Abstract
Background
Fragile X–associated tremor/ataxia syndrome (FXTAS) is a recently described, underrecognized neurodegenerative disorder of aging fragile X mental retardation 1 (FMR1) premutation carriers, particularly men. Core motor features are action tremor, gait ataxia, and parkinsonism. Carriers have expanded CGG repeats (55 to 200); larger expansions cause fragile X syndrome, the most common heritable cause of mental retardation and autism. This study determines whether CGG repeat length correlates with severity and type of motor dysfunction in premutation carriers.
Methods
Persons aged ≥50 years with a family history of fragile X syndrome underwent structured videotaping. Movement disorder neurologists, blinded to carrier status, scored the tapes using modified standardized rating scales. CGG repeat length analyses for women incorporated the activation ratio, which measures the percentage of normal active chromosome X alleles.
Results
Male carriers (n = 54) had significantly worse total motor scores, especially in tremor and ataxia, than age-matched male noncarriers (n = 51). There was a trend toward a difference between women carriers (n = 82) and noncarriers (n = 39). In men, increasing CGG repeat correlated with greater impairment in all motor signs. In women, when activation ratio was considered, increasing CGG correlated with greater ataxia.
Conclusions
CGG repeat size is significantly associated with overall motor impairment in premutation carriers. Whereas this association is most pronounced for men and covers overall motor impairment—tremor, ataxia, and parkinsonism—the association exists for ataxia among women carriers. This is the first report of a significant correlation between the premutation status and a motor feature of fragile X–associated tremor/ataxia syndrome in women.
Fragile X–associated tremor/ataxia syndrome (FXTAS)1 occurs in premutation carriers of the fragile X mental retardation 1 (FMR1) gene, predominantly in men over age 50 years. Premutations are expanded CGG repeats (55 to 200) and are also associated with premature ovarian failure.2 Larger expansions (>200) result in transcriptional inactivation and fragile X syndrome,3 the most common heritable form of mental retardation and autism.
Common manifestations of FXTAS are progressive intention tremor, cerebellar gait ataxia, parkinsonism, working memory impairment, and frontal executive dysfunction.1,4–11 Other signs include hyperintensity of the middle cerebellar peduncles on MRI,11 peripheral neuropathy,6,12 and autonomic dysfunction.6,13 The mediantimetodeathis 21years, butlifeexpectancy is variable.14 Some female carriers have classic FXTAS,15–17 but most are less affected,7,8 likely secondary to a diluting effect from the second normal X chromosome.18 Neuropathology shows generalized brain atrophy and intranuclear inclusions,19,20 perhaps related to increased levels of abnormal FMR1 messenger RNA (mRNA).21,22
The focus of this article is to study variables associated with the major motor signs in FXTAS. Previous studies found that increasing age and male sex strongly correlated with increased risk of FXTAS,7,8 and that increasing CGG repeat length correlated with earlier age at onset.23 A recent study suggested carriers of low premutation range (<70) repeats have atypical or milder FXTAS signs.24 Now we report a prospective, quantitative, cross-sectional study of the association between specified variables (CGG repeat length, mRNA level, age, and sex) and the severity of major motor signs of FXTAS in a large cohort of premutation carriers.
METHODS
Patient recruitment and selection
The only inclusion criteria were family history of fragile X syndrome and age ≥ 50 years. Eligible family members were encouraged to participate regardless of the presence of neurologic symptoms and carrier status; those with normal FMR1 repeat sizes served as controls. Families were recruited from local and national fragile X syndrome support groups in the United States or from fragile X clinics associated with one of three centers—the University of California at Davis, the University of Colorado at Denver and Health Sciences Center, and the Rush University Medical Center in Chicago—from 2002 to 2005. All eligible subjects who agreed to participate were included (n = 233). Carriers were not categorized into published diagnostic categories of possible, probable, and definite FXTAS, because these criteria are based on MRI findings, which many of our subjects did not undergo. All study procedures were conducted according to approved institutional review board protocols at each participating institution.
