and (C) TEM (negative staining with 1% uranyl acetate) shows the morphology and size (scale at the bottom of panel) of individual AMT-MNP particles. The particle solution was diluted and prepared to avoid aggregation of the particles. Most particles measured between 10 and 20 nm. Bottom, phantom studies: (D) plastic phantom constructed from two concentric cylinders. The inner cylinder contains 1 μl of magnetonanoparticles in 1 ml of tap water. The outer shell contains only tap water; (E–H) show the signal drop-out (negative enhancement) as TE time was increased from 5.5 ms to 13 ms in 2.5 ms steps in T2* image acquisition sequences. This effect is due to shortening of the T2 relaxation time in presence of the iron-oxide particles.
Epilepsia © ILAE
MRI phantom studies
Figs. 1D–1H show the signal decrease in the inner cylinder that results from T2* decrease due to the presence of paramagnetic iron-oxide. The measured T2* decreased from 170 to 130 ms.
Animal experiments
Two rats were used for MRI studies during the acute stage, and five were used for MRI and electrophysiological studies during the chronic stage, four with spontaneous behavioral seizures and one without. We will use the term hippocampus when describing MRI data and dentate gyrus when describing electrophysiological data because all recording electrodes were located in the dentate gyrus, confirmed by evoked responses to perforant path stimulation (Bragin et al., 2002) and histological sections.
Rats 1 and 2 were studied during the acute stage 3 days after KA injection; rats 3–7 were studied after 2–8 months of video monitoring. Rats 3–6 displayed multiple behavioral seizures (Racine scale, stage 3–5; Racine, 1972a, 1972b) during video monitoring, while rat 7 did not display any behavioral seizures during 8 months of continuous video monitoring before the MRI studies.
MRI studies: acute period
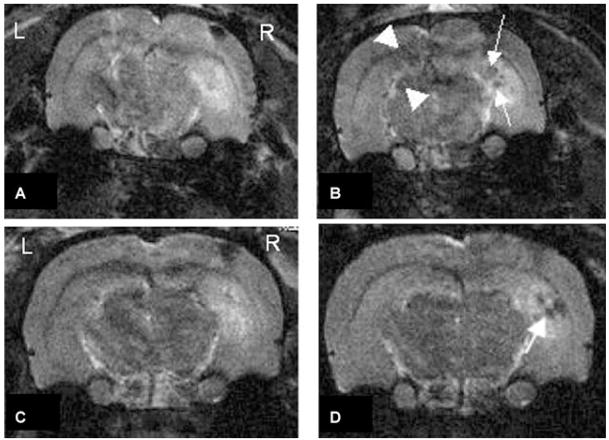
Rat 1 was injected with AMT-MNP; rat 2 was injected with unconjugated (plain) MNP. Prior to particle injection, the baseline MRIs showed areas of positive contrast enhancement (increased signal intensity) consistent with edema in the right hippocampus near the sites of KA injection as well as areas of cortical negative enhancement immediately below the skull where the burr hole was drilled prior to KA injection (Figs. 2A–C). Placement of the microinjection cannula resulted in a defect in the cortex. This small hole then filled with blood, which is dark on T2 sequences.
Figure 2.
Top, MR images of rat 1, 78 h after injection with KA in the right hippocampus. (A) baseline MRI prior to IV injection with AMT-MNP. (B) MR image acquired 6 h post AMT-MNP injection. Two areas of “negative enhancement” are visible: near the site of KA injection (arrow), and in CA1 and dentate gyrus contralateral to the site of KA injection (arrowheads) where no injury occurred. Bottom, MR images of rat 2, 78 h after injection with KA in the right hippocampus. (C) baseline MRI prior to IV injection with plain MNP, (D) MR images acquired 6 h post MNP injection. Area of “negative enhancement” is visible at the site of acute inflammation ipsilateral to the location of KA injection (arrow).
Epilepsia © ILAE
The second MR images 6 h after AMT-MNP injection in rat 1 (Fig. 2B), showed areas of negative enhancement (arrows) in the ipsilateral hippocampus near the site of KA injection, and also in the CA1 (upper arrowhead) and dentate gyrus (lower arrowhead) contralateral to the site of KA injection, away from the site of injury (Jupp et al., 2006). The normalized densitometry values, right and left respectively, of the areas of uptake in CA1 and dentate gyrus were 1.1 and 1.0 before, and 0.8 and 0.65 after injection with particles; these corresponding values at the site of edema were 1.6 before, and 0.9 after injection.
MR images 6 h after plain MNP injection in rat 2 (Fig. 2D) showed areas of negative enhancement in the injected hippocampus only (arrow). The normalized densitometry values, right and left CA1 and dentate gyrus respectively, were 0.98 and 1.0 before, and 1.0 and 0.98 after injection with plain particles; these corresponding values at the site of edema were 1.7 before, and 0.7 after injection.
