Abstract
Activity within fronto-striato-temporal regions during processing of unattended auditory deviant tones and an auditory target detection task was investigated using event-related functional magnetic resonance imaging. Activation within the middle frontal gyrus, inferior frontal gyrus, anterior cingulate gyrus, superior temporal gyrus, thalamus, and basal ganglia were analyzed for differences in activity patterns between the two stimulus conditions. Unattended deviant tones elicited robust activation in the superior temporal gyrus; by contrast, attended tones evoked stronger superior temporal gyrus activation and greater frontal and striatal activation. The results suggest that attention enhances neural activation evoked by auditory pitch deviance in auditory brain regions, possibly through top-down control from the dorsolateral prefrontal cortex involved in goal-directed selection and response generation.
Keywords: Auditory oddball, Automatic attention, Deviance detection, Prefrontal cortex, Superior temporal cortex
INTRODUCTION
Detecting a deviant auditory event within a background of homogeneous auditory events is an ostensibly simple process that is performed rapidly and with relative ease by the human brain. This capacity has evolved in organisms to detect sudden changes in the natural environment that may signal threats to their physical integrity. The neural correlates of this involuntary change detection can be indexed by the event-related potential component, — the mismatch negativity (MMN), and by a distinct hemodynamic response (HDR) in the auditory cortex [1]. On the other hand, the neural correlates of voluntary auditory attention to subjectively relevant information are well studied using the oddball paradigm, which requires detection of, and voluntary response to, infrequent task-relevant targets in a train of frequent task-irrelevant stimuli. Cognitive processing of task-relevant deviant tones under voluntary attention has been associated with a later event-related potential component, the P300. This cognitive process has a well-studied correlate in hemodynamic activity within the prefrontal, medial frontal, midbrain, and parietal regions [2–5].
The neurofunctional systems involved in processing deviant stimuli in the auditory modality are notably different from those in the visual modality [6,7]. Novelty detection in the visual modality tends toward greater top-down executive prefrontal control [4,8] than the auditory modality, where change detection is partially resolved by primary and possibly secondary auditory cortices [2,7,9]. Because deviance detection is quickly resolved in the auditory cortex, it is generally considered to be automatic and independent of attention. However, prior studies have documented both stimulus and task-related variations in neural activity evoked by auditory deviance [2,10,11].
The aim of the present study was to elucidate the effects of voluntary attention on the processing of pitch-deviant tones. We hypothesized that voluntary attention would enhance the processing of pitch-deviant tones by both modulating neural activity in sensory-specific cortical regions and recruiting higher-order fronto-striatal regions. We predicted that frontal regions would be recruited for executive control and response generation to the task-relevant auditory target stimuli and that activation in primary and secondary auditory cortical regions would be further potentiated. We used functional magnetic resonance imaging (fMRI) to examine the processing of auditory pitch deviance in the fronto-striato-temporal system described in previous work [2,4], and to examine the effects of voluntary attention on neural activity within this system.
MATERIALS AND METHODS
Participants
Fourteen right-handed (determined by the Edinburgh Handedness Inventory) [12], neuropsychiatrically healthy participants (eight women) ranging in age from 19 to 33 years (mean±SD, 22.4±3.8) were paid for their participation. Participants provided written informed consent to take part in procedures approved by DUMC and the UNC-CH Institutional Review Boards.
Stimuli and tasks
Participants performed two tasks. During three runs of a forced-choice attended auditory oddball task, participants responded with a unique button press to infrequent pitch-deviant tones (targets) and an alternate button press to frequent standard pitched tones. During seven runs of a passive unattended auditory oddball paradigm, participants performed a visual discrimination task and were instructed to ignore simultaneous presentations of the infrequent pitch-deviant tones and frequent standard tones. Participants responded with their right hand using a fiberoptic response box.
During both conditions, frequently occurring (90%) standard tones (1000 Hz) and infrequently occurring (10%) pitch-deviant tones (1064 Hz) with durations of 68 ms were delivered through headphones at ~85 dB with a stimulus onset asynchrony of 1500 ms. Button assignment was counterbalanced across participants. Time-locked behavioral and fMRI responses were recorded to the deviant tones in both conditions.
