Abstract
Osteoporosis in men is an increasingly recognized problem with associated fracture morbidity and mortality. Treatment is limited, with the bisphosphonates being the mainstay of therapy. Risedronate has demonstrated fracture efficacy in women and efficacy has been recently been investigated in men. In men, risedronate either maintains or increases bone mineral density. In placebo controlled trials it has been shown to be safe and effective in preventing fractures.
Keywords: male osteoporosis, risedronate, bone mineral density, fractures
Osteoporosis in men
Osteoporosis is increasingly recognized in men as a condition that is associated with significant morbidity and mortality. Data from the third National Health and Nutrition Examination Survey (NHANES III) indicate that 3%–6% of men in the US have osteoporosis and 28%–47% have osteopenia when using male cut-offs for bone mineral density (BMD) (Looker et al 1997). In a population-based sample Melton et al compared osteoporosis in men with women (Melton et al 1998). By World Health Organization criteria, the age-adjusted prevalence of osteoporosis at the hip, spine, or wrist was 35% among women 50 years of age or older. A similar approach produced an osteoporosis prevalence rate in men 50 years of age and older of 19%. They showed that bone density predicts fracture risk in men as it does in women, and the prevalence of osteoporosis in men, using sex-specific normal values, is substantial. They concluded that there was a need for better prevention and treatment strategies for men (Melton et al 1998).
There are several differences in the clinical presentation of osteoporosis between men and women (Poor et al 1995a; Vanderschueren et al 2000; Diamond 2005). Men achieve approximately 1% greater peak bone mass than women at the spine and 5% at the femoral neck (Looker et al 1997; Tenenhouse et al 2000). Although the incidence of osteoporosis-related fractures is much less in men, osteoporotic fractures are associated with greater morbidity and mortality in men compared with women (Jiang et al 2005). Among men and women >50 years of age, the incidence of spinal fractures was estimated at 5% and 16% and that of hip fractures at 6% and 18%, respectively (Diamond 2005). Men with hip fractures have greater mortality (Poor et al 1995b; Jiang et al 2005; Hawkes et al 2006) and at least in one study were twice likely to die compared with women with hip fractures (Poor et al 1995a). Among men with hip fractures who survive, 80% will not regain their prefracture functional status and up to 50% will be institutionalized (Poor et al 1995b).
Observational data showed that men referred to tertiary care osteoporosis centers had higher mean baseline femoral neck and lumbar spine bone mineral densities than women, yet they had double the rate of prevalent vertebral fractures compared with women and triple the rate of multiple prevalent vertebral fractures compared with women (Sawka et al 2004). Furthermore, men were twice as likely as women to sustain a fracture within 2 years of starting treatment during the observation period. It was suggested that osteoporosis may be under-recognized in men until the condition is at an advanced stage and that a form of gender bias may exist in the recognition and treatment or referral for treatment of osteoporosis in men.
In male patients presenting with osteoporotic fractures, major causes of skeletal fragility, such as hypogonadism, glucocorticoid excess, primary hyperparathyroidism and alcohol abuse, can often be identified (Vanderschueren et al 2000). In as many as 50% of osteoporotic men, however, no etiology can be found (Vanderschueren et al 2000). The loss of skeletal integrity in aging men may be partially related to endocrine deficiencies, including vitamin D, androgen, and/or estrogen deficiency. While the consequences of vitamin D or estrogen deficiency in women have been well established, the skeletal impact of these (partial) age-related deficiencies in men remains to be clarified. As knowledge about the prevalence and etiology of osteoporosis in men increases, it will be recognized and treated in more men, in hopes of preventing fracture.
Risedronate
Risedronate [1-hydroxy-2-(3-pyridinyl)ethylidene bisphosphonic acid monosodium salt], (Cincinnati), is a potent pyridinyl bisphosphonate that has been shown to decreases the risk of vertebral and nonvertebral fractures in postmenopausal women with osteoporosis (Harris et al 1999; Reginster et al 2000; McClung et al 2001). Recently it has been studied in men under a variety of clinical circumstances (Table 1).
