Abstract
In nonphagocytic cells, Rac1 is a component of NADPH oxidase that produces reactive oxygen species [Ushio-Fukai M (2006) Sci STKE 2006:re8]. Rac1 is expressed abundantly in mammalian retinal photoreceptors, where it is activated in response to light stimuli [Balasubramanian N, Slepak VZ (2003) Curr Biol 13:1306–1310]. We used Cre-LoxP conditional gene targeting to knock down Rac1 expression in mouse rod photoreceptors and found protection against light-induced photoreceptor death compared with WT litter-mates. We also found a similar protective effect on rods using apocynin, which inhibits NADPH oxidase activity. These results implicate both neuronal Rac1 and NADPH oxidase in cell death in this model of CNS degeneration. Studies in which dominant-mutants of Rac1 were expressed in transgenic Drosophila species demonstrated that Rac1 is a key regulator of photoreceptor morphogenesis and polarity [Chang HY, Ready DF (2000) Science 290:1978–1980]. However, we found that diminished Rac1 expression in mouse rods had no effect on retinal structure or function examined by light microscopy, electron microscopy, rhodopsin measurement, electroretinogram activity, and visual acuity, indicating rod outer segment morphogenesis proceeded normally in Rac1 conditional knockout mice. The lack of structural or functional effect of Rac1 depletion on photoreceptors, but protection under conditions of stress, indicate that the Rac1 pathway warrants exploration as a target for therapy in retinal neurodegenerative diseases.
Keywords: apoptosis, retinal degeneration, Rho GTPases, retinal light damage, superoxide anions
Rac1 is a member of the Rho GTPases, which are important regulators of cellular functions including morphogenesis, polarity, and apoptosis (1). In response to extracellular stimuli via various types of membrane receptors, Rac1 can act as an intracellular molecular switch by cycling between a GDP-bound inactive state and a GTP-bound active state. When bound to GTP, activated Rac1 stimulates various downstream effectors to elicit a variety of biological activities.
Transgenic expression of dominant-negative and dominant-active Rac1 has revealed many important tissue-specific functions of Rac1. For example, studies in transgenic Drosophila species that express dominant-active Rac1 in rhodopsin-null mutants and dominant-negative Rac1 in WT showed that it is a key regulator of rhodopsin-mediated photoreceptor morphogenesis (2). However, recent studies indicate that analysis of dominant mutants may be ambiguous and may not always represent a specific activation or inhibition of Rac1 (3). Although systemic deletion of Rac1 is lethal in embryonic mice (4), conditional gene-targeting studies can be used to dissect the tissue-specific roles of mammalian Rac1 in vivo. Using this method, Rac1 has been shown to regulate diverse cellular functions in different cell types. Rac1 has an important role in stem cell renewal in the adult mouse epidermis (5), and it is essential for the axon guidance in the mouse telencephalon (6). Rac1 acts as a component of NADPH oxidase in the adult mouse cardiomyocytes, and is critical in the development of cardiac hypertrophy via production of reactive oxygen species (7).
Rac1 expression is localized with rhodopsin-bearing transport carriers in frog photoreceptors, suggesting that Rac1 is involved with photoreceptor polarity through the post-Golgi trafficking of rhodopsin (8). Rac1 expression has been reported in mammalian rod photoreceptors based on immunohistochemistry, but the expression pattern of Rac1 in rods is still under debate (9–11). Rac1 is activated in response to light stimuli in the mammalian retina (10, 11), but the specific role of Rac1 in mammalian photoreceptors remains to be elucidated.
To study the function of Rac1 in mammalian photoreceptors, we used a Cre-LoxP conditional gene-targeting strategy to delete Rac1 in rod photoreceptors. Contrary to previous studies implicating Rac1 in Drosophila photoreceptor morphogenesis and polarity (2), we found that diminished Rac1 expression in mouse rod photoreceptors had no apparent effect on the maintenance of rod structure or function. Because light-induced photoreceptor death by apoptosis is accompanied by increased toxic superoxide levels (12), we wondered whether depleting Rac1, a key component of superoxide producing NADPH oxidase in some tissues, would affect light toxicity in the retina. Depleting Rac1 reduced rod susceptibility to light-induced degeneration, implicating neuronal Rac1 in this model of CNS degeneration.
Results
Generation of Rac1 Conditional Knockout (Rac1 CKO) in Mouse Rod Photoreceptors.
Mice homozygous for the floxed Rac1 gene (13) were crossed with transgenic mice expressing Cre recombinase under the control of the long (4.1 kb) mouse opsin promoter (LMOP-Cre mice) (14) to generate Rac1 CKO mice (Rac1flox/flox, LMOP-Cre+/−). Rac1 CKO mice were compared with WT litter-mates (Rac1flox/flox, LMOP-Cre−/−) to minimize the influence of the mixed genetic background. The LMOP-Cre mouse line exhibits efficient and selective Cre-mediated recombination in rod photoreceptors (14).
To estimate the degree of conversion of the Rac1flox allele to Rac1null by the LMOP-Cre transgene in rod photoreceptors, we isolated genomic DNA from the neural retinas of Rac1 CKO mice and performed real-time PCR with specific primers to the Rac1null allele. This indicated a 37.5% ± 7.2% conversion (n = 5) of the Rac1flox allele to Rac1null in Rac1 CKO retinas. Because rod photoreceptors comprise approximately 70% of neural retinal cells (15), we estimate that approximately 50% of Rac1flox allele was converted to Rac1null in rod photoreceptors.