Acquisition of magnetic resonance imaging data
Images were acquired on a 1.5 T General Electric Signa scanner with a birdcage-type standard quadrature head coil and an advanced nuclear magnetic resonance echoplanar system. The participants’ heads were positioned along the cantho-meatal line and immobilized using a vacuum cushion and a forehead strap. T1-weighted sagittal scans were used to select 16 contiguous oblique axial slices in plane with the anterior and posterior commissure. Functional images were acquired using a gradient echoplanar sequence (TR=1500 ms, TE=40, flip angle=90°, NEX=1, voxel dimensions 3.75 × 3.75 × 5 mm, imaging matrix 64 ×64 voxels). Functional imaging runs 1–7 (unattended condition) consisted of 200 time points and runs 8–10 (attended condition) consisted of 160 time points. High-resolution T1-weighted anatomical images (3D SPGR, TR=22 ms, TE=5 ms, flip angle=20°, FOV=24 cm voxel dimensions 0.9375 ×0.9375 × 1.5 mm, 256 × 256 voxels, 124 images) were acquired for coregistration and normalization of functional images.
Functional image analysis
Quantitative image analysis was performed using custom MATLAB software (Duke-UNC BIAC, Durham, North Carolina, USA), image normalization using SPM99 (Wellcome Department of Cognitive Neurology, University College London, UK), and image rendering using MRIcro (School of Psychology, University of Nottingham, UK). Head motion was detected by center of mass measurements in three orthogonal planes and all data collected met criteria of less than 1 mm movement in the x, y, and z directions.
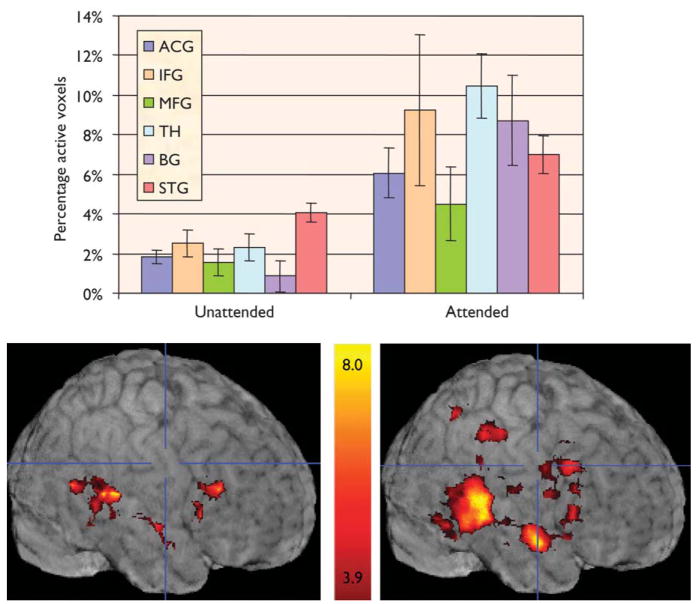
Two methods of image analysis were performed independently. In a whole brain voxel-based approach, individual participant t-maps were generated for each stimulus type (auditory targets and auditory pitch-deviants) by identifying voxels whose average activity correlated with an empirically derived HDR template [13]. For the purposes of t-map creation, 15-image epochs of five images preceding, nine images following, and the image coincident with each target presentation were excised from the functional runs. These epochs were averaged and the mean of the five prestimulus images across all epochs was subtracted from each of the subsequent 10 images to generate baseline adjusted 15-image epochs. Thus, the five images preceding each event (pitch-deviant tone) served as the baseline for the HDR. This baseline controlled for neural activity associated with simple button-press responses accompanying the presentation of the standard tones. The resulting maps from all participants were averaged and these group-averaged t-maps were displayed on a group composite anatomical image (Fig. 1).
Fig. 1.
Percentage of activated voxels across regions and conditions. ACG, anterior cingulate gyrus; IFG, inferior frontal gyrus; MFG, middle frontal gyrus;TH, thalamus; BG, basal ganglia; STG, superior temporal gyrus.
