Abstract
Cigarette craving, one hallmark sign of nicotine dependence, is often measured in laboratory settings using cue reactivity methods. How lab measures of cue reactivity relate to real world smoking behavior is unclear, particularly among non-treatment seeking smokers. Within a larger study of hormonal effects on cue reactivity (N=78), we examined the predictive relationship of cue reactivity to smoking, each measured in several ways. Results indicated that cue-evoked craving in response to stressful imagery, and to a lesser extent, in vivo smoking cues, significantly predicted smoking behavior during the week following testing. However, this predictive relationship was absent upon controlling for reactivity to neutral cues. Nicotine dependence may moderate the relationship between cue reactivity and actual smoking, such that this predictive relationship is less robust among highly dependent smokers than among smokers low in nicotine dependence. The question of whether cue-elicited craving predicts smoking among smokers not in treatment is best answered with a qualified yes, depending on how craving is manipulated and measured. Our findings highlight important methodological and theoretical considerations for cue reactivity research.
Keywords: cue reactivity, craving, dependence, smoking
I. Introduction
Cue-elicited craving for tobacco has long been considered one driving influence on continued smoking (Shiffman, 1991; Tiffany, 1990). One method to assess cue-elicited craving is via real-time assessment within the smokers’ own environment. Such methods often reveal that cue- elicited craving is a strong predictor of smoking (Shiffman et al., 2002; Shiffman, Paty, Gnys, Kassel, & Hickcox, 1996), thus supporting that methodology as a viable tool to measure craving in the real world, and its relationship to actual smoking behavior.
Another common method to assess cue-elicited craving is based on the cue reactivity paradigm. Within lab-based studies of cue reactivity, current users of a drug are presented with stimuli that, over time, have been repeatedly paired with drug use. Cues can be related to the substance itself (in vivo cues of the touch or smell of a cigarette, sight of a lighter), situationally associated cues (alcohol, social situations of smoking, time of day), or affective cues (positive or negative emotions, stress). One advantage of lab-based cue reactivity procedures is that they offer greater potential for experimental manipulation of tobacco-specific cues. A meta-analysis of cue-reactivity studies found robust effects for self-report of craving in response to cues, and a somewhat smaller, though still meaningful effect size for physiological responses to cues (Carter & Tiffany, 1999).
One question that persists about lab-based measures of cue-elicited craving is whether they have any meaningful relationship with smoking behavior. Evidence for the link between cue reactivity and smoking behavior comes from a small number of studies that have examined cue reactivity and treatment outcome. These studies generally suggest a small but predictive relationship between cue reactivity and smoking relapse (Abrams, Monti, Carey, Pinto, & Jacobus, 1988; Niaura, Abrams, Demuth, Pinto, & Monti, 1989; Niaura et al., 1988; Shadel et al., 1998; Waters et al., 2004), time to first lapse (Waters et al., 2004), or changes in smoking level (Payne, Smith, Adams, & Diefenbach, 2006).
We are aware of few studies that have examined the relationship between cue reactivity and smoking behavior among non-treatment seeking smokers, though some have examined analogs of smoking behavior, such as desire to smoke (Payne, Smith, Sturges, & Holleran, 1996) or a behavioral choice task for smoking (Sayette, Martin, Wertz, Shiffman, & Perrott, 2001). One recent study examined the relationship between smoking, nicotine dependence and craving among a large sample of non-treatment seeking regular smokers, pooled across a number of smaller studies (Donny, Griffin, Shiffman, & Sayette, 2008). Results indicated that nicotine dependence, but not craving, to be related to smoking behavior, measured distally in time as the number of cigarettes per day prior to any cue exposure. In a much smaller study (n=10) that focused on brain activity during cue exposure (Smolka et al., 2006), investigators noted an association between craving and nicotine dependence, but failed to find an association between craving and smoking behavior (i.e., cigarettes per day prior to any cue exposure).
The present study builds upon the literature above with an examination of cue-elicited craving, its potential interaction with nicotine dependence, and their predictive relationship with smoking behavior. In contrast to studies noted above, we examine two indices of smoking behavior that are prospective and more proximal to cue reactivity testing: 1) smoking on the day of cue reactivity assessment, and 2) smoking in the days following testing. Our primary hypothesis was that cue-reactivity would predict both measures of smoking behavior. The current study is based on an ongoing larger study examining gender and hormonal influences on cue reactivity, portions of which of have been reported elsewhere (LaRowe, Saladin, Carpenter, & Upadhyaya, 2007).
