Abstract
Gonad-stimulating substance (GSS) of starfish is the only known invertebrate peptide hormone responsible for final gamete maturation, rendering it functionally analogous to the vertebrate luteinizing hormone (LH). Here, we purified GSS of starfish, Asterina pectinifera, from radial nerves and determined its amino acid sequence. The purified GSS was a heterodimer composed of 2 different peptides, A and B chains, with disulfide cross-linkages. Based on its cysteine motif, starfish GSS was classified as a member of the insulin/insulin-like growth factor (IGF)/relaxin superfamily. The cDNA of GSS encodes a preprohormone sequence with a C peptide between the A and B chains. Phylogenetic analyses revealed that starfish GSS was a relaxin-like peptide. Chemically synthesized GSS induced not only oocyte maturation and ovulation in isolated ovarian fragments, but also unique spawning behavior, followed by release of gametes shortly after the injection. Importantly, the action of the synthetic GSS on oocyte maturation and ovulation was mediated through the production of cAMP by isolated ovarian follicle cells, thereby producing the maturation-inducing hormone of this species, 1-methyladenine. In situ hybridization showed the transcription of GSS to occur in the periphery of radial nerves at the side of tube feet. Together, the structure, sequence, and mode of signal transduction strongly suggest that GSS is closely related to the vertebrate relaxin.
Keywords: gonadotropin, gonad-stimulating substance, insulin-like growth factor/relaxin superfamily, 1-methyladenine, in situ hybridization
Gonadotropins play important regulatory roles in reproduction in both vertebrates and invertebrates. The vertebrate gonadotropins—follicle-stimulating hormone (FSH) and luteinizing hormone (LH)—are structurally and functionally conserved across various species, whereas no such molecule has been identified in invertebrates. The insect hormones parsins have been assumed as the physiological counterpart of LH and FSH in mammals (1). Some gonadotropic hormones, such as egg development neurosecretory hormone (2), egg-laying hormone (3), and androgenic gland hormone (4), have been found in a few invertebrate species. More recently, an insulin-like peptide was reported to be responsible for the regulation of egg maturation in the mosquito, Aedes aegypti (5), demonstrating the involvement of insulin signaling in egg maturation among the invertebrates.
The gonad-stimulating substance (GSS) of an echinoderm, the starfish, was the very first gonadotropin to be identified in invertebrates. GSS mediates oocyte maturation in starfish by acting on the ovary to produce the maturation-inducing hormone (MIH) 1-methyladenine (1-MeAde), which in turn induces the maturation of the oocytes (6). In this sense, GSS is functionally identical to vertebrate LH, especially piscine and amphibian LHs, acting on the ovarian follicle cells to produce MIH to induce the final maturation or meiotic resumption of the oocyte (7). Considering the functional similarity that GSS shares with vertebrate LH, it is very important from an evolutionary point of view to know the chemical and molecular structure of GSS. In this study, we purified GSS from radial nerves of the starfish, Asterina pectinifera, and analyzed the activity of the synthesized GSS. Further, we cloned the gene encoding GSS and determined the site of its transcription. Interestingly, phylogenetic analyses revealed that it belonged to the insulin/insulin-like growth factor (IGF)/relaxin superfamily and, more precisely, to the subclass of relaxin/insulin-like peptides.
Results
Purified GSS Comprises 2 Polypeptide Chains; Namely, A and B Chains.
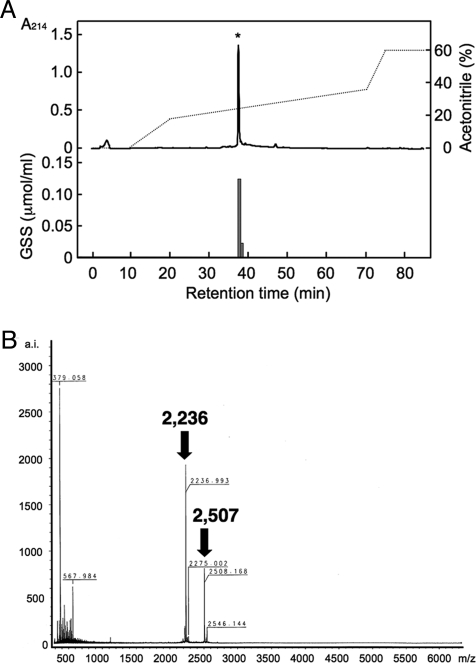
The crude nerve extract was prepared from radial nerves of ≈5,500 starfish, A. pectinifera, collected just before the spawning period. The final yield of GSS was 12.6 pmol per 0.18 mL. The nerve extract was deproteinized before RP-HPLC. GSS was purified by 4 steps of RP-HPLC. GSS activity at each step was assessed by a spawning assay by using ovarian fragments of A. pectinifera. The first RP-HPLC showed a single peak, the activity of which is shown in the histogram in Fig. 1A. The final fraction also contained a single peak with a molecular weight of 4,737. Further chemical reduction of the GSS fraction by using DTT brought about 2 different signals on MS: 2,507 and 2,236 (Fig. 1B). This result shows that the 4,737 form was a heterodimeric complex composed of 2 different peptides with molecular weights of 2,507 (A chain) and 2,236 (B chain). These fractions were analyzed by a protein sequencer, and their peptides were separately sequenced de novo by liquid chromatography-MS/MS. The A chain was composed of 24 aa (SEYSGIASYCCLHGCTPSELSVVC); the B chain, 19 aa (EKYCDDDFHMAVFRTCAVS). These 2 peptides were found to contain 6 cysteines that probably form 3 pairs of disulfide bonds, thus losing 6 hydrogen atoms to give a total molecular weight of 4,737, instead of the sum value of the A and B chains, 4,743.
