Abstract
The availability of genetically altered animal models of human disease for basic research has generated great interest in new imaging methodologies. Digital subtraction angiography (DSA) offers an appealing approach to functional imaging in small animals because of the high spatial and temporal resolution, and the ability to visualize and measure blood flow. The micro-injector described here meets crucial performance parameters to ensure optimal vessel enhancement without significantly increasing the total blood volume or producing overlap of enhanced structures. The micro-injector can inject small, reproducible volumes of contrast agent at high flow rates with computer-controlled timing synchronized to cardio-pulmonary activity. Iterative bench-top and live animal experiments with both rat and mouse have been conducted to evaluate the performance of this computer-controlled micro-injector, a first demonstration of a new device designed explicitly for the unique requirements of DSA in small animals. Injection protocols were optimized and screened for potential physiological impact. For the optimized protocols, we found that changes in the time-density curves for representative regions of interest in the thorax were due primarily to physiological changes, independent of micro-injector parameters.
Index Terms: contrast agent, digital subtraction angiography functional imaging, rodent, x-ray
I. INTRODUCTION
The increasing use of small animals in basic research has generated great interest in new approaches to small animal imaging—particularly magnetic resonance microscopy, microPET, microSPECT, optical imaging, and microCT. Functional imaging in small animals can be addressed particularly well using x-ray digital subtraction angiography (DSA), given the ease of use and its ability to capture rapid physiological changes in blood flow [1]. Extensive work ranging from initial studies first suggested by Mistretta et al. in the 1970s to evaluating the efficacy of subtraction angiography in clinical diagnosis has been done in canines, porcines, and humans [2–12]. Scaling DSA to the higher temporal and spatial resolutions encountered in the rodent requires unique approaches for an optimal small animal DSA imaging system. A 100 kg human is approximately 4000 times larger than a 25 gm mouse. Thus, one must scale the spatial resolution accordingly from millimeters to microns. In addition, the mouse heart beats at ~600 beats per minute (bpm), nearly ten times more rapid than the human heart. The drive for higher spatial and temporal resolution to capture rapid physiologic changes has led to the development of the micro power contrast injector described in this paper. Prior work in mice and rats [2, 13–21] has not fully optimized the methods for small animals. For example, previous images were acquired asynchronous with cardiac or ventilatory cycles, resulting in limited precision in measuring physiologic changes and significant subtraction artifacts. Contrast injections were given manually at low or varying flow rates. These deficiencies in prior work made quantitative measurements of blood flow unreliable. In addition, some studies used significant contrast injection volumes—up to 50% of the total blood volume and would alter the physiology unfavorably.
This study explores a computer-controlled power injector that allows quantitative blood flow measurements through functional DSA imaging of mice and rats with minimal physiological impact. A number of injection parameters are crucial to produce a quality subtraction angiogram. These include a tight (temporal) bolus with a small volume and high flow rate combined with appropriate catheter placement [3, 9, 10, 22]. Large contrast volumes with slow flow at peripheral catheter locations result in spread and dilution of the contrast bolus. The bolus dilution contributes to increase in blood volume from contrast because one must inject more contrast agent to achieve adequate enhancement. This can lead to retrograde flow and the inability to separate left/right heart and lung [2, 4, 5, 15, 17]. The computer-controlled system described here can inject small volumes of contrast agent at high flow rates with high reproducibility at very precise times in the physiological cycles. Injection volumes and flow rates were optimized to produce DSA images capable of distinguishing flow in overlapping vessels. Pulmonary flow could be measured with contrast volumes as low as 1% of the total blood volume in the mouse and at less than 1% of the volume in the rat. This opens the possibility of novel DSA methods to quantify real-time changes in blood flow. The injector has already been applied to a number of x-ray and MRI studies for vasculature imaging, perfusion, and flow measurements [23–27].
II. MATERIALS AND METHODS
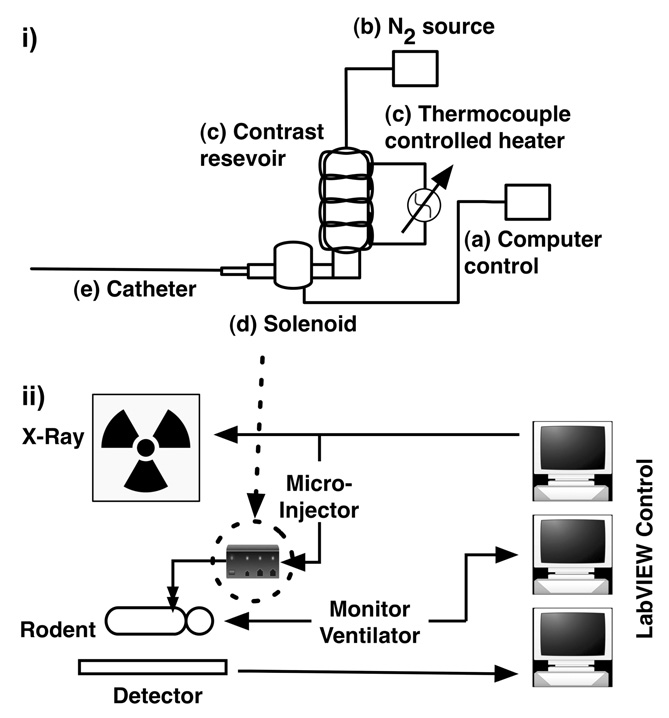
The system (Figure 1-i) is designed to inject precisely controlled amounts of contrast (Isovue 370, 370mg iodine/mL, Bracco Diagnostics, Princeton, NJ) at high rate and at specific points in the breathing and cardiac cycles. Timing is controlled through LabVIEW software (National Instruments, Austin, TX) (Figure 1-ii) that allows reproducible event-driven sequences that links image acquisition, contrast injection, and physiologic control and monitoring. When a trigger is received (Figure 1-i, a), a pressurized N2 supply (b) pushes contrast through a zero dead volume direct lift solenoid valve (d) (Cole Parmer, Vernon Hills, IL). The trigger is provided by the LabVIEW software and a TTL generating PCI-6602 counter/timer board (National Instruments, Austin, TX). This specific solenoid was chosen because it had five important characteristics: 1) it is able to withstand high pressures (up to 100 PSI); 2) it is easy to clean; 3) it supports high flow rates; 4) it holds no contrast volume in the valve chamber, which eliminates dead volume; and 5) it has a rapid (milliseconds) response time. These solenoid characteristics allow repeatable delivery of small injection volumes at high flow rates. A custom tapered catheter was constructed for the mouse. A commercial catheter was used for the rat. Isovue 370 has a viscosity 20.9-times that of water [28] making injections at high flow rates in rodent-sized catheters a challenge. To address this challenge, catheters were chosen for maximum lumen size, while still being small enough for cannulization [29]. We developed tapered catheters to support the required higher flow rates. Catheter lengths were shortened as much as possible to reduce flow resistance, while still allowing adequate mobility for the surgical cannulization procedure. For the mouse studies, the custom catheter (Figure 2) was made from polyethylene (PE) tubing of various interconnecting sizes (PE-190, -90, -50, -10). In the rat studies, a shortened (13.5 cm from catheter tip to the end of the stub adapter) polyurethane catheter was used (PU-50 Chronic-Cath, CC-3P, Access Technologies, Skokie, IL).