Table 1.
Summary of randomized controlled trials of risedronate for the treatment of male osteoporosis
| Condition | Design | n | RIS dose (mg) | BMD LS (%) | BMD FN (%) | Vert fracture | Hip fracture |
|---|---|---|---|---|---|---|---|
| Primary OP (Boonen 2006) 24 months | RCT | 191 | 35 per wk | 4.5 | NR | NR | NR |
| 93 | Pla | NR | NR | NR | NR | ||
| Primary and Secondary OP (Ringe 2003) 12 months | RCT | 158 | 5 per day | 4.7 | 1.8 | 5.1% | NR |
| 158 | Pla | 1.0 | 0.2 | 12.7% | NR | ||
| CIOP prevention (Reid 2000, 2001) 12 months | RCT | 184 | 2.5 or 5 perday | 0.8a | 1.5a | Ris 9%
Pla 24% |
NR |
| Pla | −3.4 | −3.3 | Pooled | NR | |||
| CIOP treatment (Reid 2001; Cohen 1999) 12 months | 2.5 or 5 perday | 4.8a | 2.1a | 2.5/5 mg vs placebo 82.4% reduction (p = 0.008) | NR | ||
| Pla | 1.2 | −0.2 | NR | ||||
| Post-stroke (Sato 2005) 18 months | RCT | 140 | 2.5/day | 2.5b | NR | NR | 1.4 |
| 140 | Pla | −3.5b | NR | NR | 7.1 | ||
| Hyperthyroidism (Majima 2006) 12 months | RCT | 14 | 2.5 | 6.07 | 4.41 | NR | NR |
| 13 | Pla | 1.90 | 2.61 | NR | NR | ||
| IBD (Henderson 2006) 12 months | RCT | total 61 | 5 | 2.9 | 3.1 | NR | NR |
| male 25 | Pla | 1.04 | 0.1 | NR | NR | ||
| Transplantation (Tauchmanova 2003) 12 months | RCT | total 34 | 5 | 5.9 | 1.3 | NR | NR |
| male 16 | Pla | 1.1 | −4.2 | NR | NR | ||
| Leprosy (Kanaji 2006) 9 months | RCT | 12 | 2.5 | 4.8c | NR | 0.82** | NR |
| 11 | Pla | 0.9c | NR | 1.88 | NR | ||
| Stress fractures (Milgrom 2004) 3 months | RCT | 165 | 30 for 2 weeks then 30 weekly | N/A | N/A | N/A | 14.5 |
| 159 | Pla | N/A | N/A | N/A | 13.2 |
BMD change with 5 mg dose
metacarpal BMD
estimate from graph
p < 0.05
Abbreviations: BMD, bone mineral density; CIOP, corticosteroid-induced osteoporosis; IBD, inflammatory bowel disease; NR, not reported; OP, osteoporosis; Pla, placebo; RIS, risedronate; RCT, randomized controlled trial.
Primary osteoporosis
Boonen et al recently reported in abstract from, a 2-year, double-blind, randomized, placebo-controlled, parallel group, multicenter study to determine the efficacy and safety of risedronate 35 mg once a week (Boonen et al 2006), compared with placebo in men with osteoporosis. Men between the ages of 36 and 84 years, inclusive, who had osteoporosis defined as either a lumbar spine T-score ≤–2.5 and femoral neck T-score ≤–1 SD or a lumbar spine T-score ≤–1 and femoral neck T-score ≤–2 SD were randomized (2:1) to either risedronate 35 mg once a week (n = 191) or placebo (n = 93). All received daily supplementation of calcium (1000 mg) and vitamin D (400–500 IU) for 2 years. Efficacy was assessed by percentage change from baseline in lumbar spine and total proximal femur, femoral neck, and femoral trochanter bone mineral density (BMD) and bone turnover markers (BTMs) including type I collagen C- telopeptide (Ctx), type I collagen N-telopeptide/creatinine (Ntx/cr), and bone specific alkaline phosphatase (BSAP). BMD was measured at months 6, 12, 24, and end point.