Immunohistochemistry of retinal sections using an anti-Rac1 monoclonal antibody indicated that Rac1 expression was selectively decreased in the rod photoreceptors of Rac1 CKO mice. Rac1 expression was evident in the outer nuclear layer (ONL) and photoreceptor inner segments but not the outer segments of control mice by immunohistochemical staining (Fig. 1A) or in isolated rod outer segments (ROS) by immunoblot analysis (Fig. S1). In Rac1 CKO mice, Rac1 was decreased in the ONL compared with controls but was unchanged in the proximal retinal layers (Fig. 1A).
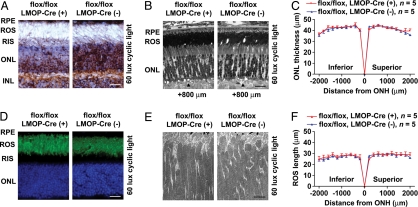
Fig. 1.
Photoreceptors of Rac1 CKO mice have normal structure. (A) Representative immunohistochemical staining of Rac1 (brown) in the retina from Rac1 CKO mice and WT litter-mates. Sections were stained with Mayer hematoxylin to show cell nuclei (light blue). (Scale bar: 20 μm.) (B) Morphology of the photoreceptor layer is normal in light microscopy of retinal sections cut along the vertical meridian containing the ONH. (Scale bar: 20 μm.) Arrowheads placed at 800 μm superior to ONH. (C and F) ONL thickness and ROS length of Rac1 CKO mice are indistinguishable from WT litter-mates at 20 points across the retina. (D) Representative immunohistochemical staining of rhodopsin (green) in the retina from Rac1 CKO mice and WT litter-mates. Nuclei in cells were stained with DAPI (blue). (Scale bar: 20 μm.) (E) Transmission electron micrographs showing the ROS structure of Rac1 CKO mice and WT litter-mates. (Scale bar: 2 μm.) RPE, retinal pigment epithelium; RIS, rod inner segments; INL, inner nuclear layer.
Structure and Function of Rac1 CKO Mouse Rods.
Transgenic expression of dominant-active Rac1 in Drosophila rescued rhabdomere morphogenesis in rhodopsin-null mutants, whereas expression of dominant-negative Rac1 caused degeneration of rhabdomeres (2). Because Drosophila rhabdomeres are homologous to the mammalian ROS, we analyzed photoreceptor structure in retinal sections from Rac1 CKO mice and control litter-mates. Rac1 CKO mice had normal ROS length and structure by light microscopy at 8 weeks of age (Fig. 1 B and F), and no ROS structural change was evident by transmission electron microscopy (Fig. 1E). No difference was seen in the expression pattern of rhodopsin between Rac1 CKO mice and controls by immunohistochemistry using an anti-rhodopsin monoclonal antibody (Fig. 1D). The quantity of rhodopsin in the whole retina of Rac1 CKO mice was not different from controls (Table S1). Rac1 promotes either cell survival or cell death depending on cell type and cell conditions (16). However, there was no difference in ONL thickness (reflecting rod cell numbers) between Rac1 CKO and control mice (Fig. 1 B and C), and Rac1 CKO retinas showed no degeneration up to 6 months of age (Fig. S2).
Visual function of Rac1 CKO mice was assessed by using optomotor responses to determine visual acuity (17) and was indistinguishable from control animals (Rac1 CKO: 0.404 cycles/degree ± 0.005, n = 5 animals; control: 0.405 cycles/degree ± 0.006, n = 5 animals; Fig. 2 A and B). Rod responses to light stimuli in Rac1 CKO were evaluated with the dark adapted electroretinogram (ERG) (Fig. 2 C–G). The intensity-response curves of the dark-adapted ERG a-wave, which directly reflects rod photoreceptor circulating dark-current (18) and transduction pathway activity (19), indicated there was no difference between Rac1 CKO and WT mice in amplitude or implicit time (Fig. 2 D and E). The lower portion of the Rac1 CKO amplitude-versus-intensity curve of the ERG b-wave, reflecting bipolar cell activity postsynaptic to rods, was shifted to higher intensity (i.e., lower sensitivity) by 0.26 log without a change in maximum amplitude (Fig. 2 F and G). A nearly twofold reduction in the number of responding rods uniformly distributed across the retina could produce this effect (20). However, log-K values from Naka-Rushton fits to the data indicated this shift was not statistically significant (F test, P = 0.153, n = 5). Thus, rod-specific depletion of Rac1 did not affect rod structure, number, visual pigment level or kinetics, or neural response to light.
Fig. 2.
Photoreceptors of Rac1 CKO mice have normal visual function. (A) Visual acuities were evaluated by increasing the spatial frequency of the rotating grating until an optomotor response could not be detected. (B) Visual acuities of Rac1 CKO mice were indistinguishable from WT litter-mates. (C) Retinal responses as measured by the dark-adapted ERG. (D and E) The a-wave of Rac1 CKO mice has normal amplitude and implicit time across a 2.5-log-unit intensity range. (F and G) The b-wave of Rac1 CKO mice has normal amplitude and implicit time across a 4.5-log-unit intensity range.
Rac1 Depletion Protects Photoreceptors from Phototoxic Insult.