MRI studies: chronic period
AMT-MNP was injected in four rats that developed recurrent behavioral seizures after KA injection and one rat that did not develop seizures. Fig. 3 shows the MR images of rats 3–7 before (left column) and after (middle column) AMT-MNP injection. All rats showed KA-induced hippocampal atrophy, as well as negative signal enhancement corresponding to the site of the injection cannula placement, in the baseline MRIs prior to particle injection. No significant within animal differences were seen at various time intervals after AMT-MNP injection, which ranged from 2 to 24 h. Rats 3 and 4 with behavioral seizures showed bilateral uptake of AMT-MNP particles. In rat 3, the normalized densitometry values, right and left respectively, of the areas of uptake were 0.86 and 0.77 before, and 0.68 and 0.69 after injection with particles. The corresponding values for rat 4 were 0.9 and 1.0 before, and 0.64 and 0.55 after injection with the particles.
Figure 3.
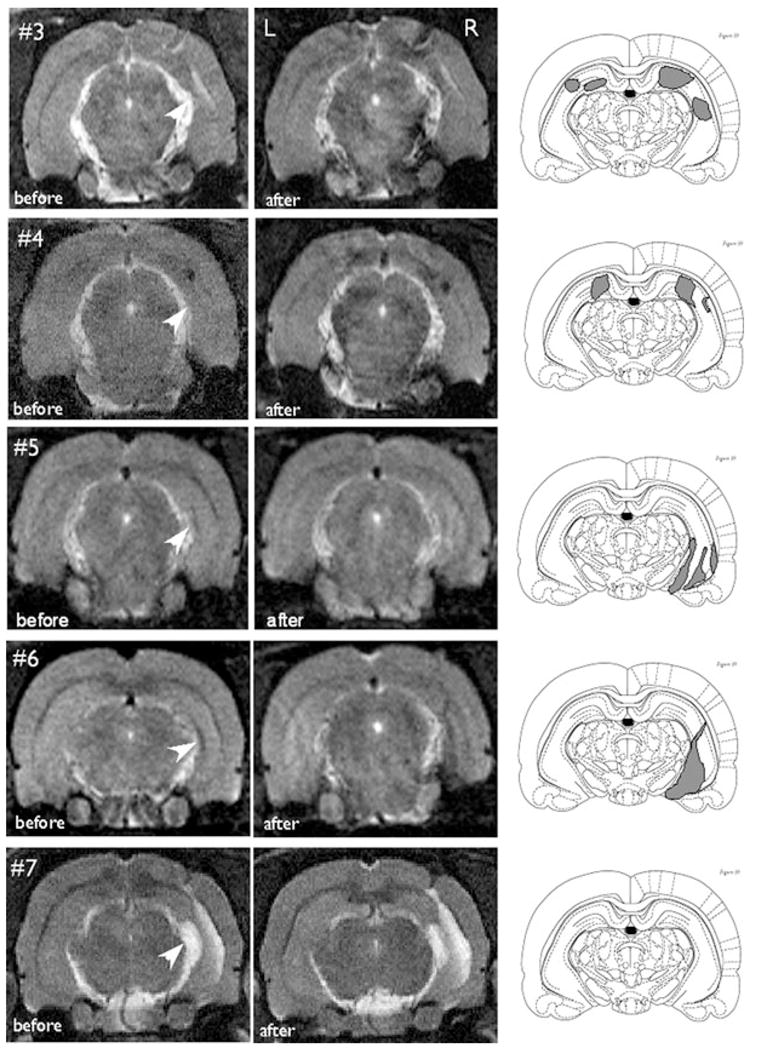
MR images of rats 3, 4, 5, 6 and 7 (top to bottom) are shown before (left column) and after (middle column) tail vein injection of AMT-MNP particles (15 mg/kg). Rats 3 and 4 showed bilateral uptake of particles; rats 5 and 6 showed unilateral uptake, and rat 7 did not show any particle uptake. Arrows in the left column show the areas of hippocampal atrophy due to KA injection. Rats 3–6 had spontaneous behavioral seizures; rat 7 did not show any behavioral seizure activity. The location of areas with AMT-MNP uptake is shown in the right column on sections from Paxinos (Paxinos, 1997). Images were taken 6 h after AMT-MNP injection for rats 3, 4, and 7, 2 h after injection for rat 5, and 4 h after injection for rat 6.