Fig. 1.
Purification of starfish GSS. (A) The final semimicrochromatography with a SMART system by using a reverse-phase semimicrocolumn (1.5 × 150 mm) at a flow rate of 0.1 mL/min. The activity was recovered at 37–39 min, as shown by the asterisk (*). The activity of this fraction is shown in the histogram in the bottom half of the graph. (B) Mass spectrogram of purified starfish GSS after DTT treatment.
GSS Is a Relaxin-like Peptide.
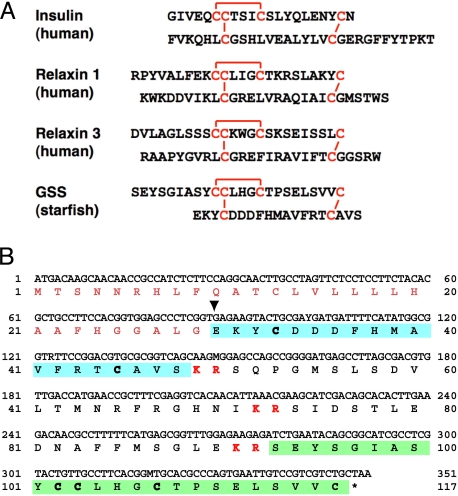
The A chain of GSS harbored a cysteine motif, identical to the signature pattern (CCxxxCxxxxxxxxC) of the insulin/IGF/relaxin superfamily (Fig. 2A). To confirm that GSS belongs in this superfamily, we deduced the nucleotide sequences possibly coding for the amino acids in the A and B chains and determined the positions of degenerate codons. By using pairs of degenerate primers based on the B chain and A chain, cDNA fragments were obtained from the mRNA of A. pectinifera radial nerves by RT-PCR. A cDNA sequence encoding GSS was determined by using the SMART RACE cDNA Amplification Kit (Clontech). The cDNA (351 bp) consisted of an ORF encoding a peptide of 116 aa, including a signal peptide of 29 aa (Fig. 2B). This analysis revealed that the signal peptide at the N terminus was followed by the B chain, and the A chain was located at the C terminus. Sandwiched between the A and B chains was an intermediate C peptide with 44 aa carrying 3 proteolytic sites, of which 2 were located at either end of the peptide and 1 in the middle. The deduced amino acid sequences of A and B chains were identical to those determined for purified natural GSS.
Fig. 2.
Amino acid sequence of starfish GSS. (A) Comparison of the heterodimeric structure of starfish GSS with those of various representative members of the insulin superfamily. The cysteine bridges are shown in red. (B) Coding DNA sequence and predicted amino acid sequences of GSS. Sequences of A and B chains are shown in green and blue boxes, respectively. Characters shown in red boldface indicate basic dipeptides that are the sites of proteolytic cleavage. Inverted triangle shows the deduced cleavage site of the signal peptide.