Excessive bright light exposure leads to rhodopsin-mediated stimulation of apoptotic cell death of rod photoreceptors in several species, including mouse (21). Rac1 is a potent proapoptotic factor (16), and is activated in mammalian retinas in response to light (10, 11). We explored a role for Rac1 in light-induced photoreceptor apoptosis by exposing mice to 15,000 lux illumination for 24 h and evaluating rod survival 7 d later. Rac1 CKO retinas had a greater number of rod nuclei surviving and better preserved outer segments than the WT litter-mates (Fig. 3 A and B). Photoreceptor apoptosis was studied by TUNEL assay after 24 h in dark after light exposure. Retinal sections of Rac1 CKO mice had fewer TUNEL-positive photoreceptors compared with WT litter-mates, indicating that Rac1 depletion protected rod photoreceptors from apoptosis (Fig. S3).
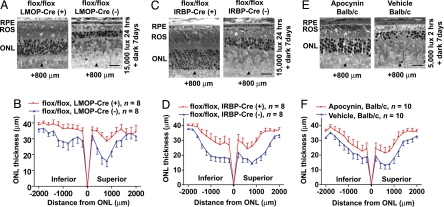
Fig. 3.
Rod-specific Rac1 depletion and administration of NADPH oxidase inhibitor protect photoreceptors from light-induced degeneration. (A and B) Rac1 CKO mice (Rac1flox/flox, LMOP-Cre+/−) had a greater average ONL thickness (P = 0.0024, Student t test) and better preserved ROS structure compared with WT litter-mates (Rac1flox/flox, LMOP-Cre−/−), which were severely disorganized and shortened. (C and D) Rac1 CKO mice (Rac1flox/flox, IRBP-Cre+/−) had a greater average ONL thickness (P = 0.0107, Student t test) compared with WT litter-mates (Rac1flox/flox, IRBP-Cre−/−). (E and F) Albino BALB/c mice were injected i.p. with NADPH oxidase inhibitor apocynin or vehicle only for controls. BALB/c mice were used because of their increased susceptibility to light damage, and the highly inbred strain minimized genetic variability. Apocynin-injected BALB/c mice had a greater average ONL thickness (P = 0.0027, Student t test) compared with vehicle-injected controls. Mice were exposed to either 15,000 lux illumination for 24 h (A–D) or 5,000 lux for 2 h (E and F) after 16 h in darkness, and then kept in the dark for 7 d. Photoreceptor cell survival was evaluated by measuring ONL thickness in retinal sections cut along the vertical meridian containing the ONH. (Scale bars: 20 μm.) Arrowheads are placed at 800 μm superior to ONH.
Possible Mechanisms of Light Damage Protection.
Overexpression of Cre recombinase is toxic to photoreceptor cells (22) and hence does not explain the neuroprotection in Rac1 CKO mice. We excluded neuroprotection resulting from an insertional effect of the LMOP-Cre transgene by generating a second set of Rac1 CKO mice using transgenic mice expressing Cre recombinase under the control of the photoreceptor-specific interphotoreceptor matrix retinoid binding protein (IRBP) promoter (23). These Rac1 CKO mice (Rac1flox/flox, IRBP-Cre+/−) showed similar protection from light damage (Fig. 3 C and D), indicating that Rac1 deficiency conveys protection against light-induced photoreceptor degeneration, and it is not caused by an insertional effect of the Cre transgene.
Susceptibility to phototoxic insult depends on the total amount of rhodopsin in the retina and the rate of rhodopsin recycling after bleach (21, 24–26). We found no difference between Rac1 CKO and controls in total amount (Rac1 CKO: 0.54 nmol/retina ± 0.01, n = 8 retinas from 4 animals; control: 0.55 nmol/retina ± 0.02, n = 7 retinas from 4 animals; Table S1) or regeneration kinetics of rhodopsin (Rac1 CKO: 0.011/min ± 0.001; control: 0.011/min ± 0.001; Table S1).
Acute phototoxic insult involves the induction of AP-1 and members of the IL-6 family of cytokines in the neural retina and phosphorylation of STAT3 in Müller glial cells (21, 27). Activated Rac1 stimulates AP-1 activity through phosphorylation of c-jun (28) and indirectly phosphorylates STAT3 via production of IL-6 (29). However, no difference was observed between Rac1 CKO mice and control litter-mates in the expression pattern of any of these molecules or in the expression of c-fos, a potent component of AP-1 (Fig. 4).
Fig. 4.
Analyses of potential downstream pathways of Rac1 and NADPH oxidase components. Rac1 CKO mice and control litter-mates were either kept in dim light (dark) or 6 h in dark after exposure to 15,000 lux for 24 h (light). Total cell lysates or membrane proteins were prepared from fresh isolated neural retinas and were analyzed for the expression of the indicated proteins by immunoblotting.
Inhibition of NADPH Oxidase Protects Photoreceptors from Light-Induced Degeneration.
Rac1 in nonphagocytic cells is a component of the multi-subunit enzyme NADPH oxidase, which can generate superoxide anions (30). Although Rac2 is a component of NADPH oxidase in some tissues (13), it is not present in retina (Fig. S4). Superoxide anions are involved in light-induced degeneration (12), and anti-oxidants have a protective effect (31). NADPH oxidase subunits in forebrain include gp91phox and p22phox (32). Increased membrane gp91phox and p22phox are associated with NADPH oxidase activation by ketamine in forebrain (32). In the neural retina, which develops from the forebrain, we found induced expression of these subunits, as well as Rac1, in membranes after light stress (Fig. 4). In addition, lower Rac1 levels were present in retinal membranes from Rac1-CKO mice versus controls after light stress (Fig. S5).