2. Methods
2.1 Participants
Adult smokers (≥ 10 cigs/day) ages 18–40 were recruited from general community for a laboratory-based cue reactivity study. Once individuals completed a brief phone screen, those who were potentially eligible presented at the laboratory for informed consent and final assessment of eligibility. Exclusion criteria (and numbers excluded at the baseline visit on the basis of each) were as follows: a) any major psychiatric disorder within past month, including substance use disorders, assessed via semi-structured clinical interviews (First, Spitzer, Gibbon, & Williams, 2002) (10), b) use of psychotropic medication within one month (1), and c) medical condition (e.g., hypertension, arrhythmia) or use of medications (e.g., beta-blockers) that could affect physiological assessment of craving or cue reactivity (1). In addition, as the parent study sought to evaluate the effects of menstrual phase on cue reactivity, women had the added criteria of: d) not currently taking contraception or hormone replacement, e) being devoid of Pre-Menstrual Dysphoric Disorder (PMDD), f) being post menarche and pre-menopausal, g) having a regular menstrual cycle between 25 and 35 days, h) not post hysterectomy, and i) not currently pregnant, with at least three months post parturition and breast feeding for any prior pregnancy.
2.2 Procedure
Eligible participants participated in four laboratory-based cue reactivity sessions, each held approximately one week apart and timed to coincide with distinct phases of the menstrual cycle (Carpenter, Upadhyaya, LaRowe, Saladin, & Brady, 2006). Male participants were yoked to women and also participated in all four sessions. For the remainder of this report, however, we present data based on the first session only, as this time-point offers the greatest number of participant observations for the current research question.
The cue reactivity sessions were held in the outpatient General Clinical Research Center (GCRC). All participants were required to have smoked prior to each session (i.e., within 45–60 minutes of the cue reactivity procedure) in an effort to control time since last cigarette. Average carbon monoxide levels taken prior to initiation of study procedures indicate that this manipulation was effective (mean = 21.6; SD = 14.5). Urine drug screens and pregnancy tests were completed to confirm that the participant had neither used illicit drugs nor was pregnant, after which participants initiated the cue reactivity procedure. Participants were exposed to each of four cues (discussed below), presented in counterbalanced order. Each cue administration lasted 90 seconds and was separated by a 10-minute nature show designed to diminish any carry-over effects. Subjective measures of craving were assessed pre and post each cue administration. Physiological measures of craving were collected prior to cue presentation and during the entire cue administration. The entire cue reactivity session took approximately 120 minutes.
2.3 Cues
Two active cues were administered with two parallel inactive/control cues (4 cues total). Smoking cues consisted of in vivo manipulation of the participants’ own brand of cigarettes and a lighter. Standardized instructions for cue manipulation were delivered via headphones (e.g., handling, smelling the cigarette). The relevant neutral cue consisted of a similar manipulation of pencils/eraser. Negative affective cue presentation consisted of a personalized stressful script composed in advance of the procedure by the participant him/herself (e.g., a recollection of a recent, stress inducing event at work or home), again presented via headphones. The relevant control for the affective manipulation was a neutral relaxed script, again personalized to each participant and delivered via headphones.
2.4 Measures
Cue reactivity assessment included both subjective and physiological measures. Subjective craving was assessed via the Questionnaire of Smoking Urges – Brief (Cox, Tiffany, & Christen, 2001), a 10-item scale with 7-point Likert response format. The QSU includes two factors: Factor 1assesses positive reinforcement (i.e., hedonic craving) and Factor 2 assesses negative reinforcement (i.e., alleviation of negative affect/withdrawal). For total QSU and factor scores, items were averaged to produce an average score (range 1–7). Physiological assessment of craving included both heart rate and skin conductance, measured prior to and during each cue manipulation. Details of physiological assessment can be found in a prior paper (LaRowe et al., 2007).
Throughout the study period, participants maintained a diary of smoking. They were provided with a calendar in which they prospectively tracked the number of cigarettes smoked daily. Diaries were collected and reviewed at the cue reactivity sessions. Thus, participants returning for a subsequent study visit provided detailed smoking data for the day of and week following the initial cue reactivity assessment (see analyses, below). Participants who failed to return their completed diary (estimated to be <15% of entire sample) were asked to recall CPD (cigarettes per day) for each of the seven days prior, an approach consistent with general time-line follow back (TLFB) procedures (Sobell & Sobell, 1996).
2.5 Analyses
Analyses were restricted to those participants who completed the first cue reactivity session and who provided complete smoking diaries (N=78). Subjective and physiological measures of craving were analyzed as predictors of smoking behavior in two ways. First, each measure in response to the two active cues (smoking in vivo; stressful script) each in isolation (i.e., without any control for neutral or relaxing cues; hereafter called ‘absolute craving’) was analyzed in a regression model predicting smoking. Thus, average QSU scores post smoking cue and post stressful cue served as measures of subjective craving. For physiological data, we examined the percent change from baseline in both heart rate and skin conductance, as defined in the following formula: ([maximum value during cue − pre-cue value]/pre-cue value) × 100. Second, these same analyses were conducted again, this time controlling for response to the appropriate control cue (neutral or relaxed cue); hereafter called ‘differential cue reactivity’. All analyses controlled for presentation order of cues.