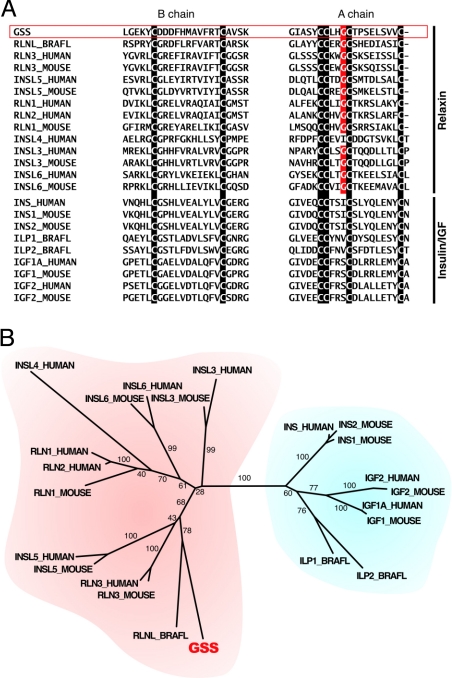
Having determined the identity of GSS, the full-length amino acid sequence of GSS was aligned by using MAFFT (8) to other members of the insulin/IGF/relaxin superfamily of human and mouse. We also searched amphioxus (Branchiostoma floridae) genomic [Joint Genome Institute (JGI)] and EST [National Center for Biotechnology Information (NCBI)] databases and could identify 1 predicted relaxin-like (RLNL_BRAFL, BRAFLDRAFT_100967; GenBank accession no.: XM_002238502) and 2 insulin/IGF-like (insulin-like peptide 1; ILP1_BRAFL, 2; ILP2_BRAFL) genes (Fig. S1). A comparison of the GSS sequence with other members of the insulin/IGF/relaxin superfamily, including amphioxus sequences, is outlined in Fig. 3A. The third cysteine in the A chain of GSS was preceded by a glycine residue, as in other relaxins except for INSL 4. Furthermore, the C terminus of GSS was identical to relaxins 1–3, INSL 5, and RLNL. However, the first cysteine residue in the B chains of GSS was not followed by a glycine residue. Thus, GSS differed from vertebrate relaxins in not having the relaxin-specific receptor-binding motif, RxxxRxxI/V (9). A phylogenetic tree was generated (Fig. 3B). Our phylogenetic analysis revealed that GSS and other insulin/IGF/relaxin superfamily sequences were divided into 2 distinct clusters: One for insulin/IGF and another for relaxin/insulin-like clusters. The GSS sequence belonged to the latter cluster. A BLAST search also confirmed that the starfish GSS was similar to the relaxin/relaxin-like members from the scores and e-values (Fig. S2). Further, we did a phylogenetic analysis of GSS after removing the glycine residue preceding the third cysteine in the A chain to confirm the precise position of GSS in the insulin/IGF/relaxin superfamily. Even after the removal of the glycine residue from the A chain, GSS clustered with the members of relaxin family but not with those of the insulin family (Fig. S2).
Fig. 3.
Alignment of the peptide sequences and the phylogenetic tree of the GSS and insulin/IGF/relaxin superfamily. The descriptions of the abbreviations and the sequence sources are given in Fig. S2. (A) Two parts of the whole peptide sequence alignment (Fig. S2) around the B and A chain regions. The cysteine residues that distinguish the insulin/IGF/relaxin superfamily peptides are indicated by the white characters on the black background. The glycine residues that are very common in the A peptides of the relaxin subfamily, but not in that of the insulin/IGF subfamily, are indicated by the white characters on the red background. (B) The phylogenetic tree constructed from the alignment by using the neighbor-joining method as described in this work (Fig. S3). The number located beside each branch is the bootstrap score. The relaxin and the insulin/IGF subfamilies are indicated by red and blue backgrounds, respectively.
Synthetic GSS Induces Oocyte Maturation and Ovulation in Vitro and Spawning in Vivo.
We examined whether synthetic GSS could induce oocyte maturation and ovulation in vitro and spawning in vivo in A. pectinifera. Synthetic GSS could induce oocyte maturation and ovulation in the ovarian fragments of A. pectinifera within 30 min of incubation at the median effective concentration (EC50) of 2 nM, similar to natural GSS (Table 1). In contrast, neither oocyte maturation nor ovulation could be seen when the ovarian fragments were incubated with either of the 2 peptides alone or in combination, freshly mixed even at an excess concentration of 1 μM. Additionally, spawning behavior and subsequent spawning occurred when the males and females of A. pectinifera with fully grown testes and ovaries, respectively, were injected with synthetic GSS. Interestingly, starfish injected with GSS moved upward along the walls of the aquarium and then shed the gametes within 30 min (oocyte maturation in vitro and spawning behavior in vivo are available in Movie S1, Movie S2, and Movie S3).
Table 1.
Effects of various synthetic peptides on the induction of oocyte maturation in vitro
Starfish ovarian fragments were incubated with or without various concentrations of A chain alone, B chain alone, a mixture of A and B chains, or a dimeric form (synthetic GSS) for 1 h. The effective dose for inducing gamete spawning in 50% of ovarian fragments was determined (mean ± SEM) from 4 experiments.
*Peptides used in single or mixed forms were examined up to 1 μM.
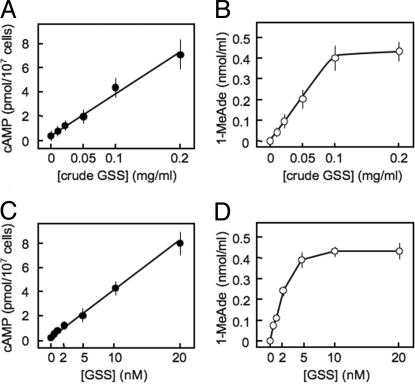
We then examined whether crude radial nerve extracts and synthetic GSS could stimulate cAMP and 1-MeAde production. A dose-dependent increase in intracellular cAMP levels was observed in the follicle cells treated with either nerve extracts or the heterodimeric form of synthetic GSS over the entire range of concentrations tested (Fig. 4 A and C). Similarly, a dose-dependent increase in the levels of 1-MeAde was observed in the follicle cells treated with either nerve extracts up to 0.1 mg/mL or synthetic GSS up to 10 nM (Fig. 4 B and D). We estimated from these experiments that a single radial nerve contained ≈50 pmol/mg (wet weight) GSS.