To test the hypothesis that reduced NADPH oxidase activity has a protective effect on rod photoreceptors, we injected the selective NADPH oxidase inhibitor apocynin into BALB/c mice before exposure to acute phototoxic insult. Apocynin inhibits NADPH oxidase activity by impeding subunit assembly (33). Apocynin injection protected rod photoreceptors from light damage compared with vehicle (Fig. 3 E and F), implicating NADPH oxidase and oxidative stress in light-induced retinal degeneration.
Discussion
Acute retinal phototoxic insult up-regulates or activates a number of molecules, including Rac1 (34), but these may represent part of normal visual transduction and neuronal signaling in addition to prodeath (35) or prosurvival (36) responses. Absorption of light by rhodopsin is the first step in these biochemical pathways, which may include the generation of reactive oxygen species. Rac1 deletion in mouse rod photoreceptors allowed us to dissect the functional and structural roles of Rac1 specifically in these cells. Rac1 depletion reduced the rod susceptibility to light-induced death but did not affect rod structure or function under appropriate levels of cyclic light.
Photoreceptors are vulnerable to oxidative damage because of their high oxygen consumption (37) and high content of polyunsaturated fatty acids (38) but low levels of antioxidants in the outer segments (39). In light-induced retinal degeneration, photoreceptor apoptosis is tightly linked to induced production of nitric oxide and superoxide anions (12), leading to neuronal death (40). There is strong evidence that superoxide anions participate in the photoreceptor death, but the mechanisms that regulate superoxide generation are poorly understood. In nonphagocytotic cells, Rac1 is critical for assembly and function of NADPH oxidase, which produces superoxide anions upon stimulation. Our results show that rod-specific Rac1 deletion was associated with lower levels of light-stimulated Rac1 expression in retinal membranes and protected rod photoreceptors from light-induced degeneration. The NADPH inhibitor apocynin had a similar protective effect. Since Rac1 is a component of NADPH oxidase in other tissues, these results are consistent with a role for reduced NADPH oxidase activity in light damage protection in Rac1 CKO mice.
Rho GTPases, including Rac1, have emerged as key regulators of the neuronal morphogenesis and polarity (41). Although transgenic expression of a dominant-negative Rac1 in Drosophila inhibits rhabdomere morphogenesis (2), our histomorphometric analysis and electron microscopic observation of the ROS in Rac1 CKO mice indicated that Rac1 is not essential for maintaining normal morphology of mammalian photoreceptors. Rods maintain proper cell polarity by post-Golgi trafficking of rhodopsin to the ROS (8). C-terminal mutations in rhodopsin interfere with this directed trafficking and lead to aberrant rhodopsin localization and severe retinal degeneration (42). Although Rac1 may participate in rhodopsin trafficking in frog photoreceptors (8), our Rac1 CKO mice showed neither mis-localized expression of rhodopsin nor signs of retinal degeneration. This indicates that, if Rac1 functions in mammalian photoreceptor morphogenesis and polarity, other Rac1-related Rho GTPases may compensate for the role of Rac1 in Rac1 CKO mice. Alternatively, because both opsin and IRBP promoters lead to relatively late expression of Cre recombinase and depletion of Rac1, our study may not recapitulate the results published earlier on the effects of dominant-negative Rac1 in Drosophila photoreceptors.
Rac1 inhibition warrants further study as a strategy for neuroprotection. It protects rod photoreceptors from light damage without causing functional loss as seen in some other genetic manipulations. For example, in mice that lack functional rhodopsin (i.e., rhodopsin-null and Rpe65-null mice), rods are protected from light damage (26) but have minimal or no function (43, 44). Further, c-fos-null mice show resistance to light damage (35), but they suffer attenuated retinal function (45). In contrast, reducing Rac1 expression did not affect rod structure or function but protected against neuronal death by phototoxic insult.
Materials and Methods
Animals.
Experiments were conducted in accordance with the ARVO statement for the Use of Animals in Ophthalmic and Vision Research, and protocols were approved by the National Eye Institute Animal Care and Use Committee. Rac1 CKO mice (Rac1flox/flox, LMOP-Cre+/−) were generated by mating the floxed Rac1 mice (mixed background of C57BL/6, SV/129, and BALB/c) (13) with the LMOP-Cre mice (mixed background of FVB/N and C57BL/6) (14). Litter-mates (Rac1flox/flox, LMOP-Cre−/−) were used as the WT controls. We also obtained Rac1 CKO mice (Rac1flox/flox, IRBP-Cre+/−) and control litter-mates (Rac1flox/flox, IRBP-Cre−/−) by mating the floxed Rac1 mice with IRBP-Cre mice (mixed background of C57BL/6 and CBA, and has been backcrossed to C57BL/6 for more than 5 generations) (23). BALB/c mice were purchased from The Jackson Laboratory. Mice were reared in dim white fluorescent light (60 lux) in a 12-h dark/light cycle.
Genotyping.