Epilepsia © ILAE
Rats 5 and 6 with behavioral seizures showed unilateral particle uptake in the right hippocampus, the site of KA injection. In rat 5, the normalized densitometry values of the area of uptake on the right were 0.95 before and 0.81 after injection. The corresponding values for Rat 6 were 0.97 and 0.64. These values for the contralateral left hippocampus were 0.86 and 0.88 for rat 5 and 1.1 and 1.1 for rat 6. Rat 7 did not have any particle uptake despite presence of hippocampal atrophy.
The grand mean values of signal intensity for all four groups were compared and showed a significant difference among the groups (F = 9.51, p = 0.0001, single factor ANOVA). A specific correction for multiple post hoc comparisons of all possible pairs was made using the conservative Scheffe’s procedure. For 4 means and N = 40, A = 0.713, the postinjection area of signal enhancement was significantly different from all other groups while there was no significant difference between the other groups at p = 0.01 level.
Correlation between uptake of AMT-MNP in chronic rats and subsequent intracranial EEG recordings
Interictal activity
Rats 3 and 4 with bilateral uptake of AMT-MNP showed IIS in both the right and left dentate gyri at similar rates (Figs. 4A and 4B). These IIS occurred simultaneously within a 20 ms window and also independently in each side. This is illustrated by the perievent time histogram in Fig. 4C. The mean ratio of IIS recorded in the dentate gyrus of the KA-injected side to the IIS recorded in the homotopic area of the contralateral side in the rats with bilateral AMT-MNP uptake was 1.2 ± 0.35.
Figure 4.
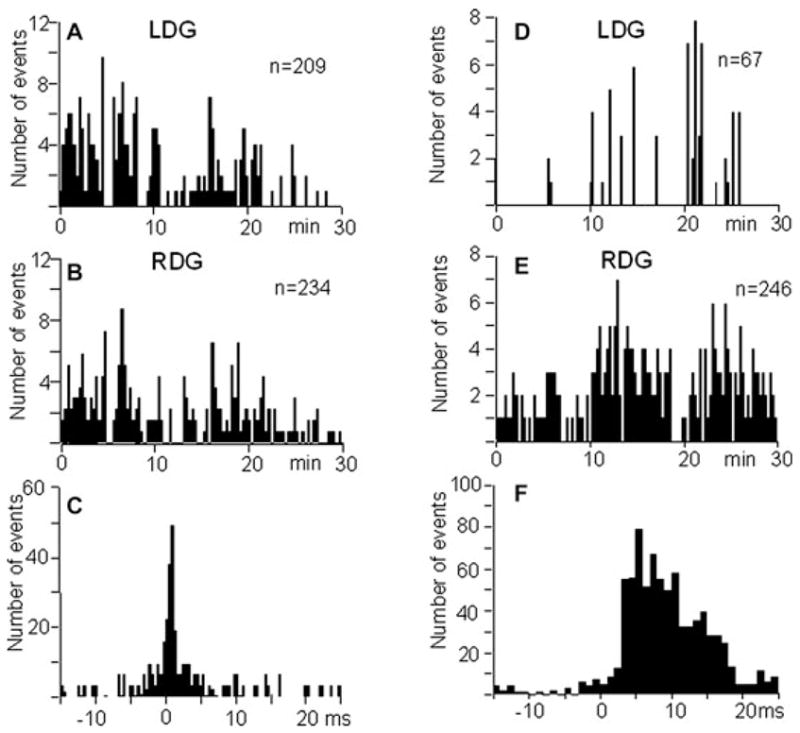
Left, rate of IIS in rat 4 with bilateral AMT-MNP uptake recorded during 30 min of immobility and slow wave sleep in the left (A) and right (B) dentate gyrus. (C) Perievent histogram of IIS recorded in the left dentate gyrus versus right dentate gyrus. Rate of IIS was the same on both sides. Right, rate histogram of IIS recorded in the left (D) and right (E) dentate gyrus during 30 min of immobility and slow wave sleep in rat 5 which showed uptake of AMT-MNP in the right hippocampus. (F) Perievent time histogram for IIS recorded in the left dentate gyrus versus right dentate gyrus. Most IIS occurred on the right, the side of AMT-MNP uptake.