Fig. 4.
Effects of crude radial nerve extract (A and B) and synthetic GSS (C and D) on cAMP and 1-MeAde production on starfish ovarian follicle cells. (A and C) Intracellular cAMP content. The amount of cAMP was measured by an EIA system. (B and D) 1-MeAde content produced by follicle cells. The amount of 1-MeAde released into the medium was estimated by in vitro maturation assays by using isolated oocytes, with an analytical curve generated with authentic 1-MeAde. Symbols and bars represent the mean ± SEM for 4 experiments.
GSS mRNA Is Expressed in the Radial Nerve of A. pectinifera.
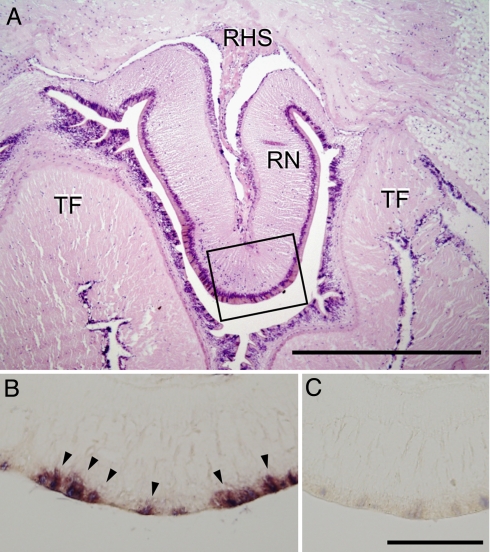
In situ hybridization was used to locate where the mRNA of GSS was synthesized in the starfish. The radial nerve is located in the arm of the starfish, surrounded by the tube feet (Fig. 5A). The mRNA of GSS was detected in the peripheral area of radial nerves proximal to the tube feet but not at the side of the hemal sinus (Fig. 5B). No significant signal above background levels was observed in control sections hybridized with sense probes (Fig. 5C).
Fig. 5.
Localization of GSS mRNA in radial nerves by in situ hybridization. (A) The tissue around radial nerves (RN) was sectioned by using Technovit 7100 resin (Heraeus Kulzer GmbH, Wehrheim) and stained by hematoxylin-eosin to show the overall structure. TF, tube foot; RHS, radial hemal sinus. (Scale bar: 500 μm.) Isolated radial nerves were sectioned by using paraffin-embedding technique and were hybridized with DIG-labeled antisense (B) or sense (C) probes. Signals were detected in the peripheral region (arrows). The positions of B and C correspond to the square in A. (Scale bar: 100 μm.)
Discussion
The chemical entity of GSS has been a mystery ever since the hormone's discovery in 1959 (10). Here, we report the purification of GSS, and additionally that GSS was chemically synthesized based on the amino acid structure we obtained. More intriguingly, cDNA cloning and phylogenetic analyses revealed that starfish GSS was a relaxin-like peptide, a member of the insulin/IGF/relaxin superfamily. Thus, the current study represents a relaxin system previously undescribed in invertebrates and points toward a possible reproductive role for this peptide in starfish.
The GSS protein that we purified had 2 polypeptide chains, A and B, with 6 cysteine atoms. The A chain of GSS harbored the cysteine motif (CCxxxCxxxxxxxxC), which is the signature pattern of the insulin/IGF/relaxin superfamily. Among the insulin/IGF/relaxin superfamily peptides, the relaxin/relaxin-like peptide family has been shown to include relaxins 1 to 3 and INSL peptides (INSLs 3 to 7). All share high structural similarity with insulin because of the presence of 6 cysteine residues, which confer 2 interchain disulfide bonds and 1 intrachain disulfide bond. Thus, relaxin and insulin were thought to be derived from a common ancestral gene and therefore grouped as the insulin superfamily, which later included IGFs I and II (IGF-1 and IGF-2) (11). Reports on the evolution of the relaxin group have postulated that relaxin existed before the emergence of the fish lineage and put forward the possibility of the existence of an ancestor of relaxin. In fact, it was previously reported that Ciona intestinalis had an RLN gene (12), but later it was found that this gene was not present in the completed genome of C. intestinalis (11). Thus, currently, fish relaxin 3 is considered the earliest form from which all of the present relaxins have evolved (11).