Mice were genotyped by PCR methods by using tail DNA as a template. The genotyping of Rac1 and Cre was performed as described in refs. 13 and 23. LMOP-Cre mice are derived from FVB/N strain, which carries a retinal degeneration mutation (Pdebrd1). We confirmed that this mutation is not inherited in all of the mice we used for the experiments by genotyping of Pdebrd1 as described in ref. 46.
Rpe65 encodes the retinoid isomerase, which is essential for rhodopsin regeneration. Resistance to light damage has been associated with a Leu-to-Met polymorphism at aa 450 of Rpe65 (24, 25). For the light damage and rhodopsin regeneration studies, we used only Rac1 CKO mice and litter-mate controls with Rpe65450Leu/Met, because our pilot study showed reduced light-induced photoreceptor degeneration in control mice with Rpe65450Met/Met. The Leu-to-Met variant of Rpe65 was tested as described in ref. 47.
Immunohistochemistry.
Rac1 immunostaining was done on 10-μm frozen sections from 8-week-old Rac1 CKO mice and control litter-mates. Sections were postfixed in 4% paraformaldehyde/2% acetic acid in PBS solution for 30 min at room temperature and incubated for 2 min in ice-cold ethanol/acetic acid (95:5). Antigen retrieval (Rodent Decloaker; Biocare Medical) was done at 80 °C for 30 min and blocked with 3% goat serum at room temperature for 30 min to reduce the background interaction between the monoclonal antibody and the mouse tissue. Sections were incubated at 4 °C overnight with an anti-Rac1 monoclonal antibody (clone 23A8, 0.5 μg/mL; Millipore). Detection was by the EnVision+ System-HRP (DAB) and Mayer hematoxylin (DAKO). For rhodopsin immunostaining, standard immunohistochemical techniques (48, 49) were used with an anti-rhodopsin monoclonal antibody (clone Rho 1D4, 2 μg/mL; Millipore).
Protein Preparation and Immunoblotting.
ROS proteins were prepared from fresh isolated neural retinas (n = 4 animals per group) on continuous sucrose gradient as described in ref. 50. ROS proteins were recovered from the 32/37% sucrose interface (band I), and other retinal proteins were collected from the 37/42% sucrose interface (band II). Total cell lysates or membrane proteins were prepared from fresh isolated neural retinas as described in ref. 51.
Aliquots were used for protein determination by using a BCA Protein Assay Kit (Pierce). Protein samples (5 μg/lane for band I and band II samples, and 50 μg per lane for total cell lysates or membrane proteins) were subjected to immunoblotting analysis with the following antibodies: anti-Rac1 specific monoclonal antibody (1:500; Cytoskeleton), anti-opsin monoclonal antibody (1:10,000, clone RET-P1; Sigma–Aldrich), anti-phospho-STAT3 (Tyr-705) polyclonal antibody (1:1,000; Cell Signaling), anti-STAT3 polyclonal antibody (1:1,000; Cell Signaling), anti-phospho-c-Jun (Ser-73) polyclonal antibody (1:1,000; Cell Signaling), anti-c-Jun/AP-1 polyclonal antibody (1:50; EMD Chemicals), anti-c-fos polyclonal antibody (1:200, Santa Cruz Biotechnology), anti-α-tubulin monoclonal antibody (1:5,000, clone DM1A; Abcam), anti-gp91-phox monoclonal antibody (1:500, clone 53; BD Biosciences), and anti-p22-phox polyclonal antibody (1:200; Santa Cruz Biotechnology).
Real-Time PCR.
Amounts of Rac1null allele in the neural retina of Rac1 CKO mice (8 weeks of age, n = 5) were estimated by real-time PCR analysis by using a 2X SYBR GreenER qPCR SuperMix (Invitrogen). Primer sequences to amplify Rac1null allele (13) were 5′-TCCAATCTGTGCTGCCCATC-3′ and 5′-CAGAGCTCGAATCCAGAAACTAGTA-3′. Each amplification reaction was done in a final volume of 25 μL with 125 ng of neural retinal DNA as a template. To allow quantification of recombination frequency, we constructed a standard curve by mixing known amounts of Rac1flox/flox DNA with Rac1null/WT DNA as reported in ref. 23. The data were collected from 3 independent experiments with each experiment performed in triplicate.
Histologic Evaluation.
Eyes from 8-week or 6-month-old Rac1 CKO and control mice were used for histologic evaluation as described in ref. 52. For light microscopy, 0.5-μm-thick sections were cut along the vertical meridian, passing through the optic nerve head (ONH), and stained with 0.1% toluidine blue. Photoreceptor cell number was evaluated by measuring ONL thickness on photomicrographs of retinal sections taken with a ×20 objective of a photomicroscope (E800; Nikon) and digital camera (DXM1200; Nikon). Measurements were made across the inferior and superior retinas every 200 μm to 2000 μm from the ONH. Rod outer segment length was measured at the same retinal locations. For transmission electron microscopy, 100-nm ultrathin sections were cut by using an Ultracut R ultramicrotome (Leica), stained with uranyl acetate and lead citrate, and examined by electron microscope (JEM 1010; JEOL).
Visual Acuity.
We evaluated the visual acuities of Rac1 CKO mice and control litter-mates at 7 weeks of age by using optokinetic responses (OptoMotry; CerebralMechanics) as described in refs. 17 and 49.
Electroretinography.