Epilepsia © ILAE
In rats 5 and 6 with unilateral uptake of AMT-MNP, IIS also were observed in both left and right dentate gyri, however, the rate of IIS contralateral to the side of AMT-MNP uptake was much lower than ipsilaterally (Figs. 4D and 4E). The majority of IIS on the contralateral side occurred with delay after IIS generated on the side with AMT-MNP uptake (Fig. 4F). Many more IIS occurred on the side of AMT-MNP uptake; the mean ratio of IIS recorded in the dentate gyrus of the KA-injected side to the IIS recorded in the homotopic area of the contralateral side in these rats was 3.05 ± 2.6.
pHFOs with a dominant frequency of 100–200 Hz were observed in all rats with bilateral and unilateral AMT-MNP uptake. In rats 3 and 4 with bilateral uptake, pHFOs occurred independently in both left and right dentate gyri without side predominance. The mean rate of recurrence was 8.4 ± 5.7 per min. Fig. 5A shows a recoding from both right and left dentate gyri of rat 4 where bilateral pHFOs were associated with IIS and small amplitude waves. In rats 5 and 6 with unilateral AMT-MNP uptake, pHFOs occurred only on the side of uptake. The mean rate of recurrence was 4.1 ± 2.0 per min. pHFOs in the fast ripple (FR) frequency range (250–500 Hz) were seen only in rat 5 (Fig. 5B), and recurred at a rate of 28 ± 1.8 per min. Rat 7 with no AMT-MNP uptake had normal EEG activity with no IIS.
Figure 5.
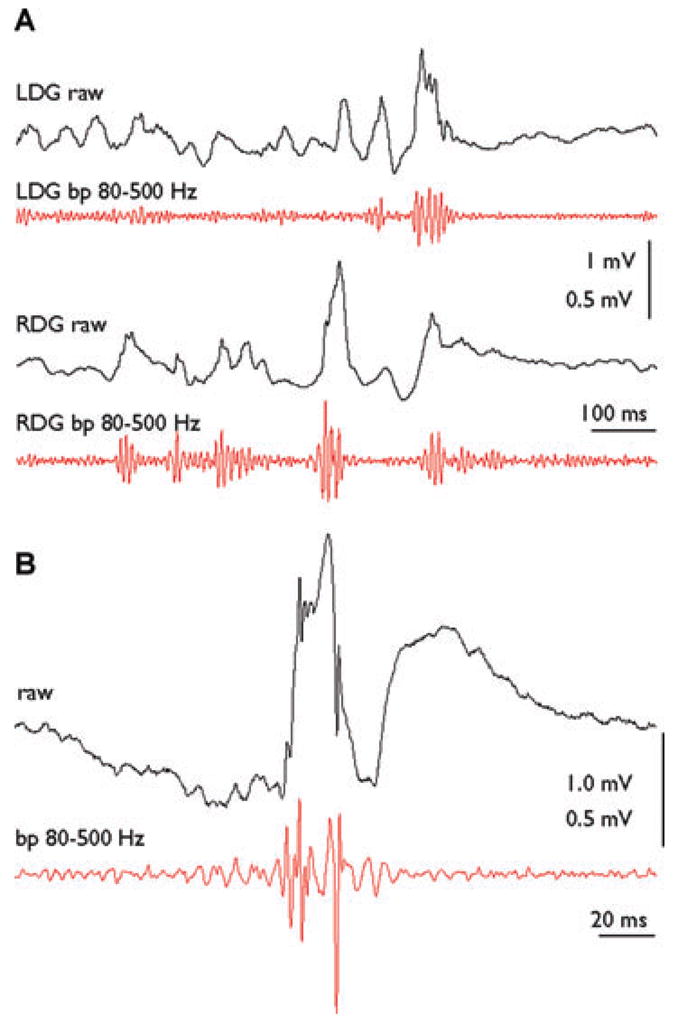
(A) An example of raw (black lines) and band pass filtered (red lines) pHFOs in the ripple frequency range recorded in the left (LDG) and right (RDG) dentate gyrus of rat 4 which showed bilateral AMT-MNP uptake. (B) Single EEG spike with superimposed pHFO (black line) and band pass filtered pHFO in the Fast Ripple frequency range (red line) in rat 5 on the side of AMT-MNP uptake.
Epilepsia © ILAE
Ictal activity
Spontaneous seizures were confirmed during electrophysiological recordings in all four rats that showed behavioral seizures during video monitoring. All seizures in these rats were low voltage fast onset (Bragin et al., 1999). In rats 3 and 4 with bilateral AMT-MNP uptake, seizures occurred simultaneously in the dentate gyri of both sides. In rats 5 and 6 with unilateral AMT-MNP uptake, seizures started unilaterally on the side of uptake and propagated to the contralateral dentate gyrus (Fig. 6). Rat 7 with no AMT-MNP uptake showed no ictal EEG activity.
Figure 6.