Sequence comparisons revealed that GSS was closer to the relaxin/insulin-like group than the insulin/IGF group. For example, the prepropeptide of GSS is organized like mammalian relaxins 1–3, INSL 5, and amphioxus relaxin-like (RLNL); i.e., signal peptide, B chain domain, and connecting peptide domain, followed by the A chain domain. GSS terminates with the cysteine residue at the C terminus of the A chain, whereas the insulins have the asparagine residue beyond cysteine. It is also important to note that the third cysteine in the A chain of GSS was preceded by a glycine residue. Substitution of GlyA14 in relaxin with isoleucine reduced the biological activity and the receptor-binding capacity of relaxin ≈100-fold (13). Despite its similarity with mammalian relaxins, the GSS sequence does not possess the vertebrate relaxin-specific receptor-binding motif RxxxRxxI/V, a distinct and well-conserved feature of the relaxin group identified thus far (9) (see below for further discussion).
Finally, our phylogenetic tree using sequences of insulin/IGF/relaxin of human, mouse, and amphioxus along with starfish GSS returned 2 distinct clusters for insulin/IGF and relaxin/insulin-like groups. Importantly, GSS was found to be closer to relaxins. It is of interest to note that the amphioxus RLNL sequence was also similar to GSS. Two other amphioxus sequences, ILP 1 and ILP 2, were grouped with mammalian insulin/IGFs. Additionally, our searches of the sea urchin Strongylocentrotus purpuratus genomic and EST databases could also identify a relaxin-like and 2 insulin/IGF-like genes [Sea Urchin Genome Resources (NCBI)]. Together, the present study has revealed that relaxin/relaxin-like peptide exists in invertebrate species also, indicating a possibility for the emergence of relaxin to be earlier than it has been thought at present.
Further support to our claim that GSS is a relaxin-like peptide is the induction of cAMP production in isolated ovarian follicle cells of starfish in response to GSS. Insulin in general binds to the receptor tyrosine kinase (RTK), known as the insulin receptor (IR), and stimulates a signaling pathway that includes phosphoinositide 3-kinase (PI3K) and serine/threonine kinase (AKT) (14). IGFs preferentially bind a related RTK and activate the MAPK pathway (15), whereas relaxins bind G protein-coupled receptors (GPCRs), stimulating cAMP production (16). A very recent report showed that an insulin-like peptide could induce egg maturation in the mosquito by binding to the mosquito insulin receptor (5). In our study, we found that all forms of GSS tested invariably induced cAMP production, indicating GPCRs to be involved in mediating the action of GSS. As discussed above, however, GSS does not possess the vertebrate relaxin-specific receptor-binding motif RxxxRxxI/V (9). The relaxin members achieve receptor specificity reportedly through this binding motif. For example, in the rat, relaxin 3 is more specific to RXFP 3 (GPCR 135), even though it can bind to RXFP 4 (GPCR 142) and RXFP 1 (LGR7) as well (17). Because GSS does not possess the relaxin-specific receptor-binding motif, we need further studies to find the receptors in starfish to which GSS would bind.
In this study, we found that the major sites of GSS mRNA expression were the supporting cells in the outer layer of the radial nerve at the side of tube feet. Based on the gamete-shedding activity, previous studies could find that GSS was localized predominantly in the neurosecretory granules on the supporting cell layer just beneath the outer cuticle of the radial nerve (18, 19). Our data not only further corroborate these findings, but also provide proof for the original site of GSS transcription. Further immunohistochemical analyses are required to clarify the localization of GSS protein and its secretory pathway.
GSS has been shown to stimulate the ovarian follicle cells in starfish to produce the MIH, 1-MeAde, which further induces the maturation of oocytes. Similarly, in our study the synthetic GSS could induce oocyte maturation within 30 min of incubation. Another interesting characteristic of GSS is the induction of spawning behavior in the starfish injected with the radial nerve extract (20). In our study, we observed that sexually mature starfish injected with the synthetic GSS moved along the walls of the aquarium before releasing the gametes. Because GSS is found in starfish during nonbreeding seasons, it is interesting to determine whether synthetic GSS can induce spawning behavior in sexually immature starfish during nonbreeding seasons. Interestingly, a very recent work (21) published during the preparation of this manuscript reported that a pentapeptide, cubifrin, induced germinal vesicle breakdown (GVBD) and spawning behavior in sea cucumber in a manner very similar to GSS in starfish. However, unlike GSS, cubifrin could induce GVBD in follicle-enclosed oocytes only in the presence of ovarian wall. Therefore, we assume that the action of GSS was more direct in inducing GVBD and spawning behavior compared with that of cubifrin.
Thus far, relaxin was shown to be a hormone important for reproduction in mammals only, but our data have shown that in starfish, relaxin-like peptide has an action analogous to gonadotropins in the vertebrates in stimulating the production of MIH, a role which has not been shown previously in any other species. Interestingly, the importance of INSL3-RXFP 2 (LGR 8) paracrine system in gonadotropin (LH)-induced oocyte maturation has been demonstrated in rats (22). These findings raise the possibility that relaxin or relaxin-like peptides may play an important role in reproduction in both vertebrates and invertebrates. Identification of a relaxin-like peptide exhibiting distinct reproductive functions in starfish will open new avenues into the study of the evolution of relaxin/relaxin-like peptides throughout the animal kingdom with reference to their highly diversified functions.