Criterion variables within the regression analyses consisted of both CPD on the day of the first cue reactivity session and the average for the 7-day period following the cue reactivity session. The former CPD measure may be viewed as a proximal measure of smoking behavior, but one that is potentially disrupted by the occurrence of the laboratory cue reactivity assessment (i.e., a two hour block in which the participant could not smoke). However, while the overall number of cigarettes smoked on the day of cue reactivity assessment may have been somewhat deflated, our measure still represents the total number of cigarettes smoked that entire day (not just up to the point of testing), as captured by the TLFB procedures. The latter CPD measure is more distal in time but serves as an estimate of smoking behavior that was unaffected by cue reactivity assessment, and thus possibly may be a more ecologically valid estimate (7 day average, unaffected by study procedures).
3. Results
Average age for the sample (n=78) was 29 (SD = 6); 60% were male, 83% were Caucasian, and 14% were African American. Almost all participants (99%) had completed high school; 62% had completed college. Participants were moderately dependent on tobacco (average FTND score = 4.8; SD = 2.0), smoking an average of 19.3 (SD = 7.9; range 10–40) cigarettes daily, with an average of 11.1 years (SD = 5.5) of regular smoking. Forty-four percent lived with another smoker, and 17% used non-cigarette tobacco in addition to cigarettes. The average number of cigarettes smoked on the day of cue reactivity testing was 18 (SD = 8.5; range 1 – 44), whereas the average cigarettes smoked per day for the week following cue reactivity testing was 19 (SD = 7.2; range 6.9 – 38.7).
We first established a basic cue reactivity effect, to ensure that our ‘active’ cues effectively elicited craving. This was the case. Participants reported significantly greater subjective craving (total QSU scores) in response to both the in vivo smoking cue (Mean = 4.6, SD = 1.4) and the stressful imagery cue (Mean = 4.4, SD = 1.2) than to either of their control cues (Mean = 3.9, SD = 1.4 for neutral in vivo cue; Mean = 3.7, SD = 1.5 for relaxed imagery cue; p <.001 for both comparisons).
Results for absolute craving (unadjusted for response to control cues) and its relationship to smoking are presented in Table 1. Two significant associations between craving and smoking on the day of cue reactivity were identified: greater QSU Factor 1 craving (hedonic craving) and higher heart rate in response to the stressful imagery cue were both predictive of greater smoking. A number of subjective craving measures were predictive of smoking in the week following the cue reactivity assessment. Total craving (QSU) scores in response to both the in vivo smoking cue and the stressful imagery cue were significantly and positively associated with increases in smoking. Specifically, for every 1-point increase in craving in total QSU in response to the stressful imagery, there was a 1.7 increase (95% CI: 0.3 – 3.0; p = .02) in average number of cigarettes/day. Whereas the relationship between responses to the in vivo cue and smoking behavior appeared to be marginally driven by QSU Factor 2 (alleviation of negative affect/withdrawal), responses to the stressful imagery and smoking behavior were driven by both QSU factors (see Table 1). As with smoking on the day of cue reactivity assessment, higher rate in response to the stressful imagery also predicted smoking for the week following assessment.
Table 1.
Absolute, Cue-Elicited Subjective and Physiological Craving as Predictors of Smoking Behavior a
| B | Std. Error | 95% CI for B | β | p | |
|---|---|---|---|---|---|
| Subjective Craving | |||||
| Smoking on Day of Cue Reactivity Assessment | |||||
| In Vivo Smoking Cue | |||||
| QSU 1 | 1.0 | .7 | −0.4 – 2.4 | .16 | .2 |
| QSU Factor 1 | 1.3 | .8 | −0.3 – 2.9 | .19 | .1 |
| QSU Factor 2 | .5 | .6 | −0.6 – 1.7 | .1 | .3 |
| Stressful Imagery Cue | |||||
| QSU | 1.0 | .8 | −0.5 – 2.6 | .15 | .2 |
| QSU Factor 1 | 1.6 | .8 | 0.1 – 3.1 | .24 | .04 |
| QSU Factor 2 | .2 | .6 | −1.0 – 1.4 | .04 | .7 |
| Smoking During Week Following Cue Reactivity Assessment | |||||
