Abstract
Using a social ecological model, this paper describes selected intra- and interpersonal factors that influence exercise behavior in women post hip fracture who participated in the Exercise Plus Program. Model testing of factors that influence exercise behavior at 2, 6 and 12 months post hip fracture was done. The full model hypothesized that demographic variables; cognitive, affective, physical and functional status; pain; fear of falling; social support for exercise, and exposure to the Exercise Plus Program would influence self-efficacy, outcome expectations, and stage of change both directly and indirectly influencing total time spent exercising. Two hundred and nine female hip fracture patients (age 81.0 ± 6.9), the majority of whom were Caucasian (97%), participated in this study. The three predictive models tested across the 12 month recovery trajectory suggest that somewhat different factors may influence exercise over the recovery period and the models explained 8 to 21% of the variance in time spent exercising. To optimize exercise activity post hip fracture, older adults should be helped to realistically assess their self-efficacy and outcome expectations related to exercise, health care providers and friends/peers should be encouraged to reinforce the positive benefits of exercise post hip fracture, and fear of falling should be addressed throughout the entire hip fracture recovery trajectory.
Keywords: hip fracture, exercise, self-efficacy, outcome expectations, recovery
Introduction
While there has been limited work in the implementation of exercise activities post hip fracture, there is some support to suggest important benefits for these individuals. Specifically, for those who have sustained a hip fracture, regular exercise (resistive and/or aerobic) improves mobility and quadriceps strength (Tinetti et al 1999; Mangione et al 2005; Tsauo et al 2005; Jones et al 2006), increases walking speed (Henderson et al 1992; Habris et al 1995; Jones et al 2006), and weight-bearing ability (Habris et al 1995). Despite the potential benefits of exercise, however, the majority of older adults do not participate in sufficient physical activity or exercise (Centers for Disease Control and Prevention Behavioral Risk Factor Surveillance System 2006), including those who have sustained a hip fracture.
A social ecological model is one of the most comprehensive approaches to explaining exercise behavior in older adults (Sallis 2003; Sallis et al 2006; United States Department of Health and Human Services 2000; Medley and Syme 2000). Specifically a social ecological model suggests that an individual’s behavior is affected by a wide sphere of influences: intrapersonal, interpersonal, institutional/organizational, public policy, and the environment.
Intrapersonal factors
Intrapersonal factors include such things as physical and cognitive status. Age-related dysfunction of frontal systems, for example, can result in deficits in planning, organization, self-control, and awareness of problems, which are likely to affect the ability to perform functional activities or engage in regular exercise (Sarkisian et al 2000; Norwalk et al 2001; Wang et al 2002).
Other intrapersonal factures, such as physical and mental health status have been noted to influence self-efficacy and outcome expectations, with low mood disturbance and better overall mental health associated with stronger self-efficacy and outcome expectations (Gecht et al 1996; Kurlowicz 1998; Perkins and Jenkins 1998). Mental health influences exercise activity such that those who were depressed were less likely to exercise (Oliver and Cronan 2002; Bonnet et al 2005; Mangione et al 2005; Forkan et al 2006). Perceived physical health status also has been associated with adherence to exercise in older adults (Sin et al 2002; Brown et al 2003; Munneke et al 2003; Lee and Laffrey 2006). Further there is evidence that such things as gait and balance, functional status, pain, or fear of falling may further influence an individuals’ willingness to engage in exercise activities (Cumming et al 2000; Bruce et al 2002; Li et al 2003; Delbacre 2004; Fletcher and Hirdes 2004; Martin et al 2005).
Interpersonal factors
Two overriding theories help explain the interpersonal interactions that can influence exercise behavior and behavior change as related to exercise. The first is social cognitive theory and the theory of self-efficacy (Bandura 1997) which suggests that the stronger the individual’s self-efficacy and outcome expectations, the more likely it is that he or she will initiate and persist with a given activity. Self-efficacy expectations are the individuals’ beliefs in their capabilities to perform a course of action to attain a desired outcome, whereas outcome expectations are the beliefs that a certain consequence will be produced by personal action. Both self-efficacy and outcome expectations play an influential role in the adoption and maintenance of exercise behavior in older adults (Brassington et al 2002; Gyurcsik et al 2003; Estabrooks et al 2005; Li et al 2005; McAuley et al 2006).
The second theory is the transtheoretical model (TTM) (Prochaska and Velicer 1997), an integrative model of intentional behavior change. The central construct of the TTM is stage of change (SOC), which describes behavior change as a progression through a series of stages. Individuals can be classified into one of the following five stages: Precontemplation, Contemplation, Preparation, Action and Maintenance. Precontemplation occurs when the individual has no intention to change behavior. Contemplation occurs when the individual is thinking about changing behavior, but not committed to the behavior change. Preparation refers to the period when the individual intends to change behavior sometime soon and is actively preparing. Action occurs when the individual has changed behavior recently (within the past six months). Maintenance occurs when the individual has maintained behavior change for a period longer than six months. These stages are directly related to exercise behavior. As individuals progress through the stages of change they report exercising more, are more fit based on physiological measures and have stronger self-efficacy expectations (Godin et al 2004; Ackerman et al 2005). Likewise, self-efficacy and outcome expectations increase from precontemplation to maintenance in older adults (Resnick and Nigg 2003; Schumann et al 2003; Godin et al 2004; Ackermann et al 2005; Riebe et al 2005).
There is a relationship between self-efficacy and outcome expectations with stage of change. Consistently, self-efficacy and outcome expectations increase from precontemplation to maintenance in older adults (Gorely and Gordon 1995). The older adult’s beliefs about his or her ability to exercise and the benefits associated with exercise influences whether or not the individual is willing to initiate and/or adhere to an exercise program (ie, stage of change). Self-efficacy and outcome expectations therefore can have both a direct and indirect effect on exercise through stage of change.
Another important interpersonal factor influencing participation in exercise is social support from friends, family, and experts. Consistent with the theory of self-efficacy, when there is encouragement to exercise from family, friends, and/or experts, older adults are more likely to participate in regular exercise activities (Resnick et al 2002; Sharma et al 2005; Greene et al 2006; Lim and Laffrey 2006; Lippke and Ziegelmann 2006; Resnick et al 2006).
Institutional/organization and environment and policy
The organizational structure and environment the older adult lives in and the policies that impact their communities can influence exercise activities as well (Takano et al 2002; Iwarsson 2005). Environments that facilitate function have been noted to be important factors in prevention of functional decline (Takano et al 2002; Crews 2005; Iwarsson 2005) and enabling people to achieve their highest level of function and well-being (Humpel et al 2002; Takano et al 2002). Unfortunately, designated exercise space is generally limited in home and facility based settings (Mihalko and Wickley 2003) and outside walkways, hallways, and common areas are seldom used to promote physical activity. While there are general guidelines to encourage all adults to engage in 30 minutes daily of physical activity (Centers for Disease Control and Prevention, Merck Institute of Aging and Health 2004; Thompson 2003; National Blueprint for Increasing Physical Activity 2002), there are no policies to promote this and no specific guidelines post hip fracture.
Despite existing knowledge on the factors and theories related to exercise, the fact remains that older adults do not frequently exercise. Encouraging exercise is especially important for a post-hip fracture population, given that this is likely to optimize recovery. In recognition of this possibility, this investigative team undertook a clinical trial to motivate exercise behavior in older adults post-hip fracture, which included three treatment arms: a home-based exercise program (Exercise), a motivational intervention (Plus), the combination of the two (Exercise Plus Program), compared with routine care.
