Abstract
In humans, structural and functional changes attributable to aging are more visibly evident in the skin than in any other organ. Estrogens have significant effects on skin physiology and modulate epidermal keratinocytes, dermal fibroblasts and melanocytes, in addition to skin appendages including the hair follicle and the sebaceous gland. Importantly, skin aging can be significantly delayed by the administration of estrogen. This paper reviews the effects of estrogens on skin and the mechanisms by which estrogens can alleviate the changes due to aging that occur in human skin. The relevance of estrogen replacement therapy (HRT) in post-menopausal women and the potential value of selective estrogen receptor modulators (SERMs) as a therapy for diminishing skin aging are also highlighted.
Keywords: estrogen receptors, skin, menopause, SERMs, HRT
Estrogens and skin biology
A number of studies have shown that estrogens have many important beneficial and protective roles in skin physiology (reviewed in Thornton 2002, 2005). They have been shown to accelerate cutaneous wound healing (Ashcroft and Ashworth 2003), while a significant number of women notice an improvement in inflammatory skin disorders such as psoriasis during pregnancy (Dunna and Finlay 1989; Boyd et al 1996; Raychaudhuri et al 2003). Estrogens also offer some degree of protection against skin photoaging (Weinstock 1994; Tsukahara et al 2001, 2004) and epidemiological studies indicate that the mortality rates from both non-melanoma skin cancers (Weinstock 1994) and melanoma (Miller and MacNeil 1997) are significantly lower in women.
Many of the effects of estrogen on human skin are based on the changes that are seen in post-menopausal women, with a number of studies documenting the differences seen following the menopause, although there is also a variation in skin thickness during the menstrual cycle, with skin thickness lowest at the start of the menstrual cycle, when estrogen and progesterone levels are low, which then increases with the rising levels of estrogen (Eisenbeiss 1998). Many women report a sudden onset of skin aging several months after menopausal symptoms begin. The menopause causes hypoestrogenism, accelerating age-related deterioration, which results in thinner skin, an increase in number and depth of wrinkles, increased skin dryness, and decreased skin firmness and elasticity (Brincat 2000). Hormone replacement therapy (HRT) has been shown to increase epidermal hydration, skin elasticity, skin thickness (Sator et al 2001), and also reduces skin wrinkles (Phillips et al 2001). Furthermore, the content and quality of collagen and the level of vascularization is enhanced (Brincat et al 1987).
The epidermis
Epidermal thinning is associated with aging, and topical estradiol has been shown to reduce epidermal thinning in aging skin and maintain skin thickness (Shah and Maibach 2001). Recent studies have confirmed that administration of estradiol to gonadectomised mice increases epidermal thickness in both sexes (Azzi et al 2005).
In women an increase in the mitotic activity of epidermal keratinocytes occurs in response to estrogens (Punnonen 1972). Furthermore, the stimulation of proliferation and DNA synthesis of human epidermal keratinocytes by estrogens has been demonstrated in vitro (Urano et al 1995; Kanda and Watanabe 2004; Verdier-Sevrain et al 2004) and cultured human epidermal keratinocytes have high affinity estrogen binding sites (Verdier-Sevrain et al 2004). Specific antibodies for the two distinct intracellular estrogen receptors (ERα and ERβ) have shown that epidermal keratinocytes from human scalp skin of both sexes predominately express ERβ in situ (Thornton et al 2003).
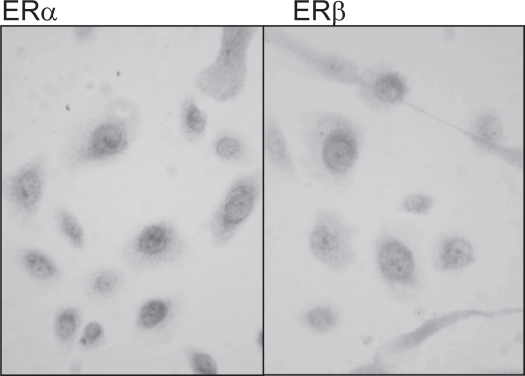
However, primary cultures of epidermal keratinocytes from female breast skin express both ERα and ERβ in vitro (see Figure 1).
Figure 1.