Full-field dark-adapted ERG responses were recorded from Rac1 CKO mice and control litter-mates at 8 weeks of age as described in ref. 52.
Light Exposure.
Eight- to 10-week-old mice were dark adapted overnight for 16 h before light exposure. Equally pigmented, sex-matched Rac1 CKO mice and control litter-mates were exposed to 15,000 lux of diffuse white fluorescent light for 24 h beginning at 10 AM. Pupils were dilated 3 times with 1% atropine sulfate topical corneal ophthalmic solution before dark adaptation, before light exposure, and at 6 PM during light exposure. Pupil dilation was confirmed at the conclusion of light exposure.
Albino male BALB/c mice (7 to 8 weeks old) were dark-adapted overnight for 16 h before light exposure. Apocynin (Sigma-Aldrich) was dissolved in PBS solution. Apocynin (50 mg/kg) was injected i.p. 16 h before and 1 h before light exposure. Control animals were injected with PBS solution only. Mice were exposed to 5,000 lux of diffuse white fluorescent light for 2 h beginning at 11 AM.
During exposure, each mouse was housed separately in a well ventilated transparent plastic cage and provided with food and water but no bedding to obscure light. Cages were illuminated from above and below, and average illuminance was measured at the cage floor. Temperature was monitored and maintained at 25 °C during light exposure. After light exposure, the mice were housed in darkness for 7 d and then euthanized, and the eyes were removed immediately for histologic examination.
Statistical Analysis.
All data are expressed as mean ± SE. The two-tailed Student t test was used to compare the average ONL thickness (from 2 mm inferior to 2 mm superior to the ONH). Naka-Rushton fits to the ERG b-wave data were compared using the extra sum-of-squares F test (GraphPad Prism). Average Western blot band densities were expressed in box and whiskers plots as mean, 25–75% (min-max) and the one-way ANOVA (GraphPad Prism) was used to compare the means. For further information see Fig. S5.
Rhodopsin Measurement, TUNEL Staining, RNA Preparation and RT-PCR.
Rhodopsin measurement, TUNEL staining, RNA preparation, and RT-PCR were performed as described in refs. 25–27. For further information see SI Text.
Supplementary Material
Acknowledgments.
We thank Maria Santos and Jinbo Li for technical assistance; Mary Crawford and Chi-Chao Chan for EM imaging; STRRMD lab members for discussion; Eric Wawrousek, Robert Fariss, Noriaki Sasai, Masayuki Akimoto, Masaru Inatani, Minoru Satoh, James Liao, and Thomas Leto for technical advice; and Deborah Carper for critical comments on the manuscript.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0808940106/DCSupplemental.
References
- 1.Jaffe AB, Hall A. Rho GTPases: Biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 2.Chang HY, Ready DF. Rescue of photoreceptor degeneration in rhodopsin-null Drosophila mutants by activated Rac1. Science. 2000;290:1978–1980. doi: 10.1126/science.290.5498.1978. [DOI] [PubMed] [Google Scholar]
- 3.Wang L, Zheng Y. Cell type-specific functions of Rho GTPases revealed by gene targeting in mice. Trends Cell Biol. 2007;17:58–64. doi: 10.1016/j.tcb.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 4.Sugihara K, et al. Rac1 is required for the formation of three germ layers during gastrulation. Oncogene. 1998;17:3427–3433. doi: 10.1038/sj.onc.1202595. [DOI] [PubMed] [Google Scholar]
- 5.Benitah SA, Frye M, Glogauer M, Watt FM. Stem cell depletion through epidermal deletion of Rac1. Science. 2005;309:933–935. doi: 10.1126/science.1113579. [DOI] [PubMed] [Google Scholar]
- 6.Chen L, et al. Rac1 controls the formation of midline commissures and the competency of tangential migration in ventral telencephalic neurons. J Neurosci. 2007;27:3884–3893. doi: 10.1523/JNEUROSCI.3509-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Satoh M, et al. Requirement of Rac1 in the development of cardiac hypertrophy. Proc Natl Acad Sci USA. 2006;103:7432–7437. doi: 10.1073/pnas.0510444103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deretic D, et al. Phosphoinositides, ezrin/moesin, and rac1 regulate fusion of rhodopsin transport carriers in retinal photoreceptors. Mol Biol Cell. 2004;15:359–370. doi: 10.1091/mbc.E03-04-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitchell DC, et al. Developmental expression of three small GTPases in the mouse eye. Mol Vis. 2007;13:1144–1153. [PMC free article] [PubMed] [Google Scholar]
- 10.Balasubramanian N, Slepak VZ. Light-mediated activation of Rac-1 in photoreceptor outer segments. Curr Biol. 2003;13:1306–1310. doi: 10.1016/s0960-9822(03)00511-6. [DOI] [PubMed] [Google Scholar]