| In Vivo Smoking Cue | |||||
| QSU | 1.2 | .6 | 0.0 – 2.5 | .23 | .05 |
| QSU Factor 1 | 1.1 | .7 | −0.3 – 2.4 | .18 | .1 |
| QSU Factor 2 | .9 | .5 | 0.0 – 1.9 | .2 | .055 |
| Stressful Imagery Cue | |||||
| QSU | 1.7 | .7 | 0.3 – 3.0 | .29 | .02 |
| QSU Factor 1 | 1.6 | .7 | 0.2 – 2.8 | .26 | .03 |
| QSU Factor 2 | 1.0 | .5 | 0.0 – 2.1 | .23 | .05 |
| Physiological Craving 2 | |||||
| Smoking on Day of Cue Reactivity Assessment | |||||
| In Vivo Smoking Cue | |||||
| Heart Rate | .2 | .1 | −0.1 – 0.4 | .17 | .2 |
| Skin Conductance | 0 | .001 | −0.004 – .002 | −0.1 | .4 |
| Stressful Imagery Cue | |||||
| Heart Rate | .3 | .1 | .03 – .57 | .26 | .03 |
| Skin Conductance | 0 | .003 | −0.008 – .006 | −0.04 | .8 |
| Smoking During Week Following Cue Reactivity Assessment | |||||
| In Vivo Smoking Cue | |||||
| Heart Rate | .1 | .1 | −0.13 – .24 | .08 | .5 |
| Skin Conductance | 0 | .001 | −.004 – .001 | −.13 | .3 |
| Stressful Imagery Cue | |||||
| Heart Rate | .3 | .1 | 0.03 – .5 | .27 | .03 |
| Skin Conductance | 0 | .003 | −.01 – .01 | −.06 | .6 |
All analyses control for presentation order of cues
QSU – Questionnaire on Smoking Urges, Brief
Physiological measures defined as percentage change from prior to cue exposure vs. peak value during cue exposure
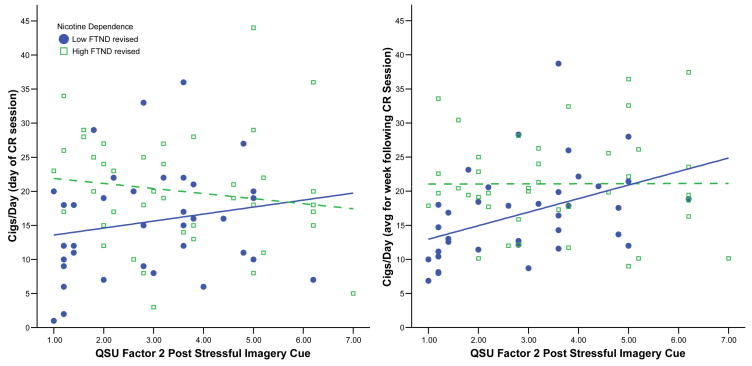
The above analyses were unaffected by gender. They were, however, moderated by level of nicotine dependence, primarily for subjective craving in response to the stressful imagery cue. We removed one item from the FTND (Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991) that pertained to cigarettes smoked per day (which would artificially confound outcome of smoking behavior, since cigarettes smoked per day was our main outcome), leaving a revised scale with a range of 0–7. The revised FTND was then entered into a similar regression, in addition to the predictor variables (and their interaction). With regard to general craving (i.e., total QSU scores), the interaction between craving in response to the stressful imagery cue and nicotine dependence was marginally significant for smoking behavior both on the day of the cue reactivity session (p = .07) and for the week following (p = .09). The interaction between craving for negative reinforcement (i.e., QSU Factor 2) and nicotine dependence was significant, for both smoking on the day of cue reactivity assessment (p = .04) and for the week following (p = .05). Figure 1 graphically depicts these relationships using a median split on the revised FTND (0–3 vs. 4–7). For both outcomes, smoking behavior increased as negative reinforcement craving increased, but only among those participants with low nicotine dependence. For participants with high levels of nicotine dependence, there was no relationship between craving and smoking. We further examined if this moderating effect of nicotine dependence was a function of differential responding to cues, and indeed it was. Participants with high levels of nicotine dependence reported significantly greater craving than did those with low levels of nicotine dependence for all but one cue (p ≤ .02). Thus, smokers high in nicotine dependence had greater ambient craving, irrespective of cue presentation.
Figure 1.
Craving and nicotine dependence as predictors of smoking behavior, both on day of cue reactivity testing (left panel) and week following cue reactivity testing (right panel). Fitted regression lines indicate low dependence (solid line) and high dependence (dashed line). CR: Cue Reactivity; FTND: Fagerström Test for Nicotine Dependence (revised to omit cigs/day item)
Results for differential cue reactivity (controlling for response to neutral cues) are presented in Table 2. There were no significant associations between cue-elicited craving and smoking behavior, either on the day of cue reactivity testing or for the week following. We examined the ability of all cues (including the control cues) to predict smoking, and in each case, the active cues accounted for a larger percent variance in smoking behavior. Neither gender nor nicotine dependence moderated the relationship between differential cue-elicited craving and smoking behavior.
Table 2.