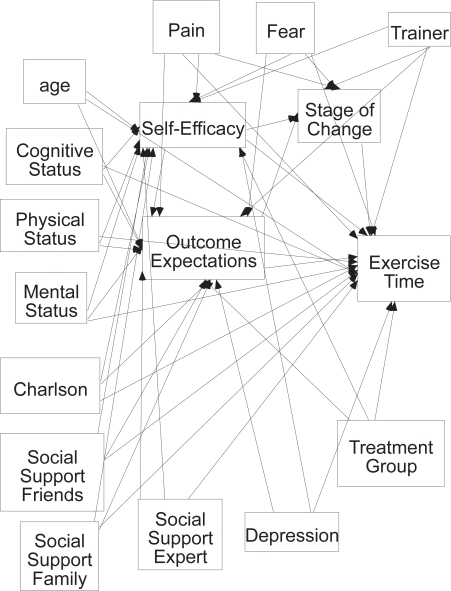
The purpose of this paper is to describe selected intra- and interpersonal factors that influence exercise behavior in women post hip fracture who participated in this project. Model testing of factors that influence exercise behavior at 2, 6, and 12 months post hip fracture was done and consideration given to consistency and differences noted between these models. The full model hypothesized that demographic variables; cognitive, affective, physical and functional status; pain; fear of falling; social support for exercise, and exposure to the Exercise Plus Program would influence self-efficacy, outcome expectations, and stage of change both directly and indirectly influencing total time spent exercising. The 89 hypothesized relationships are demonstrated in Figure 1.
Figure 1.
Full hypothesized model.
Methods
Study design
Data were derived from a randomized clinical trial using a repeated measure two by two design with participants randomized to one of four groups: exposure to the Exercise Plus Program (exercise plus motivation), the Exercise only component of the Exercise Plus Program, the Plus (or motivational) only component of the Exercise Plus Program, or routine care.
Sample
Participants were recruited from 6 hospitals in the greater Baltimore area between July 2000 and September 2004. A detailed description of eligibility and recruitment has been described elsewhere (Buie et al 2001). Briefly, eligible patients were female, 65 years of age or older, community-dwelling at the time of fracture, had a non-pathologic fracture within 72 hours preceding admission, and surgical repair of the hip fracture. Medical exclusions included evidence of symptomatic cardiovascular disease, neuromuscular conditions limiting exercise, or other conditions that increased risk when exercising home alone. Participants had to be walking without human assistance prior to the fracture and score ≥20 on the Folstein Mini Mental Status Exam (Folstein et al 1975). Also, informed consent and baseline measures had to be obtained within 15 days of the fracture to be eligible for randomization. Institutional Review Board approvals were obtained from the University of Maryland, School of Medicine as well as the study hospitals, and all enrolled subjects provided their own informed consent.
A total of 209 female hip fracture patients were consented within 15 days of the hip fracture. The majority of the participants were Caucasian (97%), and the average age of the participants was 81.0 ± 6.9. Approximately one third (34%) of the participants were married. The remaining were widowed (57%), never married (3%), or divorced or separated (6%). The average number of years in school was 12.2 ± 2.9.
The intervention: The Exercise Plus Program
The Exercise Plus Program and theoretical premise of the program has been described in detail elsewhere (Resnick et al 2002a, 2007). Briefly, the Exercise component of the Exercise Plus Program is a home based exercise intervention administered by exercise trainers which incorporates an aerobic exercise program using a Stairstep (Yu-Yahiro et al 2001; Resnick et al 2007), a comprehensive strengthening program that covers all muscles groups, and stretching exercises which are part of the warm up and cool down periods. Participants were encouraged to perform aerobic activity at least 3 days per week and strength training two days per week. The Plus component was also implemented by an exercise trainer and included a self-efficacy based intervention using education, verbal encouragement through goal setting and positive reinforcement, removal of unpleasant sensations associated with exercise, and individualized cueing (Resnick et al 2002a, 2007). In all treatment groups visits from the trainer were initially twice a week for the first three months, once a week for the next three months, and then once a month in the final six months of the program. On weeks when there was no face-to-face visit, for those exposed to the Plus component, weekly telephone calls were made to answer questions about exercise and encourage adherence.
Measures
Follow up data was collected at 2, 6, and 12 months post hip fracture. Measures addressing intrapersonal factors included demographic information, the Short Form Health Survey (SF-36), the Centers for Epidemiologic Studies and Depression Scale, a single item fear of falling question, the numeric rating scale for pain; interpersonal factors included Social Support for Exercise Scale, the Self-efficacy for Exercise Scale (SEE), the Outcome Expectations for Exercise (OEE) scale, and the Stage of Change Questionnaire. The Yale Physical Activity Survey was used to measure time spent exercising. A description of the measures and reliability and validity is provided in Table 1.
Table 1.
Description of study measures
| Measure | Description | Score range and interpretation | Reliability and validity |
|---|---|---|---|
| Self-efficacy for Exercise: (Resnick and Jenkins 2000) | A nine item measure that focuses on self-efficacy expectations related to the ability to continue to exercise in the face of barriers to exercising. | 0 (no confidence) to 10 (high confidence). Higher scores indicate stronger self-efficacy. | Evidence of internal consistency (alpha=0.93), and validity based on a significant relationship between efficacy expectations and moderate exercise, and confirmatory factor analysis. (Resnick and Jenkins 2000). |
| Outcome expectations for Exercise (Resnick et al 2000, 2001): | A nine item measure that focuses on the perceived consequences of exercise for older adults. | 1 (strongly disagree) to 5 (strongly agree). Higher scores indicate stronger outcome expectations. | Evidence of internal consistency (alphas ranging from 0.88 to 0.93), and validity based on a significant relationship between outcome expectations and moderate exercise, and confirmatory factor analysis. (Resnick and Jenkins 2000). |
| The SF-36 (Ware and Sherbourne 1992). | An eight dimension measure of health status that focuses on: physical functioning, role-physical, bodily pain, general health, vitality, social functioning, role emotional, and mental health. The 8 subscales are combined to constitute mental and physical health scores. | 0 to 14 for mental health; and 0 to 100 representing the percentage of total possible score achieved. | There is support for the reliability (Chronbach’s alpha for subscales ranging from 0.75 to 0.86) and validity of this measure (based on contrasting groups and factor analysis) when used with older adults (Stewart 1993, 1988;Walters and Munro 2004). |