The primary endpoint showed a statistically significant difference between risedronate and placebo groups in mean percent change from baseline to endpoint in lumbar spine BMD 4.53% (95% CI: 3.46%, 5.60%). The risedronate group had statistically significant increases compared with placebo in mean percentage change from baseline for lumbar spine BMD at months 6, 12, and 24, total proximal femur and femoral trochanter BMD at months 12, 24, and endpoint, and femoral neck BMD at month 24 and endpoint. The mean percentage change values for all BTMs were statistically significantly reduced in the risedronate group compared with baseline and with placebo at all time points measured.
The two treatment groups were comparable in overall percentages of patients with adverse events (AEs) (73%, placebo, 70% risedronate), serious AEs (16% placebo, 15%, risedronate), moderate to severe upper GI AEs (4% placebo, 3% risedronate) and overall musculoskeletal AEs (11% placebo, 12% risedronate). In this 2-year study, risedronate 35 mg once a week was safe and effective for the treatment of osteoporosis in men.
Ringe et al (2006) assessed the efficacy and safety of risedronate in the treatment of men with primary and secondary osteoporosis. They conducted a single-center, open label, randomized, prospective 1-year study where patients were randomized to risedronate (risedronate 5 mg/day plus calcium 1000 mg/day and vitamin D 800 IU/day) or control groups (alfacalcidol 1 μg/day plus calcium 500 mg/day or vitamin D 1000 IU/day plus calcium 800 mg/day). The mean age in the risedronate-treated group was 55.8 years and in the control group 58.0 years. Prevalent vertebral fractures were present in 53.2% of the risedronate group and 51.3% of the control group. BMD measurements, x-rays of the spine, and patient self-assessments of back pain were performed at baseline and 12 months. Blinded semi-quantitative fracture assessment was conducted by a radiologist. A total of 316 men with osteoporosis were enrolled in the trial (risedronate, n = 158; control, n = 158). At 1 year, lumbar spine BMD increased by 4.7% in the risedronate group versus an increase of 1.0% in the control group (p < 0.001). Significant increases in BMD at the total hip and femoral neck were also observed with risedronate compared with the control group. Over 1 year, 8 patients suffered new vertebral fractures (5.1%) in the risedronate-treated group, compared with 20 patients with new vertebral fractures (12.7%) in the control group, representing a 60% (p = 0.028) reduction in fractures. Nonvertebral fractures occurred in 10 patients (6.3%) in the risedronate-treated group compared with 17 patients (10.8%) in the control group. They concluded that daily treatment with risedronate for 12 months significantly increased BMD at the lumbar spine, femoral neck, and total hip and significantly reduced the incidence of new vertebral fractures.
Corticosteroid-induced bone loss
Limited information is available on the effect of bisphosphonates in men receiving corticosteroid therapy. Reid et al (2001) studied 184 men among the patients enrolled in two double-blind, placebo-controlled, 1-year studies with similar protocols. The studies evaluated the effects of risedronate in patients beginning corticosteroid treatment (prevention study) at a dose of at least 7.5 mg per day of prednisone or equivalent or continuing long-term treatment of corticosteroid (treatment study) at that dose. The men received either placebo or risedronate (2.5 mg or 5 mg) daily, along with calcium supplementation (500–1000 mg). Endpoints included differences in BMD at the lumbar spine, femoral neck, and femoral trochanter, assessment of vertebral fractures, changes in biochemical markers of bone turnover, and overall safety. The underlying diseases requiring corticosteroid treatment included rheumatoid arthritis, lung disease, polymyositis, polymyalgia rheumatica, temporal arteritis, and vasculitis. A total of 184 male patients received at least one dose of study drug (ITT population). The baseline characteristics of the 184 enrolled patients were similar between treatment groups. At baseline, the mean lumbar spine BMD was similar in the risedronate and placebo groups. The mean daily corticosteroid dose prior to enrollment was 19.4 mg (SD, 16.4) and the mean corticosteroid dose during the 1-year study was 14.2 mg (SD, 15.7); the corticosteroid doses were similar among the three treatment groups. Forty percent of men had prevalent (baseline) vertebral fractures; the prevalence was slightly higher in the risedronate 5 mg group (46%) than in the other groups.