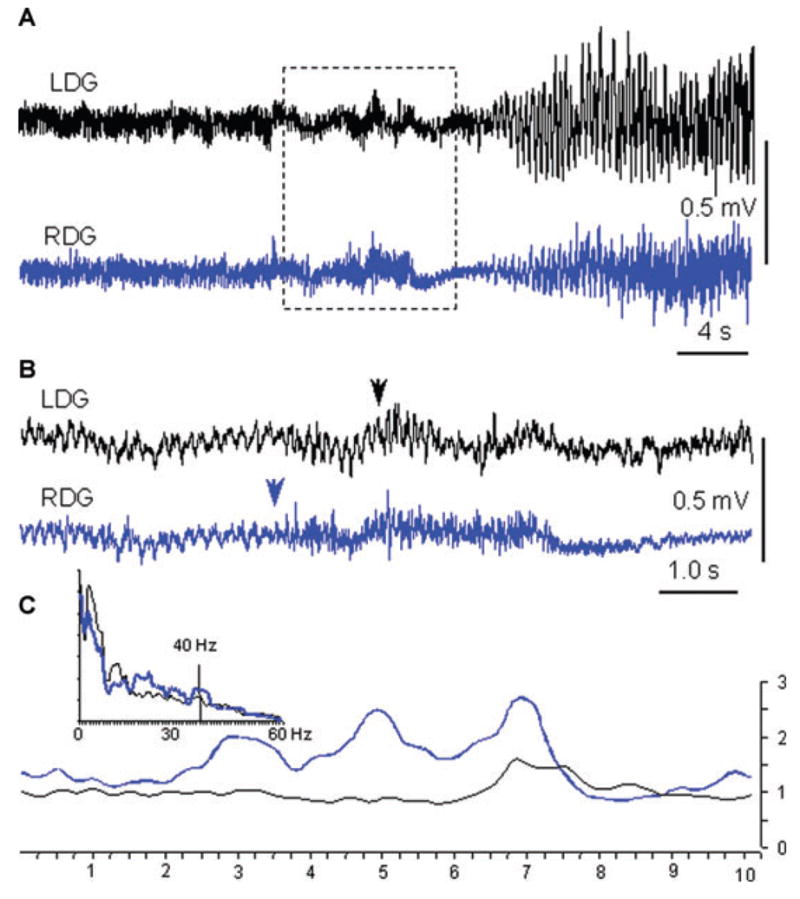
(A) An example of a seizure from rat 6, with onset in the right dentate gyrus (RDG) near the region of unilateral AMT-MNP uptake. (B) Expanded part of A outlined by the dashed box. There is prominent theta rhythm before the seizure onset, interrupted by the appearance of high frequency activity first in the RDG (arrow) and about 2 s later in the left dentate gyrus (LDG). Arrows indicated where seizure starts on the basis of visual estimation. (C) Inset represents a power spectrogram of the 10 s period presented in B., where the blue line is the RDG and black line is the LDG recording. Lines below illustrate the temporal relation of the increase in power of the 35–45 Hz frequency band at the seizure onset. The increase in power begins in the RDG ~4 s earlier than in the LDG.
Epilepsia © ILAE
Histology
All rats studied, two with bilateral AMT-MNP uptake, and one without, showed gliosis (GFAP stain) in the area of KA injection, and in the contralateral hippocampus. Iron-laden macrophages on Perl’s stain indicated bleeding along the needle tracks (kainic acid) and electrode tracks (EEG) but not in other areas of negative enhancement seen on MRI (Fig. 7).
Figure 7.
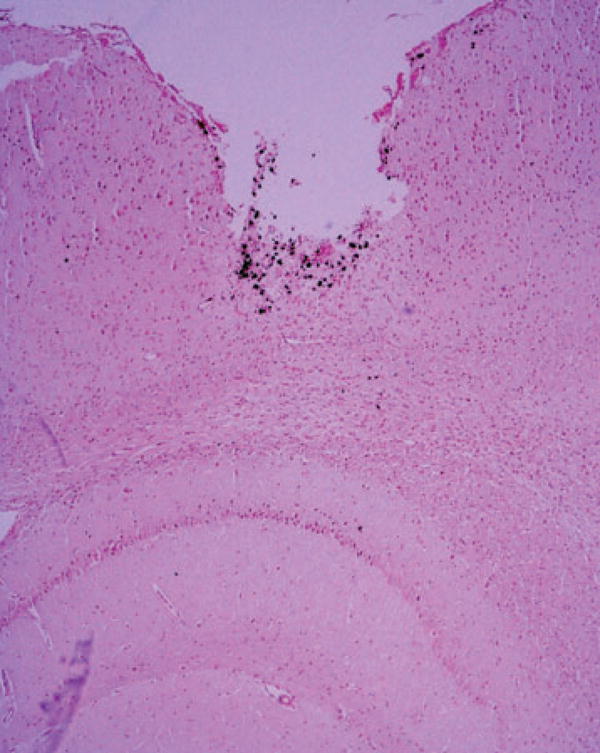
Perl’s stain of a coronal section from rat 4, corresponding to the upper right quadrant of the MRI seen in Fig. 5. The defect at the cortical surface, caused by the cannula used for the intrahippocampal kainate injection, is barely visible on the MRI scan, but bleeding in that region is identified by the Perl’s stain. The hippocampus immediately below this area shows negative enhancement on the post-AMT-MNP MRI scan, but no evidence of bleeding with Perl’s stain.