Structured videotape and FXTAS Rating Scale administration
Subjects underwent a structured videotaped examination, designed to capture the major motor features of FXTAS: tremor, cerebellar dysfunction, and parkinsonism. Participating movement disorders specialists trained study personnel to produce acceptable standardized videos at each site. These neurologists, blinded to the subjects’ premutation status, scored the videotapes using the FXTAS Rating Scale.25
The FXTAS Rating Scale was developed for this study. Initially the scale was composed of all items from the Clinical Rating Scale for Tremor,26 the International Cooperative Ataxia Rating Scale,27 and the Unified Parkinson’s Disease Rating Scale (part III).28 In addition, the tandem test,29 a sensitive and important (but not specific) test for cerebellar gait dysfunction,30 was added. This large composite scale was reduced to the final FXTAS Rating Scale by elimination of items that were repetitive and items that had unacceptable interrater reliability. Clinimetric testing of the final scale among the three movement disorders neurologists showed all items had a weighted kappa statistic of good to excellent (≥0.4).31 Three subdomain scores to measure each motor feature of interest—tremor, ataxia, and parkinsonism—were designated, and their sum represented the total FXTAS score.
Molecular analyses
Genomic DNA was isolated from peripheral blood leukocytes using standard, previously published methods.7 FMR1 DNA analysis was performed using both PCR and Southern blot as described previously,7,22 which provides determination of allele size, methylation status, and, thus, estimation of the X-activation ratio in women. The activation ratio, which measures the percentage of cells that carry the normal allele on the active chromosome X, was the ratio of the intensity of the normal FMR1 unmethylated band over the sum of the intensities of the normal unmethylated and methylated bands, as previously described.7 Quantifications of FMR1 mRNA were performed as described previously.21
Statistical analysis
The two-sample t test was used to compare the total and subdomain FXTAS Rating Scale scores between the premutation and control groups. Data from the entire cohort were analyzed, and data from men and women were studied separately, because women carriers have a second normal FMR1 allele that is predicted to dilute the expression of neurologic signs. Associations between variables of CGG repeat length, age, and mRNA level (independent variables) and the rating scale scores (dependent variable) were analyzed by multiple regression. Regression analyses included all subjects, encompassing the entire CGG range, from normal through premutation. The CGG repeat length regression analysis was controlled for age. For women, the association between CGG repeat length and rating scale scores was reanalyzed with a term to control for activation ratio (AR), with (1 − R) × (CGG), AR, and age as the independent variables. Throughout the analysis, the alpha = 0.05 nominal level was used.
Seven subjects had alleles that fell within the “gray-zone” range of 41 to 54 repeats. Data from gray-zone carriers are included in regression analyses performed across the full range of allele sizes for carriers and controls. There is uncertainty regarding the boundaries of the gray zone, e.g., 41 to 54 vs 45 to 54, and whether alleles in this range produce neurologic signs. Therefore, we used the broader category for the regression analysis, and we excluded these subjects from the control group in group-wise comparisons.
Lexin Li, PhD, Department of Statistics, North Carolina State University, conducted the statistical analysis.
RESULTS
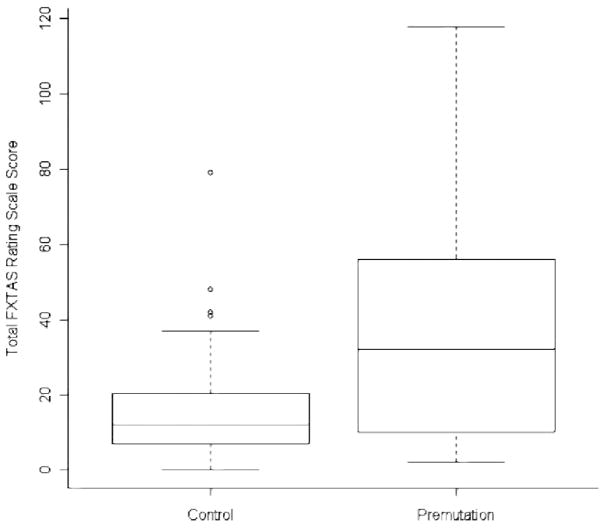
Demographic data for premutation carriers and controls are shown in table 1. The total and all subdomain FXTAS Rating Scale scores were significantly worse in the entire group of carriers compared with controls (table 2). This also held true when men were analyzed separately (figure). Whereas all three subdomain scores showed impairment, differences in parkinsonism were less marked than the other two subdomains. In contrast to the findings in men, there was only a trend toward a difference in motor scores between women carriers and controls.
Table 1.