Fig. 1.
Micro-injector components (i): (a) trigger input, (b) air supply, (c) temperature-controlled contrast reservoir, (d) zero-dead-volume direct lift solenoid, and (e) rotating Luer fitting for connection to custom injector catheters. Pane (ii) shows a schematic overview of the x-ray system A LabVIEW-based system integration allowed for reproducible event-driven imaging sequences that linked image acquisition, contrast injection, and physiologic control and monitoring.
Fig. 2.
The custom catheter (not to scale) designed for the mouse was constructed from a series of polyethylene catheter segments glued together cascading from a large lumen (PE-190) for supporting a high injection flow rate to a small diameter lumen (PE-10) so that cannulization was possible.
The contrast resides in a heated reservoir (Figure 1-i, c) between the solenoid and N2 supply. The temperature is maintained at 37°C to reduce the viscosity of Isovue 370 [28] from 20.9 to 9.4 mPa•s. The heating also alleviates temperature shock when injecting into the small animal. All the parts of the micro-injector have quick-disconnect fittings to allow quick cleaning and maintenance. The combination of the specific components allowed us to design and implement a micro-contrast power injector that could deliver small bolus injection volumes at high flow rates with computer controlled timing.
Bench-top and live animal x-ray imaging experiments were conducted to measure the injection volumes under various scenarios, the reproducibility of the injections, the smallest amount of contrast agent detectible by our system, and the physiologically-mediated effects of contrast injection. We found optimum injection volumes and flow rates that allowed us to see non-overlapping, contrast-enhanced blood flow for live animals.
A. Bench-top Experiment
Tests were conducted to characterize the micro-injector injection volumes and flow rates with various combinations of catheter lengths and lumen sizes, driving pressures, and length of time the solenoid valve was opened. Injection volumes and flow rates were determined by a mass-difference analysis. For each combination, the mass of 50–60 micro-centrifuge tubes was measured before and after the addition of injected contrast. The mass difference was divided by the contrast density (1.41 g/cm3), resulting in the volume of injected contrast. The flow rate was computed by dividing the newly found injection volume with the duration of the solenoid activation window, mathematically expressed as:
| (1) |
| (2) |
Linear regression was applied to injection volume and flow curves in respect to varying solenoid active window at a constant 80PSI driving pressure and varying driving pressure at a constant 50ms solenoid active window. Coefficient of determination (r2) values were then found for the linear fits.
B. Live Animal Experiment
The radiographic system (Figure 1-ii) constructed for this work included an 80 kW generator (Phillips CXP) with a 0.3/1.0 mm focal spot W anode tube. Images were acquired with x-ray techniques optimized for small animal DSA [23]. Typical exposure parameters were 70kVp, 200mA, and 10ms. The tube was mounted on a C arm constructed from extruded aluminum. A flexible carbon fiber table designed for rodents floated above a 95×95mm cooled CCD detector (ImageStar, Photonic Science, East Sussex, England) with a 46×46µm pitch and a 15mg/cm2 (~45µm thick) Gd2O2S scintillator. The system was controlled by two computers running custom-written control software (LabVIEW) that were linked together to support synchronization of image acquisition with physiologic control [30]. Exposures, ventilation, injection, and image capture can be triggered individually with this software to allow a variety of sequences synchronized with physiological parameters [24]. For this study, images were acquired at every heartbeat with end-expiration apnea, with one contrast injection for each sequence. For the rat studies, pixels were binned at 2×2 producing an effective resolution of 92×92 µm. No binning was used for the mouse images resulting in an effective resolution of 46 µm. Images were logarithmically subtracted [31].
Experiments were conducted on the live animal to find the appropriate injection volumes and to characterize the performance of the micro-injector. Regions of interest (ROIs) included the pulmonary artery, lung parenchyma, left ventricle, and aorta. The heart, coronary arteries, and kidney vasculature were also imaged to show other uses of the injector.
A series of injection volumes, ranging from 50 to 1000µL, was performed on the rat to define the linear range of enhancement of the blood vessels. Performance of the system was characterized with the following concerns: limited increase in total blood volume (<10%), non-enhancement of overlapping structures; no enhancement due to second pass of contrast; a contrast-to-noise-ratio (CNR) ≥ 5 (Rose criterion) [32]. The CNR was measured by:
| (3) |
where SC was the DSA signal in an enhanced blood vessel, SNC was the signal of the background, and σSNC was the standard deviation in a region of the image in which there is no structured background. This standard deviation is in part due to the Poisson statistical nature of x-ray production. The aorta was chosen for CNR measurements because in an anterior-posterior projection, this vessel does not overlap any other vessels.
Once an injection volume that produced good enhancement of the blood vessels was found, we studied variability in physiology arising from the injection parameters. Six DSA runs were performed with injection volumes increasing from 50 to 150µL and then back down to 50µL in 50µL gradations. The contrast injection was performed at the QRS of the cardiac cycle, and images were acquired at every QRS at end-expiration apnea. To measure the repeatability and any variability in imaging physiology arising from the injection parameters, a nonparametric deconvolution technique using singular value decomposition (SVD) was used to find relative pulmonary blood volumes (PBV), pulmonary blood flows (PBF), and mean transit times (MTT) of the left pulmonary artery, right lung parenchyma, left ventricle, and aorta. The SVD technique used was based on work done by Ostergaard et al. [33]. The pulmonary artery root was used as the arterial input function. Coefficient of variations (4) were calculated for PBV, PBF, and MTT from the six injections for each ROI. The coefficient of variation (Cv) was measured as:
| (4) |
where σ is the standard deviation and μ is the mean. In addition, p-values (one-way ANOVA) were determined by comparing the MTT between the various injection volumes within the same ROIs.