Materials and Methods
Animals.
Starfish, A. pectinifera, were collected from the coastal areas of Yokosuka (Kanagawa, Japan), Noto (Ishikawa, Japan), Asamushi (Aomori, Japan), and Nagashima (Kagoshima, Japan). Animals were kept in circulating seawater at 15 °C and used within 2 months of collection. The animals have been used according to the guidelines set up by National Institutes of Natural Sciences, Japan.
Preparation of Nerve Extract.
Radial nerves were manually isolated by forceps, then quickly frozen on dry ice and kept at −80 °C before use. The gross weight of the nerves from ≈5,500 starfish was 126.3 g (wet weight). Frozen nerves were ground in liquid nitrogen by using a mortar and pestle. Subsequently, 4 volumes of extraction buffer [10 mM ammonium acetate solution containing 1 mM pepstatin A, 5 mg/mL leupeptin, and 0.2 mM 4-(2-aminoethyl) benzenesulfonyl fluoride] was added, and the ground tissue was homogenized on ice by using a motorized homogenizer (physcotron; Niti-on Medical and Physical Instrument Manufacturing). The homogenates were centrifuged at 100,000 × g for 60 min at 4 °C. The supernatants were collected, the precipitates were resuspended in the extraction buffer, and the centrifugation step was repeated. The supernatants from 2 repeated extractions were pooled and preserved as the crude nerve extract.
Biological Assays.
GSS activity was biologically assayed by using ovarian fragments of A. pectinifera, as described previously (23). The ovary of a mature female was excised and cut into small fragments that contain only a few lobes with scissors. These fragments were exposed to preparations with or without GSS. The ovarian fragments responded to the preparations with GSS by shedding oocytes within 1 h. The released oocytes were observed under a microscope for GVBD so as to ascertain whether they had completed maturation or not.
Purification of GSS.
The crude nerve extract was dissolved in water and applied to a Sephadex G-50 column (50 × 250 mm) to eliminate proteins. The crude GSS fractions were lyophilized and subjected to HPLC with a system comprising a Shimadzu LC-6AD pump, an SPD-6A UV spectrophotometer, and an SLC-6B system controller equipped with a reverse-phase column (Develosil C30 Rpaqueous AR-5 column, 10 × 250 mm; Nomura Chemical). The first HPLC was carried out under neutral conditions with 10 mM sodium phosphate buffer at pH 7.0. After injection of the sample, a linear gradient of acetonitrile (0–30%) in 10 mM sodium phosphate buffer at pH 7.0 was run for 50 min. The GSS fraction obtained was subjected to a second HPLC with a reverse-phase column (Develosil C30 Rpaqueous AR-5 column, 10 × 250 mm; Nomura Chemical) under acidic conditions with 10 mM trimethylamine acetate buffer at pH 4.0. After sample injection, a linear gradient of acetonitrile (24–30%) in 10 mM trimethylamine acetate buffer at 4.0 was run for 60 min. GSS fractions were lyophilized and used for a third HPLC by using the SMART system (Amersham Pharmacia Biotech) equipped with a reverse-phase column (Develosil C30 Rpaqueous AR-3 column, 2.0 × 250 mm; Nomura Chemical). After injection of the sample, a linear gradient of acetonitrile (20–21%) in 10 mM sodium phosphate buffer at pH 6.0 was run for 125 min. GSS was finally purified by a fourth HPLC by using the SMART system equipped with a reverse-phase column (Develosil C30 Rpaqueous AR-3 column, 1.5 × 250 mm; Nomura Chemical). After injection of the sample, a linear gradient of acetonitrile (18–35%) in 10 mM sodium phosphate buffer at pH 6.0 was run for 50 min. An aliquot of the purified GSS was used for the determination of molecular weight by MALDI-TOF MS (Reflex III; Brucker Daltonics).
Amino Acid Sequencing.
The amino acid sequence of the purified GSS was analyzed with a protein sequencer (Procise 492cLC; Applied Biosystems) and electronspray ionization-tandem quadruple/orthogonal-acceleration TOF MS/MS (Micromass) equipped with a nano-HPLC system (Jasco).
Chemical Synthesis.
Peptides of A and B chains were synthesized commercially (Toray Research Center). The heterodimeric form was also produced commercially (Peptide Institute) based on the heterodimeric structure of the insulin/IGF/relaxin superfamily.
Effect of Synthetic GSS.
Follicle cells were isolated from folliculated oocytes as described previously (24). Ten million follicle cells were incubated with synthetic GSS for 2 h at 20 °C in a total volume of 1.0 mL of artificial seawater. After the incubation, the cells were spun down at 1,000 × g for 1 min and quickly frozen in liquid nitrogen. Supernatants were analyzed for the amount of 1-MeAde released from follicle cells as described previously (23). The frozen follicle cells were analyzed for the amount of intracellular cAMP with a BIOTRAK cAMP EIA system (GE Healthcare).
cDNA Cloning.