The second approach, consisting of a region of interest (ROI) analysis [13], was conducted independently from the whole brain approach. Regions were manually traced on each participant’s high-resolution coplanar images using anatomical landmarks (LONI, http://www.loni.ucla.edu/) to identify six ROIs: (1) anterior cingulate gyrus (ACG), (2) inferior frontal gyrus (IFG), (3) middle frontal gyrus (MFG), (4) superior temporal gyrus (STG), (5) basal ganglia (BG), and (6) thalamus (TH). T-maps were generated using the previously described epoch-based analysis to identify voxels significantly correlated with an empirically derived HDR template within these ROIs.
For conservative comparison across task conditions, voxels with t>2.5 (p<0.005, uncorrected) were accepted as activated. The average percentage of activated voxels (PAV) and the average percentage signal change (PSC) at each time point in the event epoch were calculated within each ROI and used as dependent measures.
Finally, in order to minimize the dependency of the results on the selected threshold, we performed a cluster-based analysis, following Bosch [14]. Using a minimum cluster size of 20, we generated small 3D ROIs from clusters of activated voxels within the six ROIs. For each individual brain in the group, we computed a mean z-value for all voxels in the corresponding cluster. Mean z-values for the group were compared between attended and unattended auditory conditions. Border voxels were eliminated to avoid systematic reduction of z-values and we defined equivalent size and 3D-shaped ROIs on the basis of the centroid of activation that did not cross anatomical landmarks. The mean z-value from a given cluster ROI from an individual’s data set served as the dependent variable for ANOVA analyses.
RESULTS
Behavioral data
Reaction times and accuracy of behavioral responses to auditory targets were recorded. The participants averaged 77% correct, 20% incorrect, and 2% missing responses [t (13)=8.7, p<0.0001]. The average latency for correct responses was 525 ms, and 318 ms for incorrect responses [t (12)=9.5, p<0.0001; one participant made no errors].
Blood oxygenation-level-dependent activation – cortical regions
Fig. 1 presents the group-averaged images of blood oxygenation-level-dependent activation elicited by unattended and attended pitch-deviant tones. Condition and hemisphere effects were tested for both PSC and PAV for each ROI. Deviant tones evoked strong activation in STG in both conditions, with greater PAV and marginally greater PSC during the attended condition than the unattended condition [PAV, F(1,13)=8.5, p=0.01; PSC, F(1,13)=3.3, p=0.09]. Analysis of only those voxels activated in the unattended condition revealed a significantly greater PSC to attended tones [F(1,13)=26.3, p<0.001]. Finally, a greater proportion of voxels were activated in the right than in the left hemisphere across conditions [F(1,13)=22.2, p<0.001], and this effect was largest for the attended condition [F(1,13)=5.7, p<0.05]. In sum, unattended deviant tones elicited activation in the STG that was enhanced when participants attended and responded to those same tones.
A different pattern of results was observed in analyses of the three frontal ROIs. An overall gyrus effect for PAV [F(2,26)=9.0, p =0.001], with greater spatial extent in the IFG than in the ACG [F(1,13)=8.0, p<0.05] and the MFG [F(1,13)=19.8, p=0.001], was observed. An overall condition effect was observed for both dependent measures, with greater activation observed during the attended condition than the unattended condition [PAV, F(1,13)=24.5, p<0.0001; PSC, F(1,13)=27.1, p<0.0001]. These two main effects interacted such that the condition effect was largest in the IFG [PAV, F(2,26)=14.3, p<0.0001; PSC, F(2,26)=7.8, p<0.01]. Finally, the right hemisphere was more strongly activated than the left hemisphere [PAV, F(1,13)=22.8, p<0.0001; PSC, F(1,13)=12.1, p<0.005], with the largest hemisphere effect observed in the IFG [PSC, F(2,26)=7.4, p<0.01; PAV, F(2,26)=9.6, p<0.01]. In sum, activation to the deviant tone within frontal regions was driven largely by task-relatedness and response generation. The greatest activation within the frontal cortex was observed in the right IFG to the attended condition.
