Abstract
Post-translational modification of the cell’s proteome by ubiquitin and ubiquitin-like proteins provides dynamic functional regulation. Ubiquitin and SUMO are well-studied post-translational modifiers that typically impart distinct effects on their targets. The recent discovery that modification by SUMO can target proteins for ubiquitination and proteasomal degradation sets a new paradigm in the field, and offers insights into the roles of SUMO and ubiquitin in genome stability.
Keywords: ubiquitin, SUMO, STUbL, proteasome, degradation, SUMO interaction motif, ubiquitin ligase, DNA repair, genome stability
1. Introduction
Historically, there have been reports of intimate interactions between the SUMO and ubiquitin pathways, including evidence that modification by SUMO could “prime” protein ubiquitination and proteasomal degradation (e.g. [1, 2]). However, ubiquitin E3 ligases able to mediate this phenomenon were unidentified and thus, SUMO-directed ubiquitination remained on the fringe of accepted SUMO functions. In this review we cover the recent identification of SUMO-targeted ubiquitin ligases (STUbLs) and highlight their impact on critical cell regulatory pathways. Despite their novelty, STUbLs are already implicated in fundamental and diverse processes such as chemotaxis and genome stability [3]. Notably, the human STUbL provides a missing piece to the long-standing puzzle of how the leukemia therapeutic agent called arsenic trioxide exerts its effects [4, 5]. Recent reviews have covered detailed functions of ubiquitin, SUMO and their various modes of interplay [3, 6-9]; so herein, we will mainly focus on the role(s) of STUbLs in the maintenance of genome stability.
2. Novel crosstalk between the ubiquitin and SUMO pathways
In a screen for genes required for cell viability in the absence of the budding yeast RecQ helicase Sgs1 (human BLM homolog), a number of so-called SLX genes were identified [10]. Two of these SLX genes encode RING domain-containing proteins, Slx5 and Slx8, which were shown to form a complex. Primary sequence homologues of Slx5 were only detectable in Saccharomycetes, whereas an Slx8 homolog was identified in fission yeast [10]. This indicated that Slx5-Slx8 complexes were likely to be functionally conserved, albeit with highly diverged Slx5 subunits. In a recent fission yeast study, a RING domain protein called Rfp1 was isolated as an interactor of the Smc5-6 DNA repair complex non-SMC subunit Nse5 [11]. Intriguingly, Slx5 also interacts with the budding yeast Nse5 homologue [12], suggesting that Rfp1 and Slx5 might be functionally related. Further analyses showed that Rfp1 forms a complex with fission yeast Slx8 and confirmed that Rfp1 and Slx5 are functionally homologous [11, 13]. Database searches using Rfp1 returned clear homologues in higher eukaryotes including human RNF4, indicating that the Slx5-Slx8 complex is functionally conserved in eukaryotes (see Fig. 1; [11, 13, 14]).
Figure 1. Schematic representation of STUbL protein architecture.
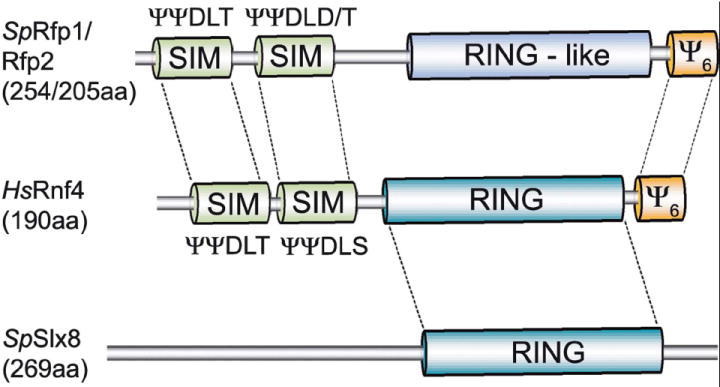
The heterodimeric yeast STUbLs are exemplified by the fission yeast Slx8-Rfp complex. Protein domains in both Slx8 and Rfp that are critical for function, including the canonical RING domain, the two SIMs and a C-terminal stretch of 6 hydrophobic amino acids (Ψ6) are incorporated into a single polypeptide chain in mammalian STUbLs, as exemplified by human RNF4. Respective protein sizes are given in brackets, and sequences of the conserved SIM are indicated above the SIM protein boxes, where Ψ is a hydrophobic amino acid.
Until recently, the function of the Slx5-Slx8 complex remained enigmatic. Early insights came from an elegant study in budding yeast that identified genomic mutations able to suppress the phenotypes of a hypomorphic allele of the transcriptional regulator Mot1 [15]. The majority of suppressor mutations were found in multiple components of the SUMO pathway, and intriguingly, in SLX5 and SLX8. Together with the increase in cellular SUMO conjugates observed in slx5Δ and slx8Δ cells [15], this suggested that Slx5-Slx8 is functionally related to the SUMO pathway. Mechanistic insight came from a number of yeast studies that identified SUMO-binding and ubiquitin E3 ligase activities associated with the Slx5 (Rfp1)-Slx8 heterodimeric complex [3, 11, 13, 16, 17]. The combination of these activities and observed deregulation of the SUMO pathway in budding and fission yeast cells lacking Slx5 (Rfp1)-Slx8 activity [3, 11, 13, 14, 16, 17] suggested that Slx5 (Rfp1)-Slx8 constituted a SUMO-targeted E3 ubiquitin ligase (STUbL). The ability of STUbLs to ubiquitinate both SUMO conjugates and proteins containing SUMO-like domains (SLDs) was confirmed both in vitro and in vivo [11, 13, 16-18]. Furthermore, STUbL-mediated ubiquitination was shown to promote the proteasomal degradation of SUMO conjugates (Fig. 2; [11, 13, 16, 17]. The human Rfp1-Slx8 homolog, RNF4, was shown to rescue the phenotypes of yeast STUbL mutants, further supporting the functional conservation of STUbLs [11, 13, 14, 16]. Indeed, the STUbL mechanism was recently confirmed in human cells and will be described in a later section [4, 5].
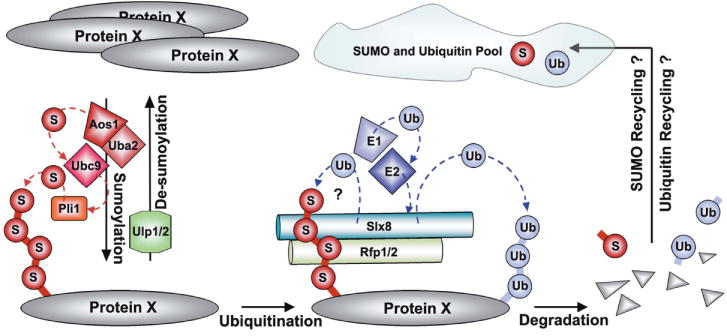
Figure 2. Proposed function of the fission yeast Slx8-Rfp STUbL complex and relationship to the SUMO and ubiquitin systems.
A specific cellular protein (protein X) exists in a sensitive balance between its sumoylated and non-sumoylated form, with only a minor fraction of its pool being SUMO-modified. This balance is maintained by the activities of SUMO modifying E1 (Fub2/Rad31), E2 (Ubc9), and E3s (Pli1), and either of two SUMO specific proteases (Ulp1/2). Slx8-Rfp recognizes sumoylated forms of protein X via Rfp-mediated non-covalent SUMO-SIM interactions. Aided by ubiquitin E1 and E2s, Slx8 ubiquitinates the sumoylated protein X, either directly and/or on the SUMO moieties, which targets protein X for degradation. After degradation, SUMO and ubiquitin likely return to their cellular pools to be re-used in a new round of SUMO and/or ubiquitin protein modification. SUMO moieties are denoted by S and ubiquitin groups by Ub.