Epilepsia © ILAE
Discussion
The results of this study provide important proof of principle for several aspects of the hypothesis that conjugated MNP, which are not radioactive, could be used to delineate specific cerebral functions on MRI. Most importantly, the MRI patterns observed before and after conjugated MNP injection provide evidence that these magnetized particles cross the BBB in this epilepsy model (Oby & Janigro, 2006), to be taken up by brain parenchyma, and that the negative enhancement observed in epileptogenic tissues after MNP injection was not due to blood products. Whether MNP uptake across the blood–brain barrier also occurs in normal nonepileptogenic rat brain remains to be demonstrated.
The fact that AMT-MNP concentration does not merely indicate nonspecific inflammatory changes associated with KA injection (Vezzani et al., 2008) is suggested by the difference between the MRI pattern seen with AMT-MNP and that with plain MNP during the acute phase. The latter, which are known to be taken up in areas of inflammation (Dousset et al., 1999a, 1999b), concentrated only unilaterally on the side of the kainate injection, while the former were concentrated bilaterally. Epileptic status in unilateral intrahippocampal kainate-injected animals, however, involves hippocampal and parahippocampal structures bilaterally, and the subsequent epileptogenic process can be bilateral or unilateral (Bragin et al., 1999). Therefore, this suggestion that plain MNPs only indicate inflammation, and not epileptogenicity, needs to be substantiated by further studies; given that only one rat was investigated with each nanoparticle, it is possible that the rat injected with plain MNP had a strictly unilateral epileptogenic process and that these particles could also identify areas of epileptic activity.
The potential for AMT-MNP to be a specific surrogate marker of brain tissue involved in spontaneous seizures was supported by the chronic studies, which demonstrated concentration of this tracer in the four rats that exhibited spontaneous seizures, but not in the rat with no spontaneous seizures. The epileptogenicity of brain tissue demonstrating AMT-MNP uptake was confirmed by the electrophysiological studies showing that these areas were capable of generating both interictal and ictal discharges, while no such epileptic abnormalities were seen in the rat that did not concentrate AMT-MNP or demonstrate spontaneous behavioral seizures.
The correlation between bilateral versus unilateral AMT-MNP uptake and bilateral versus unilateral electrophysiological evidence of epileptogenicity is suggestive that this tracer could be a reliable surrogate marker to localize the epileptogenic region; however, the results are by no means definitive. The number of rats is small and the pattern of negative enhancement on MRI could not be quantitatively measured by densitometry. Many more animals will be needed to confirm these visual MRI analyses with quantitative data that can be analyzed statistically. The electrophysiological evidence of lateralization is more convincing, but also subject to limitations.
The two rats with bilateral AMT-MNP concentration had bilateral independent IIS and pHFOs, indicating bilateral epileptogenicity. The seizures appeared to begin bilaterally as well; however, this diffuse onset pattern usually indicates that the site of ictal generation is actually some distance from the electrode contacts. It is not possible, therefore, to determine whether seizures began independently from both sides, or consistently from one side, with rapid propagation. In the two rats with unilateral AMT-MNP concentration, IIS predominated on that side, and pHFOs, including FR in one rat, were limited to that side. In these rats, seizures consistently began on the side of apparent AMT-MNP concentration, with subsequent propagation to the contralateral side.
These electrophysiological studies remain restricted, because it was not possible to sample all areas with and without AMT-MNP uptake. Consequently, a clear delineation of the spatial relationship between areas of uptake and epileptogenesis cannot be demonstrated. Further studies with a much larger sample size are necessary to define how well AMT-MNP might not only localize, but also define the extent of, the epileptogenic region.
It is curious that none of the rats in this study demonstrated the unilateral hypersynchronous ictal onsets commonly encountered in our previous studies of intrahippocampal kainate-induced epilepsy (Bragin, et al., 1999). Lewis rats were used for these experiments because they had been used for previous MNP studies, whereas all of our previous electrophysiological investigations of intrahippocampal kainate-induced epilepsy were carried out with Wistar rats. It is possible that a strain difference could have accounted for our inability to record clearly focal hypersynchronous ictal onsets. The absence of fast ripples in three of the four epileptic rats, on the other hand, is not surprising in view of the fact that only fixed electrodes were used. Because FR are generated from small, discrete clusters of neurons, they are difficult to find without moveable electrodes (Bragin, et al., 2002). The ripple frequency pHFOs recorded here, however, have the same significance as fast ripples, because ripple frequency oscillations do not occur in normal dentate gyrus (Bragin et al., 2004).