Blood oxygenation-level-dependent activation – midbrain region
Activation within the BG and TH was highly dependent upon the experimental condition. An overall condition effect [PAV, F(1,13)=13.3, p<0.01; PSC, F(1,13)=18, p=0.001] revealed that few voxels were activated by the unattended deviant tones and those voxels produced small PSC; by contrast, in the attended condition, the same tone evoked a greater PAV and PSC.
Dorsal/ventral distribution effects
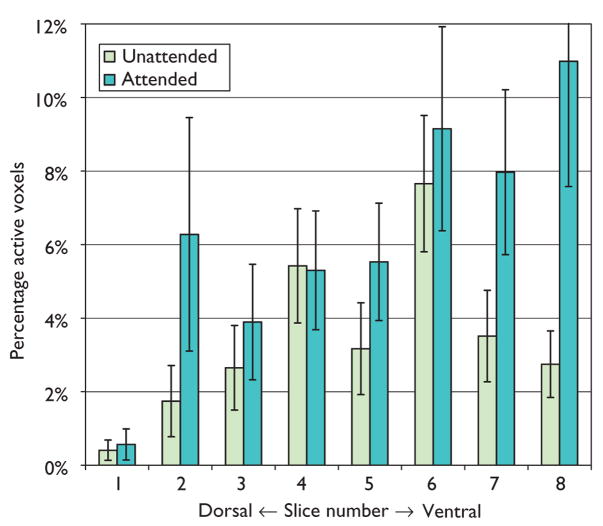
Differences in activation along the dorsal/ventral dimension were assessed by slice number/location on PAV and PSC within the STG and each of the frontal ROIs. As shown in Fig. 2, the spatial extent of activation within the STG increased in more dorsal slices for the unattended condition [slice by condition, F(7,91)=3.7, p<0.05]. For frontal regions, there were no overall slice effects but slice by condition interactions were observed for the MFG and ACG. Within the ACG, a greater PSC was observed in more dorsal slices during the attended condition [F(6,78)=7.3, p<0.001]. For the MFG, PAV decreased from ventral to dorsal slices during the unattended condition but increased from ventral to dorsal slices during the attended condition [F(6,78)=2.3, p<0.05]. Thus, results of this study are in accord with other research documenting recruitment of a dorsal network of brain regions during directed attention tasks [13,15].
Fig. 2.
Percentage of active voxels within the superior temporal gyrus, plotted by axial slice number and condition. Slice 4 is coincident with the anterior commissure; slices 1–3 are inferior to, and slices 5–8 are superior to slice 4 in 5-mm increments.
Cluster-based analysis
A cluster-based analysis was performed on each of the ROIs. Analysis of the cluster within the STG showed that deviant tones evoked strong activation across both conditions, but greater activation to the attended condition than the unattended condition [t(13)=2.4, p<0.02]. Activation in frontal clusters (ACG, IFG, MFG) was highly dependent upon the experimental condition with greater mean z-values to the attended condition [F(2, 52)=4.9, p<0.04]. Similarly, the striatal clusters had greater mean z-values to attended auditory stimuli [F(1,26)=9.3, p<0.01].
DISCUSSION
A network of frontal, striatal, and auditory ROIs was analyzed to enable an assessment of the HDR during automatic and controlled auditory change detection. Unattended pitch-deviant tones elicited reliable activation in the STG, with minimal frontal cortical engagement of the IFG. By contrast, when attended, deviant tones elicited greater activation in all regions interrogated. The condition effects observed in STG activation suggest that task-relatedness and cognitive control enhance automatic responses to deviant stimuli through feedback effects to frontal regions recruited by voluntary attention to auditory stimuli. Previous research documents increases in auditory cortex activation as attention is parametrically increased during the presentation of syllables [16]. Further, a more demanding dichotic listening task recruits a more extensive fronto-temporal network than monaural listening [17]. In sum, one of the functions of top-down attention is to enhance low-level, automatic sensory processing of novel, deviant stimuli. Combined functional connectivity analysis and diffusion tensor imaging, along with subdivision of the STG into smaller functional units relevant to attentional systems, could provide more direct evidence to support this hypothesized feedback connection.