3. Relationship of STUbLs to the SUMO pathway
To understand the role of STUbLs in genome maintenance it is useful to place STUbLs in context with the canonical SUMO pathway. Current data already reveal important insights into the relationship between STUbLs and the SUMO system. Yeast STUbLs, SUMO conjugation enzymes (E1 and E2) and SUMO proteases (Ulp1 and Ulp2) are all either essential, or their mutation causes pronounced growth defects and genomic instability (see Table 1 and Refs. therein). In hypomorphic E1 and E2 mutant cells, sumoylation is attenuated, whereas many proteins accumulate in their SUMO-conjugated states in STUbL and Ulp mutant cells. Thus, it appears that a sensitive balance between the sumoylated and un-sumoylated forms of the proteome is crucial for genome stability. Interestingly, however, abolishing more than 90% of detectable sumoylation in both fission and budding yeast has relatively minor consequences for cell growth [19-21]. For example, the sole PIAS family SUMO E3 ligase of fission yeast, Pli1, is required for the majority of sumoylation and yet its deletion causes no macroscopic growth defects [20, 21]. Closer inspection of pli1Δ cells reveals subtle genome instability phenotypes including enhanced minichromosome loss, increased telomere length, heterochromatic silencing defects at the centromere and rDNA, as well as increased sensitivity to the microtubule-destabilizing agent thiabendazole (TBZ; [20, 21]). The critical SUMO conjugates remaining in Pli1 mutant cells likely depend on the other SUMO E3 ligase of fission yeast called Nse2 [22, 23], and consistently, pli1Δ nse2-SA mutant cells appear to phenocopy a deletion of fission yeast SUMO [24]. Based on the above observations, the accumulation of SUMO conjugates in STUbL and Ulp mutant cells appears more detrimental to cell growth and genomic stability than hyposumoylation.
Table 1.
Phenotypic comparisons of SUMO isopeptidases vs. STUbLs
| ulp2Δ (Ulp1 mut) | STUbLts/Δ | References | ||
|---|---|---|---|---|
| SUMO pathway interaction | Accumulation of SUMO chains | Y (ulp2Δ) (S.c., S.p.) | Y (S.c., S.p.) | [11, 14, 15, 25, 92] |
| Growth defects suppressed by SUMO mutants defective for chain formation | Y (ulp2Δ) (S.c.) | N (S.c.) | [16, 25] | |
| Growth phenotype suppressed by deleting SUMO E3 ligase | Y (ulp2Δ) (S.c.) | N (S.c.) Y (S.p.) |
[11, 15, 25] | |
| Growth phenotype suppressed by over-expressing SUMO E3 ligase | Y (ulp2Δ2) (S.c.) | n.d. | [93] | |
| UV sensitivity suppressed by ULP2 overexpression | n.d./n.a. | Y (S.p.) | [14] | |
| Synthetic lethal with STUbL | Y (ulp1ΔN338) (S.c.) | n.a. | [27] | |
| High copy suppressor of ulp1ts | N (S.c.) | Y (S.c.) | [17] | |
| DNA damage sensitivity | HU Sensitivity | Y (ulp2Δ, ulp1ts) (S.c.) | Y (S.p., S.c.) | [10, 11, 92, 94] |
| MMS sensitivity | Y (ulp2Δ, ulp1ts) (S.c.) | N (S.c.) Y (S.p.) |
[11, 28, 92] | |
| UV sensitivity | Y (ulp2Δ, ulp1ts) (S.c.) | Y (S.c., S.p.) | [11, 17, 92, 95] | |
| CPT sensitivity | n.d. | N (S.c.) Y (S.p.) |
[11, 28] | |
| Genomic instability | Elevated DNA repair foci | Y (ulp1ts, ulp1ΔN338) (S.c.) | Y (S.c., S.p.) | [11, 27, 28, 95, 96] |
| Elevated spontaneous GCR | n.d. | Y (mainly telomere addition) (S.c.) | [28] | |
| Elevated 2μm plasmid number | Y (ulp1T490L, ulp1ΔES) (S.c.) | Y (S.c.) | [27, 97] | |
| Elevated chromosome & centromeric plasmid loss | Y (ulp2Δ2, ulp2Δ) (S.c.) | n.d. | [92, 93] | |
| Hyper-recombination | Y (ulp1ΔN338, ulp1I615N) (S.c.) | Y (S.c.) | [27, 95, 96] | |
| Elevated mutation rate | Y (ulp1I615N) (S.c.) | Y (S.c.) | [28, 95] | |
| Telomeric and rDNA silencing defect | Y and n.d. (ulp2Δ) (S.c.) | Y (S.c.) | [73] | |
| Role in telomere maintenance | Y (ulp1Δ160) | Y (S.c.) | [59, 98] | |
| Meiotic defects | Sporulation defect | Y (ulp2Δ) (S.c.) | Y (S.c.) | [10, 92] |
| Cell cycle progression | Growth reduced with G2/M arrest | Y (ulp1ts, ulp1I615N, ulp1ΔES) (S.c.) | Y (S.c., S.p.) | [11, 95, 97, 99] |
| Checkpoint constitutively activated | n.d. | Y (S.c.) | [28] | |
| Impaired checkpoint recovery/adaptation | Y (S.c.) | n.d. | [92, 94] | |
| Key genetic interactions | Suppressor of mot1ts | Y (ulp1, ulp2) (S.c.) | Y (S.c.) | [15] |
| High-copy suppressor of Smc2-6 | Y (ULP2) (S.c.) | N (S.c.) | [93] | |
| High-copy suppressor of pds5-1 | Y | N | [100] | |
| Synthetic lethal with srs2Δ | Y (ulp1I615N) (S.c.) | N (S.c.) | [95, 101] | |
| Synthetic lethal with sgs1Δ/rqh1Δ | N (S.c.) | Y (S.c., S.p.) | [10, 11] | |
| Synthetic lethal with mus81Δ/eme1Δ | N | N (S.c.) Y (S.p.) | [11, 28] | |
| Synthetic lethal/sick with recombination mutants rad51/52/54/55/50Δ or mre11Δ | Y (ulp1I615N) (S.c.) | Y (S.p.: rad51/55Δ and n.d.; S.c.1) | [11, 48, 95, 101] | |
| Synthetic sick with sir2/4Δ | Y (ulp2Δ) (S.c.) | Y (S.c.) | [73] | |
| Other | Microtubule destabilizing agent sensitivity | Y (ulp2Δ (S.c.) | Y (S.p.) | [92] & our unpublished results |
| Aberrant mitotic spindles | Y (ulp2Δ) (S.c.) | n.d. | [92] | |
| Temperature sensitivity | Y(ulp2Δ, ulp1I615N) (S.c.) | n.d. | [92, 95] | |
| Localization | Nuclear | Y Nuclear (Ulp2) and Nuclear pores (Ulp1) (S.c.) | Y (S.c., S.p.) Slx8 foci formation during S/G2 phase, colocalizing with PCNA and nucleolar protein Not1 (S.c.) | [11, 27, 93, 102, 103] |
Burgess et al. reported suppression of the nibbled growth phenotype in slx8Δ cells by deletion of homologous recombination enzymes Rad50, 52, 59 and Mre11 [27]. (S.c.), S. cerevisiae; (S.p.), S. pombe; n.a., not applicable; n.d., not done; Y, yes; N, no.
4. SUMO-directed ubiquitination: a chain reaction?
In addition to the genome stability defects shared by yeast Ulp and STUbL mutants, cells mutant for either factor also exhibit an accumulation of high molecular weight poly-SUMO chains [11, 13-17]. It is unknown whether these are mainly unanchored poly-SUMO chains or chains conjugated to target proteins. What is clear, however, is that the accumulation of SUMO chains is a major cause of ulp2Δ phenotypes [25]. SUMO contains a number of lysine residues that can be used as “acceptors” for another SUMO moiety in the process of poly-SUMO chain formation [26]. Conceptually, these same lysine residues can also be modified by other post-translational modifications, including ubiquitin. SUMO in which all lysine residues are mutated to arginine (SUMOK-R) prevents SUMO chain formation and notably, supports near normal vegetative growth of both fission and budding yeast [11, 25]. Interestingly, the SUMOK-R mutant suppresses the sensitivity of ulp2Δ cells to elevated temperature, HU, TBZ, and caffeine, indicating that these ulp2Δ phenotypes are the result of compromised SUMO chain processing [25].