Additional imaging studies were performed to demonstrate synchronization across the phases of the cardiac cycle. In the first study, imaging was performed at different intervals (systole, diastole, and diastasis) with contrast injections at the same point (QRS) in the cardiac cycle. In the second study, instead of injecting during a fixed time point in the cardiac cycle, contrast injections were first made at the QRS, and then 30, 50, 60, and 75% of the R-R interval after the QRS with imaging at a fixed time point, the QRS. The anatomic target was the coronary arteries. Finally, DSA images of the mouse kidney were acquired to demonstrate the utility of the micro-injector in the smaller (25 gm) model. The target organ was the kidney with injection via the iliac artery.
All animal studies were conducted with approval of the Duke Institutional Animal Care and Use Committee. Right jugular catheters (tapered 2F at tip for mice (Figure 2) and 3F for rats were placed in female mice (25–30g, C57BL/6) and rats (160–190g, Fischer 344) for cardio-pulmonary studies. 3F catheters were placed in the carotid artery at the level of the aortic arch for rat coronary vessel imaging. The kidney vasculature in the mouse was visualized using the tapered catheter inserted through the left iliac artery so that the tip was at the level just distal to the left renal artery. Animals were anesthetized with Nembutal (50mg/kg, IP, Abbott Laboratories, North Chicago, IL) and butorphanol (2mg/kg IP, Fort Dodge Animal Health, Fort Dodge, IA), perorally intubated, and mechanically ventilated at 60 (rat) and 90 (mouse) breaths per minute with a tidal volume of 0.3–0.4ml for mice and 1.5–1.7ml for rats. Anesthesia was maintained with Isoflurane (1–3%, Halocarbon Products Corporation, River Edge, NJ). Body temperature was measured with a rectal thermocouple and was maintained at constant levels (37±0.1°C) with a heat lamp controlled via feedback from the thermocouple. Solid-state transducers on the breathing valve measured airway pressure and flow [34, 35]. Pediatric electrodes were taped on the footpads for ECG. All physiologic signals were continuously collected (Coulbourn Instruments, Allentown, PA) and displayed on a computer using custom LabVIEW software for the duration of the experiment. These signals were also used to control the cardio-ventilatory gating described earlier. At the conclusion of the studies, the animals were euthanized with an overdose of anesthesia.
III. RESULTS
A. Bench-top Experiment
The characteristics of the micro-injector found in the bench-top experiments are shown in Table 1. Figure 3 and Figure 4 graphically show injection volumes and flow rates as a function of the solenoid active window at constant driving pressure and the performance when changing the driving pressure while keeping a constant solenoid active window. Each point is the mean of 50–60 measurements. The r2 was > 0.99 for the linear regressions of injection volume vs. solenoid active time windows at a constant driving pressure. The flow rate remained constant from 50 to 200ms. The r2 for the linear regressions that mapped injection volume and flow rate vs. driving pressure at a constant injection duration was also > 0.99. Figure 4 demonstrates this linearity.
Table 1.
Micro-injector injection volume and flow rates for rat and mouse catheter, as a function of injection time, and driving pressure. Figure 3 and Figure 4 graphically represent these data
| Mouse DSA | Rat DSA | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| ID (mm) | 0.28 at tip | 0.6 | |||||||
| Length (mm) | 63 | 130 | |||||||
| Window (ms) | 50 | 100 | 150 | 50 | 50 | 50 | 50 | 100 | 200 |
| Pressure (PSI) | 80 | 80 | 80 | 5 | 10 | 20 | 80 | 80 | 80 |
| Volume (µL) | 9.2 ±1.9 |
12.5 ±1.4 |
18.4 ±4.4 |
5.7 ±0.9 |
7.5 ±1.4 |
15.4 ±2 |
47.1 ±3.9 |
91.7 ±11.5 |
190.2 ±20.5 |
| Rate (µL/ms) | 0.18 ±0.038 |
0.12 ±0.014 |
0.12 ±0.022 |
0.11 ±0.002 |
0.15 ±0.003 |
0.31 ±0.006 |
0.94 ±0.019 |
0.92 ±0.009 |
0.95 ±0.005 |
Fig. 3.
Mouse and rat catheter injection volumes and flow rates for various solenoid active window durations at constant driving pressure (80 PSI). Each data point consisted of 50–60 measurements. The curves include ±1 standard deviation for each data point (only the rat injection volume and mouse injection flow rate had standard deviation bars that were visible. For the other cases, the absolute variances were too small and are not visible). There is a linear response of injection volumes and flow rates to the solenoid active windows as seen by the r2 values (>0.99) in a linear fit.
Fig. 4.
Injection volumes and flow rates for 5, 10, 20 and 80 PSI driving pressures at constant 50ms solenoid active window are shown for the rat. Each data point consisted of 50–60 measurements. The curves include ±1 standard deviation for each data point. There is a linear response of injection volumes and flow rates to the driving pressure as seen by the r2 values (>0.99) in a linear fit.
B. Live Animal Experiment
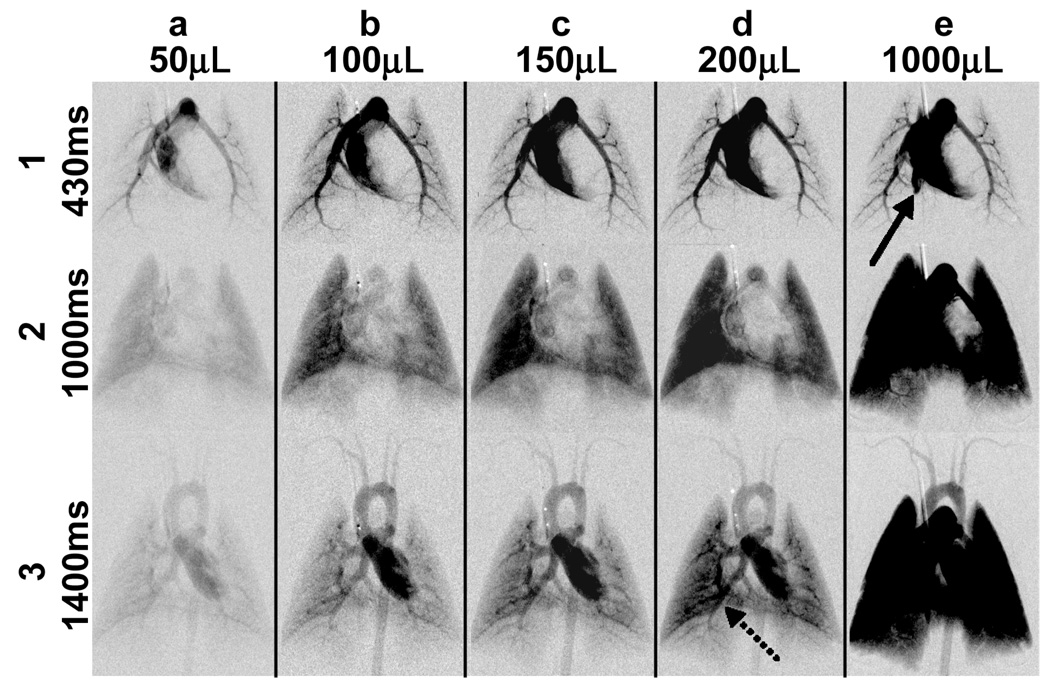
Figure 5 shows enhancement of the pulmonary circulation in the rat, especially in the parenchyma, with increasing volumes of contrast. Note that injection volumes larger than 150µL created overlap of enhanced vessels that can inhibit accurate measures of circulation. As the injection duration becomes longer than a heart cycle, there is an increase in the duration of the bolus, which increases overlap of enhanced vessels.