The expression of ERα and ERβ in human epidermal keratinocytes cultured from human female breast skin. Both nuclear and cytoplasmic expression of ERα and ERβ, with intense staining in the perinuclear region was seen in epidermal keratinocytes derived from female breast skin.
Another study has demonstrated mRNA and protein for ERβ and not ERα is expressed in cultured human epidermal keratinocytes (Kanda and Watanabe 2003a, 2003b), while a different group have shown that both ERα and ERβ are expressed in cultured human neonatal foreskin keratinocytes (Verdier-Sevrain et al 2004). These differences may be due to differences in anatomical site, since the number of estrogen receptors has been shown to differ with anatomical site, with face skin homogenates having a greater number of receptors than breast or thigh skin (Hasselquist et al 1980). A more recent study has shown that there are marked differences in the expression of the two estrogen receptors in human skin derived from different anatomical sites including scalp, breast and abdomen (Nelson 2006); in contrast to scalp, which predominantly expresses ERβ (Thornton et al 2003), breast skin predominantly expresses ERα in situ (Nelson 2006).
Recently, it has been reported that 17β-estradiol conjugated with BSA can stimulate proliferation and DNA synthesis of cultured human keratinocytes (Kanda and Watanabe 2004), suggesting that estradiol can stimulate cell proliferation via a cell membrane receptor. This is further supported by another group who demonstrated that incubation of cultured human epidermal keratinocytes with estradiol increased phosphorylation levels of ERK1 and ERK2 kinases within 15 minutes (Verdier-Sevrain et al 2004).
A protective role for estrogens on the human epidermis may also exist since estradiol prevents apoptosis induced by hydrogen peroxide in cultured epidermal keratinocytes by promoting the expression of bcl-2 (Kanda and Watanabe 2003a). Furthermore, estradiol inhibits the production of chemokines involved in the attraction of macrophages, which are important in inflammation (Kanda and Watanabe 2003b).
The dermis
Autoradiography following administration of systemic or topical estrogen has demonstrated that radio-labeled estradiol binds to dermal fibroblasts in rat and mouse skin (Stumpf et al 1974; Bidmon et al 1990). More recently, specific antibodies have demonstrated ERβ, but not ERα is expressed by dermal fibroblasts in the papillary dermis of human scalp skin in both sexes (Thornton et al 2003), whereas primary cultures of human dermal fibroblasts from female skin have been shown to express both mRNA and protein for ERα and ERβ (Haczynski et al 2002). Although dermal fibroblasts co-express both estrogen receptors, immunocytochemistry showed some variation in their expression; ERβ was predominately nuclear, while ERα was expressed in both the cytoplasm and the nucleus. Furthermore, mRNA levels for ERβ were higher than levels of ERα. A later study by the same group has demonstrated that estradiol up-regulates the expression of ERβ in dermal fibroblasts cultured from postmenopausal women (Surazynski et al 2003), thereby altering the ratio of ERα to ERβ.
Estrogens have been shown to influence skin thickness by stimulating collagen synthesis, maturation and turnover in rats (Smith and Allison 1966) and female guinea pigs (Henneman 1968). In mice, estrogen administration increased hyaluronic acid synthesis by 70% in two weeks (Sobel 1965), leading to increased dermal water content. In wound healing models, estrogen reduces wound size and stimulates matrix deposition in both human and murine skin (Ashcroft et al 1999; Ashcroft and Ashworth 2003), highlighting the effects of estrogens on the dermal fibroblasts.
Estrogen is also believed to provide some protection against photoaging. Ultraviolet (UV)-B exposure is associated with up-regulation of matrix metalloproteinase (MMP) production, leading to an increase in collagen degradation and is also thought to decrease type I and type III collagen synthesis (reviewed in Baumann 2005). Tsukahara and colleagues (2001) demonstrated that ovariectomized rats exposed to UVB radiation display an increased formation of deep wrinkles, decreased skin elasticity and marked and advanced curling of dermal elastic fibers compared to rats with normal estrogen levels. Furthermore, the same authors demonstrated that in hairless mice ovariectomy alone decreases skin elasticity, with a further significant increase in skin sagging in the ovariectomized animals exposed to 12 weeks of UV-A irradiation and a significant increase in skin wrinkling following UV-B irradiation (Tsukahara et al 2004). In each case this was associated with an increase in elastase activity. This led the authors to suggest that ovariectomy causes an increase in elastase mediated degradation of dermal elastic fibers with a subsequent reduction in skin elasticity, thus explaining the skin changes seen in post-menopausal women.