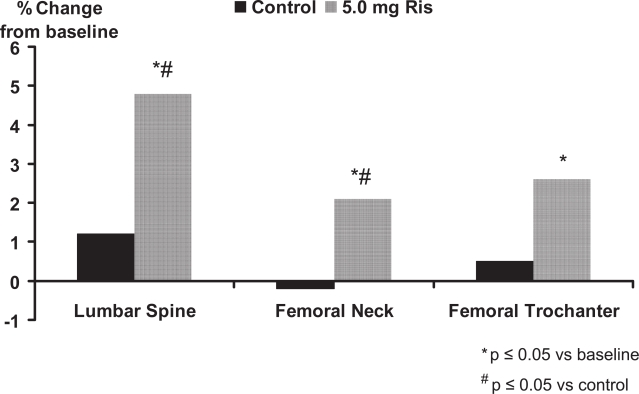
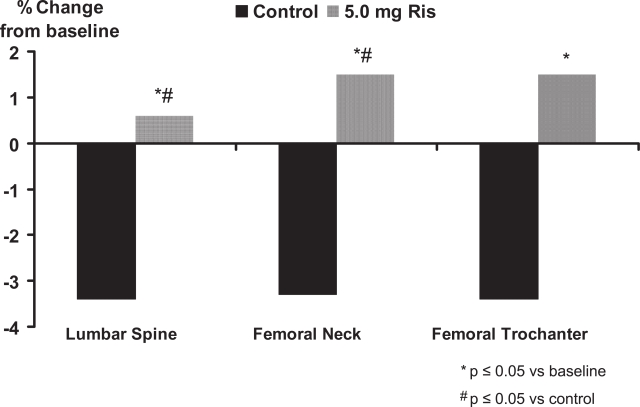
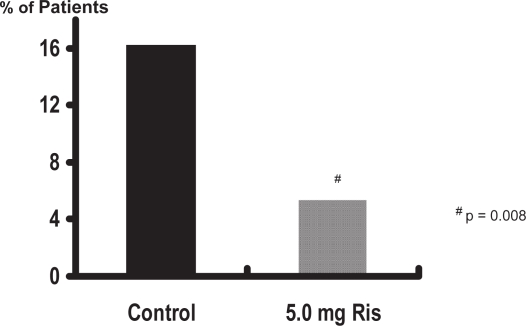
In the treatment study, risedronate 5 mg significantly (p < 0.01) increased lumbar spine BMD by 4.8% at the lumbar spine, 2.1% at the femoral neck, and 2.6% at the femoral trochanter compared with baseline values (Figure 1). In the prevention study, bone loss was prevented with risedronate 5 mg; in the placebo group, BMD decreased significantly (p < 0.01) by 3.4%, 3.3%, and 3.4% in the lumbar spine, femoral neck, and trochanter, respectively, at 1 year (Figure 2). The differences between risedronate 5 mg and placebo groups were significant at all skeletal sites in the prevention study (p < 0.01) and at the lumbar spine in the treatment study (p < 0.001). The 2.5 mg dose also had a positive effect on BMD, although of a lesser magnitude than the 5 mg dose. When the data from the two studies were combined, the incidence of vertebral fractures decreased 82.4% (95% CI: 36.6%, 95.1%) in the pooled risedronate groups compared with placebo (p = 0.008). Risedronate was well tolerated in men, with a similar incidence of upper gastrointestinal adverse events in the placebo and treatment groups. They concluded that daily treatment with risedronate increases bone density and decreases vertebral fracture risk within 1 year in men receiving corticosteroid therapy.
Figure 1.
Risedronate in the treatment of corticosteroid-induced osteoporosis.
Figure 2.
Risedronate in the prevention of corticosteroid-induced osteoporosis.