In light of the following results, SUMO chains are intuitive STUbL targets: (i) STUbLs contain multiple SUMO-interacting motifs (SIMs), which can support simultaneous non-covalent binding to multiple SUMO moieties (e.g. poly-SUMO; [3, 5, 11]); (ii) STUbL mutant cells accumulate poly-SUMO conjugates and (iii) STUbL ubiquitin ligase activity and substrate affinity are increased towards poly-SUMO conjugates in vitro [3, 5, 16, 18]. However, unlike in ulp2Δ cells, expression of SUMOK-R in STUbL mutant fission or budding yeast cells is highly toxic ([11, 16]; J.P. & M.N.B. unpublished observations). Thus, unexpectedly, SUMO chains are not a significant toxic species in STUbL mutant cells; rather, SUMO chains and STUbLs may have partially redundant functions. For example, proteins modified by SUMO chains might be sequestered in the absence of STUbL activity, thereby partially “detoxifying” this pool of SUMO conjugates. That the SUMOK-R mutant is viable and supports largely wild-type vegetative growth has other important ramifications for STUbL function [11, 16, 25]. In fission yeast, the inviability of STUbL mutants is suppressed by concomitant deletion of the SUMO E3 ligase Pli1, which demonstrates the essential role of STUbLs is to regulate SUMO conjugates [11]. Therefore, healthy growth of SUMOK-R fission yeast indicates that at least in this organism, the critical STUbL targets are either mono- or multiply mono-sumoylated. Furthermore, since SUMOK-R lacks acceptor lysine residues, ubiquitination of the SUMO moiety is unlikely to be functionally crucial. The analogous SUMOK-R mutant of budding yeast is also relatively aphenotypic in comparison to STUbL mutant cells (e.g. [17, 25]). Thus, in the highly divergent budding and fission yeasts, critical STUbL targets apparently do not need to be marked by SUMO chains. How does this fit with the in vitro data and in vivo observation of SUMO chains in STUbL mutants? The in vivo accumulation of poly-SUMO conjugates in STUbL mutants might represent the engagement of futile cycles of SUMO conjugation on a substrate that was destined for degradation. The apparent in vitro stimulation of STUbLs by poly-SUMO conjugates is expected, since such chains present many more docking sites and potential ubiquitin acceptor sites (9 lysine sidechains). Further studies are required to resolve the precise nature of STUbL targets and the signals required to trigger their ubiquitination.
5. DNA repair proteins as potential STUbL targets
The genomic instability and genotoxin sensitivity of STUbL mutant yeast indicates that some STUbL clients might be DNA repair factors [10, 11, 16, 17, 27]. In addition, mutations in slx8 display genetic interactions with hypomorphic alleles of homologous recombination repair (HRR) factors, including mutants of RAD52, RAD51 (S.p. rhp51), SGS1 (S.p. rqh1), mus81 and rad60 (see Table 1 and below; [10, 11, 16, 27, 28]). Interestingly, Rad52 and Sgs1 homologues are sumoylated in both yeast and humans, and Rad60 contains SUMO-like domains (SLDs), making these factors potential STUbL targets [29-34]. Indeed, STUbLs ubiquitinate the DNA repair proteins Rad52, Rad57 and Rad60 in vitro, in a manner depending on the presence of STUbL SIMs and substrate SUMO modification (or presence of SLDs) [11, 17, 18, 35]. Despite the in vitro data, confirmation that these proteins are bona fide STUbL targets in vivo is currently lacking. Yeast Rad52 sumoylation levels increase under conditions of DNA damage and during meiosis [32]. However, whereas rad52Δ cells are highly defective in HRR, a sumoylation-deficient Rad52 mutant causes only mild HRR defects and is degraded slightly faster than wild-type Rad52 in vivo [32]. These data appear inconsistent with Rad52 being a primary STUbL target.
Rad60 modulates HRR repair at stalled and/or broken replication forks, physically and functionally associates with the Smc5/6 complex, and is regulated by the replication checkpoint kinase Cds1 (the fission yeast homolog of Chk2) [33, 36-38]. Interestingly, Rad60 contains two SLDs that are recognized by Rfp1-Slx8 (STUbL) both in vitro and in vivo [11] and this interaction appears important for cell survival following replicative stress [11]. However, no STUbL-dependent in vivo ubiquitination of Rad60 was detected in the presence or absence of replicative stress [11]. Therefore, the STUbL-Rad60 functional relationship remains undefined and Rad60 may be either a STUbL target or could modulate STUbL activity through its association with Rfp1.
Rfp1 (Slx5) interacts with the Nse5 subunit of the Smc5/6 DNA repair complex in both fission and budding yeast, indicating that although functionally enigmatic, this is a physiologically important interaction [11, 12]. Notably, the Nse2 (Mms21) subunit is a SUMO E3 ligase that sumoylates multiple components of the Smc5/6 holocomplex, other DNA repair proteins and telomere components (see below and [22, 39, 40]). Thus, Smc5/6 subunits or other Nse2 targets are attractive candidate STUbL targets in regulating genome stability.
Finally, it has been suggested that budding yeast STUbLs can modulate DNA recombination processes by promoting the sumoylation of key HRR factors [27]. STUbL dysfunction causes elevated levels of both Rad51-dependent and Rad51-independent recombination events, indicating an increased burden of DNA lesions and/or a failure to suppress potentially deleterious Rad51-independent events [27]. The sumoylation of replication protein A (RPA) appears attenuated in STUbL mutant cells [27] and consistently; STUbL-mediated conjugation of SUMO to DNA replication/repair factors, including RPA, has been detected in vitro [41]. Thus, by regulating the sumoylation status of RPA, amongst other factors, STUbLs may influence recombination repair pathway choice and thereby affect genomic stability. It should be noted that whilst STUbLs clearly promote the ubiquitination and degradation of sumoylated proteins, whether they also act as E3 SUMO ligases in vivo is somewhat controversial. In fission yeast, both the essential and DNA repair roles of STUbLs are mediated through the regulation of Pli1-dependent SUMO conjugates [11]. This appears inconsistent with a functionally important E3 SUMO ligase activity associated with STUbLs, at least in this organism. The stimulation of in vitro sumoylation by budding yeast Slx5 is RING-independent and instead likely depends on its SIMs [41]. This may reflect a specific activity, or be an artifact of the increased local concentration of SUMO and its conjugation machinery on the multiple SIMs of Slx5 [16, 17]. Defective sumoylation of certain repair factors in vivo could also be an indirect consequence of globally deregulated sumoylation in STUbL mutant cells. Interestingly, an apparently bi-functional SUMO and ubiquitin E3 ligase is not unprecedented. The p53 and Topoisomerase I-interacting protein Topors is able to stimulate both SUMO and ubiquitin conjugation to p53 [42, 43]. As for budding yeast STUbLs, the ubiquitination activity of Topors is RING-dependent, whereas sumoylation activity is not. Further studies are required to determine if STUbLs and Topors are truly bi-functional, or exclusively act as SUMO-targeted ubiquitin ligases.
6. STUbLs wrestle with SUMO for replication fork stability
As STUbLs regulate the sumoylation status of DNA replication/repair-associated factors, and modes of HRR, a negative impact of STUbL dysfunction on the sensitive process of DNA replication may be anticipated. The following synthetic genetic interactions of STUbL mutations provide strong support for this prediction. The viability of STUbL mutant cells depends on the RecQ helicase homologues Sgs1 and Rqh1 of budding and fission yeast, respectively [10, 11]. The Sgs1 and Rqh1 (human BLM) helicases function in multiple aspects of HRR, replication fork stabilization and resumption of replication from stalled forks (reviewed in [44-47]). Sgs1 was recently shown to collaborate with sumoylation and the Smc5/6 subunit Nse2 in counteracting toxic recombination at stalled replication forks [29]. Thus, the synthetic lethality of RecQ helicase and STUbL mutations indicates that stalled/damaged replication forks and other potential HRR substrates accumulate in STUbL mutant cells. This notion is supported by the large increase in spontaneous foci of the HRR protein Rad52-GFP observed in STUbL mutant cells of either fission or budding yeast [11, 27, 48]. In fission yeast, STUbL mutant viability requires the Holliday junction endonuclease Mus81-Eme1 [11], which plays a critical role in replication fork restart [49-56]. Thus, genetic interaction with mus81Δ again indicates a role for STUbLs in preventing spontaneous replication fork collapse. Such replication-associated phenomena likely account for the highly elevated mitotic recombination and mutation rates in STUbL mutant cells, and their hypersensitivity to the fork-stalling agent hydroxyurea (HU) [11, 27, 28].