Our findings of fronto-temporal activation during involuntary auditory attention are consistent with electrophysiological and magnetoencephalography studies examining the temporal and spatial properties of neural activity evoked by unattended pitch-deviant tones. Source localization of event-related potential and magnetoencephalography measures of the MMN have consistently described dual frontal and temporal sources of the component. Numerous studies have shown that the MMN may be subject to attentional control under certain conditions [18–22]. The emergence, documented in this study, of a stronger frontal and midbrain component with voluntary attention suggests the recruitment of higher order processing modules, and further supports the proposition that the frontal component primarily contributes to the attentional shifting aspects of the MMN.
One outstanding question raised by this study is how the apparently automatic response to pitch deviance might be affected by divided attention tasks. Would the automatic response increase or dissipate if the participant was engaged in a task that required attention to a different auditory channel? Results from our laboratory suggest that feedback between sensory and frontal regions can modulate the automatic processing of auditory novelty during a visual attention task (Yucel et al., submitted to Journal of Cognitive Neuroscience). Together, these results converge on a description of a network of brain regions that balance bottom-up and top-down influences on attention.
CONCLUSION
The present results are compatible with prevailing cognitive theory and empirical knowledge. They demonstrate that voluntary attention and response generation to deviant auditory stimuli result in significantly greater superior temporal cortical activation than is observed during unattended automatic processing. They demonstrate that the enhancement of activity within the superior temporal cortex that results from voluntary attention is mediated by activity within fronto-striatal brain regions involved in stimulus discrimination and task generation that feeds back to auditory brain regions.
Acknowledgments
This work was supported by grants NIMHMH58251, NINDSNS41328, and NARSAD. Special thanks to Jennifer Foster, Christopher Petty, and Joshua Bizzell for data analysis and software tools.
References
- 1.Opitz B, Rinne T, Mecklinger A, von Cramon DY, Schroger E. Differential contribution of frontal and temporal cortices to auditory change detection: fMRI and ERP results. Neuroimage. 2002;15:167–174. doi: 10.1006/nimg.2001.0970. [DOI] [PubMed] [Google Scholar]
- 2.Opitz B, Mecklinger A, Friederici AD, von Cramon DY. The functional neuroanatomy of novelty processing: integrating ERP and fMRI results. Cereb Cortex. 1999;9:379–391. doi: 10.1093/cercor/9.4.379. [DOI] [PubMed] [Google Scholar]
- 3.Menon V, Ford JM, Lim KO, Glover GH, Pfefferbaum A. Combined event-related fMRI and EEG evidence for temporal-parietal cortex activation during target detection. Neuroreport. 1997;8:3029–3037. doi: 10.1097/00001756-199709290-00007. [DOI] [PubMed] [Google Scholar]
- 4.Stevens AA, Skudlarski P, Gatenby JC, Gore JC. Event-related fMRI of auditory and visual oddball tasks. Magn Reson Imaging. 2000;18:495–502. doi: 10.1016/s0730-725x(00)00128-4. [DOI] [PubMed] [Google Scholar]
- 5.Kiehl KA, Liddle PF. Reproducibility of the hemodynamic response to auditory oddball stimuli: a six-week test–retest study. Human Brain Map. 2003;18:42–52. doi: 10.1002/hbm.10074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoshiura T, Zhong J, Shibata DK, Kwok WE, Shrier DA, Numaguchi Y. Functional MRI study of auditory and visual oddball tasks. Neuroreport. 1999;10:1683–1688. doi: 10.1097/00001756-199906030-00011. [DOI] [PubMed] [Google Scholar]