Total RNA was extracted from the radial nerves of A. pectinifera after homogenization with 350 μL of RLT buffer supplied with the RNeasy Mini Kit (Qiagen). The poly(A)+ RNA fraction was obtained by using Oligotex-dT30 Super (Nippon Gene) according to the manufacturer's instructions. First-strand cDNA was synthesized from 0.5 μg of mRNA by using PowerScript (Clontech) with oligo(dT) primers. A cDNA fragment encoding GSS was amplified with degenerate oligonucleotide primers designed from the amino acid sequence of the purified GSS by RT-PCR. The forward primer, 5′-TGYGAYGAYGAYTTYCAYATGGC-3′, corresponded to the amino acid sequence CDDDFHMA. The reverse primer, 5′-TCRCTNGGNGTRCANCCRTG-3′, corresponded to the amino acid sequence HGCTPSE. A cDNA encoding GSS was cloned with the SMART RACE cDNA Amplification Kit (Clontech).
Phylogenetic Analysis.
The alignment of the peptide sequence was constructed by using MAFFT (v6.62b; http://align.bmr.kyushu-u.ac.jp/mafft/software/). The used method was the L-INS-I (8), and the parameters were as follows: scoring matrix for amino acid sequence, BLOSUM62; gap opening penalty, 1.53; offset value, 0.00. The phylogenetic tree was described by using the “NJ or UPGMA tree” on the MAFFT result page, and the parameters are as follows: size, 23 sequences × 54 sites; method, neighbor-joining; model, JTT; alpha, infinity; bootstrap resampling, 100.
In Situ Hybridization.
To generate the probe, a GSS cDNA fragment was amplified by using the forward primer, 5′-CCATCTCTTCCAGGCAACTT-3′, and the reverse primer, 5′-TCCATTTAACAAAAACCCATGA-3′, by RT-PCR and subcloned into pGEM-T Easy vector (Promega). Antisense and sense ribonucleotide probes were in vitro-transcribed with the digoxigenin (DIG) labeling kit (Roche). The paraffin-embedded sections (5 μm in thickness) of radial nerves described above were used for in situ hybridization. After hybridization, an immunoreaction was induced in the sections by using an antibody specific to DIG labeled with alkaline phosphatase (Roche). The hybridized probes were examined with nitroblue tetrazolium/ 5-bromo-4-chloro-3-indolyl phosphate (Roche) as a substrate.
Supplementary Material
Acknowledgments.
The authors wish to express their admiration for the late Professor Haruo Kanatani's meritorious achievements in the study of starfish reproduction and express their sincere gratitude for his impassioned encouragement. We thank Dr. H. Shirai for sharing valuable experience and insights. We thank Dr. H. Tousuji (Kagoshima University, Kagoshima City, Japan), Dr. Y. Sasayama and the staff of Noto Marine Laboratory (Kanazawa University, Ishikawa, Japan), Dr. S. Nemoto and the staff of the Marine and Coastal Research Center (Ochanomizu University, Tokyo), and Dr. H. Katow and the staff of the Research Center for Marine Biology (Graduate School of Life Sciences, Tohoku University, Sendai, Japan) for help in collecting starfish. Analyses of the purified peptide were performed by the National Institute for Basic Biology (NIBB) Center for Analytical Instruments. We thank Ms. Y. Makino for help with protein sequencing and mass spectrometry. This study was supported by NIBB Cooperative Research Program Grants 1-109, 2-122, 3-104, 4-108, 5-106, and 6-106. This study was supported in part by Ministry of Education, Culture, Sports, Science, and Technology (MEXT) Grants-in-aid for Scientific Research (C) 18570056 (to M.M.) and 14540620 and 16570059 (to M.Y.) and by the academic research support program from the Daiko Foundation, Grant 9092 (to M.Y.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0900243106/DCSupplemental.