7. Potential roles for STUbLs in HR-dependent telomere maintenance
Sgs1 facilitates alternative telomere lengthening (ALT) through Rad52-mediated HR [57-59]. Consequently, deletion of Sgs1 or Rad52 speeds up senescence in cells that lack telomerase (Tlc1) and depend on ALT for telomere maintenance [59]. Rapid senescence is also observed in the budding yeast telomerase and STUbL double mutants, slx8Δ tlc1Δ and slx5Δ tlc1Δ, indicating that STUbLs might facilitate ALT [59]. Intriguingly, the Smc5/6 complex promotes the recruitment of telomeres to promyelocytic leukemia nuclear bodies (PML-NBs) in tumor cells that rely on ALT to escape senescence [39]. The Smc5/6 subunit Nse2 sumoylates telomere-binding proteins Trf1/2, which are members of the shelterin complex that inhibits inappropriate HR at telomeres [60, 61]. Trf1/2 sumoylation is required for both telomere recruitment into PML-NBs and for ALT, as knockdown of Smc5 or Nse2 results in telomere delocalization and erosion [39]. Elements of the ALT pathway and sumoylation of the Trf1 ortholog, Taz1, are conserved in fission yeast [62, 63]. Taz1 inhibits HR-dependent telomere elongation in telomerase mutants (trt1Δ), since taz1Δ trt1Δ double mutants maintain linear chromosomes in an HR-dependent manner, in contrast to the chromosome circularization (end fusion) observed in trt1Δ cells [63]. In light of the above data, it is possible that STUbLs facilitate ALT by degrading sumoylated telomere-binding factors, including Trf1/2 (Taz1). Consistently, the human STUbL RNF4 localizes to PML-NBs [64], the sites of HR-dependent telomere maintenance in ALT cells [39].
8. STUbLs, arsenic trioxide and leukemia
The most mechanistically defined and exciting STUbL function to date is that of mediating the major therapeutic effect of arsenic trioxide (ATO) in treatment of acute promyelocytic leukemia (APL) [4, 5, 65]. In 95% of cases, APL is caused by a reciprocal chromosomal translocation between the retinoic acid receptor-alpha gene on chromosome 17 (RARα), with the PML gene on chromosome 15 (reviewed in [66]). Normally, PML forms dynamic subnuclear structures termed PML-nuclear bodies (PML-NBs), which are implicated in regulating critical cellular pathways such as apoptosis and those linked to genome stability through DNA repair and transcription [67]. In PML-RARα expressing APL cells, PML-NBs are disrupted by the formation of heterodimers between PML-RARα and PML. Strikingly, treatment with ATO restores normal PML-NBs in APL cells, releases the block to cellular differentiation and induces disease remission in patients [2, 66, 68, 69]. Several years ago, an elegant study demonstrated that ATO treatment induces the sumoylation of PML and PML-RARα at Lys160 of PML, which in turn results in their ubiquitination and proteasomal degradation [2]. Spurred by the recent discovery of STUbLs, human RNF4 was tested and confirmed as the critical mediator of ubiquitination and degradation of sumoylated PML/PML-RARα following ATO treatment [4, 5, 65]. Interestingly, all three studies determined that SUMO-2/3 modification of PML is required for RNF4-mediated degradation. Since SUMO-2/3 but not SUMO-1 form chains (see [70]), this observation supports that at least for PML, SUMO chains might be a critical signal for RNF4-dependent ubiquitination (see Section 3). SUMO-2/3 chains can form on PML and PML-RARα in vivo, as indicated by the accumulation of high molecular weight SUMO conjugates in siRNA RNF4 experiments [5].
The importance of STUbL-mediated PML degradation in cancer biology likely extends beyond APL, given the diverse regulatory functions of PML in the nucleus. For example, PML was recently demonstrated to be critical for the maintenance of a rare subpopulation of leukemic cells termed leukemia-initiating cells (LICs) [71]. LICs are quiescent, pluripotent, and self-renewing cells that propagate leukemia [72]. Accordingly, LICs are inherently difficult to eradicate by conventional cancer therapies that are tailored toward rapidly cycling cells. Ito et al. demonstrated that PML maintains quiescence of chronic myeloid leukemia (CML) blasts [71]. Intriguingly, ATO treatment combined with the chemotherapeutic agent cytosine arabinoside (Ara-C) leads to LIC exhaustion in mouse transplantation experiments, whereas Ara-C treatment itself does not. LIC exhaustion is likely due to ATO-dependent degradation of PML, which forces LICs out of their quiescent state and allows for Ara-C mediated DNA damage to occur during S-phase, leading to cell death [71]. Based on these findings and recent published studies [4, 5], SUMO, ubiquitin and RNF4 are apparently collaborating to promote PML degradation in ATO-treated LICs. Cancer stem cells are critical targets in the treatment of multiple tumors, including those of the breast and brain [72]. Thus, it will be important to determine the generality of PML-dependent cancer stem cell maintenance. Overall, RNF4 and its associated regulatory pathways likely represent both important biomarkers for treatment choice and novel therapy targets for improved cancer treatment.
9. STUbLs in transcriptional regulation
Compelling support for STUbL roles in transcriptional regulation comes from two studies in budding yeast [15, 73]. Wang et al. discovered Slx5 and Slx8 (STUbLs) as loss-of-function suppressors of the transcriptional defects in mot1 mutant cells. Mot1 is an essential ATPase that regulates transcription (positively and negatively) via modulating TATA-binding protein (TBP) occupancy at promoters (e.g. [74-76]). The specific target(s) of Slx5-Slx8 in rescuing mot1 mutant phenotypes was not identified; however, Mot1 contains consensus sumoylation sites and thus, in STUbL mutant cells, any sumoylated forms of Mot1 might be stabilized. Although global Mot1 protein levels were unaffected in slx5/8Δ cells [15], it is possible that only the fraction of Mot1 at promoters is sumoylated and thus sensitive to STUbL regulation. In another study, Slx5 was identified as a factor that interacts with the NAD-dependent protein deacetylase Sir2 [73]. Furthermore, slx5Δ cells exhibit silencing defects at telomeres and rDNA, phenomena also observed in sir2Δ cells [73]. Sir2 function, localization and silencing signatures were largely unaffected in slx5Δ cells, indicating that STUbLs likely act downstream of Sir2 in an undefined later step of transcriptional silencing.
The human STUbL RNF4 (or SNURF) appeared in the literature a decade ago, identified as a novel RING-finger protein and transcriptional co-activator [77, 78]. RNF4 is ubiquitously expressed in adult human and mouse tissue, but predominates in the adult testis [78, 79], and similar to yeast STUbLs, RNF4 localizes to the nucleus [77, 79]. RNF4 enhances the activity of, and interacts with a variety of steroid receptor and non-steroid transcription factors (TFs), and also relieves Trps1-mediated repression of GATA-driven genes [77, 79-83]. How RNF4 promotes these apparently disparate effects is currently unknown; however, the finding that RNF4 is a STUbL suggests plausible roles in transcriptional regulation. A common thread is that many TFs co-regulated by RNF4 are also modified by SUMO and ubiquitin and undergo proteasomal degradation (reviewed in [84]). Notably, where investigated, RNF4 interactions with and co-activation of TFs depend on RNF4 regions encompassing the two SIMs and/or a functional RING domain [77, 81-83, 85]. Thus, RNF4 might bind to sumoylated TFs and promote their ubiquitination/degradation. Consistently, the ubiquitin-proteasome system can have a positive effect on transcription [84, 86]. The ubiquitin E2 UBCH7 stimulates the activity of the androgen, progesterone and glucocorticoid receptors (AR, PR and GR) and the retinoic acid receptor (RAR) [86]. In addition, PR and the estrogen receptor alpha (ERα), TFs that are co-activated by RNF4, undergo dynamic re-cycling by the proteasome. Interruption of this cycle by proteasomal inhibition reduces transcriptional activity, possibly by preventing binding of ‘fresh’ TF to the promoter [87, 88]. RNF4-mediated degradation might also remove transcriptional repressors such as Trps1, whose repressive activity is relieved by RNF4 [81]. The apparent link between RNF4 co-activation and proteasomal degradation is, however, not always positive. Similar to ERα and PR, RNF4 co-activated GR undergoes proteasomal re-cycling [89], yet proteasomal inhibition enhances rather than attenuates GR mediated transcription [89]. Thus, specific effects of potential RNF4-mediated degradation on transcription may vary between TFs and/or be context-dependent.