- 11.Belmonte MA, Santos MF, Kihara AH, Yan CY, Hamassaki DE. Light-induced photoreceptor degeneration in the mouse involves activation of the small GTPase Rac1. Invest Ophthalmol Vis Sci. 2006;47:1193–1200. doi: 10.1167/iovs.05-0446. [DOI] [PubMed] [Google Scholar]
- 12.Donovan M, Carmody RJ, Cotter TG. Light-induced photoreceptor apoptosis in vivo requires neuronal nitric-oxide synthase and guanylate cyclase activity and is caspase-3-independent. J Biol Chem. 2001;276:23000–23008. doi: 10.1074/jbc.M005359200. [DOI] [PubMed] [Google Scholar]
- 13.Glogauer M, et al. Rac1 deletion in mouse neutrophils has selective effects on neutrophil functions. J Immunol. 2003;170:5652–5657. doi: 10.4049/jimmunol.170.11.5652. [DOI] [PubMed] [Google Scholar]
- 14.Le YZ, et al. Mouse opsin promoter-directed Cre recombinase expression in transgenic mice. Mol Vis. 2006;12:389–398. [PubMed] [Google Scholar]
- 15.Jeon CJ, Strettoi E, Masland RH. The major cell populations of the mouse retina. J Neurosci. 1998;18:8936–8946. doi: 10.1523/JNEUROSCI.18-21-08936.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murga C, Zohar M, Teramoto H, Gutkind JS. Rac1 and RhoG promote cell survival by the activation of PI3K and Akt, independently of their ability to stimulate JNK and NF-kappaB. Oncogene. 2002;21:207–216. doi: 10.1038/sj.onc.1205036. [DOI] [PubMed] [Google Scholar]
- 17.Prusky GT, Alam NM, Beekman S, Douglas RM. Rapid quantification of adult and developing mouse spatial vision using a virtual optomotor system. Invest Ophthalmol Vis Sci. 2004;45:4611–4616. doi: 10.1167/iovs.04-0541. [DOI] [PubMed] [Google Scholar]
- 18.Penn RD, Hagins WA. Signal transmission along retinal rods and the origin of the electroretinographic a-wave. Nature. 1969;223:201–204. doi: 10.1038/223201a0. [DOI] [PubMed] [Google Scholar]
- 19.Breton ME, Schueller AW, Lamb TD, Pugh EN., Jr Analysis of ERG a-wave amplification and kinetics in terms of the G-protein cascade of phototransduction. Invest Ophthalmol Vis Sci. 1994;35:295–309. [PubMed] [Google Scholar]
- 20.Hood DC, Shady S, Birch DG. Understanding changes in the b-wave of the ERG caused by heterogeneous receptor damage. Invest Ophthalmol Vis Sci. 1994;35:2477–2488. [PubMed] [Google Scholar]
- 21.Wenzel A, Grimm C, Samardzija M, Reme CE. Molecular mechanisms of light-induced photoreceptor apoptosis and neuroprotection for retinal degeneration. Prog Retin Eye Res. 2005;24:275–306. doi: 10.1016/j.preteyeres.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Jimeno D, et al. Analysis of kinesin-2 function in photoreceptor cells using synchronous Cre-loxP knockout of Kif3a with RHO-Cre. Invest Ophthalmol Vis Sci. 2006;47:5039–5046. doi: 10.1167/iovs.06-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marszalek JR, et al. Genetic evidence for selective transport of opsin and arrestin by kinesin-II in mammalian photoreceptors. Cell. 2000;102:175–187. doi: 10.1016/s0092-8674(00)00023-4. [DOI] [PubMed] [Google Scholar]
- 24.Danciger M, et al. A QTL on distal chromosome 3 that influences the severity of light-induced damage to mouse photoreceptors. Mamm Genome. 2000;11:422–427. doi: 10.1007/s003350010081. [DOI] [PubMed] [Google Scholar]
- 25.Wenzel A, Reme CE, Williams TP, Hafezi F, Grimm C. The Rpe65 Leu450Met variation increases retinal resistance against light-induced degeneration by slowing rhodopsin regeneration. J Neurosci. 2001;21:53–58. doi: 10.1523/JNEUROSCI.21-01-00053.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grimm C, et al. Protection of Rpe65-deficient mice identifies rhodopsin as a mediator of light-induced retinal degeneration. Nat Genet. 2000;25:63–66. doi: 10.1038/75614. [DOI] [PubMed] [Google Scholar]
- 27.Samardzija M, et al. Differential role of Jak-STAT signaling in retinal degenerations. FASEB J. 2006;20:2411–2413. doi: 10.1096/fj.06-5895fje. [DOI] [PubMed] [Google Scholar]
- 28.Coso OA, et al. The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell. 1995;81:1137–1146. doi: 10.1016/s0092-8674(05)80018-2. [DOI] [PubMed] [Google Scholar]
- 29.Faruqi TR, Gomez D, Bustelo XR, Bar-Sagi D, Reich NC. Rac1 mediates STAT3 activation by autocrine IL-6. Proc Natl Acad Sci USA. 2001;98:9014–9019. doi: 10.1073/pnas.161281298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ushio-Fukai M. Localizing NADPH oxidase-derived ROS. Sci STKE. 2006;2006:re8. doi: 10.1126/stke.3492006re8. [DOI] [PubMed] [Google Scholar]
- 31.Organisciak DT, Darrow RM, Jiang YI, Marak GE, Blanks JC. Protection by dimethylthiourea against retinal light damage in rats. Invest Ophthalmol Vis Sci. 1992;33:1599–1609. [PubMed] [Google Scholar]