Fig. 5.
DSA time sequence images for the rat after contrast injection using 50, 100, 150, 200, and 1000µL injection volumes (columns a to e) at the same points in its physiologic cycles of 430, 100, and 1400ms (rows 1–3) in the same animal show increasing opacification at all time points with larger injection volumes. There was more enhancement of the distal vessels and parenchyma with larger injection volumes. However, too large a volume created retrograde flow (solid arrow in e1) and significant overlapping of enhanced structures (dotted arrow in d-3, and also e-2 and e-3). In addition, there was significant increase in total blood volume. These factors suggested injections volumes between 50–150µL (columns a to c). This also allowed the contrast to be injected within one heartbeat.
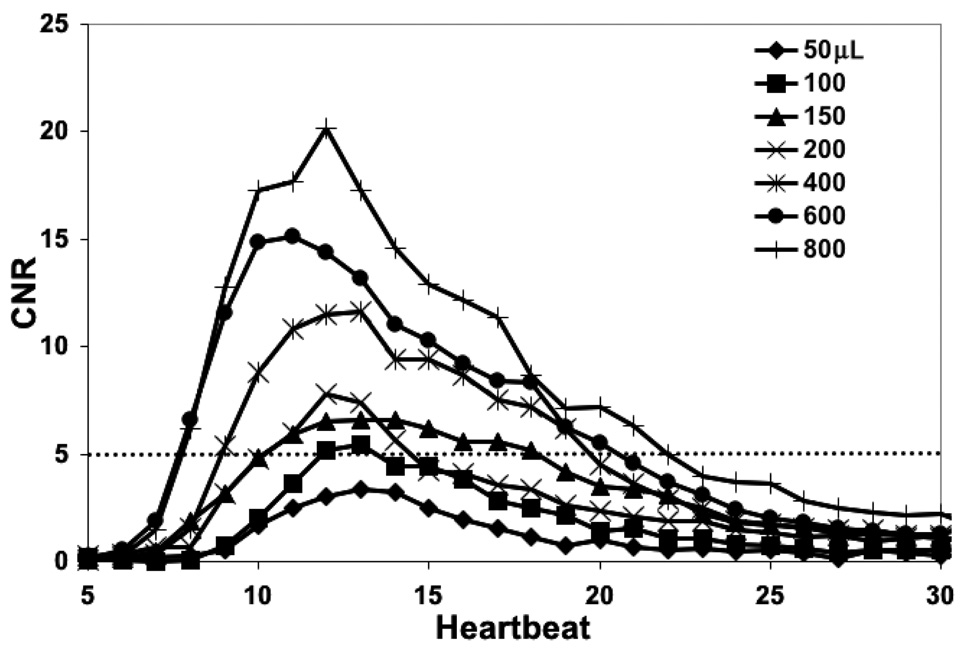
The CNR between the aorta of the rat and the background as a function of time is shown for various injection volumes in Figure 6. As expected, the CNR increases with larger contrast volume. The rise and fall in the individual CNR curves is observed as the contrast enters and passes the region of interest in the aorta. Injection volumes ≥ 00µL meet the Rose criterion for detection at their peak, i.e. a contrast to noise ratio ≥ 5 [32].
Fig. 6.
The contrast-to-noise-ratio (CNR) in the subtracted image of the rat aorta peaks above 5 for injection volumes greater than 100µL. The aorta was chosen because there is no overlap of contrast-enhanced vessels in the projection image. The dotted horizontal line represents the Rose criterion. This suggested injection volumes ≥ 100µL would be suitable in the rat.
Time-density curves of the left pulmonary artery, right lung parenchyma, left ventricle, and aorta seen in Figure 7 show that the changes in vessel enhancement for injection volumes ranging from 50–150µl (Figure 8). Note the almost identical overlap of the time-density curves for the same pairs of injection volumes when systematically increasing the injection volume from 50µL to 150µL and then back down from 150µL to 50µL. The coefficient of variation measured from the SVD-derived PBV, PBF, and MTTs (Table 2) showed little change with the three pairs of injection volumes. The greatest Cv was < 22% with the majority being between 0.04–8.2%. There was no statistically significant difference in the MTT between injection volumes as measured by one-way ANOVAs: p-value = 0.817 (left pulmonary artery) and 0.816 (right lung parenchyma, left ventricle, and aorta). The peak gray intensity value enhancement of the time-density curve scaled linearly with increasing injection volumes.
Fig. 7.
Minimum intensity projection of a typical rat DSA run that included 30 images with regions of interest used for the time-density curve measurements shown in Figure 8 and singular value decomposition (SVD) based calculation of pulmonary blood volume, blood flow, and mean transit time. The pulmonary artery root was used as the arterial input function for the SVD calculations.
Fig. 8.
Time density curves of the rat in the left pulmonary artery (a), right lung parenchyma (b), left ventricle (c), and aorta (d). The regions of interest used are shown in Figure 7. As expected, there was a shift in the curves as the contrast moves further along the vasculature. Note the repeatability and scalability of the injector with injection volumes starting from 50µL (i), increasing to 100 (ii), and then 150µL (iii), and then going from 150µL back down to 100, and finally 50µL.