10. STUbLs directly regulate a MAP kinase signaling cascade
In addition to possibly controlling TFs directly (see above), STUbLs affect transcription via regulation of signaling pathways upstream of TF activity. MAP kinase signaling cascades are evolutionary conserved phosphorelay systems that transmit information from cell surface receptors to the nucleus and function to regulate gene expression in response to extracellular stimuli. Mammalian MAP kinases control cell survival, proliferation, and differentiation [90]. In the slime mold Dictyostelium, MAP kinase signaling is essential for chemotaxis toward cAMP and involves the MAP kinase kinase Mek1 and the MAP kinase Erk1 (see [91]). Upon cAMP stimulation, Mek1 becomes rapidly sumoylated, translocates from the nucleus to the cell’s leading edge, and is later ubiquitinated. Mek1 sumoylation is required for proper chemotaxis, and correlates with Mek1 translocation from the nucleus to the cytoplasm in response to cAMP [91]. Sobko et al. demonstrated that MIP1, a potential STUbL homologue based on sequence similarity [3], negatively regulates Mek1 function and affects its subcellular localization [91]. Mek1 and Mip1 interact in vivo [91], via a region in Mip1 that contains putative SIMs [3]. Cells deleted for Mip1 largely phenocopy constitutively active Mek1, whereas cells overexpressing Mip1 resemble mek1Δ cells [91]. These results are consistent with Mip1 acting as a STUbL, ubiquitinating and downregulating Mek1 activity/stability. Interestingly, however, Sobko et al. reported that non-sumoylated Mek1 was still susceptible to Mip1-dependent ubiquitination. If confirmed, this could modify the gamut of STUbL functions.
11. Concluding remarks
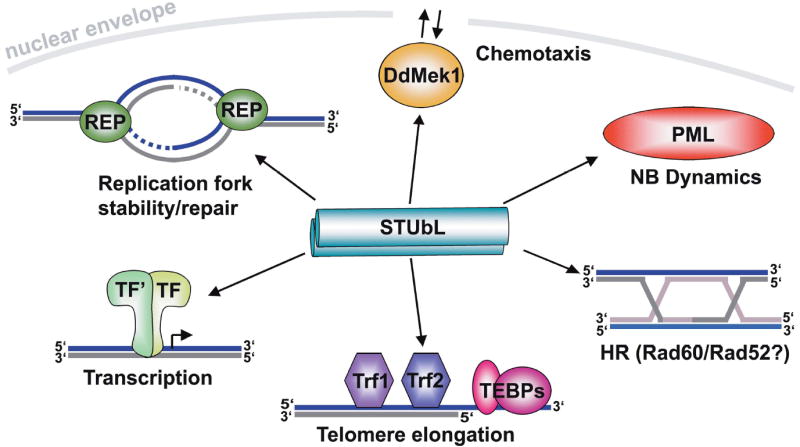
The STUbL discovery has revealed unexpected complexity in the modes of crosstalk between SUMO and ubiquitin. Furthermore, STUbLs are opening up multiple new avenues for determining the roles and regulation of SUMO in transcription, DNA repair, genome stability and human disease (Fig. 3). Finally, STUbL-focused research promises to yield new approaches towards improved therapies in cancer and perhaps other diseases.
Figure 3. Overview of STUbL functions in genome stability and transcription.
See text for details. The replisome is denoted by REP, and telomere binding proteins are denoted by TEBP.
Acknowledgments
We thank the Scripps Cell Cycle Groups for support and encouragement. Work in the laboratory of M.N.B. is supported by NIH grants GM068608 and GM081840, and that in J.J.P.P. by NIH grant CA104660.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bailey D, O’Hare P. Comparison of the SUMO1 and ubiquitin conjugation pathways during the inhibition of proteasome activity with evidence of SUMO1 recycling. Biochem J. 2005;392:271–281. doi: 10.1042/BJ20050873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lallemand-Breitenbach V, Zhu J, Puvion F, Koken M, Honore N, Doubeikovsky A, Duprez E, Pandolfi PP, Puvion E, Freemont P, et al. Role of promyelocytic leukemia (PML) sumolation in nuclear body formation, 11S proteasome recruitment, and As2O3-induced PML or PML/retinoic acid receptor alpha degradation. J Exp Med. 2001;193:1361–1371. doi: 10.1084/jem.193.12.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perry JJ, Tainer JA, Boddy MN. A SIM-ultaneous role for SUMO and ubiquitin. Trends Biochem Sci. 2008;33:201–208. doi: 10.1016/j.tibs.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Lallemand-Breitenbach V, Jeanne M, Benhenda S, Nasr R, Lei M, Peres L, Zhou J, Zhu J, Raught B, de The H. Arsenic degrades PML or PML-RARalpha through a SUMO-triggered RNF4/ubiquitin-mediated pathway. Nat Cell Biol. 2008;10:547–555. doi: 10.1038/ncb1717. [DOI] [PubMed] [Google Scholar]
- 5.Tatham MH, Geoffroy MC, Shen L, Plechanovova A, Hattersley N, Jaffray EG, Palvimo JJ, Hay RT. RNF4 is a poly-SUMO-specific E3 ubiquitin ligase required for arsenic-induced PML degradation. Nat Cell Biol. 2008;10:538–546. doi: 10.1038/ncb1716. [DOI] [PubMed] [Google Scholar]
- 6.Geiss-Friedlander R, Melchior F. Concepts in sumoylation: a decade on. Nat Rev Mol Cell Biol. 2007;8:947–956. doi: 10.1038/nrm2293. [DOI] [PubMed] [Google Scholar]
- 7.Kerscher O, Felberbaum R, Hochstrasser M. Modification of proteins by ubiquitin and ubiquitin-like proteins. Annu Rev Cell Dev Biol. 2006;22:159–180. doi: 10.1146/annurev.cellbio.22.010605.093503. [DOI] [PubMed] [Google Scholar]
- 8.Ulrich HD. Mutual interactions between the SUMO and ubiquitin systems: a plea of no contest. Trends Cell Biol. 2005;15:525–532. doi: 10.1016/j.tcb.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Wilson VG, Heaton PR. Ubiquitin proteolytic system: focus on SUMO. Expert Rev Proteomics. 2008;5:121–135. doi: 10.1586/14789450.5.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mullen JR, Kaliraman V, Ibrahim SS, Brill SJ. Requirement for three novel protein complexes in the absence of the Sgs1 DNA helicase in Saccharomyces cerevisiae. Genetics. 2001;157:103–118. doi: 10.1093/genetics/157.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prudden J, Pebernard S, Raffa G, Slavin DA, Perry JJ, Tainer JA, McGowan CH, Boddy MN. SUMO-targeted ubiquitin ligases in genome stability. EMBO J. 2007;26:4089–4101. doi: 10.1038/sj.emboj.7601838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hazbun TR, Malmstrom L, Anderson S, Graczyk BJ, Fox B, Riffle M, Sundin BA, Aranda JD, McDonald WH, Chiu CH, et al. Assigning function to yeast proteins by integration of technologies. Mol Cell. 2003;12:1353–1365. doi: 10.1016/s1097-2765(03)00476-3. [DOI] [PubMed] [Google Scholar]
- 13.Sun H, Leverson JD, Hunter T. Conserved function of RNF4 family proteins in eukaryotes: targeting a ubiquitin ligase to SUMOylated proteins. EMBO J. 2007;26:4102–4112. doi: 10.1038/sj.emboj.7601839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kosoy A, Calonge TM, Outwin EA, O’Connell MJ. Fission yeast Rnf4 homologs are required for DNA repair. J Biol Chem. 2007;282:20388–20394. doi: 10.1074/jbc.M702652200. [DOI] [PubMed] [Google Scholar]
- 15.Wang Z, Jones GM, Prelich G. Genetic analysis connects SLX5 and SLX8 to the SUMO pathway in Saccharomyces cerevisiae. Genetics. 2006;172:1499–1509. doi: 10.1534/genetics.105.052811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uzunova K, Gottsche K, Miteva M, Weisshaar SR, Glanemann C, Schnellhardt M, Niessen M, Scheel H, Hofmann K, Johnson ES, et al. Ubiquitin-dependent proteolytic control of SUMO conjugates. J Biol Chem. 2007;282:34167–34175. doi: 10.1074/jbc.M706505200. [DOI] [PubMed] [Google Scholar]
- 17.Xie Y, Kerscher O, Kroetz MB, McConchie HF, Sung P, Hochstrasser M. The yeast Hex3.Slx8 heterodimer is a ubiquitin ligase stimulated by substrate sumoylation. J Biol Chem. 2007;282:34176–34184. doi: 10.1074/jbc.M706025200. [DOI] [PubMed] [Google Scholar]
- 18.Mullen JR, Brill SJ. Activation of the Slx5-Slx8 ubiquitin ligase by poly-small ubiquitin-like modifier conjugates. J Biol Chem. 2008;283:19912–19921. doi: 10.1074/jbc.M802690200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson ES, Gupta AA. An E3-like factor that promotes SUMO conjugation to the yeast septins. Cell. 2001;106:735–744. doi: 10.1016/s0092-8674(01)00491-3. [DOI] [PubMed] [Google Scholar]