In vitro, murine dermal fibroblasts have been shown to increase collagen synthesis by 76% in response to estrogen (Hosokawa et al 1981). Similarly, incubation of cultured human dermal fibroblasts with 17β-estradiol increased type I procollagen secretion (Son et al 2005). This effect was reversed when estrogen was administered with TGF-β1 antibodies, suggesting that TGF-β1 may play a role in increasing collagen secretion in response to estrogen. Other authors have also demonstrated an increase in collagen biosynthesis in vitro in response to 17β-estradiol by measuring radio-labeled proline incorporation (Surazynski et al 2003).
The hair follicle
Hair follicles have a built in rhythm of activity that results in the periodic regeneration of new hairs and the molting of old ones. This rhythm appears to be intrinsic, although it can be influenced by hormonal or other systemic factors. Estrogens significantly influence the hair cycle in a number of mammalian species by inhibiting hair growth (reviewed in Thornton 2002, 2005; Ohnemus et al 2006). However, the effects of estrogens on the human hair cycle are less clear.
In contrast to the inhibition observed in other mammals, limited studies suggest that estrogens may have a stimulatory effect on human hair growth. During pregnancy there is an increase in the ratio of hair follicles in the growing phase of the cycle; after parturition, these follicles enter the resting phase, resulting in increased hair shedding and a transient thinning of the hair (Lynfield 1960). These events have been ascribed to the changes in the level of circulating estrogen during, and immediately following pregnancy. However, it is difficult to accredit this phenomenon entirely to the rise in plasma estrogen as several other hormones and growth factors that modulate hair growth are also altered at this time.
There is some limited trichogram data to suggest that estrogens decrease the resting phase and prolong the growing phase of the hair cycle, hence estrogens are used in the treatment of female pattern hair loss in some countries (Sinclair 1999). Further evidence for a stimulatory effect of estrogens on human hair growth comes from the treatment of women with tamoxifen which may result in scalp hair thinning or recession in some women (Gateley and Bundred 1997; Ayoub et al 1997). Likewise, a common treatment-related side effect of aromatase inhibitors, which inhibit the synthesis of estrogen, is scalp hair thinning in women (Buzdar et al 2001; Simpson et al 2004).
Recent in vitro studies have shown that 17β-estradiol inhibits female scalp hair shaft elongation (Nelson 2006), although stimulation occurs in hair follicles derived from frontotemporal male scalp (Conrad et al 2004; Conrad and Paus 2004). In addition, in female hair follicles the phytoestrogen, genistein inhibits hair shaft elongation to a similar extent as 17β-estradiol. Since genistein preferentially binds to ERβ, this opens the possibility that the inhibition of hair growth in response to 17β-estradiol may be mediated via ERβ rather than ERα (Nelson 2006). Therefore the development of selective estrogen receptor ligands may provide important clinical applications for the prevention and treatment of disorders of hair growth.
Melanocytes and melanoma
Chloasma is a common hyperpigmentation of the face seen in pregnant women, often accompanied by increased pigmentation in other areas including the areolae, linea alba and perineal skin, all of which usually fade following parturition (Kroumpouzos and Cohen 2001). Oral contraceptives containing estrogen can also result in hyperpigmentation of the face (Wade et al 1978) and ointments containing estrogen can produce intense pigmentation of the genitals, mammary areola and linea alba of the abdomen in male and female infants (Beas et al 1969).
The mean age of presentation of malignant melanoma in women is the early fifties, which correlates with the onset of the menopause (Durvasula et al 2002). Melanoma has traditionally been considered to be an estrogen receptor-positive tumor, whose prognosis is adversely affected by estrogen, whether during pregnancy or in association with the oral contraceptive pill or HRT. Recent evidence now disputes this and the relationship between estrogens and malignant melanoma remains controversial.
There is a significant lack of information in relation to HRT and melanoma and the use of steroid hormones in the management of melanoma is limited. Steroid hormone binding activity has been demonstrated in some human melanomas, but only a small percentage of melanomas respond to hormonal manipulation (Neifeld 1996). The relative expressions of the different estrogen receptors in malignant melanoma have yet to be explored, which may be of significance since alterations in the ratio of ERα and ERβ have been suggested in the development and progression of other cancers.