Baseline characteristics of the study participants*
| Premutation carriers | Controls | |
|---|---|---|
| Men, n | 54 | 51 |
| Women, n | 82 | 39 |
| Men, age, y (SD)† | 66.9 (8.3) | 64.6 (9.7) |
| Women, age, y (SD) † | 63.0 (10.0) | 64.9 (9.2) |
| Men, CGG repeat size (SD) | 89.8 (19.1) | 27.6 (5.0) |
| Women, CGG repeat size (SD) | 82.4 (16.4) | 29.9 (3.5) |
These data do not include the seven subjects with repeat sizes in the gray zone.
There was no difference in age between the male carrier and control groups (p = 0.20) or the female carrier and control groups (p = 0.31).
Table 2.
Group comparisons of Fragile X-Associated Tremor/Ataxia Syndrome Rating Scale scores*
| Premutation carriers, mean (SD) | Controls, mean (SD) | p Value | |
|---|---|---|---|
| All subjects | |||
| Total score | 23.5 (22.9) | 14.6 (13.4) | < 0.001 |
| Tremor score | 8.3 (8.8) | 4.9 (4.7) | < 0.001 |
| Ataxia score | 7.8 (8.4) | 4.6 (4.6) | < 0.001 |
| Parkinsonism score | 6.8 (8.6) | 4.7 (5.8) | 0.016 |
| Men | |||
| Total score | 36.2 (28.2) | 16.1 (14.7) | < 0.001 |
| Tremor score | 13.2 (10.9) | 5.4 (5.3) | < 0.001 |
| Ataxia score | 12.4 (10.4) | 4.9 (4.8) | < 0.001 |
| Parkinsonism score | 10.3 (10.9) | 5.3 (6.4) | 0.003 |
| Women | |||
| Total score | 15.1 (13.3) | 12.6 (11.2) | 0.14 |
| Tremor score | 5.0 (4.8) | 4.3 (3.7) | 0.19 |
| Ataxia score | 4.8 (4.9) | 4.2 (4.5) | 0.24 |
| Parkinsonism score | 4.5 (5.5) | 4.0 (4.9) | 0.29 |
These comparisons were not corrected for age because there was no significant difference in ages of carrier and control groups for either sex.
Figure. Box plot showing the distribution of total Fragile X–Associated Tremor/Ataxia Syndrome Rating Scale scores (score range 0 to 226) in male controls vs carriers.
The horizontal line in the middle of each box indicates the median, whereas the top and bottom borders of the box mark the 75th and 25th percentiles. The vertical lines above and below the box extend to the 90th and 10th percentiles. The single points are outliers beyond the 90th and 10th percentiles.
CGG repeat length, with control for age, was positively and highly correlated with impairment on motor scores in the entire cohort, ranging from noncarriers through premutation subjects (table 3). This correlation was primarily driven by the strong correlation between increasing CGG and motor impairment in men, and the correlation was more robust for tremor and ataxia than for parkinsonism. Women carriers showed only a trend toward worsening of motor scores with increasing CGG repeat length. However, when the activation ratio (percent of normal alleles that are active) was included in the analyses, ataxia strongly correlated with increasing CGG repeat length (p = 0.03).
Table 3.
P values for regression analyses of motor scores with CGG repeat length and age
| CGG repeat length* |
Age |
|||
|---|---|---|---|---|
| Regression coefficient | p Value | Regression coefficient | p Value | |
| All subjects | ||||
| Total score | 0.180 | < 0.001 | 0.927 | <0.001 |
| Tremor score | 0.068 | < 0.001 | 0.263 | < 0.001 |
| Ataxia score | 0.072 | < 0.001 | 0.306 | < 0.001 |
| Parkinsonism score | 0.039 | 0.006 | 0.360 | <0.001 |
|
Men | ||||
| Total score | 0.270 | < 0.001 | 1.302 | < 0.001 |
| Tremor score | 0.110 | < 0.001 | 0.390 | < 0.001 |
| Ataxia score | 0.109 | < 0.001 | 0.404 | < 0.001 |
| Parkinsonism score | 0.055 | 0.016 | 0.490 | < 0.001 |
|
Women | ||||
| Total score | 0.064 | 0.10 | 0.466 | < 0.001 |
| Tremor score | 0.016 | 0.28 | 0.091 | 0.03 |
| Ataxia score | 0.025 | 0.10 | 0.165 | < 0.001 |
| Parkinsonism score | 0.017 | 0.29 | 0.221 | < 0.001 |
|
Women, with incorporation of the activation ratio | ||||
| Total score | 0.43 | 0.15 | 0.43 | 0.03 |
| Tremor score | 0.08 | 0.50 | 0.06 | 0.47 |
| Ataxia score | 0.25 | 0.03 | 0.15 | 0.05 |
| Parkinsonism score | 0.11 | 0.34 | 0.26 | 0.001 |
Regressions of scores against CGG repeat length were controlled for age.