Differential Cue Reactivity (controlling for response to neutral cues) as Predictors of Smoking Behavior a
| B | Std. Error | 95% CI for B | β | p | |
|---|---|---|---|---|---|
| Subjective Cue Reactivity | |||||
| Smoking on Day of Cue Reactivity Assessment | |||||
| In Vivo Smoking Cue 1 | |||||
| QSU | .6 | 1.1 | −1.6 – 2.8 | .1 | .6 |
| QSU Factor 1 | .8 | 1.1 | −1.4 – 2.9 | .1 | .5 |
| QSU Factor 2 | .6 | 1.0 | −1.3 – 2.5 | .13 | .5 |
| Stressful Imagery Cue 1 | |||||
| QSU | .3 | 1.1 | −1.9 – 2.6 | .05 | .8 |
| QSU Factor 1 | .6 | 1.1 | −1.6 – 2.7 | .09 | .6 |
| QSU Factor 2 | .2 | .9 | −1.7 – 2.0 | .03 | .9 |
| Smoking During Week Following Cue Reactivity Assessment | |||||
| In Vivo Smoking Cue 1 | |||||
| QSU | .3 | .9 | −1.6 – 2.1 | .05 | .8 |
| QSU Factor 1 | .1 | .9 | −1.7 – 1.9 | .02 | .9 |
| QSU Factor 2 | .4 | .8 | −1.2 – 2.0 | .1 | .6 |
| Stressful Imagery Cue 1 | |||||
| QSU | .2 | .9 | −1.6 – 2.1 | .05 | .8 |
| QSU Factor 1 | .01 | .9 | −1.8 – 1.8 | .002 | .9 |
| QSU Factor 2 | .1 | .8 | −1.4 – 1.6 | .02 | .9 |
| Physiological Cue Reactivity | |||||
| Smoking on Day of Cue Reactivity Assessment | |||||
| In Vivo Smoking Cue 2 | |||||
| Heart Rate | .1 | .12 | −.14 – .35 | .12 | .4 |
| Skin Conductance | 0 | .002 | −.005 – .001 | −.18 | .2 |
| Stressful Imagery Cue 2 | |||||
| Heart Rate | .2 | .18 | −.19 – .53 | .15 | .3 |
| Skin Conductance | .0 | .004 | −.007 – .009 | .04 | .8 |
| Smoking During Week Following Cue Reactivity Assessment | |||||
| In Vivo Smoking Cue 2 | |||||
| Heart Rate | .03 | .1 | −.18 – .25 | .05 | .8 |
| Skin Conductance | 0 | .001 | −.005 – .001 | −.22 | .1 |
| Stressful Imagery Cue 2 | |||||
| Heart Rate | .2 | .16 | −.08 – .56 | .24 | .1 |
| Skin Conductance | 0 | .004 | −.007 – .008 | .02 | .9 |
All analyses control for presentation order of cues
QSU craving post active cue (smoking invivo stressful imagery) cue controlling post inactive cue (neutral invivo or relaxed imagery)
Physiological craving, defined as percentage change from prior to cue exposure vs. peak value during cue exposure, controlling for percentage change in response to inactive cue (neutral invivo or relaxed imagery)
4. Discussion
This study examined cue-elicited craving and smoking behavior among non-treatment seeking smokers. Our primary findings can be summarized as follows: 1) cue-elicited craving, particularly when measured in response to stressful cues, predicted smoking, 2) however, differential cue reactivity (i.e., craving in response to active cues adjusted for response to inactive cues), is not predictive of smoking, 3) these relationships may be moderated by nicotine dependence, and 4) physiological cue reactivity is less predictive of smoking than is subjective craving. Interpretation of these results rests on several key measurement issues, many of which have been highlighted previously (Sayette et al., 2000).
First, there was little indication that cue reactivity was predictive of smoking behavior when that behavior was operationally defined as number of cigarettes smoked on the day of cue reactivity testing. The two-hour testing procedure may have interrupted the natural rhythm of smoking for that day: 21% of participants smoked 10 cigarettes or less on the day of cue reactivity testing vs. 8% of participants who averaged 10 CPD or less for the week following. In contrast, cue reactivity (unadjusted for control cues) in the lab was generally predictive of smoking in the days following testing.
Second, reactivity to the in vivo smoking cue had little predictive effect. Previous studies, including those within our lab using identical methodology, have established that in vivo cues are potent elicitors of craving even among non-treatment seeking smokers (Shadel, Niaura, & Abrams, 2001; Upadhyaya, Drobes, & Thomas, 2004; Upadhyaya, Drobes, & Wang, 2006). Though our in vivo cue was an effective elicitor of craving, it may have been less potent relative to a lit cigarette (institutional constraints on smoking precluded the use of a lit cigarette cue). A less potent smoking cue may ultimately diminish any relationship to smoking behavior. In contrast, the personalized stress cue (again, unadjusted for control cues) was a significant predictor of smoking behavior. Prior research on affective manipulation is mixed, with some studies showing that such cues reliably elicit craving (Niaura et al., 1998) and others not (Conklin & Tiffany, 2001).