Table 2.
Pulmonary blood volume (PBV), pulmonary blood flow (PBF), and mean transit time (MTT) averages, ±1 standard deviations, and coefficients of variation (Cv) of all injection volumes (50, 100, and 150µL pairs) for each ROI in Figure 7. The PBV, PBF, and MTT values were calculated using SVD of the time density curves in Figure 8. The pulmonary artery root was used as the arterial input function. Note how the coefficients of variation are all < 22%, with most being between 0.04–8.2%. In addition, the change in MTTs between the same ROIs for each injection volume was statistically insignificant (all p-values ≥ 0.816)
| PBV, PBF, and MTT values: avg ± 1 SD |
PBV (µL) | Cv (%) | PBF (µL/100µL/sec) |
Cv (%) | MTT (sec) | Cv (%) |
|---|---|---|---|---|---|---|
| 50µL inj. | ||||||
| Left pul. artery | 0.86±0.012 | 1.5 | 1.88±0.12 | 6.4 | 0.47±0.030 | 6.4 |
| R. lung tissue | 1.01±0.078 | 7.7 | 1.09±0.05 | 4.8 | 0.96±0.025 | 2.6 |
| Left ventricle | 1.61±0.89 | 5.5 | 1.58±0.0.13 | 7.9 | 1.04±0.140 | 13.5 |
| Aorta | 0.71±0.034 | 4.7 | 0.77±0.13 | 17.1 | 0.95±0.207 | 21.9 |
| 100µL inj. | ||||||
| Left pul. artery | 1.00±0.005 | 0.5 | 2.36±0.042 | 1.8 | 0.44±0.014 | 3.3 |
| R. lung tissue | 1.02±0.003 | 0.3 | 1.20±0.022 | 1.9 | 0.89±0.010 | 1.2 |
| Left ventricle | 1.82±0.110 | 6.0 | 1.84±0.032 | 1.7 | 1.00±0.079 | 7.9 |
| Aorta | 0.69±0.022 | 3.1 | 0.76±0.0002 | 0.04 | 0.92±0.029 | 3.1 |
| 150µL inj. | ||||||
| Left pul. artery | 1.05±0.025 | 2.4 | 2.38±0.196 | 8.2 | 0.46±0.053 | 11.5 |
| R. lung tissue | 1.02±0.013 | 1.2 | 1.22±0.032 | 2.6 | 0.87±0.048 | 5.5 |
| Left ventricle | 1.85±0.004 | 0.2 | 1.76±0.017 | 0.9 | 1.06±0.013 | 1.2 |
| Aorta | 0.69±0.017 | 2.5 | 0.83±0.043 | 5.2 | 0.84±0.022 | 2.6 |
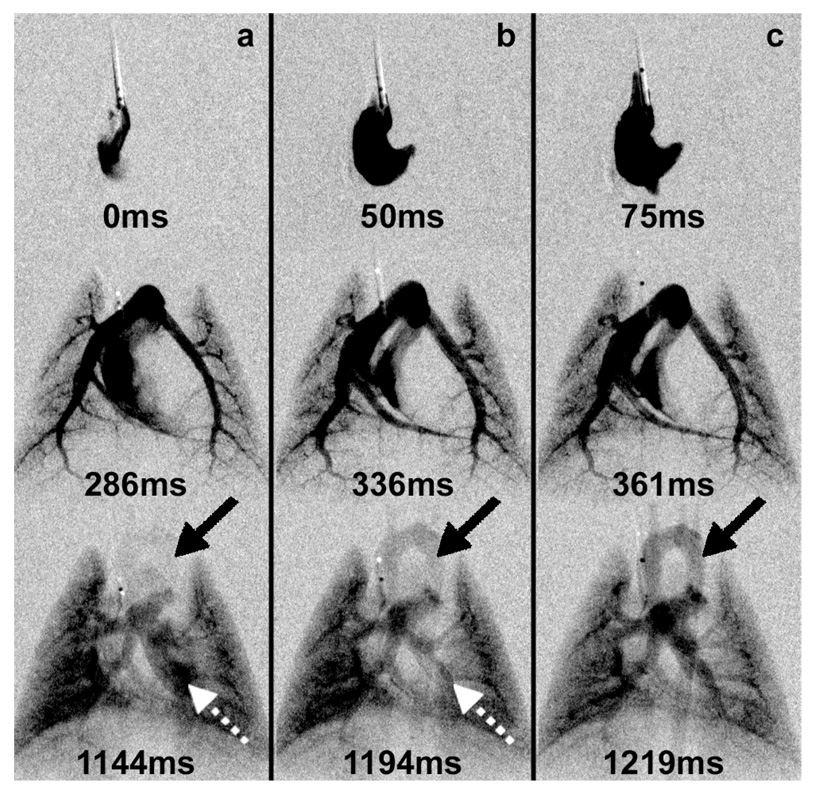
The response time of the injector is a critical element of its performance. The R-R interval of a rat is ~150ms and the readout for the camera is ~100ms. While each radiographic exposure is ~10ms, the camera readout might limit the temporal resolution to 100ms. Yet the reproducibility of the injection allows us to make three different injections at the same point in the cardiac cycle with the 10ms radiographic exposure adjusted to capture three time points separated by as little as 25ms. Thus, row 1-a (Figure 9) shows the right ventricle for injection 1 at the QRS (systole). Row 1-b shows the right ventricle 50ms (diastole) after the QRS, for the second injection. And row 1-c shows the right ventricle 75ms (diastasis) into the R-R- interval for the third injection. The reproducibility of the injector and the biological sequence allow us to view the physiology at temporal resolution of 25ms—even though the camera readout is 100ms. Changes in contrast enhancement were seen in the left ventricle (dashed arrow) and aorta (solid arrow) when the time of injection (at the QRS) was kept constant and images were acquired at systole, diastole, and diastasis.
Fig. 9.
Multiple images acquired at fixed times in the rat during (a) ventricular systole, (b) diastole, and (c) diastasis with single 100µL contrast injection initiated at the QRS. The contrast bolus transit in the circulation can be followed across time. Note how the enhancement of the left ventricle (dashed arrow) diminishes over time, while the aorta becomes more enhanced (solid arrow).