Increasing age also correlated strongly with worsening of all motor scores in the entire cohort (table 3), and analyses of each sex separately showed that the correlation was highly significant in both, more so in men than in women. In the latter, the degree of correlation was parkinsonism > ataxia > tremor. Last, there was no relationship between mRNA level and rating scale scores.
DISCUSSION
The current data confirm previous reports5,7,8 and find that male premutation carriers have markedly more tremor and ataxia, and moderately more parkinsonism, than age-matched, noncarrier controls. This study shows that in men, increasing CGG repeat length is a powerful risk factor for the motor signs common in FXTAS. In addition, increasing age correlates with the severity of motor features of FXTAS.
Women carriers, conversely, have relatively few motor signs compared with male carriers. However, when the percent of active mutant alleles is factored into the analysis, increasing CGG repeat length is a significant risk factor for ataxia in women. This intriguing observation suggests that a threshold number of neural cells with elevated mRNA levels—those in which the abnormal allele is active—may be sufficient to trigger the neurologic dysfunction underlying FXTAS. Previous work15–18 had shown clear cases of FXTAS in women, but a controlled study7 found that symptoms were generally milder in women carriers compared with men. Besides the activation ratio, there may be other sex-related effects on phenotype, including hormonal status. Although women have milder FXTAS motor signs than men, they are more common than in noncarriers, and their children are at high risk for fragile X syndrome. Because the classic syndrome was first described in men, the focus has been on tremor, ataxia, and parkinsonism. Further study of the clinical expression of the premutation in women may reveal other features of diagnostic importance specific to this sex.
Leukocyte FMR1 mRNA levels did not correlate with motor symptoms in this study. At first glance, the finding seems surprising because FMR1 mRNA has been identified within the brain intranuclear inclusions,32 high levels of abnormal mRNA are postulated to play an important role in the pathophysiology of FXTAS,1,7 and one study found a correlation between leukocyte mRNA levels and psychological features in premutation carriers.33 However, other studies have not reported similar associations between phenotype and leukocyte mRNA level.10,34–36 These observations likely reflect the fact that although regional brain levels of mRNA are elevated in FXTAS,22 blood levels do not closely correlate with brain levels. More specifically, because brain levels of FMR1 mRNA vary widely across different brain regions,22 leukocyte values may not accurately reflect expression levels in the relevant brain regions. However, the significant associations between CGG repeat length and the severity of movement disorder likely reflect the overall trend in brain mRNA levels.
Correct diagnosis of FXTAS is vital because ensuing generations are at high risk for fragile X syndrome. However, the first clinical description of FXTAS was published only 6 years ago,1 and physicians are just gradually learning about the syndrome. A recent study37 showed that only 4% of persons diagnosed with FXTAS had been previously seen by a movement disorders neurologist; the majority had been followed by general neurologists or primary care physicians. Increasing knowledge of the disorder by the latter physicians would prompt them to ask appropriate patients (table 4)20 about a family history of fragile X syndrome. Although it is important to obtain a family history, doing so may not be helpful in making the diagnosis, because the affected man may not have daughters or the daughters may not have children with a correct diagnosis of fragile X syndrome. Another obstacle to diagnosis of FXTAS is that the presentation may be nonspecific, e.g., progressive balance difficulty, cognitive dysfunction, and mild tremor in an aging male, and these findings are often ascribed to multiple strokes or simply aging. Our experience and studies suggest that typical FXTAS patients will not seek medical care; instead, they are brought into the clinic by a spouse frustrated by the patient’s change in behavior (e.g., agitation, poor insight) or cognitive decline. On examination, patients generally at least have impaired tandem gait. Many affected persons deny tremor and have minor tremor on examination.
Table 4.
Phenotypic groups recommended for FXTAS testing*
| 1. Unexplained cerebellar gait ataxia, onset ≥ 50 years |
| 2. Unexplained action tremor in person with parkinsonism or dementia, onset ≥ 50 years |
| 3. MCP sign on MRI, family history of FMR1 mutation, or premature menopause in self or family if have signs consistent with FXTAS† onset ≥ 50 years |
| 4. Probable multiple system atrophy, cerebellar subtype |
Fragile X–associated tremor/ataxia syndrome (FXTAS) is less common in women.