- 20.Watts FZ, Skilton A, Ho JC, Boyd LK, Trickey MA, Gardner L, Ogi FX, Outwin EA. The role of Schizosaccharomyces pombe SUMO ligases in genome stability. Biochem Soc Trans. 2007;35:1379–1384. doi: 10.1042/BST0351379. [DOI] [PubMed] [Google Scholar]
- 21.Xhemalce B, Seeler JS, Thon G, Dejean A, Arcangioli B. Role of the fission yeast SUMO E3 ligase Pli1p in centromere and telomere maintenance. EMBO J. 2004;23:3844–3853. doi: 10.1038/sj.emboj.7600394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andrews EA, Palecek J, Sergeant J, Taylor E, Lehmann AR, Watts FZ. Nse2, a component of the Smc5-6 complex, is a SUMO ligase required for the response to DNA damage. Mol Cell Biol. 2005;25:185–196. doi: 10.1128/MCB.25.1.185-196.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDonald WH, Pavlova Y, Yates JR, 3rd, Boddy MN. Novel essential DNA repair proteins Nse1 and Nse2 are subunits of the fission yeast Smc5-Smc6 complex. J Biol Chem. 2003;278:45460–45467. doi: 10.1074/jbc.M308828200. [DOI] [PubMed] [Google Scholar]
- 24.Xhemalce B, Riising EM, Baumann P, Dejean A, Arcangioli B, Seeler JS. Role of SUMO in the dynamics of telomere maintenance in fission yeast. Proc Natl Acad Sci USA. 2007;104:893–898. doi: 10.1073/pnas.0605442104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bylebyl GR, Belichenko I, Johnson ES. The SUMO isopeptidase Ulp2 prevents accumulation of SUMO chains in yeast. J Biol Chem. 2003;278:44113–44120. doi: 10.1074/jbc.M308357200. [DOI] [PubMed] [Google Scholar]
- 26.Tatham MH, Jaffray E, Vaughan OA, Desterro JM, Botting CH, Naismith JH, Hay RT. Polymeric chains of SUMO-2 and SUMO-3 are conjugated to protein substrates by SAE1/SAE2 and Ubc9. J Biol Chem. 2001;276:35368–35374. doi: 10.1074/jbc.M104214200. [DOI] [PubMed] [Google Scholar]
- 27.Burgess RC, Rahman S, Lisby M, Rothstein R, Zhao X. The Slx5-Slx8 complex affects sumoylation of DNA repair proteins and negatively regulates recombination. Mol Cell Biol. 2007;27:6153–6162. doi: 10.1128/MCB.00787-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang C, Roberts TM, Yang J, Desai R, Brown GW. Suppression of genomic instability by SLX5 and SLX8 in Saccharomyces cerevisiae. DNA Repair. 2006;5:336–346. doi: 10.1016/j.dnarep.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 29.Branzei D, Sollier J, Liberi G, Zhao X, Maeda D, Seki M, Enomoto T, Ohta K, Foiani M. Ubc9- and mms21-mediated sumoylation counteracts recombinogenic events at damaged replication forks. Cell. 2006;127:509–522. doi: 10.1016/j.cell.2006.08.050. [DOI] [PubMed] [Google Scholar]
- 30.Eladad S, Ye TZ, Hu P, Leversha M, Beresten S, Matunis MJ, Ellis NA. Intra-nuclear trafficking of the BLM helicase to DNA damage-induced foci is regulated by SUMO modification. Hum Mol Genet. 2005;14:1351–1365. doi: 10.1093/hmg/ddi145. [DOI] [PubMed] [Google Scholar]
- 31.Ho JC, Warr NJ, Shimizu H, Watts FZ. SUMO modification of Rad22, the Schizosaccharomyces pombe homologue of the recombination protein Rad52. Nucleic Acids Res. 2001;29:4179–4186. doi: 10.1093/nar/29.20.4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sacher M, Pfander B, Hoege C, Jentsch S. Control of Rad52 recombination activity by double-strand break-induced SUMO modification. Nat Cell Biol. 2006;8:1284–1290. doi: 10.1038/ncb1488. [DOI] [PubMed] [Google Scholar]
- 33.Boddy MN, Shanahan P, McDonald WH, Lopez-Girona A, Noguchi E, Yates IJ, Russell P. Replication checkpoint kinase Cds1 regulates recombinational repair protein Rad60. Mol Cell Biol. 2003;23:5939–5946. doi: 10.1128/MCB.23.16.5939-5946.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Novatchkova M, Bachmair A, Eisenhaber B, Eisenhaber F. Proteins with two SUMO-like domains in chromatin-associated complexes: the RENi (Rad60-Esc2-NIP45) family. BMC Bioinformatics. 2005;6:22. doi: 10.1186/1471-2105-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ii T, Fung J, Mullen JR, Brill SJ. The yeast Slx5-Slx8 DNA integrity complex displays ubiquitin ligase activity. Cell Cycle. 2007;6:2800–2809. doi: 10.4161/cc.6.22.4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ampatzidou E, Irmisch A, O’Connell MJ, Murray JM. Smc5/6 is required for repair at collapsed replication forks. Mol Cell Biol. 2006;26:9387–9401. doi: 10.1128/MCB.01335-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raffa GD, Wohlschlegel J, Yates JR, 3rd, Boddy MN. SUMO-binding motifs mediate the Rad60-dependent response to replicative stress and self-association. J Biol Chem. 2006;281:27973–27981. doi: 10.1074/jbc.M601943200. [DOI] [PubMed] [Google Scholar]
- 38.Morishita T, Tsutsui Y, Iwasaki H, Shinagawa H. The Schizosaccharomyces pombe rad60 gene is essential for repairing double-strand DNA breaks spontaneously occurring during replication and induced by DNA-damaging agents. Mol Cell Biol. 2002;22:3537–3548. doi: 10.1128/MCB.22.10.3537-3548.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Potts PR, Yu H. The SMC5/6 complex maintains telomere length in ALT cancer cells through SUMOylation of telomere-binding proteins. Nat Struct Mol Biol. 2007;14:581–590. doi: 10.1038/nsmb1259. [DOI] [PubMed] [Google Scholar]
- 40.Zhao X, Blobel G. A SUMO ligase is part of a nuclear multiprotein complex that affects DNA repair and chromosomal organization. Proc Natl Acad Sci USA. 2005;102:4777–4782. doi: 10.1073/pnas.0500537102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ii T, Mullen JR, Slagle CE, Brill SJ. Stimulation of in vitro sumoylation by Slx5-Slx8: evidence for a functional interaction with the SUMO pathway. DNA Repair. 2007;6:1679–1691. doi: 10.1016/j.dnarep.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rajendra R, Malegaonkar D, Pungaliya P, Marshall H, Rasheed Z, Brownell J, Liu LF, Lutzker S, Saleem A, Rubin EH. Topors functions as an E3 ubiquitin ligase with specific E2 enzymes and ubiquitinates p53. J Biol Chem. 2004;279:36440–36444. doi: 10.1074/jbc.C400300200. [DOI] [PubMed] [Google Scholar]
- 43.Weger S, Hammer E, Heilbronn R. Topors acts as a SUMO-1 E3 ligase for p53 in vitro and in vivo. FEBS Lett. 2005;579:5007–5012. doi: 10.1016/j.febslet.2005.07.088. [DOI] [PubMed] [Google Scholar]
- 44.Sharma S, Doherty KM, Brosh RM., Jr Mechanisms of RecQ helicases in pathways of DNA metabolism and maintenance of genomic stability. Biochem J. 2006;398:319–337. doi: 10.1042/BJ20060450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cobb JA, Bjergbaek L. RecQ helicases: lessons from model organisms. Nucleic Acids Res. 2006;34:4106–4114. doi: 10.1093/nar/gkl557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bachrati CZ, Hickson ID. RecQ helicases: guardian angels of the DNA replication fork. Chromosoma. 2008;117:219–233. doi: 10.1007/s00412-007-0142-4. [DOI] [PubMed] [Google Scholar]
- 47.Cheok CF, Bachrati CZ, Chan KL, Ralf C, Wu L, Hickson ID. Roles of the Bloom’s syndrome helicase in the maintenance of genome stability. Biochem Soc Trans. 2005;33:1456–1459. doi: 10.1042/BST0331456. [DOI] [PubMed] [Google Scholar]