Third, physiological measures of cue reactivity were less predictive of smoking behavior than were subjective measures. This is largely consistent with the greater cue reactivity literature, which suggests that cue reactivity effects on physiological measures are small (Carter & Tiffany, 1999). A number of criticisms have been drawn against physiological assessment of craving (see Sayette et al., 2000). Foremost, physiological processes are not exclusive to motivation/craving for drug use. It is unclear what clinical and theoretical meaning should be assigned to observed changes in physiological processes. Future research in cue reactivity will need to carefully consider if and how to include physiological assessment of craving. The results of this study would suggest that physiological proxies of craving are not predictive of smoking.
Fourth, consistent with some previous research (Donny et al., 2008; Payne et al., 1996), the present study suggests that nicotine dependence may moderate the relationship between cue-elicited craving and smoking behavior. At least among non-treatment seekers, cue reactivity procedures may be most sensitive among smokers with low to moderate nicotine dependence. Since highly dependent engage in smoking behavior under very diverse stimulus conditions, they are likely to have higher levels of ambient craving, irrespective of environmental cues (Shiffman & Paty, 2006), and thus may be less responsive to cues presented in a laboratory-based cue reactivity protocol. Our data support this interpretation.
Fifth, how craving and cue reactivity are defined is a central conceptual issue. It is unclear if craving should be measured a) in isolation to a specific cue alone, b) relative to an independent control/neutral cue (both of which we did in this study), or c) relative to a baseline control (i.e., pre/post assessment), as done in prior studies (Tiffany, Cox, & Elash, 2000; Waters et al., 2004). A legitimate rationale can be made for any or all of these measures of cue-elicited craving and reactivity. Measures that contain some element of control, either via an independent or a baseline control cue, may have the effect of diminishing the measurement of cue-provoked craving. Diminished measurement of craving ultimately undermines any relationship to smoking behavior. Our data clearly demonstrate this point: only unadjusted craving was related to smoking behavior, while adjusted measures were not. The literature lacks a consistent operational definition of craving and cue reactivity, and one recommendation is that future studies define this clearly. Ideally, studies should analyze cue-elicited craving and reactivity using multiple methods, as done here and elsewhere (Donny et al., 2008).
Each of the above interpretations should be considered in the context of our study limitations. Responses to cue reactivity assessment were modest and lower than expected based on a prior review (Carter & Tiffany, 1999). Greater responding to cues, or at least greater between-cue variability of craving, may have led to stronger associations with cigarette smoking. Our methods were such that each participant smoked a cigarette just prior to cue testing, and while this had the benefit of equalizing all participants on time since last cigarette, it too may have diminished cue reactivity. Deprivation prior to cue reactivity assessment can result in larger effects (Carter et al., 2006). Further, while our sample size is large in the context of existing cue reactivity studies, it may not have been large enough to adequately assess the predictive relationship between cue reactivity and smoking behavior. Finally, readers should be mindful that the current analysis was based on a larger parent study that focused on gender and menstrual phase influences on craving and smoking. As such, study eligibility was fairly constrained, and our study sample may not be representative of the larger population of non-treatment seeking smokers.
There are abundant data on real-time assessment of craving and its association with naturally occurring smoking behavior (Shiffman et al., 2002; Stone & Shiffman, 1994). Investigations examining similar relationships between lab-based measures of craving/cue reactivity and real-world smoking are more challenging. On one hand, the value of the cue reactivity paradigm rests in part on its potential to prospectively predict important clinical markers of smoking behavior (or other substance use). Thus, among treatment seekers, cue reactivity should reliably predict relapse (Drummond, 2000a, 2000b). Evidence to date suggests this is the case (Niaura et al., 1988; Shadel et al., 1998; Waters et al., 2004). Among non-treatment seekers, however, the literature is less clear. Our results indicate the relationship between cue reactivity and smoking behavior among non-treatment seeking smokers is complex and difficult to ascertain. By definition, cue reactivity research isolates cues both in context and time, removing the individual from his/her real-world smoking environment. Examination of the link between cue reactivity at time 1 and smoking at time 2 is based to a great degree on the lag between these observations. Additionally, it is possible that smokers who are not seeking treatment may exhibit diminished cue reactivity as compared to smokers who are seeking treatment and who may be at heightened sensitivity to environmental cues. We are unaware of any previous tests of cue reactivity between treatment seekers vs. non-treatment seekers.