This proof-of-principle study involves only a few animals, and much more research is necessary to demonstrate that AMT-MNP, or MNPs conjugated with other ligands, could eventually be used to image localized cerebral function with MRI in humans. The pharmacokinetics of these particles, their metabolism and elimination, will need to be defined. In particular, the fate of maghemite in the brain needs to be determined, particularly if this technique is to be used in people with epilepsy, given that iron is known to be epileptogenic (Wilmore et al., 1978). Although the particles could still be visualized on MRI 24 h after injection, they were not seen with Perl’s iron stain on tissue samples obtained weeks after the injection. This could mean that the particles were completely eliminated by this time, but they could be too small to be detected with routine histological methods. Electron microscopic studies are in progress to answer this question.
Reliable surrogate markers of epileptogenesis (the development of an epileptic abnormality) and epileptogenicity (the presence and severity of an epileptogenic abnormality) would greatly enhance our ability to diagnose, treat, and prevent epilepsy, and this is an area of active research. AMT is only one of a number of putative tracers that could eventually be useful in this regard. Although AMT PET has demonstrated that this compound is concentrated in epileptogenic tissue in a few forms of human epilepsy (Fedi et al., 2001; Duchowny, 2003; Juhasz et al., 2003; Natsume et al., 2003; Juhasz, 2004; Kagawa et al., 2005), its value as a reliable surrogate marker remains controversial, and very few clinical centers have access to this positron tracer. Our results are preliminary; however, if they lead to the ability to perform similar investigations with MRI, it could greatly facilitate this research in centers that may not have PET or the AMT tracer, and greatly reduce the cost of the extensive clinical investigations that would be necessary to fully examine the potential value of AMT or any of the other putative tracers, as reliable surrogate markers for epilepsy. More importantly, the proof of principle with respect to the ability of conjugated MNPs to cross the BBB, and their potential for identifying localized cerebral biochemical processes in a manner similar to that of PET, and to a certain extent SPECT, means that this functional MRI approach could be available not only to study other potential surrogate markers for epilepsy, but theoretically, any bioactive molecules that might be tracers for imaging normal and abnormal localized cerebral functions with high structural resolution MRI.
Acknowledgments
The authors are greatly indebted to Drs. Gevorg Karapetian and Ira Harutyunyan for their invaluable help and input with acquisition of the MR images. The authors are also indebted to Dr. Howard Soule of the Milken Family Foundation and Dr. Leonard Rome of the UCLA School of Medicine Dean’s Office for their foresight and support of this project. This work was supported by generous grants from the Stein/Oppenheimer Award, Epilepsy foundation of America, NIH P01 NS02808-42, and RO1 NS33310. Drs. Akhtari and Engel are the coinventors of the technology described in this manuscript and are two of the cofounders of Epinano Inc., which has licensed this technology from the University of California, Los Angeles.
Footnotes
Conflict of interest: We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- Akhtari M, Engel J. EU06739214.2 Patents PCT/US06/10334. 2006
- Bragin A, Engel J, Jr, Wilson CL, Vizentin E, Mathern GW. Electrophysiologic analysis of a chronic seizure model after unilateral hippocampal KA injection. Epilepsia. 1999;40(9):1210–1221. doi: 10.1111/j.1528-1157.1999.tb00849.x. [DOI] [PubMed] [Google Scholar]
- Bragin A, Mody I, Wilson CL, Engel J., Jr Local generation of fast ripples in epileptic brain. J Neuroscience. 2002;22:2012–2021. doi: 10.1523/JNEUROSCI.22-05-02012.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragin A, Wilson CL, Almajano J, Mody I, Engel J., Jr High-frequency oscillations after status epilepticus: epileptogenesis and seizure genesis. Epilepsia. 2004;45:1017–1023. doi: 10.1111/j.0013-9580.2004.17004.x. [DOI] [PubMed] [Google Scholar]