- 48.Alvaro D, Lisby M, Rothstein R. Genome-wide analysis of Rad52 foci reveals diverse mechanisms impacting recombination. PLoS Genet. 2007;3:e228. doi: 10.1371/journal.pgen.0030228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Doe CL, Ahn JS, Dixon J, Whitby MC. Mus81-Eme1 and Rqh1 involvement in processing stalled and collapsed replication forks. J Biol Chem. 2002;277:32753–32759. doi: 10.1074/jbc.M202120200. [DOI] [PubMed] [Google Scholar]
- 50.Gaskell LJ, Osman F, Gilbert RJ, Whitby MC. Mus81 cleavage of Holliday junctions: a failsafe for processing meiotic recombination intermediates? EMBO J. 2007;26:1891–1901. doi: 10.1038/sj.emboj.7601645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Osman F, Dixon J, Doe CL, Whitby MC. Generating crossovers by resolution of nicked Holliday junctions: a role for Mus81-Eme1 in meiosis. Mol Cell. 2003;12:761–774. doi: 10.1016/s1097-2765(03)00343-5. [DOI] [PubMed] [Google Scholar]
- 52.Blais V, Gao H, Elwell CA, Boddy MN, Gaillard PH, Russell P, McGowan CH. RNA interference inhibition of Mus81 reduces mitotic recombination in human cells. Mol Biol Cell. 2004;15:552–562. doi: 10.1091/mbc.E03-08-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boddy MN, Gaillard PH, McDonald WH, Shanahan P, Yates JR, 3rd, Russell P. Mus81-Eme1 are essential components of a Holliday junction resolvase. Cell. 2001;107:537–548. doi: 10.1016/s0092-8674(01)00536-0. [DOI] [PubMed] [Google Scholar]
- 54.Chen XB, Melchionna R, Denis CM, Gaillard PH, Blasina A, Van de Weyer I, Boddy MN, Russell P, Vialard J, McGowan CH. Human Mus81-associated endonuclease cleaves Holliday junctions in vitro. Mol Cell. 2001;8:1117–1127. doi: 10.1016/s1097-2765(01)00375-6. [DOI] [PubMed] [Google Scholar]
- 55.Hanada K, Budzowska M, Davies SL, van Drunen E, Onizawa H, Beverloo HB, Maas A, Essers J, Hickson ID, Kanaar R. The structure-specific endonuclease Mus81 contributes to replication restart by generating double-strand DNA breaks. Nat Struct Mol Biol. 2007;14:1096–1104. doi: 10.1038/nsmb1313. [DOI] [PubMed] [Google Scholar]
- 56.Roseaulin L, Yamada Y, Tsutsui Y, Russell P, Iwasaki H, Arcangioli B. Mus81 is essential for sister chromatid recombination at broken replication forks. EMBO J. 2008;27:1378–1387. doi: 10.1038/emboj.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lundblad V, Blackburn EH. An alternative pathway for yeast telomere maintenance rescues est1- senescence. Cell. 1993;73:347–360. doi: 10.1016/0092-8674(93)90234-h. [DOI] [PubMed] [Google Scholar]
- 58.Le S, Moore JK, Haber JE, Greider CW. RAD50 and RAD51 define two pathways that collaborate to maintain telomeres in the absence of telomerase. Genetics. 1999;152:143–152. doi: 10.1093/genetics/152.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Azam M, Lee JY, Abraham V, Chanoux R, Schoenly KA, Johnson FB. Evidence that the S.cerevisiae Sgs1 protein facilitates recombinational repair of telomeres during senescence. Nucleic Acids Res. 2006;34:506–516. doi: 10.1093/nar/gkj452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Palm W, de Lange T. How Shelterin Protects Mammalian Telomeres. Annu Rev Genet. 2008 doi: 10.1146/annurev.genet.41.110306.130350. [DOI] [PubMed] [Google Scholar]
- 61.de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19:2100–2110. doi: 10.1101/gad.1346005. [DOI] [PubMed] [Google Scholar]
- 62.Spink K, Ho JC, Tanaka K, Watts FZ, Chambers A. The telomere-binding protein Taz1p as a target for modification by a SUMO-1 homologue in fission yeast. Biochem Genet. 2005;43:103–117. doi: 10.1007/s10528-005-1503-4. [DOI] [PubMed] [Google Scholar]
- 63.Subramanian L, Moser BA, Nakamura TM. Recombination-based telomere maintenance is dependent on Tel1-MRN and Rap1 and inhibited by telomerase, Taz1, and Ku in fission yeast. Mol Cell Biol. 2008;28:1443–1455. doi: 10.1128/MCB.01614-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hakli M, Karvonen U, Janne OA, Palvimo JJ. SUMO-1 promotes association of SNURF (RNF4) with PML nuclear bodies. Exp Cell Res. 2005;304:224–233. doi: 10.1016/j.yexcr.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 65.Weisshaar SR, Keusekotten K, Krause A, Horst C, Springer HM, Gottsche K, Dohmen RJ, Praefcke GJ. Arsenic trioxide stimulates SUMO-2/3 modification leading to RNF4-dependent proteolytic targeting of PML. FEBS Lett. 2008 doi: 10.1016/j.febslet.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 66.Zhu J, Lallemand-Breitenbach V, de The H. Pathways of retinoic acid- or arsenic trioxide-induced PML/RARalpha catabolism, role of oncogene degradation in disease remission. Oncogene. 2001;20:7257–7265. doi: 10.1038/sj.onc.1204852. [DOI] [PubMed] [Google Scholar]
- 67.Bernardi R, Pandolfi PP. Structure, dynamics and functions of promyelocytic leukaemia nuclear bodies. Nat Rev Mol Cell Biol. 2007;8:1006–1016. doi: 10.1038/nrm2277. [DOI] [PubMed] [Google Scholar]
- 68.Muller S, Ledl A, Schmidt D. SUMO: a regulator of gene expression and genome integrity. Oncogene. 2004;23:1998–2008. doi: 10.1038/sj.onc.1207415. [DOI] [PubMed] [Google Scholar]
- 69.Zhang TD, Chen GQ, Wang ZG, Wang ZY, Chen SJ, Chen Z. Arsenic trioxide, a therapeutic agent for APL. Oncogene. 2001;20:7146–7153. doi: 10.1038/sj.onc.1204762. [DOI] [PubMed] [Google Scholar]
- 70.Vertegaal AC. Small ubiquitin-related modifiers in chains. Biochem Soc Trans. 2007;35:1422–1423. doi: 10.1042/BST0351422. [DOI] [PubMed] [Google Scholar]
- 71.Ito K, Bernardi R, Morotti A, Matsuoka S, Saglio G, Ikeda Y, Rosenblatt J, Avigan DE, Teruya-Feldstein J, Pandolfi PP. PML targeting eradicates quiescent leukaemia-initiating cells. Nature. 2008;453:1072–1078. doi: 10.1038/nature07016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang JC, Dick JE. Cancer stem cells: lessons from leukemia. Trends Cell Biol. 2005;15:494–501. doi: 10.1016/j.tcb.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 73.Darst RP, Garcia SN, Koch MR, Pillus L. Slx5 promotes transcriptional silencing and is required for robust growth in the absence of Sir2. Mol Cell Biol. 2008;28:1361–1372. doi: 10.1128/MCB.01291-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Auble DT, Hahn S. An ATP-dependent inhibitor of TBP binding to DNA. Genes Dev. 1993;7:844–856. doi: 10.1101/gad.7.5.844. [DOI] [PubMed] [Google Scholar]
- 75.Auble DT, Hansen KE, Mueller CG, Lane WS, Thorner J, Hahn S. Mot1, a global repressor of RNA polymerase II transcription, inhibits TBP binding to DNA by an ATP-dependent mechanism. Genes Dev. 1994;8:1920–1934. doi: 10.1101/gad.8.16.1920. [DOI] [PubMed] [Google Scholar]
- 76.Prelich G. Saccharomyces cerevisiae BUR6 encodes a DRAP1/NC2alpha homolog that has both positive and negative roles in transcription in vivo. Mol Cell Biol. 1997;17:2057–2065. doi: 10.1128/mcb.17.4.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Moilanen AM, Poukka H, Karvonen U, Hakli M, Janne OA, Palvimo JJ. Identification of a novel RING finger protein as a coregulator in steroid receptor-mediated gene transcription. Mol Cell Biol. 1998;18:5128–5139. doi: 10.1128/mcb.18.9.5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chiariotti L, Benvenuto G, Fedele M, Santoro M, Simeone A, Fusco A, Bruni CB. Identification and characterization of a novel RING-finger gene (RNF4) mapping at 4p16.3. Genomics. 1998;47:258–265. doi: 10.1006/geno.1997.5105. [DOI] [PubMed] [Google Scholar]