The menopause
Post-menopausal skin has been shown to have increased dryness (Sator et al 2004), decreased elasticity (Henry et al 1997; Sumino et al 2004), and increased wrinkling (Dunn et al 1997). Indeed, many of the effects of estrogen on the human skin have been described based on the changes that are seen following the menopause. Estrogen receptor expression has been shown to be reduced following the menopause (Punnonen et al 1980; Nelson and Bulun 2001).
In postmenopausal women skin thickness decreases by 1.13% per postmenopausal year, with an associated decrease in collagen content (2% per post-menopausal year) (Brincat et al 1987). The collagen content (types I and III) of skin is thought to decrease by as much as 30% in the first five years following the menopause (Brincat et al 1985; Affinito et al 1999). Interestingly, this decrease in skin collagen content parallels the reduction in bone mass seen in post-menopausal women (Brincat et al 1987). The decrease in skin thickness and collagen content seen in elderly females appears to correlate more closely with the period of estrogen deficiency than with chronological age (Brincat et al 1985, 1987; Affinito et al 1999).
In contrast, another study has demonstrated a closer relationship between chronological age and reduction in skin collagen, than time since menopause (Castelo-Branco et al 1992). However, for the patients in this study the time spent post-menopause was much shorter, therefore the long-term effects of estrogen deficiency may not have become apparent.
A difference in collagen subtypes has also been documented in post-menopausal women. When evaluated by immunohistochemistry, compared to pre-menopausal women, post-menopausal women demonstrate a decrease in collagen types I and III and a reduction in the typeIII/type I ratio within the dermis. Again this correlates more closely with period of estrogen deficiency than with chronological age (Affinito et al 1999). Similar findings have been reported in the arcus tendineous fasciae pelvis, where the collagen type I and type I/type III ratio was decreased in postmenopausal women compared to premenopausal women (Moalli et al 2004). Interestingly, despite these changes the total amount of collagen was not altered.
Hormone replacement therapy
Further understanding of estrogen effects on human skin is derived from comparisons between post-menopausal women taking estrogen replacement therapy and those who have not. An early study reported an increase in epidermal thickness in human female skin in response to six months of oral estrogens (Punnonen 1972). Although a different study did not demonstrate an increase in epidermal thickness, Maheux et al (1994) demonstrated an increased keratinocyte volume and more defined rete ridges in response to oral estrogen.
More recent studies have confirmed that topical estrogen increases epidermal thickness (Son et al 2005; Patriarca et al 2007). Furthermore, Son et al (2005) demonstrated an increase in keratinocyte proliferation and epidermal thickness in response to only two weeks of topical estrogen in the skin of elderly males and females. HRT has also been shown to improve skin hydration when estrogen is administered systemically (Pierard-Franchimont et al 1995) or topically (Sator et al 2001). Topical application is an efficient method, since due to its small molecular size; estrogen easily penetrates the stratum corneum (Draelos 2005). The increased hydration is believed to be due in part to an increase in the water-holding capacity of the stratum corneum (Pierard-Franchimont et al 1995). Additionally, dermal hyaluronic acid and acid mucopolysaccharide levels are increased, which will also improve hydration (reviewed in Shah and Maibach 2001).
In a randomized, double-blind, placebo-controlled trial, Maheux et al (1994), demonstrated that after 12 months of oral estrogen therapy, dermal thickness was increased by 30%, resulting in increased skin thickness in post-menopausal nuns. Skin biopsies were taken from the thigh thus eliminating confounding factors such as sun exposure and smoking (Maheux et al 1994). Other authors have also demonstrated increased skin thickness following HRT (Brincat et al 1985; Callens et al 1996; Sator et al 2001).