| Yale Physical Activity Survey (YPAS) (DiPietro et al 1993) | A five category physical activity survey that focuses on time spent in: housework, caregiving, yardwork, exercise, and recreational activities performed during a typical week. Only the exercise subscale was utilized in this study. | 0 to 1440 minutes per week. | Evidence of test-retest reliability (r = 0.63, p < 0.001), and validity based on significant correlations with physiological variables that are indicative of habitual activity (Dipietro et al 1993; Pescatello et al 1994; Kolbe-Alexander et al 2006). |
| Center for Epidemiological Studies Depression Scale (CESD) (Radloff 1977; Turk and Okifuji 1994). | The possible range of scores is 0 to 60. | 0 to 5. Higher scores indicate more depressive symptoms. | Prior use of these measures provides evidence of their reliability and validity when used with older adults (Radloff 1977; Turk and Okifuji 1994,Caracciolo and Giaquinto 2002; Bohannon et al 2003;). |
| Numeric Rating Scale (NRS) for Pain (Herr and Mobily 1991) | A single item measure that focuses on pain over the previous week. | 0 (no pain) to 10 (the worst pain). Higher scores indicate more pain. | Evidence of test-retest reliability (Spearman rank correlations from 0.67 to 0.85) (Taylor et al 2005), and concurrent validity with other pain measures (r = 0.56 to 0.90) (Herr and Mobily 1993; Herr et al 2004; Ware et al 2006) |
| Fear of Falling (Jorstad et al 2005,Resnick 1998) | A single item measure that focuses on fear of falling. | 0 (no fear) to 4 (a lot of fear). Higher scores indicate greater fear of falling. | Evidence of validity with fear of falling significantly associated with functional performance in older adults (Resnick 1998,Jorstad et al 2005) |
| The Tinetti Mobility Scale (Tinetti 1986) | A 17 item performance measure that focuses on mobility and includes: nine balance maneuvers and eight assessments related to gait. | 0 to 26. Higher scores indicate better mobility. | Evidence of inter-rater reliability (r = 0.90), and construct validity with a significant relationship between mobility and falls (Tinetti 1986). |
| The Social Support for Exercise Habits Scale (Sallis et al 1987) | Includes three separate subscales of the same 15 items that reflect social interactions that might influence exercise behavior from friends, family, and experts. | Possible ranges from 23 to 67. Lower scores reflect lower social support | Evidence of internal consistency (alphas ranging from 0.61 to 0.91) and test retest reliability (r = 0.55 to 0.79). Evidence of validity was based on statistically significant relationships between the social support scale and exercise behavior (Sallis et al 1986) (Resnick et al 2002b). |
Data analysis
Descriptive statistics were done to describe the participants. Model testing was completed to establish the factors that influence exercise behavior at 2, 6, and 12 months post hip fracture using structural equation modeling and the Amos statistical program. The sample covariance matrix was used as input and a maximum likelihood solution sought. The chi-square statistic, the normed fit index (NFI), and Steigers Root Mean Square Error of Approximation (RMSEA) were used to estimate model fit. The larger the probability associated with the chi-square, the better the fit of the model to the data (Bollen 1989; Loehlin 1998). Since the chi-square statistic is sample size dependent the chi-square divided by degrees of freedom (df) was utilized to control for sample size effects (Bollen 1989). The NFI tests the hypothesized model against a baseline model and should be 1.0 if there is perfect model fit. The NFI is “normed” so that the values cannot be below 0 or above 1. The RMSEA is a population based index and consequently is insensitive to sample size. An RMSEA of <0.10 is considered good, and <0.05 is very good (Loehlin 1998). Path significance (ie, significance of the Lambda values) was based on the Critical Ratio (CR), which is the parameter estimate divided by an estimate of the standard error. A CR >2 in absolute value was considered significant (Arbuckle 1997).
Results
Of the 209 participants initially recruited, 165 women (79%) were available for 2-month assessments, 169 (81%) were available for 6-month follow up, and 155 (75%) were available for the 12-month follow up visits. One case was deleted post-randomization due to being ineligible (no surgery was performed post hip fracture). Reasons for loss to follow up have been reported elsewhere (Resnick et al pers comm). The mean age of the participants was 80.7 (SD = 6.9), mean MMSE was 26.7 (SD = 2.8), and the majority were Caucasian (96%).
The time from fracture to first intervention visit from the trainer ranged from 28 to 200 days. While attempts were made on the part of the trainers via weekly telephone calls to initiate the intervention, participants generally were not willing to have a visit occur prior to 2 months post fracture. Only one participant had her first visit at 28 days post fracture. By two months, 22 (31%) of the participants had their first visit, by three months 44 (62%) of the participants had their first visit, and by four months 58 (82%) of the participants had their first visit.
Table 2 provides descriptive statistics of the variables under study by treatment (any of the three intervention arms) versus control group. Generally the participants had some confidence they could exercise, believed in the benefits of exercise and exercised about 1.5 to 2 hours weekly. Overall they were not depressed and reported fair mental and physical health, minimal pain and some fear of falling.
Table 2.
Means (SE) for selected outcome measures by treatment group (total n = 208; treatment group n = 157; control = 51)
| Variable | Mean | Std. Deviation | |
|---|---|---|---|
| Stage of change 2 months | Control | 1.8 | 1.6 |
| Treatment | 1.9 | 1.6 | |
| Stage of change 6 months | Control | 1.2 | 1.7 |
| Treatment | 1.9 | 1.8 | |
| Stage of change 12 months | Control | 1.0 | 1.5 |
| Treatment | 2.2 | 1.9 | |
| Outcome expectations 2 months | Control | 3.9 | 0.56 |
| Treatment | 3.9 | 0.64 | |
| Outcome expectations 6 months | Control | 3.8 | 0.64 |
| Treatment | 3.9 | 0.59 | |
| Outcome expectations 12 months | Control | 3.7 | 0.66 |
| Treatment | 3.9 | 0.61 | |
| Self-efficacy expectations 2 months | Control | 6.5 | 2.3 |
| Treatment | 6.5 | 2.8 | |
| Self-efficacy expectations 6 months | Control | 5.8 | 3.1 |
| Treatment | 7.2 | 2.5 | |
| Self-efficacy expectations 12 months | Control | 6.3 | 3.2 |
| Treatment | 7.4 | 2.4 | |
| CESD score at 2 months (larger = depressed) | Control | 12.2 | 9.3 |