Other conditions
Risedronate has been studied in a number of other conditions including post-stroke (Sato et al 2005), hyperthyroidism (Majima et al 2006), inflammatory bowel disease (Henderson et al 2006), transplantation (Tauchmanova et al 2003), leprosy (Kanaji et al 2006), and stress fractures in infantry recruits (Milgrom et al 2004).
Post-stroke
There is a high incidence of hip fractures in patients after hemiplegic stroke. Bone mineral density is decreased on the hemiplegic side in patients after stroke, correlating with the immobilization-induced bone resorption, the degree of paralysis, and hypovitaminosis D. Sato et al (2005) evaluated the effectiveness of risedronate on osteoporosis and the risk of hip fractures in men 65 years or older after stroke. They conducted an 18-month, randomized, double-blind trial of 280 male patients 65 years or older who were post-stroke, with 140 receiving a daily dose of 2.5 mg risedronate and the other 140 receiving placebo. The mean age in both groups was 76.3 years. There were no significant differences between the placebo group and risedronate group in Barthel Index, degree of hemiplegia, number of fallers, type of stroke, BMD, or biochemical indices of bone metabolism. Although the values of BMD on both hemiplegic and nonhemiplegic sides were within the reference range compared with the elderly general Japanese population, BMD on the hemiplegic side was significantly reduced compared with that of the nonhemiplegic side. Compared with the reference range of the elderly general Japanese population, both groups of patients had high levels of serum ionized calcium and urinary D-Pyr and low serum concentrations of PTH, 25-OHD, and 1,25-(OH)2D at baseline. Serum 25-OHD was outside of the reference range in both groups: 114.6 ± 21.7 ng/mL (45.9 ± 8.7 nmol/L) in the placebo group and 114.6 ± 16.7 ng/mL (45.9 ± 6.7 nmol/L) in the risedronate group.
During the study, a total of 269 falls occurred in the former group and 278 in the latter group, or 4.2 falls per patient per year for both groups. In the risedronate group, 28 (20%) of 140 patients fell at least once, and 15 (80%) fell twice or more; in the placebo group, 29 (21%) of 140 patients fell at least once, and 15 (79%) fell twice or more. The number of falls among fallers ranged from 1 to 12 in both groups.
Ten patients sustained hip fractures in the placebo group, and 2 hip fractures occurred in the risedronate group. The relative risk of a hip fracture was reduced with risedronate to 0.19 (95% CI: 0.04–0.89). Bone mineral density increased by 2.5% in the risedronate group and decreased by 3.5% in the placebo group (p < 0.001). Urinary deoxypyridinoline, a bone resorption marker, decreased by 58.7% in the risedronate group and by 37.2% in the placebo group. They concluded that treatment with risedronate increases bone mineral density and reduces hip fractures in elderly men who are post-stroke (Sato et al 2005).
Hyperthyroidism
It has been well established that hyperthyroidism leads to diminished BMD, and that a previous history of hyperthyroidism remains a risk factor for fractures. However, little is known about how to manage the reduction in BMD caused by hyperthyroidism. This study evaluated the efficacy of risedronate for the treatment of osteoporosis/osteopenia in patients with Graves’ disease (Majima et al 2006). Of 34 Japanese male patients with newly diagnosed Graves’ disease, 27 with osteoporosis/osteopenia were included in this study. They were randomly divided into two groups by therapeutic regimen. Fourteen patients were treated with an antithyroid drug and risedronate and the control group consisted of 13 patients treated with the same antithyroid drug only. The mean age and BMI was 43.6 years and 22.86 in the risedronate group and 45.5 years and 22.34 respectively in the control group. BMD at the lumber spine, femoral neck, and distal radius were measured at baseline, and at 6 and 12 months after the trial. Bone-specific alkaline phosphatase and urinary N-terminal telopeptide of type I collagen normalized by creatinine were significantly more reduced in the risedronate treated group than in the control group after both 6 and 12 months. The percentage increases in BMD at the lumbar spine femoral neck and distal radius at 1 year were 6.6%, 4.2%, and 2.4% respectively for risedronate and were greater than in the control group. These beneficial effects of risedronate for patients with osteoporosis/osteopenia caused by Graves’disease may reduce risk of future fractures. They concluded that risedronate should be considered for the treatment of decreased bone mass associated with Graves’ disease.