Signs consistent with FXTAS include cerebellar gait ataxia, action tremor, parkinsonism, cognitive decline, neuropathy, and autonomic dysfunction.
MCP =middle cerebellar peduncle.
This study used a newly developed instrument, the FXTAS Rating Scale,25 to effectively discriminate between the type and severity of major motor signs in carriers vs controls. One advantage of the scale is its reliance on items of interest selected directly from scales that are already familiar to movement disorder neurologists. The scale is also easily applied with standard neurologic tests (quiet observation, eye movements, simple motor tasks, and walking) that are feasible to rate from a videotape or in person. In its current form, however, the scale has 44 items. Further clinimetric study may allow elimination of duplicative items. Our long-term goal is to provide a comprehensive, but practical, tool for conduct of therapeutic and longitudinal trials in FXTAS.
This report helps to further define the clinical features of FMR1 carriers, in particular, showing that motor signs are associated with increasing CGG repeat length in men and with increasing percentage of active mutant FMR1 alleles in women. Further research in defining the clinical manifestations of FMR1 carriers is needed, because FXTAS often goes unrecognized. The disorder is estimated to be similar in prevalence to atypical parkinsonian disorders such as progressive supranuclear palsy (6 per 100,000) and multiple system atrophy (2 to 5 per 100,000) and is a noteworthy cause of ataxia38 (1 to 2 per 6,000) in aging men.39
Acknowledgments
Supported by grants from the National Institute of Neurological Disorders and Stroke (NS43532, P.J.H.; and NS044299, J.G.), the National Institute of Child Health and Development (HD36071 and HD02274, R.J.H.), and the American Academy of Neurology (PN:0407-045, D.A.H.).
GLOSSARY
- AR
activation ratio
- FXTAS
fragile X-associated tremor/ataxia syndrome
- MCP
middle cerebellar peduncle
- mRNA
messenger RNA
Footnotes
The online version of this article, along with updated information and services, is located on the World Wide Web at: http://www.neurology.org/cgi/content/full/70/16_Part_2/1397
Disclosure: The authors report no conflicts of interest.
References
- 1.Hagerman RJ, Leehey M, Heinrichs W, et al. Intention tremor, parkinsonism, and generalized brain atrophy in male carriers of fragile X. Neurology. 2001;57:127–130. doi: 10.1212/wnl.57.1.127. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz CE, Dean J, Howard-Peebles PN, et al. Obstetrical and gynecological complications in fragile X carriers: a multicenter study. Am Med J Genet. 1994;51:400–402. doi: 10.1002/ajmg.1320510419. [DOI] [PubMed] [Google Scholar]
- 3.Verkerk AJ, Pieretti M, Sutcliffe JS, et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65:905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- 4.Leehey MA, Hagerman RJ, Landau WM, Grigsby J, Tassone F, Hagerman PJ. A tremor/ataxia syndrome in fragile X carrier males. Mov Disord. 2002;17:744–745. [Google Scholar]
- 5.Rogers C, Partington MW, Turner GM. Tremor, ataxia and dementia in older men may indicate a carrier of the fragile X syndrome. Clin Genet. 2003;64:54–56. doi: 10.1034/j.1399-0004.2003.00089.x. [DOI] [PubMed] [Google Scholar]
- 6.Jacquemont S, Hagerman RJ, Leehey M, et al. Fragile X premutation tremor/ataxia syndrome: molecular, clinical, and neuroimaging correlates. Am J Hum Genet. 2003;72:869–878. doi: 10.1086/374321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berry-Kravis E, Lewin F, Wuu J, et al. Tremor and ataxia in fragile X premutation carriers: blinded videotape study. Ann Neurol. 2003;53:616–623. doi: 10.1002/ana.10522. [DOI] [PubMed] [Google Scholar]