| Treatment | 9.9 | 9.1 | |
| CESD score at 6 months (larger = depressed) | Control | 11.8 | 9.2 |
| Treatment | 9.2 | 8.7 | |
| CESD score at 12 months (larger = depressed) | Control | 9.0 | 7.7 |
| Treatment | 9.2 | 7.9 | |
| Summary gait and balance score 2 months | Control | 18.5 | 6.9 |
| Treatment | 20.6 | 4.5 | |
| Summary gait and balance score 6 months | Control | 17.3 | 5.7 |
| Treatment | 17.5 | 6.8 | |
| Summary gait and balance score 12 months | Control | 20.2 | 5.2 |
| Treatment | 20.3 | 5.4 | |
| Physical health status 2 months | Control | 31.3 | 11.8 |
| Treatment | 35.7 | 11.7 | |
| Physical health status 6 months | Control | 36.9 | 14.6 |
| Treatment | 40.8 | 13.6 | |
| Physical health status 12 months | Control | 40.3 | 15.6 |
| Treatment | 43.3 | 14.0 | |
| Mental health status 2 months | Control | 40.9 | 13.8 |
| Treatment | 45.9 | 9.9 | |
| Mental health status 6 months | Control | 47.8 | 12.0 |
| Treatment | 50.3 | 9.8 | |
| Mental health status 12 months | Control | 49.7 | 10.1 |
| Treatment | 50.9 | 9.3 | |
| Yale: total exercise time 2mo, hrs/wk | Control | 1.7 | 2.3 |
| Treatment | 1.8 | 2.2 | |
| Yale: total exercise time 6mo, hrs/wk | Control | 2.6 | 3.2 |
| Treatment | 2.2 | 2.9 | |
| Yale: total exercise time 12mo, hrs/wk | Control | 0.92 | 1.4 |
| Treatment | 3.1 | 3.8 | |
| Pain 2 months | Control | 3.7 | 2.2 |
| Treatment | 4.1 | 2.8 | |
| Pain 6 months | Control | 4.0 | 3.0 |
| Treatment | 3.6 | 3.0 | |
| Pain 12 months | Control | 3.7 | 2.9 |
| Treatment | 3.1 | 2.9 | |
| Fear 2 months | Control | 2.6 | 1.4 |
| Treatment | 2.3 | 1.4 | |
| Fear 6 months | Control | 2.4 | 1.4 |
| Treatment | 2.0 | 1.5 | |
| Fear 12 months | Control | 2.1 | 1.4 |
| Treatment | 1.9 | 1.4 | |
| Social support experts 2 months | Control | 17.5 | 6.4 |
| Treatment | 18.9 | 4.1 | |
| Social support experts 6 months | Control | 17.8 | 4.5 |
| Treatment | 26.1 | 8.2 | |
| Social support experts 12 months | Control | 17.5 | 6.4 |
| Treatment | 18.9 | 18.9 | |
| Social support friends 2 months | Control | 17.4 | 2.6 |
| Treatment | 17.6 | 3.6 | |
| Social support friends 6 months | Control | 18.2 | 3.8 |
| Treatment | 18.0 | 3.6 | |
| Social support friends 12 months | Control | 17.4 | 2.6 |
| Treatment | 17.6 | 3.6 | |
| Social support family 2 months | Control | 22.2 | 7.8 |
| Treatment | 22.2 | 6.0 | |
| Social support family 6 months | Control | 19.6 | 4.7 |
| Treatment | 20.9 | 6.5 | |
| Social support family 12 months | Control | 21.1 | 8.9 |
| Treatment | 19.8 | 5.6 |
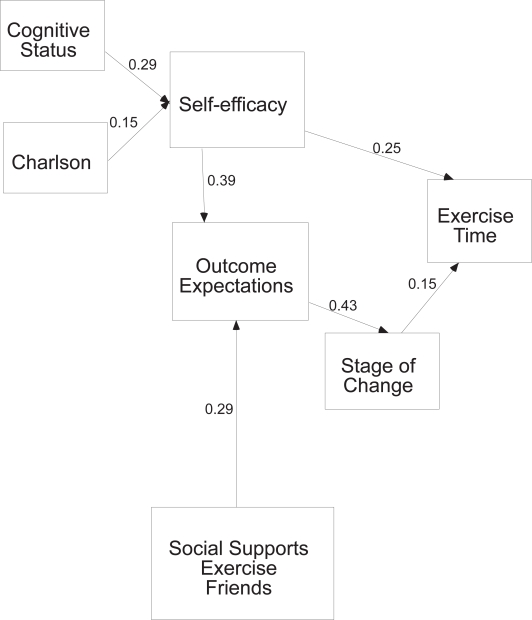
Testing of the full 2 month model indicated that out of the 89 paths hypothesized only 7 were statistically significant (Figure 2). Path coefficients for all models are shown in Table 3. Cognitive status and comorbidities related to self-efficacy expectations such that those who had better cognitive status and fewer comorbidities had higher self-efficacy expectations. Self-efficacy expectations and social support for exercise from friends related to outcome expectations such that those with higher self-efficacy expectations and more support from friends to exercise had stronger outcome expectations. Outcome expectations directly related to stage of change such that those with stronger outcome expectations were more likely to be exercising. Self-efficacy and stage of change directly related to time spent in exercise, as those with stronger self-efficacy and a higher stage of change (eg, in maintenance versus precontemplation) spent more time exercising. While this model showed a good fit to the data (χ2 = 22.6, df =14, p = 0.07, ratio 1.6; NFI = 0.84, and RMSEA of 0.05), it explained only 10% of the variance of exercise behavior at two months post hip fracture.
Figure 2.
Two month model significant paths only.
Table 3.
Path coefficients for significant paths in hypothesized models
| Path Tested | 2 Month Model | 6 Month Model | 12 Month Model |
|---|---|---|---|
| Cognitive status → Self-efficacy | 0.29(0.00) | ||
| Comorbidities → Self-efficacy | −0.15(0.04) | ||
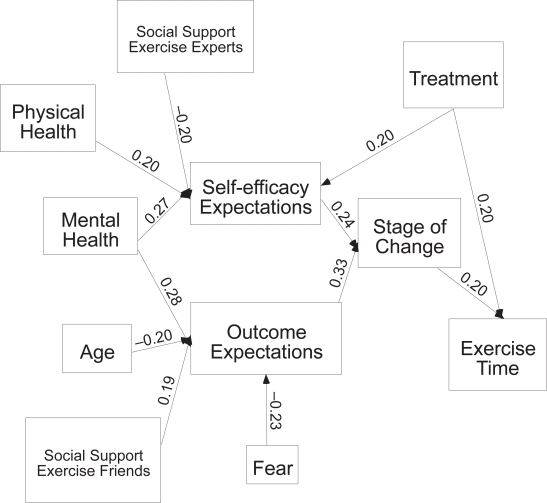
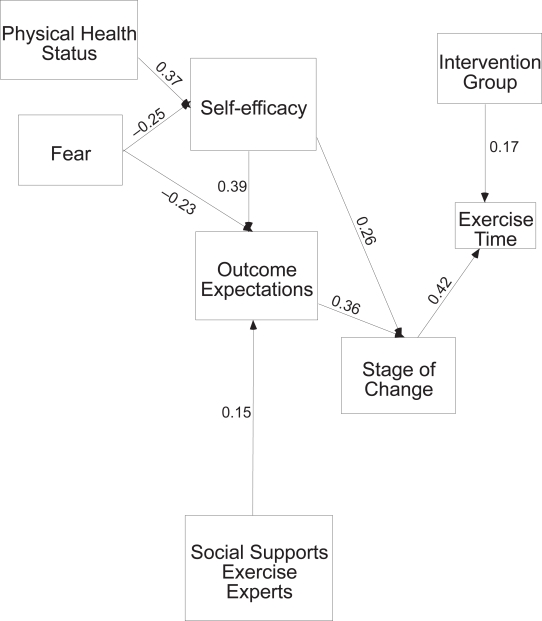
| Physical health → Self-efficacy | 0.20 (0.04) | 0.37(0.00) | |
| Mental health → Self-efficacy | 0.27(0.01) | ||
| Social Support Experts → Self-efficacy | −0.20(0.01) | ||
| Treatment group → Self-efficacy | 0.20(0.01) | ||
| Fear → Self-efficacy | −0.25(0.00) | ||
| Age → Outcome expectations | −0.20 (0.01) | ||
| Mental Health → Outcome expectations | 0.28(0.01) | ||
| Social Support Friend → Outcome expectations | 0.29(0.00) | 0.19(0.01) | |
| Social Support Experts → Outcome expectations | 0.15(0.04) | ||
| Fear → Outcome expectations | −0.23(0.00) | −0.23(0.00) | |
| Self-efficacy → Outcome expectations | 0.39(0.00) | 0.39(0.00) | |
| Self-efficacy → Stage of change | 0.24(0.00) | 0.26(0.00) | |
| Outcome expectations → Stage of change | 0.44(00) | 0.33(0.00) | 0.36(0.00) |
| Self-efficacy → Exercise time | 0.25(0.00) | ||
| Stage of change → Exercise time | 0.15(0.04) | 0.20(0.01) | 0.42(0.00) |
| Treatment group*→ Exercise time | 0.20(0.01) | 0.17(0.02) |
Exposure to any component of the intervention (Exercise only, Motivation only, Exercise Plus Motivation) versus routine care
At six months post hip fracture (Figure 3), 12 of the 89 hypothesized paths were significant. Physical and mental health, social support from an expert, and treatment group all related to self-efficacy expectations such that those who were exposed to any of the treatment groups, had better health, and less support from an expert to exercise, had stronger self-efficacy expectations. Age, mental health, fear of falling and social support from friends related to outcome expectations for exercise. Those who were younger, had better mental health, more support from friends for exercise, and less fear of falling had stronger outcome expectations for exercise. Self-efficacy and outcome expectations were associated with stage of change such that those with stronger efficacy expectations were more likely to be in higher stages of change such as action or maintenance. Stage of change and treatment group were the only variables to directly relate to time in exercise, with higher stages of change and exposure to treatment being associated with more time spent in exercise. All the other significant variables indirectly related to exercise time through self-efficacy or outcome expectations and then stage of change. There was a fair fit of the model to the data (χ2 = 110.6, df = 38, p = 0.00, ratio 2.9, NFI = 0.74, and RMSEA of 0.09), it explained 8% of the variance of exercise behavior at six months post hip fracture.