Inflammatory bowel disease
Low bone density and fractures are common in patients with inflammatory bowel disease (IBD). Henderson et al (2006) conducted a study to determine whether risedronate is safe and effective in preserving bone mass compared with calcium alone in IBD patients with low bone mass. They enrolled 61 ambulatory patients, 25 of whom were males, with Crohn’s disease (n = 31) or ulcerative colitis (n = 30) and low bone density in a double-blind placebo-controlled trial. Patients were randomized to 12 months of therapy with risedronate 5 mg or placebo. All received a 600 mg calcium supplement. Bone density using dual energy x-ray absorptiometry was performed at baseline and at 12 months. Disease activity, use of corticosteroid, and adverse events were noted. Forty-eight patients completed the trial. Compared with the placebo group, risedronate resulted in a 2.0% (95% CI: 0.02–3.97) and 1.9% (95% CI: 0.21–3.62) improvement in bone density at the spine and hip, respectively. IBD diagnosis, gender, therapy, and disease status had no effect on the results. There were no significant differences in the adverse events. They concluded that risedronate improved bone density at the spine and hip in patients with either Crohn’s disease or ulcerative colitis and low bone mass. These data suggest that risedronate is a safe and effective therapy to improve bone mass in these patients (Henderson et al 2006).
Transplantation
In this prospective randomized study risedronate was evaluated in patients who had undergone allogeneic stem cell transplant (SCT) for hematological malignancies (Tauchmanova et al 2003). Thirty-four patients (18 females, 16 males, age 32 ± 10 years) with BMD T-score ≤–1.5 SD at least 6 months after SCT were treated with calcium 1 g/day and vitamin D 800 IU/day and randomized to receive (n = 17) or not receive (n = 17) oral risedronate 5 mg/day. The duration of treatment was 12 months. After 12 months, lumbar BMD increased (5.9 ± 1.7%, p < 0.05), compared with baseline in risedronate-treated patients and increased slightly (+1.1 ± 1.4%) in the placebo group. They concluded that treatment with risedronate for 12 months increased BMD significantly at the lumbar spine and prevented further bone loss at the femoral neck in long-term survivors after allogeneic stem cell transplantation.
Leprosy
Kanaji et al (2006) evaluated the therapeutic effect of risedronate in male osteoporotic patients with leprosy. Twenty-three male patients with leprosy, 63–87 years of age, were randomly divided into two groups, risedronate, 2.5 mg/day or placebo. The BMD of the lumbar spine (L2–L4) was measured by dual-energy X-ray absorptiometry (DXA), and urinary N-telopeptide cross-links (NTX) were assessed at baseline, 6 months, and 12 months after treatment. There were no significant differences in age, body mass index, BMD, or urinary NTX levels at baseline between the two groups. They showed that oral administration of risedronate prevented vertebral fractures. There were a mean of 0.82 new vertebral fractures per patient during the 12 months’ treatment in the risedronate group and 1.88 per patient in the placebo group. The number of incident vertebral fractures per patient in the risedronate group was significantly lower than those in the placebo group (p < 0.05), suggesting that risedronate treatment prevented incident vertebral fractures. Increased lumbar BMD and significant reductions of bone turnover as measured by urinary NTX levels were seen with risedronate compared with placebo. These findings suggest that oral administration of risedronate contributes to the prevention of vertebral fractures by suppressing bone resorption and increasing in lumbar BMD in the elderly male patients with leprosy.
Stress fractures
A randomized, double-blind, placebo controlled trial of 324 new infantry recruits known to be at high risk for stress fracture was conducted (Milgrom et al 2004). Recruits were given a loading dose of 30 mg of risedronate or placebo daily for 10 doses during the first 2 weeks of basic training and then a once a week maintenance dose for the following 12 weeks. Recruits were monitored by biweekly orthopedic examinations during 15 weeks of basic training for stress fractures. Bone scans for suspected tibial and femoral stress fractures and radiographs for suspected metatarsal stress fractures were used to verify stress fracture occurrence. No statistically significant difference in the tibial, femoral, metatarsal, or total stress fracture incidence between the treatment group and the placebo group was seen and it was concluded that prophylactic treatment with risedronate in a training population at high risk for stress fracture using a maintenance dosage for the treatment of osteoporosis does not lower stress fracture risk.