- 8.Jacquemont S, Hagerman RJ, Leehey MA, et al. Penetrance of the fragile X-associated tremor/ataxia syndrome in a premutation carrier population. JAMA. 2004;291:460–469. doi: 10.1001/jama.291.4.460. [DOI] [PubMed] [Google Scholar]
- 9.Grigsby J, Brega AG, Jacquemont S, et al. Impairment in the cognitive functioning of men with fragile X-associated tremor/ataxia syndrome (FXTAS) J Neurol Sci. 2006;248:227–233. doi: 10.1016/j.jns.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 10.Cohen S, Masyn K, Adams J, et al. Molecular and imaging correlates of the fragile X-associated tremor/ataxia syndrome. Neurology. 2006;67:1426–1431. doi: 10.1212/01.wnl.0000239837.57475.3a. [DOI] [PubMed] [Google Scholar]
- 11.Brunberg JA, Jacquemont S, Hagerman RJ, et al. Fragile X premutation carriers: characteristic MR imaging findings of adult male patients with progressive cerebellar and cognitive dysfunction. Am J Neuroradiol. 2002;23:1757–1766. [PMC free article] [PubMed] [Google Scholar]
- 12.Berry-Kravis E, Goetz CG, Leehey MA, et al. Neuropathic features in fragile X premutation carriers. Am J Med Genet A. 2007;143:19–26. doi: 10.1002/ajmg.a.31559. [DOI] [PubMed] [Google Scholar]
- 13.Pugliese P, Annesi G, Cutuli N, et al. The fragile X premutation presenting as postprandial hypotension. Neurology. 2004;63:2188–2189. doi: 10.1212/01.wnl.0000145709.61117.08. [DOI] [PubMed] [Google Scholar]
- 14.Leehey MA, Berry-Kravis E, Min SJ, et al. Progression of tremor and ataxia in male carriers of the FMR1 pre-mutation. Mov Disord. 2007;22:203–206. doi: 10.1002/mds.21252. [DOI] [PubMed] [Google Scholar]
- 15.Hagerman RJ, Leavitt BR, Farzin F, et al. Fragile-X-associated tremor/ataxia syndrome (FXTAS) in females with the FMR1 premutation. Am J Hum Genet. 2004;74:1051–1056. doi: 10.1086/420700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biancalana V, Toft M, Le Ber I. FMR1 premutations associated with fragile X-associated tremor/ataxia syndrome in multiple system atrophy. Arch Neurol. 2005;62:962–966. doi: 10.1001/archneur.62.6.962. [DOI] [PubMed] [Google Scholar]
- 17.Zuhlke C, Budnik A, Gehlken U, et al. FMR1 premutation as a rare cause of late onset ataxia: evidence for FXTAS in female carriers. J Neurol. 2004;251:1418–1419. doi: 10.1007/s00415-004-0558-1. [DOI] [PubMed] [Google Scholar]
- 18.Berry-Kravis E, Potanos K, Weinberg D, Zhou L, Goetz CG. Fragile X-associated tremor/ataxia syndrome in sisters related to X-inactivation. Ann Neurol. 2005;57:144–147. doi: 10.1002/ana.20360. [DOI] [PubMed] [Google Scholar]
- 19.Greco CM, Berman RF, Martin RM, et al. Neuropathology of fragile X-associated tremor/ataxia syndrome (FXTAS) Brain. 2006;129:243–255. doi: 10.1093/brain/awh683. [DOI] [PubMed] [Google Scholar]
- 20.Jacquemont S, Hagerman RJ, Hagerman PJ, Leehey MA. Fragile-X syndrome and fragile X-associated tremor/ataxia syndrome: two faces of FMR1. Lancet Neurol. 2007;6:45–55. doi: 10.1016/S1474-4422(06)70676-7. [DOI] [PubMed] [Google Scholar]
- 21.Tassone F, Hagerman RJ, Taylor AK, Gane LW, God-frey TE, Hagerman PJ. Elevated levels of FMR1 mRNA in carrier males: a new mechanism of involvement in the fragile-X syndrome. Am J Hum Genet. 2000;66:6–15. doi: 10.1086/302720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tassone F, Hagerman RJ, Garcia-Arocena D, Khandjian EW, Greco CM, Hagerman PJ. Intranuclear inclusions in neural cells with premutation alleles in fragile X associated tremor/ataxia syndrome. J Med Genet. 2004;41:e43. doi: 10.1136/jmg.2003.012518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tassone F, Adams J, Berry-Kravis EM, et al. CGG correlates with age of onset of motor signs of the fragile X-associated tremor/ataxia syndrome (FXTAS) Am J Med Genet B Neuropsychiatr Genet. 2007;144:566–569. doi: 10.1002/ajmg.b.30482. [DOI] [PubMed] [Google Scholar]