- 32.Behrens MM, et al. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science. 2007;318:1645–1647. doi: 10.1126/science.1148045. [DOI] [PubMed] [Google Scholar]
- 33.Williams HC, Griendling KK. NADPH oxidase inhibitors: New antihypertensive agents? J Cardiovasc Pharmacol. 2007;50:9–16. doi: 10.1097/FJC.0b013e318063e820. [DOI] [PubMed] [Google Scholar]
- 34.Choi S, Hao W, Chen CK, Simon MI. Gene expression profiles of light-induced apoptosis in arrestin/rhodopsin kinase-deficient mouse retinas. Proc Natl Acad Sci USA. 2001;98:13096–13101. doi: 10.1073/pnas.201417498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hafezi F, et al. The absence of c-fos prevents light-induced apoptotic cell death of photoreceptors in retinal degeneration in vivo. Nat Med. 1997;3:346–349. doi: 10.1038/nm0397-346. [DOI] [PubMed] [Google Scholar]
- 36.Li G, et al. Nonredundant role of Akt2 for neuroprotection of rod photoreceptor cells from light-induced cell death. J Neurosci. 2007;27:203–211. doi: 10.1523/JNEUROSCI.0445-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu DY, Cringle SJ. Oxygen distribution and consumption within the retina in vascularised and avascular retinas and in animal models of retinal disease. Prog Retin Eye Res. 2001;20:175–208. doi: 10.1016/s1350-9462(00)00027-6. [DOI] [PubMed] [Google Scholar]
- 38.Bush RA, Reme CE, Malnoe A. Light damage in the rat retina: The effect of dietary deprivation of N-3 fatty acids on acute structural alterations. Exp Eye Res. 1991;53:741–752. doi: 10.1016/0014-4835(91)90109-r. [DOI] [PubMed] [Google Scholar]
- 39.Winkler BS. An hypothesis to account for the renewal of outer segments in rod and cone photoreceptor cells: Renewal as a surrogate antioxidant. Invest Ophthalmol Vis Sci. 2008;49:3259–3261. doi: 10.1167/iovs.08-1785. [DOI] [PubMed] [Google Scholar]
- 40.Brown GC. Mechanisms of inflammatory neurodegeneration: iNOS and NADPH oxidase. Biochem Soc Trans. 2007;35:1119–1121. doi: 10.1042/BST0351119. [DOI] [PubMed] [Google Scholar]
- 41.Luo L. Rho GTPases in neuronal morphogenesis. Nat Rev Neurosci. 2000;1:173–180. doi: 10.1038/35044547. [DOI] [PubMed] [Google Scholar]
- 42.Deretic D. A role for rhodopsin in a signal transduction cascade that regulates membrane trafficking and photoreceptor polarity. Vision Res. 2006;46:4427–4433. doi: 10.1016/j.visres.2006.07.028. [DOI] [PubMed] [Google Scholar]
- 43.Redmond TM, et al. Rpe65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nat Genet. 1998;20:344–351. doi: 10.1038/3813. [DOI] [PubMed] [Google Scholar]
- 44.Humphries MM, et al. Retinopathy induced in mice by targeted disruption of the rhodopsin gene. Nat Genet. 1997;15:216–219. doi: 10.1038/ng0297-216. [DOI] [PubMed] [Google Scholar]
- 45.Kueng-Hitz N, et al. The retina of c-fos−/− mice: Electrophysiologic, morphologic and biochemical aspects. Invest Ophthalmol Vis Sci. 2000;41:909–916. [PubMed] [Google Scholar]
- 46.Gimenez E, Montoliu L. A simple polymerase chain reaction assay for genotyping the retinal degeneration mutation (Pdeb(rd1)) in FVB/N-derived transgenic mice. Lab Anim. 2001;35:153–156. doi: 10.1258/0023677011911525. [DOI] [PubMed] [Google Scholar]
- 47.Kim SR, et al. Rpe65 Leu450Met variant is associated with reduced levels of the retinal pigment epithelium lipofuscin fluorophores A2E and iso-A2E. Proc Natl Acad Sci USA. 2004;101:11668–11672. doi: 10.1073/pnas.0403499101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haruta M, et al. Induction of photoreceptor-specific phenotypes in adult mammalian iris tissue. Nat Neurosci. 2001;4:1163–1164. doi: 10.1038/nn762. [DOI] [PubMed] [Google Scholar]
- 49.Haruta M, et al. In vitro and in vivo characterization of pigment epithelial cells differentiated from primate embryonic stem cells. Invest Ophthalmol Vis Sci. 2004;45:1020–1025. doi: 10.1167/iovs.03-1034. [DOI] [PubMed] [Google Scholar]
- 50.Organisciak DT, et al. Adaptive changes in visual cell transduction protein levels: Effect of light. Exp Eye Res. 1991;53:773–779. doi: 10.1016/0014-4835(91)90113-s. [DOI] [PubMed] [Google Scholar]
- 51.Vijayasarathy C, Takada Y, Zeng Y, Bush RA, Sieving PA. Retinoschisin is a peripheral membrane protein with affinity for anionic phospholipids and affected by divalent cations. Invest Ophthalmol Vis Sci. 2007;48:991–1000. doi: 10.1167/iovs.06-0915. [DOI] [PubMed] [Google Scholar]
- 52.Kjellstrom S, Bush RA, Zeng Y, Takada Y, Sieving PA. Retinoschisin gene therapy and natural history in the Rs1h-KO mouse: Long-term rescue from retinal degeneration. Invest Ophthalmol Vis Sci. 2007;48:3837–3845. doi: 10.1167/iovs.07-0203. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.