In addition to increased skin thickness, estrogen has also been shown to increase the collagen content of the skin. A randomized, double-blind, placebo-controlled trial carried out by Sauerbonn et al (2000) demonstrated an increase of 6.49% in skin collagen fiber content after six months of treatment with an oral combination of estrogen and cytoproterone acetate. Other authors have demonstrated an increase in overall collagen content (Brincat et al 1987) and specifically collagen type III (Savvas et al 1993; Schmidt et al 1996). Another study found no difference in skin thickness or collagen content in upper arm or abdominal skin following oral estrogen therapy (Haapasaari et al 1997); however the patients in this study had only a short period of estrogen deficiency (median time post-menopause 12 months), which may explain this finding. Importantly, the increase in skin collagen content in response to estrogen therapy appears to be related to the initial collagen content. Brincat et al (1987) demonstrated that in women with low skin collagen content, estrogen is initially therapeutic, but later becomes purely prophylactic, whereas for women in the early years post-menopause with only mild collagen loss, estrogen is of prophylactic value only. Indeed, optimum collagen content was seen following two years of treatment in a study which examined the effect of estradiol implants over a period ranging from two to ten years (Brincat et al 1987).
The use of topical estrogen has also been shown to increase skin collagen. Varila et al (1995) demonstrated an increase in collagen content following topical estrogen administration. They also demonstrated an increase in collagen synthesis as shown by increased type I and type III procollagen levels. However, with topical administration, the effect was limited to the area to which the estrogen was directly applied (Varila et al 1995; Schmidt et al 1996). A more recent study examining the effect of estrogen on human skin has demonstrated an increase in collagen content in buttock skin from elderly males and females treated with topical estradiol (Son et al. 2005). Type I procollagen mRNA and protein were significantly increased in males and females, with a significantly greater increase in females compared to males. Similarly, immunohistochemical expression for type I procollagen protein was increased following estradiol treatment. The same group also demonstrated an increase in tropoelastin and fibrillin mRNA and protein in response to estradiol and suggested this can be equated to an increase in elastic fibers. Furthermore, they demonstrated an increase in TGF-β and TGF-β type II receptor mRNA and protein expression in response to estrogen therapy, although TGF-β type-I receptor expression was not increased. Since TGF-β is a growth factor known to stimulate fibroblast proliferation and extracellular matrix (ECM) secretion, they suggested that this is the mechanism by which estrogen increases ECM secretion (Son et al 2005). Furthermore, a decrease in MMP-1 expression was demonstrated in response to topical estrogen, which may also explain increased collagen content in estrogen treated skin. Interestingly, when topical estradiol was applied to the skin of young males, similar effects were also demonstrated (Son et al 2005).
Skin wrinkling is synonymous with aging, but is also affected by environmental and hormonal factors. It occurs due to decreased skin elasticity as a result of elastic degeneration and loss of connective tissue (reviewed in Shah and Maibach 2001). In early postmenopausal women monitored for five years, skin elasticity has been shown to decrease by 1.5% per year, a change not seen in women on HRT (Henry et al 1997). Furthermore, conjugated equine estrogen has been shown to increase forearm skin elasticity in postmenopausal women (Sumino et al 2004). Punnonen et al (1987) demonstrated that topical estrogen thickens elastic fibers in the papillary dermis, increases their number and improves their orientation, although this effect has not been shown by other authors (Brincat et al 2005). This led to the suggestion that estrogen deficiency plays a role in wrinkle formation and in a large cohort study it was shown that wrinkling is reduced in postmenopausal women administered estrogen (Dunn et al 1997).
In post-menopausal women skin wrinkling has also been shown to be improved by topical estrogen therapy after a 24-week treatment period (Creidi et al 1994). However, other authors have demonstrated an improvement in skin elasticity, although there was no improvement in wrinkling in smokers (Castelo-Branco et al 1998). Furthermore, estrogen was not shown to consistently improve the appearance of facial skin over a five-year period, suggesting the effects may be reduced in sun-exposed skin (Pierard-Franchimont et al 1999).
More recently, Wolff et al (2005) demonstrated decreased facial wrinkling and increased skin rigidity following long-term oral estrogen administration. Women who were at least five years post-menopause and had either taken oral estrogen continuously, or had never used HRT, were examined by a single blind observer who assessed wrinkle formation using an objective photographic wrinkle score and measured skin rigidity using a durometer. They demonstrated that the average wrinkle score was significantly lower in the hormone treated group. Skin rigidity was also significantly lower in the group using HRT (Wolff et al 2005). This suggests that estrogen therapy has long-term benefits on skin and supports the use of early and continuous HRT in preventing detrimental skin changes (Naftolin 2005).