Figure 3.
Six month model significant paths only.
At 12 months post hip fracture, nine of the 89 hypothesized paths were significant (Figure 4). Physical health and fear of falling related to self-efficacy expectations. Those with better health and less fear had stronger self-efficacy expectations. Self-efficacy expectations, social support from an expert, and fear of falling all related to outcome expectations for exercise. Those who had stronger self-efficacy, more support from an expert, and less fear of falling had stronger outcome expectations for exercise. As noted in the 6 month model, self-efficacy and outcome expectations related to stage of change, and those with stronger self-efficacy and outcome expectations were more likely to be in higher stages of change for exercise. Stage of change and exposure to treatment were the only variables directly related to time spent in exercise. All other variables indirectly related to time in exercise through self-efficacy and outcome expectations. There was a fair fit of the model to the data (χ2 = 59.7, df = 19, p = 0.00, ratio 3.1, NFI = 0.76, and RMSEA of 0.10), and the model explained 21% of the variance of exercise behavior at twelve months post hip fracture.
Figure 4.
Twelve month model significant paths only.
Discussion
The findings from this study support prior findings and add to the understanding of the factors that relate to exercise behavior in older adults, particularly those who have sustained a hip fracture. The three predictive models tested across the 12 month recovery trajectory suggest that somewhat different factors may influence exercise over the recovery period. At two months post hip fracture the participants were just beginning to be exposed to the intervention, which may explain why treatment group status was not related to exercise behavior. However, 6 and 12 months post fracture the exposure to treatment did relate to time spent doing exercise; this finding speaks well to the effort of encouraging exercise. Although there were five different trainers providing treatment during the course of the study, there was no evidence of trainer effect during any of the testing time points. Thus, the benefits of encouraging exercise are not trainer-specific, and the skills to be an effective trainer may be easily learned.
Similar to prior studies with community dwelling older adults (Litt et al 2002; Resnick and Nigg 2003; Benjamin et al 2005; Stiggelbout et al 2006), self-efficacy and outcome expectations related to stage of change for exercise, which directly related to exercise behavior. However, with the exception of the two month testing time point, self-efficacy and outcome expectations had no direct relationship with exercise. Instead, they indirectly related to exercise through stage of change. Although there have been multiple studies supporting a direct relationship between self-efficacy and/or outcome expectations with exercise (Booth et al 2000; Rhodes et al 2001; Brassington et al 2002; Litt et al 2002; Conn et al 2003a, 2003b; O’Connor 2004; Cress M 2005; Sharma et al 2005; Taylor-Piliae and Froelicher 2005; Lee and Laffrey 2006; Wilcox et al 2006), this relationship has not been consistent among older adults. In a recent study (Stiggelbout et al 2006) of community dwelling older adults involved in exercise programs, self-efficacy influenced intention to exercise but not actual exercise behavior. The lack of relationship between self-efficacy and actual exercise behavior was also noted in a sample of older adults participating in a home-based exercise program viewed on television (Hopman-Rock et al 2005) and among older adults post stroke (Resnick pers comm). Thus, the results of this study add to the evidence suggesting that interventions might best be targeted at encouraging self-efficacy related to readiness to adopt exercise behavior, after which time doing exercise will increase.
The lack of a direct relationship between outcome expectations and exercise behavior in older adults post hip fracture may be due to the sample studied and a ceiling effect of the measure. That is, these individuals had all volunteered to participate in an exercise intervention study and therefore were likely to have high outcome expectations related to exercise. Indeed, at baseline (2 months post hip fracture), the majority of participants agreed with the positive benefits associated with exercise on the outcome expectations measure and had a high mean score of 3.9 (SD = 0.69 and range of 1 to 5) (Resnick 2006).
Age, cognitive status, and comorbidities had a limited indirect relationship with exercise behavior. The participants were, however, all older adults and had to meet specific cognitive criteria and be free of a large number of comorbidities to be eligible to participate in the study. Consequently, the homogeneity of the sample may have influenced findings. Nonetheless, results suggest that in a similar population of older adults with hip fractures, age, cognition, and health are not a deterrent to exercise. Perceptions of physical and mental health status were noted to relate to self-efficacy and/or outcome expectations at 6 and 12 months post fracture, with those in better perceived health having stronger self-efficacy or outcome expectations, which then indirectly influenced time spent in exercise. Clinically, it is important to recognize that those with poorer health are likely to have lower self-efficacy and outcome expectations associated with exercise and may benefit from interventions to strengthen those beliefs. In particular it is critical that individuals with perceptions of poor health status understand and believe that it is safe for them to exercise and that there will be a benefit to doing so (Resnick et al 2005).
The relationship between self-efficacy and outcome expectations for exercise and fear of falling noted in this study has not been reported in prior research. The study findings suggest that the relationship between fear and exercise may be mediated by self-efficacy and outcome expectations, as was demonstrated at 6 and 12 months post hip fracture. It is of note that the impact of fear seems most prevalent at 12 months post fracture rather than in the more immediate post fracture period (eg, 2 months post fracture). It seems likely that individuals further along in the recovery trajectory may be engaging in more activity, and thereby reconsidering their fear in the face of that activity. This suggests that ongoing efforts might be made to address the fear of falling experienced by individuals well after their initial fracture.
Exposure to the intervention did not directly relate to self-efficacy and outcome expectations, as was anticipated (Resnick et al 2002a) although there were non-significant trends of an increase or maintenance of efficacy expectations in the treatment group and a decline in the control group. The lack of a significant impact on self-efficacy and outcome expectations may in part be due, as indicated previously, to the sample included in the study (ie, volunteers in an exercise intervention study) and ceiling effects of these measures. It is also possible that, post hip fracture, older adults may evaluate their self-efficacy and outcome expectations based on their prior health status, not current status post hip fracture. Consequently, as noted in this study, a self-efficacy based intervention improved exercise behavior but did not influence self-efficacy or outcome expectations in the first year post hip fracture.