- 79.Galili N, Nayak S, Epstein JA, Buck CA. Rnf4, a RING protein expressed in the developing nervous and reproductive systems, interacts with Gscl, a gene within the DiGeorge critical region. Dev Dyn. 2000;218:102–111. doi: 10.1002/(SICI)1097-0177(200005)218:1<102::AID-DVDY9>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 80.Wu SM, Kuo WC, Hwu WL, Hwa KY, Mantovani R, Lee YM. RNF4 is a coactivator for nuclear factor Y on GTP cyclohydrolase I proximal promoter. Mol Pharmacol. 2004;66:1317–1324. doi: 10.1124/mol.66.5.. [DOI] [PubMed] [Google Scholar]
- 81.Kaiser FJ, Moroy T, Chang GT, Horsthemke B, Ludecke HJ. The RING finger protein RNF4, a co-regulator of transcription, interacts with the TRPS1 transcription factor. J Biol Chem. 2003;278:38780–38785. doi: 10.1074/jbc.M306259200. [DOI] [PubMed] [Google Scholar]
- 82.Saville B, Poukka H, Wormke M, Janne OA, Palvimo JJ, Stoner M, Samudio I, Safe S. Cooperative coactivation of estrogen receptor alpha in ZR-75 human breast cancer cells by SNURF and TATA-binding protein. J Biol Chem. 2002;277:2485–2497. doi: 10.1074/jbc.M109021200. [DOI] [PubMed] [Google Scholar]
- 83.Lyngso C, Bouteiller G, Damgaard CK, Ryom D, Sanchez-Munoz S, Norby PL, Bonven BJ, Jorgensen P. Interaction between the transcription factor SPBP and the positive cofactor RNF4. An interplay between protein binding zinc fingers. J Biol Chem. 2000;275:26144–26149. doi: 10.1074/jbc.M003405200. [DOI] [PubMed] [Google Scholar]
- 84.Faus H, Haendler B. Post-translational modifications of steroid receptors. Biomed Pharmacother. 2006;60:520–528. doi: 10.1016/j.biopha.2006.07.082. [DOI] [PubMed] [Google Scholar]
- 85.Poukka H, Aarnisalo P, Santti H, Janne OA, Palvimo JJ. Coregulator small nuclear RING finger protein (SNURF) enhances Sp1- and steroid receptor-mediated transcription by different mechanisms. J Biol Chem. 2000;275:571–579. doi: 10.1074/jbc.275.1.571. [DOI] [PubMed] [Google Scholar]
- 86.Verma S, Ismail A, Gao X, Fu G, Li X, O’Malley BW, Nawaz Z. The ubiquitin-conjugating enzyme UBCH7 acts as a coactivator for steroid hormone receptors. Mol Cell Biol. 2004;24:8716–8726. doi: 10.1128/MCB.24.19.8716-8726.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lonard DM, Nawaz Z, Smith CL, O’Malley BW. The 26S proteasome is required for estrogen receptor-alpha and coactivator turnover and for efficient estrogen receptor-alpha transactivation. Mol Cell. 2000;5:939–948. doi: 10.1016/s1097-2765(00)80259-2. [DOI] [PubMed] [Google Scholar]
- 88.Reid G, Hubner MR, Metivier R, Brand H, Denger S, Manu D, Beaudouin J, Ellenberg J, Gannon F. Cyclic, proteasome-mediated turnover of unliganded and liganded ERalpha on responsive promoters is an integral feature of estrogen signaling. Mol Cell. 2003;11:695–707. doi: 10.1016/s1097-2765(03)00090-x. [DOI] [PubMed] [Google Scholar]
- 89.Deroo BJ, Rentsch C, Sampath S, Young J, DeFranco DB, Archer TK. Proteasomal inhibition enhances glucocorticoid receptor transactivation and alters its subnuclear trafficking. Mol Cell Biol. 2002;22:4113–4123. doi: 10.1128/MCB.22.12.4113-4123.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Krishna M, Narang H. The complexity of mitogen-activated protein kinases (MAPKs) made simple. Cell Mol Life Sci. 2008 doi: 10.1007/s00018-008-8170-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sobko A, Ma H, Firtel RA. Regulated SUMOylation and ubiquitination of DdMEK1 is required for proper chemotaxis. Dev Cell. 2002;2:745–756. doi: 10.1016/s1534-5807(02)00186-7. [DOI] [PubMed] [Google Scholar]
- 92.Li SJ, Hochstrasser M. The yeast ULP2 (SMT4) gene encodes a novel protease specific for the ubiquitin-like Smt3 protein. Mol Cell Biol. 2000;20:2367–2377. doi: 10.1128/mcb.20.7.2367-2377.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Strunnikov AV, Aravind L, Koonin EV. Saccharomyces cerevisiae SMT4 encodes an evolutionarily conserved protease with a role in chromosome condensation regulation. Genetics. 2001;158:95–107. doi: 10.1093/genetics/158.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schwartz DC, Felberbaum R, Hochstrasser M. The Ulp2 SUMO protease is required for cell division following termination of the DNA damage checkpoint. Mol Cell Biol. 2007;27:6948–6961. doi: 10.1128/MCB.00774-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Soustelle C, Vernis L, Freon K, Reynaud-Angelin A, Chanet R, Fabre F, Heude M. A new Saccharomyces cerevisiae strain with a mutant Smt3-deconjugating Ulp1 protein is affected in DNA replication and requires Srs2 and homologous recombination for its viability. Mol Cell Biol. 2004;24:5130–5143. doi: 10.1128/MCB.24.12.5130-5143.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Palancade B, Liu X, Garcia-Rubio M, Aguilera A, Zhao X, Doye V. Nucleoporins prevent DNA damage accumulation by modulating Ulp1-dependent sumoylation processes. Mol Biol Cell. 2007;18:2912–2923. doi: 10.1091/mbc.E07-02-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dobson MJ, Pickett AJ, Velmurugan S, Pinder JB, Barrett LA, Jayaram M, Chew JS. The 2 μm plasmid causes cell death in Saccharomyces cerevisiae with a mutation in Ulp1 protease. Mol Cell Biol. 2005;25:4299–4310. doi: 10.1128/MCB.25.10.4299-4310.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen XL, Silver HR, Xiong L, Belichenko I, Adegite C, Johnson ES. Topoisomerase I-dependent viability loss in Saccharomyces cerevisiae mutants defective in both SUMO conjugation and DNA repair. Genetics. 2007;177:17–30. doi: 10.1534/genetics.107.074708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li SJ, Hochstrasser M. A new protease required for cell-cycle progression in yeast. Nature. 1999;398:246–251. doi: 10.1038/18457. [DOI] [PubMed] [Google Scholar]
- 100.Stead K, Aguilar C, Hartman T, Drexel M, Meluh P, Guacci V. Pds5p regulates the maintenance of sister chromatid cohesion and is sumoylated to promote the dissolution of cohesion. J Cell Biol. 2003;163:729–741. doi: 10.1083/jcb.200305080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pan X, Ye P, Yuan DS, Wang X, Bader JS, Boeke JD. A DNA integrity network in the yeast Saccharomyces cerevisiae. Cell. 2006;124:1069–1081. doi: 10.1016/j.cell.2005.12.036. [DOI] [PubMed] [Google Scholar]
- 102.Takahashi Y, Mizoi J, Toh EA, Kikuchi Y. Yeast Ulp1, an Smt3-specific protease, associates with nucleoporins. J Biochem. 2000;128:723–725. doi: 10.1093/oxfordjournals.jbchem.a022807. [DOI] [PubMed] [Google Scholar]
- 103.Li SJ, Hochstrasser M. The Ulp1 SUMO isopeptidase: distinct domains required for viability, nuclear envelope localization, and substrate specificity. J Cell Biol. 2003;160:1069–1081. doi: 10.1083/jcb.200212052. [DOI] [PMC free article] [PubMed] [Google Scholar]