Although estrogen administration has been shown to have positive effects on skin by delaying or preventing skin aging manifestations (Brincat 2000; Sator et al 2004), understanding the effects of estrogen on skin is complicated by a number of factors. Firstly, when interpreting the results of studies looking at the effects of estrogen on skin it is important to remember that few randomized trials have been carried out and that the majority of trials have been observational studies. The use of different estrogen preparations and doses with, or without, the concomitant use of progesterone or testosterone also complicates this issue. Progestagens are combined with estrogen compounds and given cyclically or continuously since estrogen therapy alone may result in endometrial hyperplasia. Estrogen monotherapy can be used following hysterectomy (Sator et al 2004). Thus, making true comparisons and judgments of the isolated effects of estrogen are difficult. Indeed, topical progesterone cream (2%) alone has been shown to increase skin elasticity and decrease wrinkling in peri- and post-menopausal women (Holzer et al 2005).
Controversies surrounding the risks associated with the use of hormone therapy also complicate the issue. HRT was initially introduced to improve menopausal symptoms, reduce the risk of osteoporosis and was thought to reduce the risk of cardiovascular events (Stampfer et al 1991). However, a recent study has suggested that HRT may increase the risk of cardiovascular events (Grady et al 2002). It has also been reported that HRT may increase the risk of breast cancer (Rossouw et al 2002) and this has led to a significant reconsideration of the risks and benefits of systemic HRT. In response to this, recommendations for the use of HRT have become more stringent and it is now generally accepted that HRT should only be used to relieve menopausal symptoms, in the short-term (Hall and Phillips 2004). This means that justifying the use of randomized clinical trials to assess the effects of systemic estrogen on skin is difficult from a risk-benefit point of view.
Despite the controversy that surrounds the use of HRT in post-menopausal women; many authors suggest that the benefits of HRT in skin necessitate the need for further studies of its effects, not only in skin, but in other organ systems (Draelos 2005; Naftolin 2005; Wolff et al 2005). Furthermore, as the visual effects that are seen in the skin following the menopause may physiologically mirror the internal effects in other tissues (bone, cardiovascular system, cancer), skin may represent an easily accessible model. The use of topical estrogen could also be considered, but again this requires further investigation to determine the optimal dose for local benefits, while ensuring that systemic effects are avoided (Verdier-Sevrain et al 2006).
Skin and aging
Although estrogen deficiency is associated with skin changes, intrinsic aging also affects skin physiology. Skin undergoes profound changes associated with aging. This is most apparent in the face and other sun-exposed areas. Aging is associated with a decrease in skin thickness due to atrophy of the epidermis, dermis and subcutaneous fat. This is associated with dryness, wrinkling and an increased incidence of proliferative lesions. In the epidermis, aging is associated with a decrease in epidermal thickness, flattening of the dermal papilla and a decrease in melanocyte and Langerhans cell density. Within the dermis, increasing age leads to reduced fibroblast activity, reduced collagen and hyaluronic acid content, more fragmented elastin fibers and decreased vascularity (Ashcroft et al 1995). Aging skin has also been shown to have increased pro-enzyme MMP-2 expression (Ashcroft et al 1997), suggesting that aged skin is primed for tissue breakdown. A number of environmental factors such as sun exposure and smoking also affect the rate at which skin changes.
Mechanism of estrogen action
Estrogens are the terminal ligand in the biosynthetic pathway of gonadal steroid hormones and are synthesized from androgens by the loss of the C-19 angular methyl group and the formation of an aromatic A ring by the aromatase complex (Payne and Hales 2004). Estrone is derived from androstenedione, whereas estradiol is formed from testosterone. Estrone and estradiol are interconvertable due to different isoenzymes of 17β-hydroxysteroid dehydrogenase (Labrie 2004).
The principle source of estrogen biosynthesis is the ovary in females of reproductive age. In men, estradiol can be produced in peripheral tissues by the actions of aromatase on testosterone (Simpson 1998). Humans, along with some other primates are unusual in that the adrenal cortex secretes large quantities of adrenal androgens, including dehydroepiandrosterone (DHEA), which can then be converted into active steroids in peripheral tissues providing they have the appropriate enzymes (Labrie 2004). DHEA synthesized in the adrenal zona reticularis serves as the main precursor of active estrogens in post-menopausal women. Notwithstanding, the production of DHEA also decreases with age. Serum concentrations are low prior to puberty, reaching a peak in adulthood. However, throughout adult life, levels decline and by the 7th decade are reduced to only 10%–20% of the peak concentrations in both sexes (Parker et al 1997). Therefore, with aging the precursor steroids for peripheral estrogen biosynthesis are reduced.