Ongoing research is needed to explore the measurement of self-efficacy and outcome expectations post hip fracture, and establish ways to help older adults carefully evaluate their self-efficacy and outcome expectations related to exercise in the face of an acute clinical change. This is important because self-efficacy based interventions may be even more effective when the participant realistically appreciates his or her true efficacy expectations.
Social support for exercise from friends related to self-efficacy for exercise at 2 and 6 months post hip fracture. This finding has been inconsistent in prior research with social support for exercise from friends relating to exercise behavior among some samples of community dwelling older adults (Booth et al 2000; Resnick et al 2002b), but not others (Eyler et al 1999; Brassington et al 2002). It is possible that interactions with peers, possibly peers who themselves exercise (and may have experienced a hip fracture), has a positive influence on self-efficacy related to exercise post hip fracture. Practitioners should consider the use of peers to strengthen beliefs and thereby improve exercise behavior in older adults post hip fracture as was done in a recent study testing a group based exercise program for older adults post hip fracture led by an older adult trainer (Jones et al 2006).
Social support for exercise from experts (anyone perceived by the participant to be an expert) was negatively related to self-efficacy expectations at 6 months post hip fracture. Although it was anticipated that social support on the part of the experts would increase time spent in exercise there are several possible explanations for the negative relationship. Social support for exercise did increase from two months to six months post fracture in the treatment group (18.9 to 26.1) while staying essentially unchanged in the control group (17.5 to 17.8). It is possible that this increase in social support from the experts was not related to exercise behavior, with other factors taking on a greater precedence. It is also possible, since the intervention did not control the interactions between the participants and any of their health care providers (primary care physicians, nurses, nurse practitioners, or surgeons), that some negative interchanges related to exercise may have occurred. We had experiences, for example, in which some participants were told not to exercise by their orthopedist or primary medical doctor (Resnick 2005). Future research should seek to understand the ongoing exchanges between patients and providers for this reason.
While the revised models with significant paths had a fair to good fit with the data, they only explained a small amount of the variance in exercise behavior (8% to 20%). The many non-significant hypothesized predictors further support the challenges associated with increasing exercise activity among older adults, particularly those who have sustained a hip fracture. Specifically, pain, depressive symptoms, and gait and balance consistently had no direct or indirect influence on exercise behavior. Using the social ecological model of behavior, possible factors that might influence exercise behavior among older adults but were not considered in this study could be added to future work, including environmental and policy considerations (eg, providing financial incentives for participation in exercise or establishing safe walking paths within communities) (Booth et al 2000), whether or not the individual had to stop exercise for a period due to an acute event (Stiggelbout et al 2006), or consideration of life events such as the death of a loved one, pet, or change in location (Wilcox and King 2004).
Limitations
This study was limited in that the sample size was small and homogenous which influenced model fit results and the likely replicability of the findings. However, despite these limitations, the findings provide some guidance for future work in the area of developing interventions to increase exercise post hip fracture, as well as measurement challenges for social cognitive constructs post hip fracture (ie, accurate assessment of self-efficacy and outcome expectations). In addition to helping older adults post hip fracture realistically readjust their self-efficacy and outcome expectations related to exercise, the research team recommends that health care providers and friends/peers reinforce the positive benefits of exercise post hip fracture, and continue to address fear of falling throughout the entire hip fracture recovery trajectory, as well as explore additional factors that may influence time spent in exercise post hip fracture.
Acknowledgments
Support for this project was provided by National Institute on Aging (NIA) grants R37 AG09901, R01-AG18668, R01 AG17082, and the Claude D. Pepper Older Americans Independence Center P60-AG12583. Authors also would like to thank Thera-Band Academy for their generous contribution of Thera-Band® resistive bands used by study participants; hospitals and personnel participating in the Baltimore Hip Studies; and research staff who worked with study patients and their families. Authors also would like to thank hip fracture patients and their families for volunteering their time and information for this work.
References
- Arbuckle J. Amos users’ guide version 36. Chicago: Small Waters Corporation; 1997. [Google Scholar]
- Bandura A. Self-efficacy: The Exercise of Control, New York. New York: W.H. Freeman and Company; 1997. [Google Scholar]
- Benjamin K, Edwards NC, Bharti VK. Attitudinal, perceptual, and normative beliefs influencing the exercise decisions of community-dwelling physically frail seniors. Journal of Aging and Physical Activity. 2005;13:276–93. doi: 10.1123/japa.13.3.276. [DOI] [PubMed] [Google Scholar]
- Bohannon RW, Maljanian R, Goethe J. Screening for depression in clinical practice: reliability and validity of a five-item subset of the CES-Depression. Perceptions and Motor Skills. 2003;97:855–63. doi: 10.2466/pms.2003.97.3.855. [DOI] [PubMed] [Google Scholar]
- Booth ML, Owen N, Bauman A, et al. Social-cognitive and perceived environment influences associated with physical activity in older Australians. Preventive Medicine. 2000;31:15–22. doi: 10.1006/pmed.2000.0661. [DOI] [PubMed] [Google Scholar]
- Brassington GS, Atienza A, Perczek, et al. Intervention-related cognitive versus social mediators of exercise adherence in the elderly. American Journal of Preventive Medicine. 2002;23:80–6. doi: 10.1016/s0749-3797(02)00477-4. [DOI] [PubMed] [Google Scholar]
- Bruce DG, Devine A, Prince RL. Recreational physical activity levels in healthy older women: the importance of fear of falling. Journal of the American Geriatrics Society. 2002;50:84–9. doi: 10.1046/j.1532-5415.2002.50012.x. [DOI] [PubMed] [Google Scholar]
- Buie V, Orwig B, Resnick B, et al. Frail Elderly women post-hip fracture: Recruitment and retention into a 12-month exercise intervention study. The Gerontologist. 2001;41:41–57. [Google Scholar]
- Caracciolo B, Giaquinto S. Criterion validity of the center for epidemiological studies depression (CES-D) scale in a sample of rehabilitation inpatients. Journal of Rehabilitation Medicine. 2002;34:221–5. doi: 10.1080/165019702760279215. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention 2006. behavioral risk factor surveillance system 2006 [online]. Accessed on December 12, 2006. URL:https://www.cdc.gov/brfss
- Centers for Disease Control and Prevention, Merck Institute of Aging and Health The State of Aging and Health in America. 2004;2004:21–6. [Google Scholar]