References
- 1.Kuczer M, Rosinski G, Konopinska D. Insect gonadotropic peptide hormones: Some recent developments. J Pept Sci. 2007;13:16–26. doi: 10.1002/psc.792. [DOI] [PubMed] [Google Scholar]
- 2.Masler EP, Hagedorn HH, Petzel DH, Borkovec AB. Partial purification of egg development neurosecretory hormone with reverse-phase liquid chromatographic techniques. Life Sci. 1983;33:1925–1931. doi: 10.1016/0024-3205(83)90677-x. [DOI] [PubMed] [Google Scholar]
- 3.Chiu AY, et al. Purification and primary structure of the neuropeptide egg-laying hormone of Aplysia californica. Proc Natl Acad Sci USA. 1979;76:6656–6660. doi: 10.1073/pnas.76.12.6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hasegawa Y, Haino-Fukushima K, Katakura Y. Isolation and properties of androgenic gland hormone from the terrestrial isopod, Armadillidium vulgare. Gen Comp Endocrinol. 1987;67:101–110. doi: 10.1016/0016-6480(87)90209-7. [DOI] [PubMed] [Google Scholar]
- 5.Brown MR, et al. An insulin-like peptide regulates egg maturation and metabolism in the mosquito Aedes aegypti. Proc Natl Acad Sci USA. 2008;105:5716–5721. doi: 10.1073/pnas.0800478105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanatani H, Shirai H, Nakanishi K, Kurokawa T. Isolation and identification of meiosis-inducing substance in starfish, Asterias amurensis. Nature. 1969;221:273–274. doi: 10.1038/221273a0. [DOI] [PubMed] [Google Scholar]
- 7.Nagahama Y, Yoshikuni M, Yamashita M, Tokumoto T, Katsu Y. Regulation of oocyte growth and maturation in fish. Curr Top Dev Biol. 1995;30:103–145. doi: 10.1016/s0070-2153(08)60565-7. [DOI] [PubMed] [Google Scholar]
- 8.Katoh K, Kuma K, Toh H, Miyata T. MAFFT version 5: Improvement in accuracy of multiple sequence alignment. Nucleic Acids Res. 2005;20:511–518. doi: 10.1093/nar/gki198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ross AD, et al. Human relaxin gene 3 (H3) and the equivalent mouse relaxin (M3) gene. J Biol Chem. 2002;277:1148–1157. doi: 10.1074/jbc.M107882200. [DOI] [PubMed] [Google Scholar]
- 10.Chaet AB, McConnaughy RA. Physiologic activity of nerve extracts. Biol Bull. 1959;117:407–408. [Google Scholar]
- 11.Wilkinson TN, Speed TP, Tregear GW, Bathgate RAD. Evolution of the relaxin-like peptide family. Evol Biol. 2005;5:14. doi: 10.1186/1471-2148-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Georges D, Schwabe C. Porcine relaxin, a 500 million-year-old hormone? The tunicate Ciona intestinalis has porcine relaxin. FASEB J. 1999;13:1269–1275. doi: 10.1096/fasebj.13.10.1269. [DOI] [PubMed] [Google Scholar]
- 13.Bullensbach EE, Schwabe C. Functional importance of the A chain loop in relaxin and insulin. J Biol Chem. 1994;269:13124–13128. [PubMed] [Google Scholar]
- 14.McDonald N, Murray-Rust J, Blundell T. Structure-function relationships of growth factors and their receptors. Br Med Bull. 1989;45:554–569. doi: 10.1093/oxfordjournals.bmb.a072342. [DOI] [PubMed] [Google Scholar]
- 15.Halls ML, van der Westhuizen ET, Bathgate RAD, Summers RJ. Relaxin family peptide receptors: Former orphans reunite with their parent ligands to activate multiple signaling pathways. Br J Pharmacol. 2007;150:677–691. doi: 10.1038/sj.bjp.0707140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu C, et al. INSL5 is a high-affinity-specific agonist for GPCR142-GPR100. J Biol Chem. 2005;280:292–300. doi: 10.1074/jbc.M409916200. [DOI] [PubMed] [Google Scholar]
- 17.Kuei C, et al. R3 (BΔ23–27) R/I5 chimeric peptide, a selective antagonist for GPCR135 and GPCR142 over relaxin receptor LGR7: In vitro and in vivo characterization. J Biol Chem. 2007;282:25425–25435. doi: 10.1074/jbc.M701416200. [DOI] [PubMed] [Google Scholar]
- 18.Imlay MJ, Chaet AB. Microscopic observations of gametes shedding substance obtained from radial nerves. Science. 1967;146:1177–1179. doi: 10.1126/science.146.3648.1177. [DOI] [PubMed] [Google Scholar]
- 19.de Angelis, et al. Presence of granules containing gonad-stimulating substance in starfish radial nerve. Annot Zool Jpn. 1972;45:16–21. [Google Scholar]
- 20.Kanatani H. On the substances controlling certain reproductive phenomena in starfishes. Prontif Acad Sci Commentarii. 1970;2:1–36. [Google Scholar]
- 21.Kato S, et al. Neuronal peptides induce oocyte maturation and gamete spawning of sea cucumber, Apostichopus japonicas. Dev Biol. 2009;326:169–176. doi: 10.1016/j.ydbio.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 22.Kuwamura K, et al. Paracrine regulation of mammalian oocyte maturation and male germ cell survival. Proc Natl Acad Sci USA. 2004;101:7323–7328. doi: 10.1073/pnas.0307061101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shirai H. In: Methods in Cell Biology. Schroeder TE, editor. Vol 27. Orlando, FL: Academic; 1986. pp. 73–88. [Google Scholar]
- 24.Mita M. Effect of cysteine and its derivatives on 1-methyladenine production by starfish follicle cells. Dev Growth Differ. 1985;27:563–572. doi: 10.1111/j.1440-169X.1985.00563.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.