Figure 10 shows the impact of timing in one of the most critical imaging studies, i.e. demonstration of the coronary arteries. In this experiment, a 150µL injection was made at the QRS. Subtraction was performed on the next cardiac cycle at the QRS. The same experiment was repeated with delays between the QRS and injection ranging from 30–75% of the R-R interval. The injection at the QRS occurs at systole during which the aortic valves are open and allows visualization of the coronary arteries. During this period, the contrast can flow retrograde and then enter the coronary arteries upon normal flow. At all other times, the retrograde flow is not possible, so the left ventricle (LV) does not fill. Timing differences of the injection was < 100ms. This made the difference between seeing the coronaries (@ 0% delay) and seeing only the ascending aorta.
Fig. 10.
Coronary artery imaging (arrows) of the rat at the same time point in the cardiac cycle, at the QRS, with a 150µL contrast injection at the QRS, 30, 50, 60, and 75% of the R-R interval after the QRS (left to right).
The mouse @ 25 grams is yet 10-times smaller than the rat with a heart rate roughly 3-times that of a rat, which poses even greater challenges for the injector. Figure 11 shows a DSA image @ 46 µm resolution of the renal vessels of a live mouse acquired with a 150ms (20µL) bolus injected at the QRS. Images were acquired at end systole on every heartbeat (R-R interval of 136ms). The catheter was placed in the iliac artery so that the tip was at the level just distal to the left renal artery. During the first heartbeat, the right renal artery fills some of the feeding vessels to the renal cortex. The second heartbeat demonstrates more complete filling of the right renal vasculature. The vasculature in the left kidney fills later due to the relative displacement (3.5 mm) of the left and right renal arteries.
Fig. 11.
Mouse renal DSA at 46µm projection resolution. Note the vasculature of the renal cortex and adrenals (white arrows) and the enhancement of the renal arteries (black arrows). The injection catheter was just distal to the left renal artery to allow enhancement of both kidneys. Note delayed enhancement of the left renal cortex due to the arterial offset (3.5 mm) of right and left renal arteries.
IV. DISCUSSION
A number of considerations were taken in developing a power contrast injector for small animals that allowed low volume injections with high flow rates and computer-controlled injection synchronization with biological signals. Bench-top and live animal tests were performed to characterize the injection volume and reproducibility, amount of enhancement in blood vessels, and any injector-induced physiological effects.
A key factor in achieving the linear response is the design of the custom catheters. The catheters were designed for maximum flow rate, while still being small enough for cannulization. This was achieved by using the largest lumen possible for the vessel being cannulated followed by successively larger segments approaching the injector. The overall catheter lengths were as short as possible to reduce flow resistance, while still being long enough for surgical handling. At constant driving pressure, a linear increase in injection volumes occurs for both catheters with increasing injection time (Figure 3). The flow rate at constant driving pressure is independent of the solenoid active window. Figure 4 shows linear increases in injection volume and flow rate with increasing driving pressure at a constant injection time. Here, only the rat catheter was characterized because lowering the driving pressure for the mouse catheter decreases the injection volume to a point where the standard deviation is > 25% of the injection volume.
In the rat, injection volumes between 50–150µL produced significant enhancement of the pulmonary vasculature with injection volumes and durations that were physiologically reasonable and had limited enhancement overlap (Figure 5). Larger injection volumes created significant overlap of enhanced structured and increased total blood volume (dotted arrow in d-3, and also e-2 and e-3). However, Figure 6 suggests injection volumes ≥ 100µL, where the CNR meets the Rose criterion. Therefore, a 100–150µL contrast injection is recommended for the rat. The total blood volume in a 200g rat is ~12mL. Thus, this injection volume is ≤ 1.25% of the total blood volume [36–39]. For the mouse, we were able to visualize the renal vasculature with a 20µL injection. The blood volume of the mouse is ~2mL, so the injection volume is ~1% of the total blood volume.
In the subsequent in-vivo tests, we found that the changes in vessel enhancement are due only to physiological effects, not injector-related parameters. Larger injection volumes in the left pulmonary artery, right lung parenchyma, left ventricle, and aorta (Figure 7) created only amplitude shifts in the time-density plots; no shift was seen in the transit time (Figure 8). The flow metrics (relative measurements) derived from the SVD technique agree with this, as seen in the low coefficient of variation values (Table 2). There was no statistically significant difference (all p-values ≥ 0.816) in the MTT as measured by one-way ANOVAs. The MTT was independent of the injection volumes (50, 100, and 150µL).
The precision in timing of the injections has an advantage for imaging the vessels in the heart. The custom LabVIEW application allowed us to place the contrast injection at specific points in the cardiac and ventilatory cycles, which is critical in imaging rapid physiologic changes such as the one seen in Figure 9 and Figure 10. Figure 9 shows we are able to repeatedly inject while adjusting the x-ray exposure to delineate functional flow changes with 25ms temporal resolution. Figure 10 shows that the response time of the injector is sufficient to allow us to inject with such precision that we can fill the left ventricle during systole when the aortic valve is open and allows for visualization of the coronary arteries upon resuming normal blood flow, demonstrating what we believe to be the first coronary DSA images in a rat.
Placement of the catheter to enhance only the vessels of interest is critical in producing a quality subtraction angiogram, as seen in Figure 11. Contrast can easily flowed into the iliolumbar artery (just distal to the left renal artery) because it has a larger diameter. This was especially true for the mouse imaging, where the distance between the left renal and iliolumbar arteries connect to the descending aorta at very close proximity (1.4mm). Careful placement of the injection catheter allowed visualization of the renal arteries, cortex, and adrenal glands. This opens the door to renal blood flow imaging experiments.
To our knowledge, this study represents the first demonstration of a computer-controlled injector for vascular imaging in the small animal. The injector described supports response times, injection rates, and injection volumes appropriate for both rats and mice. For the mouse, the injection rate is limited to 0.2µL/ms by the bore of the catheter. The larger catheter used in the rat allowed us to vary the injection rate linearly from 0.1 to 1.0µL/ms by adjusting the driving pressure. Injection volumes of 100–150µL (0.83–1.25% of the blood volume) provided reproducible opacification of the cardio-pulmonary vasculature with minimal impact on the physiology. Injection volumes of 20µL (1% of the blood volume) allowed visualization of the renal vasculature of the mouse. Changes in the time-density curve shapes of selected regions of interest were due primarily to physiological changes, independent of micro-injector parameters.