- Dousset V, Gomez C, Petry KG, Delalande C, Caille J-M. Dose and scanning delay using USPIO for central nervous system macrophage imaging. MAGMA. 1999a;8:185–189. doi: 10.1007/BF02594597. [DOI] [PubMed] [Google Scholar]
- Dousset V, Ballarino L, Delalande C, Coussemacq M, Canioni P, Petry K, CailIe JM. Comparison of ultrasmail particles of iron oxide (USPIO)-enhanced T2-weighted, conventional T2-weighted, and gadolinium-enhanced T1-weighted MR images in rats with experimental autoimmune encephalomyelitis. AJNR Am J Neuronadiol. 1999b;20:223–227. [PMC free article] [PubMed] [Google Scholar]
- Duchowny MS. A potential role for alpha-methyl-l-tryptophan PET in seizure localization in patients with intractable epilepsy. Epilepsy Curr. 2003;3(5):184–186. doi: 10.1046/j.1535-7597.2003.03512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedi M, Reutens D, Okazawa H, Andermann F, Boling W, Dubeau F, White C, Nakai A, Gross DW, Andermann E, Diksic M. Localizing value of alpha-methyl-L-tryptophan PET in intractable epilepsy of neocortical origin. Neurology. 2001;13;57(9):1629–1636. doi: 10.1212/wnl.57.9.1629. [DOI] [PubMed] [Google Scholar]
- Jacobs MP, Fischbach GD, Davis MR, Dichter MA, Dingledine P, Lowenstein DH, Morrell MJ, Noebels JL, Rogawski MA, Spencer SS, Theodore WH. Future directions for epilepsy research. Neurology. 2001;13;57(9):1536–1542. doi: 10.1212/wnl.57.9.1536. [DOI] [PubMed] [Google Scholar]
- Juhasz C, Chugani HT. Imaging the epileptic brain with positron emission tomography. Neuroimaging Clin N Am. 2003;13(4):705–716. viii. doi: 10.1016/s1052-5149(03)00090-x. [DOI] [PubMed] [Google Scholar]
- Juhasz C, Chugani DC, Muzik O, Shah A, Asano E, Mangner TJ, Chakraborty PK, Sood S, Chugani HT. Alpha-methyl-L-tryptophan PET detects epileptogenic cortex in children with intractable epilepsy. Neurology. 2003;25;60(6):960–968. doi: 10.1212/01.wnl.0000049468.05050.f2. [DOI] [PubMed] [Google Scholar]
- Juhasz C, Chugani DC, Padhye UN, Muzik O, Shah A, Asano E, Mangner TJ, Chakraborty PK, Sood S, Chugani HT. Evaluation with alpha-[IIC]methyl-L-tryptophan positron emission tomography for reoperation after failed epilepsy surgery. Epilepsia. 2004;45(2):124–130. doi: 10.1111/j.0013-9580.2004.30303.x. [DOI] [PubMed] [Google Scholar]
- Jupp B, Williams JP, Tesiram YA, Vosmansky M, O’Brien TJ. Hippocampal T2 signal change during amygdala kindling epileptogenesis. Epilepsia. 2006;47(1):41–46. doi: 10.1111/j.1528-1167.2006.00368.x. [DOI] [PubMed] [Google Scholar]
- Kagawa K, Chugani DC, Asano E, Juhasz C, Muzik O, Shah A, Shah J, Sood S, Kupsky WJ, Mangner TJ, Chakraborty PK, Chugani HT. Epilepsy surgery outcome in children with tuberous sclerosis complex evaluated with alpha-[11C]methyl-L-tryptophan positron emission tomography (PET) J Child Neurol. 2005;20(5):399. doi: 10.1177/08830738050200050701. [DOI] [PubMed] [Google Scholar]
- Molday HS. (1984) US Pat, 2, 452, 773.
- Natsume J, Kumakura Y, Bernasconi N, Soucy JP, Nakai A, Rosa P, Fedi M, Dubeau F, Andermann F, Lisbona R, Bernasconi A, Diksic M. Alpha-[11C] methyl-L-tryptophan and glucose metabolism in patients with temporal lobe epilepsy. Neurology. 2003;11;60(5):756–761. doi: 10.1212/01.wnl.0000052682.99812.f5. [DOI] [PubMed] [Google Scholar]
- Oby E, Janigro D. The Blood–Brain Barrier and Epilepsy. Epilepsia. 2006;47(11):1761–1774. doi: 10.1111/j.1528-1167.2006.00817.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; San Diego, New York: 1997. [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. I. Afterdischarge threshold. Electroencephalogr Clin Neurophysiol. 1972a;32:269–279. doi: 10.1016/0013-4694(72)90176-9. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation: motor seizure. Electroencephalogr Clin Neurophysiol. 1972b;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Peltola J, Janigro D. Inflammation. In: Engel J Jr, Pedley TA, editors. Epilepsy: A Comprehensive Textbook. 2. Lippincott-Raven; Philadelphia: 2008. pp. 267–276. [Google Scholar]
- Wilmore LS, Sypert GW, Munson JB. Recurrent seizures induced by cortical iron injection: a model of post-traumatic epilepsy. Ann Neurol. 1978;4:329–336. doi: 10.1002/ana.410040408. [DOI] [PubMed] [Google Scholar]