Summary
Osteoporosis in men is a significant cause of morbidity and mortality. Risedronate is effective in increasing BMD in primary and corticosteroid-induced osteoporosis in men and more importantly the prevention of vertebral fractures within the first year of therapy in corticosteroid-treated men. Risedronate is also effective in the prevention of hip fractures post stroke and vertebral fractures in those with leprosy. Risedronate maintained BMD in those with Graves’ disease, inflammatory bowel disease, cystic fbrosis and transplantation. Risedronate was ineffective in the prevention of stress fractures in infantry recruits.
Figure 3.
Vertebral fracture incidence.
References
- Boonen S, Delmas PD, Wenderoth DH, et al. Risedronate shown to be safe and effective in men with osteoporosis in a 2-year, double-blind, randomized, placebo-controlled, multicentre study. Osteoporos Int. 2006;17:S230–31. [Google Scholar]
- Cohen S, Levy RM, Keller M, et al. Risedronate therapy prevents corticosteroid-induced bone loss: a twelve-month, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheum. 1999;42:2309–18. doi: 10.1002/1529-0131(199911)42:11<2309::AID-ANR8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Diamond TH. Pharmacotherapy of osteoporosis in men. Expert Opin Pharmacother. 2005;6:45–58. doi: 10.1517/14656566.6.1.45. [DOI] [PubMed] [Google Scholar]
- Harris ST, Watts NB, Genant HK, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA. 1999;282:1344–52. doi: 10.1001/jama.282.14.1344. [DOI] [PubMed] [Google Scholar]
- Hawkes WG, Wehren L, Orwig D, et al. Gender differences in functioning after hip fracture. J Gerontol A Biol Sci Med Sci. 2006;61:495–99. doi: 10.1093/gerona/61.5.495. [DOI] [PubMed] [Google Scholar]
- Henderson S, Hoffman N, Prince R. A double-blind placebo-controlled study of the effects of the bisphosphonate risedronate on bone mass in patients with inflammatory bowel disease. Am J Gastroenterol. 2006;101:119–23. doi: 10.1111/j.1572-0241.2006.00372.x. [DOI] [PubMed] [Google Scholar]
- Jiang HX, Majumdar SR, Dick DA, et al. Development and initial validation of a risk score for predicting in-hospital and 1-year mortality in patients with hip fractures. J Bone Miner Res. 2005;20:494–500. doi: 10.1359/JBMR.041133. [DOI] [PubMed] [Google Scholar]
- Kanaji A, Higashi M, Namisato M, et al. Effects of risedronate on lumbar bone mineral density, bone resorption, and incidence of vertebral fracture in elderly male patients with leprosy. Lepr Rev. 2006;77:147–53. [PubMed] [Google Scholar]
- Looker AC, Orwoll ES, Johnston CC, Jr, et al. Prevalence of low femoral bone density in older U.S. adults from NHANES III. J Bone Miner Res. 1997;12:1761–68. doi: 10.1359/jbmr.1997.12.11.1761. [DOI] [PubMed] [Google Scholar]
- McClung MR, Geusens P, Miller PD, et al. Effect of risedronate on the risk of hip fracture in elderly women. Hip intervention program study group. N Engl J Med. 344:333–40. doi: 10.1056/NEJM200102013440503. [DOI] [PubMed] [Google Scholar]
- Majima T, Komatsu Y, Doi K, et al. Clinical significance of risedronate for osteoporosis in the initial treatment of male patients with Graves’ disease. J Bone Miner Metab. 2006;24:105–13. doi: 10.1007/s00774-005-0655-y. [DOI] [PubMed] [Google Scholar]