On the other hand, one might question the rationale for any prediction between lab-based measures of cue reactivity and the quantity of cigarettes smoked. Cue reactivity may not be a reflection of how much one smokes, but merely rather a marker of stimulus control; i.e., the degree to which environmental cues trigger craving. Indeed, the ambiguous link between craving, cue reactivity and smoking behavior among non-treatment seeking smokers does not diminish the value of cue reactivity research as a whole. The cue reactivity model remains a viable methodology to test treatments for smoking cessation (and other substances of abuse) (cf. Tiffany et al., 2000; Waters et al., 2004). Prior to large and costly clinical trials, novel treatments can be tested on the degree to which they effectively diminish craving. In addition, the cue reactivity model can be used as a treatment modality in and of itself, as in the case of cue-exposure treatment (Conklin & Tiffany, 2002a, 2002b). Readers are cautioned not to interpret the current study as a validity test of cue reactivity. Rather, this study should be considered in the larger context of cue reactivity research, which has a long and sizable literature of support.
Acknowledgments
The authors thank Christine Horne, Ashley McCullough, and Gina Frattarolli with assistance with study procedures. This research was supported by NIDA grants P50 DA016511-02 (Dr. Upadhyaya, PI, component #3; Drs. Kathleen Brady and Ron See, center PIs), K23 DA020482 (Dr. Carpenter), K12 DA000357 (Dr. Gray), and MUSC GCRC grant #M01 RR01070.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrams D, Monti P, Carey K, Pinto R, Jacobus S. Reactivity to smoking cues and relapse: Two studies of discriminant validity. Behavioural Research and Therapy. 1988;26:225–233. doi: 10.1016/0005-7967(88)90003-4. [DOI] [PubMed] [Google Scholar]
- Carpenter M, Upadhyaya H, LaRowe S, Saladin M, Brady K. Menstrual cycle phase effects on nicotine withdrawal and cigarette craving: A review. Nicotine and Tobacco Research. 2006;8:627–638. doi: 10.1080/14622200600910793. [DOI] [PubMed] [Google Scholar]
- Carter B, Robinson J, Lam C, Wetter D, Tsan J, Day S, et al. A psychometric evaluation of cigarette stimuli used in a cue reactivity study. Nicotine and Tobacco Research. 2006;8:361–369. doi: 10.1080/14622200600670215. [DOI] [PubMed] [Google Scholar]
- Carter B, Tiffany S. Meta-analysis of cue reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- Conklin C, Tiffany S. The impact of imagining personalized versus standardized urge scenarios on cigarette craving and autonomic reactivity. Experimental and Clinical Psychopharmacology. 2001;9:399–408. doi: 10.1037//1064-1297.9.4.399. [DOI] [PubMed] [Google Scholar]
- Conklin C, Tiffany S. Applying extinction research and theory to cue-exposure addiction treatments. Addiction. 2002a;97:155–167. doi: 10.1046/j.1360-0443.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- Conklin C, Tiffany S. Cue-exposure treatment: Time for change. Addiction. 2002b;97:1219–1221. doi: 10.1046/j.1360-0443.2002.00205.x. [DOI] [PubMed] [Google Scholar]
- Cox L, Tiffany S, Christen A. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine and Tobacco Research. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Donny E, Griffin K, Shiffman S, Sayette M. The relationship between cigarette use, nicotine dependence, and craving in laboratory volunteers. Nicotine and Tobacco Research. 2008;10:934–942. doi: 10.1080/14622200802133681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond D. Craving research: Future directions. Addiction. 2000a;95(Suppl 2):S247–S255. doi: 10.1080/09652140050111816. [DOI] [PubMed] [Google Scholar]
- Drummond D. Addiction. What does cue-reactivity have to offer clinical research? 2000b;95(Suppl 2):S129–S144. doi: 10.1080/09652140050111708. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for Axis I Disorders, Patient Edition. New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Heatherton T, Kozlowski L, Frecker R, Fagerstrom KO. The Fagerstrom test for nicotine dependence: A revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- LaRowe S, Saladin M, Carpenter M, Upadhyaya H. Reactivity to nicotine cues over repeated cue reactivity sessions. Addictive Behaviors. 2007;32:2888–2899. doi: 10.1016/j.addbeh.2007.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niaura R, Abrams D, Demuth B, Pinto R, Monti P. Responses to smoking-related stimuli and early relapse to smoking. Addictive Behaviors. 1989;14:419–428. doi: 10.1016/0306-4603(89)90029-4. [DOI] [PubMed] [Google Scholar]