- 24.Jacquemont S, Leehey MA, Hagerman RJ, Beckett LA, Hagerman PJ. Size bias of fragile X premutation alleles in late-onset movement disorders. J Med Genet. 2006;43:804–809. doi: 10.1136/jmg.2006.042374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leehey M, Goetz C, Berry-Kravis E. Development of the FXTAS Rating Scale for quantitative motor analysis. Presented at the 10th International Congress of Parkinson’s Disease and Movement Disorders; October 30, 2006; Kyoto, Japan. [Google Scholar]
- 26.Fahn S, Tolosa E, Marin C. Clinical Rating Scale for tremor. In: Jankovic J, Tolosa E, editors. Parkinson’s disease and movement disorders. Baltimore, Munich: Urban & Schwartzenberg; 1987. pp. 225–234. [Google Scholar]
- 27.Trouillas P, Takayanagi T, Hallett M, et al. International Cooperative Ataxia Rating Scale for pharmacological assessment of the cerebellar syndrome. The Ataxia Neuropharmacology Committee of the World Federation of Neurology. J Neurol Sci. 1997;145:205–211. doi: 10.1016/s0022-510x(96)00231-6. [DOI] [PubMed] [Google Scholar]
- 28.Fahn S, Elton R . members of the UPDRS Development Committee. Unified Parkinson’s Disease Rating Scale. In: Fahn S, Marsden C, Calne D, Goldstein M, editors. Recent development in Parkinson’s disease. Florham Park, NJ: Macmillan Health Care Information; 1987. pp. 153–164. [Google Scholar]
- 29.Huntington Study Group. Unified Huntington’s disease rating scale: reliability and consistency. Mov Disord. 1996;11:136–142. doi: 10.1002/mds.870110204. [DOI] [PubMed] [Google Scholar]
- 30.Cho BL, Scarpace D, Alexander NB. Tests of stepping as indicators of mobility, balance, and fall risk in balance-impaired older adults. J Am Geriatr Soc. 2004;52:1168–1173. doi: 10.1111/j.1532-5415.2004.52317.x. [DOI] [PubMed] [Google Scholar]
- 31.Landis J, Loch G. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 32.Tassone F, Iwahashi C, Hagerman P. FMR1 RNA within the intranuclear inclusions of fragile X-associated tremor/ataxia syndrome (FXTAS) RNA Biol. 2004;1:103–105. doi: 10.4161/rna.1.2.1035. [DOI] [PubMed] [Google Scholar]
- 33.Hessl D, Tassone F, Loesch DZ, et al. Abnormal elevation of FMR1 mRNA is associated with psychological symptoms in individuals with the fragile X premuta-tion. Am J Med Genet B Neuropsychiatr Genet. 2005;139:115–121. doi: 10.1002/ajmg.b.30241. [DOI] [PubMed] [Google Scholar]
- 34.Moore CJ, Daly EM, Schmitz N, et al. A neuropsychological investigation of male premutation carriers of fragile X syndrome. Neuropsychologia. 2004;42:1934–1947. doi: 10.1016/j.neuropsychologia.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 35.Loesch DZ, Churchyard A, Brotchie P, Marot M, Tassone F. Evidence for, and a spectrum of, neurological involvement in carriers of the fragile X premutation: FXTAS and beyond. Clin Genet. 2005;67:412–417. doi: 10.1111/j.1399-0004.2005.00425.x. [DOI] [PubMed] [Google Scholar]
- 36.Loesch DZ, Litewka L, Brotchie P, Huggins RM, Tassone F, Cook M. Magnetic resonance imaging study in older fragile X premutation male carriers. Ann Neurol. 2005;58:326–330. doi: 10.1002/ana.20542. [DOI] [PubMed] [Google Scholar]
- 37.Hall DA, Berry-Kravis E, Jacquemont S, et al. Initial diagnoses given to persons with the fragile X associated tremor/ataxia syndrome (FXTAS) Neurology. 2005;65:299–301. doi: 10.1212/01.wnl.0000168900.86323.9c. [DOI] [PubMed] [Google Scholar]
- 38.Brussino A, Gellera C, Saluto A. MR1 gene premutation is a frequent genetic cause of late-onset sporadic cerebellar ataxia. Neurology. 2005;64:145–147. doi: 10.1212/01.WNL.0000148723.37489.3F. [DOI] [PubMed] [Google Scholar]
- 39.Leehey M, Hagerman P. Fragile X-associated tremor/ataxia syndrome (FXTAS) In: Subramoney S, Durr A, editors. Handbook of clinical neurology. Amsterdam, New York: Elsevier; (in press) [Google Scholar]