V. CONCLUSION
The micro-injector is capable of delivering repeatable, small volumes of contrast agent at high flow rates. These are important characteristics to ensure minimal impact on altering the physiology and optimal blood vessel enhancement without significantly increasing the total blood volume or experiencing overlap of enhanced structures. The linear reproducibility of the injection volumes and flow rates, and the ability to inject at specific time points during the cardiac cycle opens doors to experiments not previously possible. The utility of this system can be found in visualizing and quantifying real-time changes in blood flow in a variety of organ systems, e.g. cardio-pulmonary and renal systems. The micro-injector is an important component in designing an optimal system for small animal digital subtraction angiography where the spatial and temporal resolutions require a unique approach. The DSA system described here can acquire projection images covering an entire rat or mouse with an effective time sample of 10ms at frame rates (7–10 fps) far beyond any of the other modalities and 2D projected spatial resolution < 100 microns. What we know to be true in the clinical domain, i.e. that each of our modern imaging modalities has utility driven by the clinical problem, is also true at the level of the small animal. We believe small animal DSA will play a major role in functional vascular phenotyping.
ACKNOWLEDGMENT
The authors wish to thank Julie Boslego Mackel, Boma Fubara, and Laurence Hedlund for animal support and surgery, Jim Pollaro for the ventilator software control interface and monitoring system, and Antonia Chen for assistance in statistical measurements. We thank Sally Zimney for editorial assistance. All work was performed at the Duke Center for In Vivo Microscopy, an NCRR/NCI National Resource (P41 RR005959/ R24 CA092656). Publishing, 1981.
This work was supported in part by NIH/NCRR and NCI Grants P41 RR005959 and R24 CA092656.
REFERENCES
- 1.Shpilfoygel SD, Close RA, Valentino DJ, Duckwiler GR. X-ray videodensitometric methods for blood flow and velocity measurement: A critical review of literature. Medical Physics. 2000;vol. 27:2008–2023. doi: 10.1118/1.1288669. [DOI] [PubMed] [Google Scholar]
- 2.Bhargava V, Hagan G, Miyamoto MI, Ono S, Ono S, Rockman H, Ross JJ. Systolic and diastolic global right and left ventricular function assessment in small animals using an automated angiographic technique. IEEE Computers in Cardiology. 1992:191–194. [Google Scholar]
- 3.Burbank FH. AUR Memorial Award. Determinants of contrast enhancement for intravenous digital subtraction angiography. Invest. Radiology. 1983;vol. 18:308–316. doi: 10.1097/00004424-198307000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Higgins CB. Quantitation of left ventricular dimensions and function by digital video subtraction angiography. Diag. Radiology. 1982;vol. 144:461–469. doi: 10.1148/radiology.144.3.7048414. [DOI] [PubMed] [Google Scholar]
- 5.Higgins CB, Buonocore E. Digital subtraction angiography. Techniques and applications for evaluating cardiac anatomy and function. Cardiology Clinics. 1983;vol. 1:413–425. [PubMed] [Google Scholar]
- 6.Hirata M, Yoshimine, Toshiki, Kato, Amami, Ito, Mamoru, Hirabuki, Norio T, Masaaki, Nakamura, Hironobu, Hayakawa, Toru Computational imaging of cerebral perfusion by real time processing of DSA images. Clinical applications. Neuro Res. 1998;vol. 20:327–332. doi: 10.1080/01616412.1998.11740526. [DOI] [PubMed] [Google Scholar]
- 7.Kalinowski M, Kress O, Wels T, Alfke H, Klose KJ, Wagner HJ. X-ray digital subtraction angiography with 1 mol/L gadobutrol: results from a comparative porcine study with iodinated contrast agents. Invest. Radiology. 2002;vol. 37:254–262. doi: 10.1097/00004424-200205000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Ludwig J, Verhoeven L, Kersbergen J, Overtoom T. Digital subtraction angiography of the pulmonary arteries for the diagnosis of pulmonary embolism. Radiology. 1983;vol. 147:639–645. doi: 10.1148/radiology.147.3.6342028. [DOI] [PubMed] [Google Scholar]
- 9.Meaney T. Digital subtraction angiography of the human cardiovascular system. American Journal of Roentgenology. 1980;vol. 135:1153–1160. doi: 10.2214/ajr.135.6.1153. [DOI] [PubMed] [Google Scholar]
- 10.Rubin DL, Burbank FH, Bradley BR, Brody WR. An experimental evaluation of central vs. peripheral injection for intravenous digital subtraction angiography (IV-DSA) . Invest. Radiology. 1984;vol. 19:30–35. doi: 10.1097/00004424-198401000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Schlueter FJ, Zuckerman DA, Horesh L, Gutierrez FR, Hicks ME, Brink JA. Digital subtraction versus film-screen angiography for detecting acute pulmonary emboli: evaluation in a porcine model. J. of Vascular and Interventional Radiology. 1997;vol. 8:1015–1024. doi: 10.1016/s1051-0443(97)70704-8. [DOI] [PubMed] [Google Scholar]
- 12.Velkova K. Digital subtraction angiopulmography in children. Folia Medica (Plovdiv) 1992;vol. 34:37–40. [PubMed] [Google Scholar]
- 13.Kobayashi SH, M, Dono K, et al. In vivo real-time microangiography of the liver in mice using synchrotron radiation. J. of Hepatology. 2004;vol. 40:405–408. doi: 10.1016/j.jhep.2003.11.029. [DOI] [PubMed] [Google Scholar]
- 14.Longo M, Blandino A, Ascenti G, Ricciardi GK, Granata F, Vinci S. Cerebral angiography in the rat with mammographic equipment: a simple, cost-effective method for assessing vasospasm in experimental subarachnoid haemorrhage. Neuroradiology. 2002;vol. 44:689–694. doi: 10.1007/s00234-002-0781-3. [DOI] [PubMed] [Google Scholar]
- 15.Ono S, Bhargava V, Mao L, Hagan G, Rockman HA, Ross J., Jr In vivo assessment of left ventricular remodeling after myocardial infarction by digital video contrast angiography in the rat. Cardiovascular Res. 1994;vol. 28:349–357. doi: 10.1093/cvr/28.3.349. [DOI] [PubMed] [Google Scholar]