- Conn VS, Burks KJ, Pomeroy SL, et al. Are there different predictors of distinct exercise components? Rehabilitation Nursing. 2003a;28:87–91. doi: 10.1002/j.2048-7940.2003.tb02039.x. [DOI] [PubMed] [Google Scholar]
- Conn VS, Tripp-Reimer T, Maas ML. Older women and exercise: theory of planned behavior beliefs. Public Health Nursing. 2003b;20:153–63. doi: 10.1046/j.1525-1446.2003.20209.x. [DOI] [PubMed] [Google Scholar]
- Cress MB, Prohaska T, Rimmer J, et al. Best practices for physical activity programs and behavior counseling in older adult populations. Journal of Aging and Physical Activity. 2005;13:61–74. doi: 10.1123/japa.13.1.61. [DOI] [PubMed] [Google Scholar]
- Crews D. Artificial environments and an aging population: Designing for age related functional losses. Journal of Physiological Anthropology. 2005;24:103–9. doi: 10.2114/jpa.24.103. [DOI] [PubMed] [Google Scholar]
- Cumming RG, Salkeld G, Thomas M, et al. Prospective study of the impact of fear of falling on activities of daily living, SF-36 Scores, and nursing home admission. Journal of Gerontology. 2000;55:299–305. doi: 10.1093/gerona/55.5.m299. [DOI] [PubMed] [Google Scholar]
- Delbacre K, Crombez G, Vanderstraeten G, et al. Fear related avoidance of activities, falls and physical frailty: A prospective community based cohort study. Age and Ageing. 2004;33:368–73. doi: 10.1093/ageing/afh106. [DOI] [PubMed] [Google Scholar]
- Dipietro L, Caspersen CJ, Ostfeld AM, et al. A survey for assessing physical activity among older adults. Medicine Science Sports and Exercise. 1993;25:628–42. [PubMed] [Google Scholar]
- Eyler AA, Brownson RC, Donatelle RJ, et al. Physical activity social support and middle- and older-aged minority women: results from a US survey. Social Science Medicine. 1999;49:781–9. doi: 10.1016/s0277-9536(99)00137-9. [DOI] [PubMed] [Google Scholar]
- Fletcher P, Hirdes J. Restriction in activity associated with fear of falling among community-based seniors using home care services. Age Ageing. 2004;33:273–9. doi: 10.1093/ageing/afh077. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, Mchugh PR. “Mini-Mental state” – A practical method for grading the cognitive state of patients for the clinician”. Journal of Psychiatric Research. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Habris R, O’Hara P, Harper D. Functional status of geriatric rehabilitation patients: a one year follow-up study. Journal of the American Geriatric Society. 1995;36:890–6. doi: 10.1111/j.1532-5415.1995.tb06242.x. [DOI] [PubMed] [Google Scholar]
- Henderson SA, Finlay OE, Murphy, et al. Benefits of an exercise class for elderly women following hip surgery. Ulster Medical Journa. 1992;61:144–50. [PMC free article] [PubMed] [Google Scholar]
- Herr K, Mobily P. Comparison of selected pain assessment tools for use with the elderly. Applied Nursing Research. 1993;6:39–46. doi: 10.1016/s0897-1897(05)80041-2. [DOI] [PubMed] [Google Scholar]
- Herr K, Spratt K, Mobily P, et al. Pain intensity assessment in older adults: use of experimental pain to compare psychometric properties and usability of selected pain scales with younger adults. Clinical Journal of Pain. 2004;20:207–19. doi: 10.1097/00002508-200407000-00002. [DOI] [PubMed] [Google Scholar]
- Herr KA, Mobily PR. Complexities of pain assessment in the elderly. Clinical considerations. Journal of Gerontological Nursing. 1991;17:12–9. doi: 10.3928/0098-9134-19910401-04. [DOI] [PubMed] [Google Scholar]
- Hopman-Rock M, Borghouts JA, Leurs MT. Determinants of participation in a health education and exercise program on television. Preventive Medicine. 2005;41:232–9. doi: 10.1016/j.ypmed.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Humpel N, Owen N, Leslie E. Environmental factors associated with adults’ participation in physical activity: A review. American Journal of Preventive Medicine. 2002;22:188–99. doi: 10.1016/s0749-3797(01)00426-3. [DOI] [PubMed] [Google Scholar]
- Iwarsson S. A long-term perspective on person-environment fit and ADL dependence among older Swedish adults. The Gerontologist. 2005;45:327–36. doi: 10.1093/geront/45.3.327. [DOI] [PubMed] [Google Scholar]
- Jones G, Jakobi J, Taylor A, et al. Community exercise program for older adults recovering from hip fracture: A pilot study. Journal of Aging and Physical Activity. 2006;14:439–55. doi: 10.1123/japa.14.4.439. [DOI] [PubMed] [Google Scholar]
- Jorstad E, Hauer K, Becker C, et al. Measuring the psychological outcomes of falling: a systematic review. Journal of the American Geriatrics Society. 2005;53:501–10. doi: 10.1111/j.1532-5415.2005.53172.x. [DOI] [PubMed] [Google Scholar]
- Kolbe-Alexander TL, Lambert EV, Harkins JB, et al. Comparison of two methods of measuring physical activity in South African older adults. Journal of Aging and Physical Activity. 2006;14:98–114. doi: 10.1123/japa.14.1.98. [DOI] [PubMed] [Google Scholar]
- Lee Y, Laffrey S. Predictors of physical activity in older adults with borderline hypertension. Nursing Research. 2006;55:110–20. doi: 10.1097/00006199-200603000-00006. [DOI] [PubMed] [Google Scholar]
- Li F, Fisher J, Harmer P, et al. Fear of falling in elderly persons: association with falls, functional ability, and quality of life. Journal of Gerontology. 2003;58:283–90. doi: 10.1093/geronb/58.5.p283. [DOI] [PubMed] [Google Scholar]
- Litt M, Kleppinger A, Judge J. Initiation and maintenance of exercise behavior in older women: Predictors from the social learning model. Journal of Behavioral Medicine. 2002;25:83–97. doi: 10.1023/a:1013593819121. [DOI] [PubMed] [Google Scholar]
- Loehlin J. Latent variable models. New Jersey: Lawrence Erlbaum Associates; 1998. [Google Scholar]
- Mangione KK, Craik RL, Tomlinson SS, et al. Can elderly patients who have had a hip fracture perform moderate- to high-intensity exercise at home? Physical Therapy. 2005;85:727–39. [PubMed] [Google Scholar]
- Martin FC, Hart D, Spector T, et al. Fear of falling limiting activity in youngold women is associated with reduced functional mobility rather than psychological factors. Age and Ageing. 2005;34:281–7. doi: 10.1093/ageing/afi074. [DOI] [PubMed] [Google Scholar]
- Mihalko S, Wickley K. Active living for assisted living: Promoting partnerships within a systems framework. American Journal of Preventive Medicine. 2003;25:193–203. doi: 10.1016/s0749-3797(03)00184-3. [DOI] [PubMed] [Google Scholar]
- National Blueprint for Increasing Physical Activity National Blueprint for increasing physical activity among adults 50 and older, Creating a strategic framework and enhancing organizational capacity for change. Journal of Aging and Physical Activity. 2002;9:S5–28. [Google Scholar]
- O’Connor BP, Rousseau FL, Maki SA. Physical exercise and experienced bodily changes: the emergence of benefits and limits on benefits. International Journal of Aging and Human Development. 2004;59:177–203. doi: 10.2190/F8EE-F9WV-GJ2D-QLQ6. [DOI] [PubMed] [Google Scholar]
- Pescatello L, Dipietro L, Fargo A, et al. The impact of physical activity and physical fitness on health indicators among older adults. Journal of Aging and Physical Activity. 1994;2:2–13. [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measures. 1977;1:385–401. [Google Scholar]
- Resnick B. Functional performance of older adults in a long term care setting. Clinical Nursing Research. 1998;7:230–46. doi: 10.1177/105477389800700302. [DOI] [PubMed] [Google Scholar]
- Resnick B.2006The Baltimore Hip Studies: Hip 4 and Hip 5University of Maryland Unpublished Data Analysis.