- Melton LJ, III, Atkinson EJ, O’Connor MK, et al. Bone density and fracture risk in men. J Bone Miner Res. 1998;13:1915–23. doi: 10.1359/jbmr.1998.13.12.1915. [DOI] [PubMed] [Google Scholar]
- Milgrom C, Finestone A, Novack V, et al. The effect of prophylactic treatment with risedronate on stress fracture incidence among infantry recruits. Bone. 2004;35:418–24. doi: 10.1016/j.bone.2004.04.016. [DOI] [PubMed] [Google Scholar]
- Poor G, Atkinson EJ, Lewallen DG, et al. Age-related hip fractures in men: clinical spectrum and short-term outcomes. Osteoporos Int. 1995a;5:419–26. doi: 10.1007/BF01626602. [DOI] [PubMed] [Google Scholar]
- Poor G, Atkinson EJ, O’Fallon WM, et al. Determinants of reduced survival following hip fractures in men. Clin Orthop Relat Res. 1995b:260–65. [PubMed] [Google Scholar]
- Reginster J, Minne HW, Sorensen OH, et al. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral efficacy with risedronate therapy (VERT) Study Group. Osteoporos Int. 2000;11:83–91. doi: 10.1007/s001980050010. [DOI] [PubMed] [Google Scholar]
- Reid DM, Adami S, Devogelaer JP, et al. Risedronate increases bone density and reduces vertebral fracture risk within one year in men on corticosteroid therapy. Calcif Tissue Int. 2001;69:242–47. doi: 10.1007/s00223-001-1060-8. [DOI] [PubMed] [Google Scholar]
- Reid DM, Hughes RA, Laan RF, et al. Efficacy and safety of daily risedronate in the treatment of corticosteroid-induced osteoporosis in men and women: a randomized trial. European corticosteroid-induced osteoporosis treatment study. J Bone Miner Res. 2000;15:1006–13. doi: 10.1359/jbmr.2000.15.6.1006. [DOI] [PubMed] [Google Scholar]
- Ringe JD. [Osteoporosis in men] Dtsch Med Wochenschr. 2003;128:925–28. doi: 10.1055/s-2003-38842. [DOI] [PubMed] [Google Scholar]
- Ringe JD, Faber H, Farahmand P, et al. Efficacy of risedronate in men with primary and secondary osteoporosis: results of a 1-year study. Rheumatol Int. 2006;26:427–31. doi: 10.1007/s00296-005-0004-4. [DOI] [PubMed] [Google Scholar]
- Sato Y, Iwamoto J, Kanoko T, et al. Risedronate sodium therapy for prevention of hip fracture in men 65 years or older after stroke. Arch Intern Med. 2005;165:1743–48. doi: 10.1001/archinte.165.15.1743. [DOI] [PubMed] [Google Scholar]
- Sawka AM, Adachi JD, Papaioannou A, et al. Are there differences between men and women prescribed bisphosphonate therapy in canadian subspecialty osteoporosis practices? J Rheumatol. 2004;31:1993–95. [PubMed] [Google Scholar]
- Tauchmanova L, Selleri C, Esposito M, et al. Beneficial treatment with risedronate in long-term survivors after allogeneic stem cell transplantation for hematological malignancies. Osteoporos Int. 2003;14:1013–19. doi: 10.1007/s00198-003-1520-2. [DOI] [PubMed] [Google Scholar]
- Tenenhouse A, Joseph L, Kreiger N, et al. Estimation of the prevalence of low bone density in canadian women and men using a population-specific DXA reference standard: the canadian multicentre osteoporosis study (CaMos) Osteoporos Int. 2000;11:897–904. doi: 10.1007/s001980070050. [DOI] [PubMed] [Google Scholar]
- Vanderschueren D, Boonen S, Bouillon R. Osteoporosis and osteoporotic fractures in men: a clinical perspective. Baillieres Best Pract Res Clin Endocrinol Metab. 2000;14:299–315. doi: 10.1053/beem.2000.0075. [DOI] [PubMed] [Google Scholar]