- 16.Ouandji F, Potter E, Chen WR, Li Y, Tang D, Liu H. Characterization of a CCD-based digital x-ray imaging system for small-animal studies: properties of spatial resolution. Applied Optics. 2002;vol. 41:2420–2427. doi: 10.1364/ao.41.002420. [DOI] [PubMed] [Google Scholar]
- 17.Rockman HA, Ono S, Ross RS, Jones LR, Karimi M, Bhargava V, Ross J, Jr, Chien KR. Molecular and physiological alterations in murine ventricular dysfunction. Proc. of the National Academy of Sciences of the USA. 1994;vol. 91:2694–2698. doi: 10.1073/pnas.91.7.2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akisada M, Hyodo K, Ando M, Maruhashi A, Konishi K, Toyofuku F, Nishimura K, Hasegawa S, Suwa A, Takenaka E. Synchrotron radiation at the Photon Factory for non-invasive coronary angiography: experimental studies. J. Cardiography. 1986;vol. 16:527–534. [PubMed] [Google Scholar]
- 19.Chen F, Sun X, De Keyzer F, Yu J, Peeters R, Coudyzer W, Vandecaveye V, Landuyt W, Bosmans H, Van Hecke P, Marchal G, Ni Y. Liver tumor model with implanted rhabdomyosarcoma in rats: MR imaging, microangiography, and histopathologic analysis. Radiology. 2006;vol. 239:554–562. doi: 10.1148/radiol.2392050277. [DOI] [PubMed] [Google Scholar]
- 20.Edelman JL, Castro MR. Quantitative image analysis of laser-induced choroidal neovascularization in rat. Experimental Eye Res. 2000;vol. 71:523–533. doi: 10.1006/exer.2000.0907. [DOI] [PubMed] [Google Scholar]
- 21.Luedemann W, Brinker T, Schuhmann MU, von Brenndorf AI, Samii M. Direct magnification technique for cerebral angiography in the rat. Invest. Radiology. 1998;vol. 33:421–424. doi: 10.1097/00004424-199807000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Yamauchi T, Furui S, Harasawa A, Ishimura M, Imai T, Hayashi T. Optimum iodine concentration of contrast material through microcatheters: hydrodynamic analysis of experimental results. Phys. Med. Bio. 2002;vol. 47:2511–2523. doi: 10.1088/0031-9155/47/14/310. [DOI] [PubMed] [Google Scholar]
- 23.Lin M, Samei E, Badea CT, Yoshizumi TT, Johnson GA. Optimized radiographic spectra for small animal digital subtraction angiography. Medical Physics. 2006;vol. 33:4249–4257. doi: 10.1118/1.2356646. [DOI] [PubMed] [Google Scholar]
- 24.Lin M, Badea CT, Johnson GA. Functional cardio-pulmonary imaging of the rodent using micro radiography. Academy of Molecular Imaging Annu. Conf; March 18–23, 2005; Orlando, FL. [Google Scholar]
- 25.Badea CT, Hedlund LW, Lin M, Boslego Mackel JF, Johnson GA. Tumor imaging in small animals with a combined micro-CT / micro-DSA system using iodinated conventional and blood pool contrast agents. Contrast Media and Molecular Imaging. 2006;vol. 1:153–164. doi: 10.1002/cmmi.103. [DOI] [PubMed] [Google Scholar]
- 26.Badea CT, Hedlund LW, Lin M, Boslego-Mackel JS, Samei E, Johnson GA. Tomographic digital subtraction angiography for lung perfusion estimation in rodent. Medical Physics. 2007 May;34(5):1546–1555. doi: 10.1118/1.2717384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mistry N, Pollaro J, Song J, Lin M, Johnson GA. pulmonary perfusion imaging in the rodent lung using dynamic contrast enhanced MRI. Magn. Reson. Med. 2007 doi: 10.1002/mrm.21353. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fischer HW. Viscosity, solubility and toxicity in the choice of an angiographic contrast medium. Angiology. 1965;vol. 16:759–766. doi: 10.1177/000331976501601206. [DOI] [PubMed] [Google Scholar]
- 29.Chuang VP, Lawrence DD, Richli WR, Lee YY, C C, Wallace S. A large lumen microcatheter for oncologic intervention. Cardiovasc. Intervent. Radiol. 1995;vol. 19:265–268. doi: 10.1007/BF00239426. [DOI] [PubMed] [Google Scholar]
- 30.Badea CT, Hedlund LW, Johnson GA. Micro-CT with respiratory and cardiac gating. Medical Physics. 2004;vol. 31:3324–3329. doi: 10.1118/1.1812604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balter S, Ergun D, Tscholl E, Buchmann F, Verhoeven L. Digital subtraction angiography: fundamental noise characteristics. Radiology. 1984;vol. 152:195–198. doi: 10.1148/radiology.152.1.6374758. [DOI] [PubMed] [Google Scholar]
- 32.Bushberg J, Seibert JA, Leidholdt EMJ, Boone JM. The Essential Physics of Medical Imaging. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2002. [Google Scholar]
- 33.Ostergaard L, Weisskoff RM, Chesler DA, Gyldensted C, Rosen BR. High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part I: Mathematical approach and statistical analysis. Magn. Reson. Med. 1996;vol. 36:715–725. doi: 10.1002/mrm.1910360510. [DOI] [PubMed] [Google Scholar]
- 34.Chen BT, Brau ACS, Johnson GA. Measurement of regional lung function in rats using hyperpolarized 3Helium dynamic MRI. Magn. Reson. Med. 2003;vol. 49:78–88. doi: 10.1002/mrm.10336. [DOI] [PubMed] [Google Scholar]
- 35.Chen BT, Yordanov AT, Johnson GA. Ventilation-synchronous MR microscopy of pulmonary structure and ventilation in mice. Magn. Reson. Med. 2005;vol. 53:69–75. doi: 10.1002/mrm.20307. [DOI] [PubMed] [Google Scholar]
- 36.Diehl K-H, Hull R, Morton D, Pfister R, Rabemampianina Y, Smith D, Vidal J-M, Vorstenbosch CVD. A good practice guide to the administration of substances and removal of blood, including routes and volumes. J. of App. Toxicology. 2001;vol. 21:15–23. doi: 10.1002/jat.727. [DOI] [PubMed] [Google Scholar]
- 37.Harkness JE, Wagner JE. The Biology and Medicine of Rabbits and Rodents. 3rd ed. Philadelphia: Lea & Febiger; 1989. [Google Scholar]
- 38.Lee H, Blaufox M. Blood volume in the rat. J. of Nuclear Medicine. 1985;vol. 26:72–76. [PubMed] [Google Scholar]
- 39.Mitruka BM, Rawnsley HM. Clinical, Biochemical and Hematological Reference Values in Normal Experimental Animals And Normal Humans. New York: Masson Publishing; 1981. [Google Scholar]