- Resnick B, Jenkins LS. Testing the reliability and validity of the Self-Efficacy for Exercise scale. Nursing Research. 2000;49:154–9. doi: 10.1097/00006199-200005000-00007. [DOI] [PubMed] [Google Scholar]
- Resnick B, Magaziner J, Orwig D, et al. Evaluating the components of the Exercise Plus Program: Rationale, theory and implementation. Health Education Research. 2002a;17:648–59. doi: 10.1093/her/17.5.648. [DOI] [PubMed] [Google Scholar]
- Resnick B, Michael K, Shaughnessy M, et al. 2007Testing a Comprehensive Conceptualization of Physical Activity in Stroke PatientsPersonal Communication (Manuscript submitted).
- Resnick B, Nigg C. Testing a theoretical model of exercise behavior for older adults. Nursing Research. 2003;52:80–8. doi: 10.1097/00006199-200303000-00004. [DOI] [PubMed] [Google Scholar]
- Resnick B, Orwig D, Magaziner J, et al. The effect of social support on exercise behavior in older adults. Clinical Nursing Research. 2002b;11:52–70. doi: 10.1177/105477380201100105. [DOI] [PubMed] [Google Scholar]
- Resnick B, Orwig D, Wehren L, et al. The Exercise Plus Program for Older Women Post Hip Fracture: Participant Perspectives. The Gerontologist. 2005;45:539–44. doi: 10.1093/geront/45.4.539. [DOI] [PubMed] [Google Scholar]
- Resnick B, Orwig D, Yu-Yahiro J, et al. Testing the Effectiveness of the Exercise Plus Program in Older Women Post Hip Fracture. Annals of Behavioral Medicine. 2007;34:67–76. doi: 10.1007/BF02879922. [DOI] [PubMed] [Google Scholar]
- Resnick B, Orwig D, Zimmerman S, et al. The Exercise Plus Program for Older Women Post Hip Fracture: Participant Perspectives. The Gerontologist. 2005;45:539–44. doi: 10.1093/geront/45.4.539. [DOI] [PubMed] [Google Scholar]
- Rhodes RE, Martin AD, Taunton JE. Temporal relationships of self-efficacy and social support as predictors of adherence in a 6-month strength-training program for older women. Perception and Motor Skills. 2001;93:693–703. doi: 10.2466/pms.2001.93.3.693. [DOI] [PubMed] [Google Scholar]
- Sallis J, Grossman R, Pinski R, et al. The development of scales to measure social support for diet and exercise behaviors. Preventive Medicine. 1986;16:825–36. doi: 10.1016/0091-7435(87)90022-3. [DOI] [PubMed] [Google Scholar]
- Sharma M, Sargent L, Stacy R. Predictors of leisure-time physical activity among African American women. American Journal of Health Behavior. 2005;29:352–9. doi: 10.5993/ajhb.29.4.7. [DOI] [PubMed] [Google Scholar]
- Stewart A, King A, Haskell W. Endurance exercise and health-related quality of life in 50–65 year-old adults. The Gerontologist. 1993;33:782–9. doi: 10.1093/geront/33.6.782. [DOI] [PubMed] [Google Scholar]
- Stewart AL, Hays RD, Ware JE. The MOS short-form general health survey: Reliability and validity in a patient population. Medical Care. 1988;26:724–735. doi: 10.1097/00005650-198807000-00007. [DOI] [PubMed] [Google Scholar]
- Stiggelbout M, Hopman-Rock M, Crone M, et al. Predicting older adults’ maintenance in exercise participation using an integrated social psychological model. Nursing Research. 2006;21:1–14. doi: 10.1093/her/cyh037. [DOI] [PubMed] [Google Scholar]
- Takano T, Nakamura K, Watanabe Urban residential environments and senior citizens’ longevity in megacity areas: the importance of walkable green spaces. Journal of Epidemiology and Community Health. 2002;56:913–8. doi: 10.1136/jech.56.12.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Piliae R, Froelicher E. Measurement properties of Tai Chi exercise self-efficacy among ethnic Chinese with coronary heart disease risk factors: a pilot study. European Journal Cardiovascular Nursing. 2005;3:287–94. doi: 10.1016/j.ejcnurse.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Taylor L, Harris J, Epps C, et al. Psychometric evaluation of selected pain intensity scales for use with cognitively impaired and cognitively intact older adults. Rehabilitation Nursing. 2005;30:55–61. doi: 10.1002/j.2048-7940.2005.tb00360.x. [DOI] [PubMed] [Google Scholar]
- Thompson P, Buchner D, Pina IL, et al. American Heart Association Council on Clinical Cardiology Subcommittee on Exercise, Rehabilitation, and Prevention; American Heart Association Council on Nutrition, Physical Activity, and Metabolism Subcommittee on Physical Activity. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity) Circulation. 2003;107:3109–16. doi: 10.1161/01.CIR.0000075572.40158.77. [DOI] [PubMed] [Google Scholar]
- Tinetti ME. Performance oriented assessment of mobility problems in elderly patients. Journal of the American Geriatrics Society. 1986;34:199–206. doi: 10.1111/j.1532-5415.1986.tb05480.x. [DOI] [PubMed] [Google Scholar]
- Tinetti ME, Baker DI, Gottschalk M, et al. Home-based multicomponent rehabilitation program for older persons after hip fracture: a randomized trial. Archives of Physical Medicine and Rehabilitation. 1999;80:916–22. doi: 10.1016/s0003-9993(99)90083-7. [DOI] [PubMed] [Google Scholar]
- Tsauo J, Leu W, Chen Y, et al. Effects on function and quality of life of postoperative home-based physical therapy for patients with hip fracture Archives of Physical Medicine and Rehabilitation. 2005;86:1953–7. doi: 10.1016/j.apmr.2005.04.020. [DOI] [PubMed] [Google Scholar]
- Turk D, Okifuji A. Detecting depression in chronic pain patients: adequacy of self-reports. Behavioral Research and Therapeutics. 1994;32:9–16. doi: 10.1016/0005-7967(94)90078-7. [DOI] [PubMed] [Google Scholar]
- Walters S, Munro J. Using the SF-36 with older adults: a cross-sectional community-based survey. Age and Ageing. 2004;30:337–43. doi: 10.1093/ageing/30.4.337. [DOI] [PubMed] [Google Scholar]
- Ware L, Epps C, Herr K, et al. Evaluation of the revised faces pain scale, verbal descriptor scale, numeric rating scale, and Iowa pain thermometer in older minority adults. Pain Management in Nursing. 2006;7:117–25. doi: 10.1016/j.pmn.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Wilcox S, Dowda M, Griffin SF, et al. Results of the first year of active for life: translation of 2 evidence-based physical activity programs for older adults into community settings. American Journal of Public Health. 2006;96:1201–9. doi: 10.2105/AJPH.2005.074690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox S, King A. The effects of life events and interpersonal loss on exercise adherence in older adults. Journal of Aging and Physical Activity. 2004;12:117–30. doi: 10.1123/japa.12.2.117. [DOI] [PubMed] [Google Scholar]
- Yu-Yahiro J, Gardner A, Resnick B, et al. Challenges of designing an exercise program for use with frail elderly hip fracture patients. The Gerontologist. 2001;41:57. [Google Scholar]