- Niaura R, Rohsenow D, Binkoff J, Monti P, Pedraza M, Abrams D. Relevance of cue reactivity to understanding alcohol and smoking relapse. Journal of Abnormal Psychology. 1988;97:133–152. doi: 10.1037//0021-843x.97.2.133. [DOI] [PubMed] [Google Scholar]
- Niaura R, Shadel W, Abrams D, Monti P, Rohsenow D, Sirota A. Individual differences in cue reactivity among smokers trying to quit: Effects of gender and cue type. Addictive Behaviors. 1998;23:209–224. doi: 10.1016/s0306-4603(97)00043-9. [DOI] [PubMed] [Google Scholar]
- Payne T, Smith P, Adams S, Diefenbach L. Pretreatment cue reactivity predicts end-of-treatment smoking. Addictive Behaviors. 2006;31:702–710. doi: 10.1016/j.addbeh.2005.05.053. [DOI] [PubMed] [Google Scholar]
- Payne T, Smith P, Sturges L, Holleran S. Reactivity to smoking cues: Mediating roles of nicotine dependence and duration of deprivation. Addictive Behaviors. 1996;21:139–154. doi: 10.1016/0306-4603(95)00043-7. [DOI] [PubMed] [Google Scholar]
- Sayette M, Martin C, Wertz M, Shiffman S, Perrott M. A multi-dimensional analysis of cue-elicited craving in heavy smokers and tobacco chippers. Addiction. 2001;96:1419–1432. doi: 10.1080/09652140120075152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayette M, Shiffman S, Tiffany S, Niaura R, Martin C, Shadel W. The measurement of drug craving. Addiction. 2000;95(Suppl 2):S189–S210. doi: 10.1080/09652140050111762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadel W, Niaura R, Abrams D. Effect of different cue stimulus delivery channels on craving reactivity: Comparing in vivo and video cues in regular cigarette smokers. Journal of Behavior Therapy and Experimental Psychiatry. 2001;32:203–209. doi: 10.1016/s0005-7916(01)00035-0. [DOI] [PubMed] [Google Scholar]
- Shadel W, Niaura R, Abrams D, Goldstein M, Rohsenow D, Sirota A, et al. Scripted imagery manipulations and smoking cue reactivity in a clinical sample of self-quitters. Experimental and Clinical Psychopharmacology. 1998;6:179–186. doi: 10.1037//1064-1297.6.2.179. [DOI] [PubMed] [Google Scholar]
- Shiffman S. Refining models of dependence: Variations across persons and situations. British Journal of Addiction. 1991;86:611–615. doi: 10.1111/j.1360-0443.1991.tb01817.x. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Gwaltney C, Balabanis M, Liu K, Paty J, Kassel J, et al. Immediate antecedents of cigarette smoking: An analysis from ecological momentary assessment. Journal of Abnormal Psychology. 2002;111:531–545. doi: 10.1037//0021-843x.111.4.531. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty J. Smoking patterns and dependence: Contrasting chippers and heavy smokers. Journal of Abnormal Psychology. 2006;115:509–523. doi: 10.1037/0021-843X.115.3.509. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty J, Gnys M, Kassel J, Hickcox M. First lapses to smoking: Within-subjects analysis of real-time reports. Journal of Consulting and Clinical Psychology. 1996;64:366–379. doi: 10.1037//0022-006x.64.2.366. [DOI] [PubMed] [Google Scholar]
- Smolka M, Buhler M, Klein S, Zimmerman U, Mann K, Heinz A, et al. Severity of nicotine dependence modulates cue-induced brain activity in regions involved in motor preparation and imagery. Psychopharmacology. 2006;184:577–588. doi: 10.1007/s00213-005-0080-x. [DOI] [PubMed] [Google Scholar]
- Sobell L, Sobell M. Alcohol Timeline Followback (TLFB) Users’ Manual. Toronto: Addiction Research Foundation: Addiction Research Foundation; 1996. [Google Scholar]
- Stone A, Shiffman S. Ecological momentary assessment (EMA) in behavioral medicine. Annals of Behavioral Medicine. 1994;16:199–202. [Google Scholar]
- Tiffany S. A cognitive model of drug urges and drug-use behavior: role of automatic and nonautomatic processes. Psychological Review. 1990;97:147–168. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- Tiffany S, Cox L, Elash C. Effects of transdermal nicotine patches on abstinence-induced and cue-elicited craving in cigarette smokers. Journal of Consulting and Clinical Psychology. 2000;68:233–240. doi: 10.1037//0022-006x.68.2.233. [DOI] [PubMed] [Google Scholar]
- Upadhyaya H, Drobes D, Thomas S. Reactivity to smoking cues in adolescent cigarette smokers. Addictive Behaviors. 2004;29:849–856. doi: 10.1016/j.addbeh.2004.02.040. [DOI] [PubMed] [Google Scholar]
- Upadhyaya H, Drobes D, Wang W. Reactivity to in vivo smoking cues in older adolescent cigarette smokers. Nicotine and Tobacco Research. 2006;8:135–140. doi: 10.1080/14622200500432112. [DOI] [PubMed] [Google Scholar]
- Waters A, Shiffman S, Sayette M, Paty J, Gwaltney C, Balabanis M. Cue-provoked craving and nicotine replacement therapy in smoking cessation. Journal of Consulting and Clinical Psychology. 2004;72:1136–1143. doi: 10.1037/0022-006X.72.6.1136. [DOI] [PubMed] [Google Scholar]