- 7.Halgren E, Marinkovic K, Chauvel P. Generators of the late cognitive potentials in auditory and visual oddball tasks. Electroencephalogr Clin Neurophysiol. 1998;106:156–164. doi: 10.1016/s0013-4694(97)00119-3. [DOI] [PubMed] [Google Scholar]
- 8.Tales A, Newton P, Troscianko T, Butler S. Mismatch negativity in the visual modality. Neuroreport. 1999;10:3363–3367. doi: 10.1097/00001756-199911080-00020. [DOI] [PubMed] [Google Scholar]
- 9.O’Leary DS, Andreasen NC, Hurtig RR, Kesler ML, Rogers M, Arndt S, et al. Auditory attentional deficits in patients with schizophrenia. A positron emission tomography study. Arch Gen Psychiatry. 1996;53:633–641. doi: 10.1001/archpsyc.1996.01830070083013. [DOI] [PubMed] [Google Scholar]
- 10.Petkov CI, Kang X, Alho K, Bertrand O, Yund EW, Woods DL. Attentional modulation of human auditory cortex. Nat Neurosci. 2004;7:658–663. doi: 10.1038/nn1256. [DOI] [PubMed] [Google Scholar]
- 11.Doeller CF, Opitz B, Mecklinger A, Krick C, Reith W, Schroger E. Prefrontal cortex involvement in preattentive auditory deviance detection: neuroimaging and electrophysiological evidence. Neuroimage. 2003;20:1270–1282. doi: 10.1016/S1053-8119(03)00389-6. [DOI] [PubMed] [Google Scholar]
- 12.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 13.McCarthy G, Luby M, Gore J, Goldman-Rakic P. Infrequent events transiently activate human prefrontal and parietal cortex as measured by functional MRI. J Neurophysiol. 1997;77:1630–1634. doi: 10.1152/jn.1997.77.3.1630. [DOI] [PubMed] [Google Scholar]
- 14.Bosch V. Statistical analysis of multi-subject fMRI data: assessment of focal activations. J Magn Reson Imaging. 2000;11:61–64. doi: 10.1002/(sici)1522-2586(200001)11:1<61::aid-jmri9>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 15.Linden DE, Prvulovic D, Formisano E, Vollinger M, Zanella FE, Goebel R, et al. The functional neuroanatomy of target detection: an fMRI study of visual and auditory oddball tasks. Cereb Cortex. 1999;9:815–823. doi: 10.1093/cercor/9.8.815. [DOI] [PubMed] [Google Scholar]
- 16.Jancke L, Mirzazade S, Shah NJ. Attention modulates activity in the primary and the secondary auditory cortex: a functional magnetic resonance imaging study in human subjects. Neurosci Lett. 1999;266:125–128. doi: 10.1016/s0304-3940(99)00288-8. [DOI] [PubMed] [Google Scholar]
- 17.Jancke L, Shah NJ. Does dichotic listening probe temporal lobe functions? Neurology. 2002;58:736–743. doi: 10.1212/wnl.58.5.736. [DOI] [PubMed] [Google Scholar]
- 18.Paavilainen P, Tiitinen H, Alho K, Naatanen R. Mismatch negativity to slight pitch changes outside strong attentional focus. Biol Psychol. 1993;37:23–41. doi: 10.1016/0301-0511(93)90025-4. [DOI] [PubMed] [Google Scholar]
- 19.Naatanen R, Paavilainen P, Tiitinen H, Jiang D, Alho K. Attention and mismatch negativity. Psychophysiology. 1993;30:436–450. doi: 10.1111/j.1469-8986.1993.tb02067.x. [DOI] [PubMed] [Google Scholar]
- 20.Alain C, Woods DL. Attention modulates auditory pattern memory as indexed by event-related brain potentials. Psychophysiology. 1997;34:534–546. doi: 10.1111/j.1469-8986.1997.tb01740.x. [DOI] [PubMed] [Google Scholar]
- 21.Woldorff MG, Hackley SA, Hillyard SA. The effects of channel-selective attention on the mismatch negativity wave elicited by deviant tones [see comment] Psychophysiology. 1991;28:30–42. doi: 10.1111/j.1469-8986.1991.tb03384.x. [DOI] [PubMed] [Google Scholar]
- 22.Woldorff MG, Hillyard SA, Gallen CC, Hampson SR, Bloom FE. Magnetoencephalographic recordings demonstrate attentional modulation of mismatch-related neural activity in human auditory cortex. Psychophysiology. 1998;35:283–292. doi: 10.1017/s0048577298961601. [DOI] [PubMed] [Google Scholar]