Estrogen receptor: non-genomic signaling
While the best described mode of estrogen signaling is mediated via two related proteins (ERα and ERβ) that belong to the nuclear receptor superfamily (see below), a further level of complexity in estrogen signaling is now apparent due to studies demonstrating rapid effects in response to estrogens that cannot be attributed to signaling via the classical intracellular nuclear receptors and their genomic pathways (Levin 2002). These rapid effects, at rates much faster than can be attributed to genomic signaling, have led to the view that cell membrane forms of estrogen receptors exist that are coupled to cytosolic signal transduction proteins able to initiate different signaling cascades via conventional second messengers (Nadal et al 2001). Estrogens have been shown to activate second messengers such as adenylate cyclase and cAMP (Aronica et al 1994), phospholipase C (Lieberherr et al 1993), protein kinase C (Marino et al 2002) and the mitogen-activated protein kinase (MAPK) (Shaul 1999; Russel et al 2000). Interestingly, estrogen can also activate ligand or voltage-gated ion channels (Pappas et al 1995; Razandi et al 1999; Nadal et al 2001) resulting in the increase in levels of intracellular calcium (Benten et al 2001).
The term “activators of nongenotropic estrogen-like signaling (ANGELS)” has been introduced to define small nonphenolic molecules that mimic the nongenotropic actions of estrogens, but lack their classical genotropic effects (Kousteni et al 2003). ANGELS appear to represent a novel class of compounds that differ mechanistically from classical estrogens. One such compound is estren, which is completely devoid of classical genotropic actions (Kousteni et al 2003), but can reverse bone loss in sex steroid-deficient mice (Kousteni et al 2002).
Classical mechanism of action: genomic signaling
Two distinct intracellular estrogen receptors (ERα and ERβ) have been identified that belong to the superfamily family of nuclear hormone receptors (Moverare et al 2002). It is now clear that estrogens have important effects on many non-reproductive tissues and the expression of the two estrogen receptors is tissue dependent. In addition to the male and female reproductive tissues, ERα and ERβ are expressed in tissues as diverse as bone, brain, lung, bladder, thymus, pituitary, hypothalamus, heart, kidney, adrenal, the cardiovascular system and the skin including the hair follicle (reviewed in Thornton 2002, 2005).
The two estrogen receptors are distinct proteins encoded by separate genes that are located on different chromosomes (Enmark et al 1997). The ERα and ERβ proteins share approximately 97% homology in the DNA binding domain, with only a few amino acids differing in this region. However, in the ligand binding domain they only share 59% homology (Gustafsson 1999), while they share little homology in the amino terminal domain, hinge domain and COOH domain (Moverare et al 2002). With such a difference in the ligand binding domain, it could be anticipated that the receptors would bind estradiol with different affinities; this however is not the case, since 17β-estradiol has a similar affinity for both receptors. Ligand binding analysis has shown an average dissociation constant of 0.5 nM for 17β-estradiol on mouse ERβ and 0.2 nM on mouse ERα (Giguere 1998), suggesting ERβ has a slightly lower affinity for 17β-estradiol. Many synthetic estrogens also bind both receptors with a similar affinity (Kuiper et al 1997).
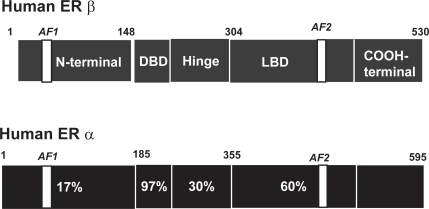
Similar to all intracellular steroid receptors, ERα and ERβ are ligand activated nuclear transcription factors that enhance target-gene transcription upon binding to chromatin. Activation of the target gene by 17β-estradiol activates both ERα and ERβ to increase transcriptional activities when dimers of the liganded receptors bind to estrogen response elements (ERE) which are specific DNA palindrome sequences located in the promoter region of estrogen-regulated target genes (Klinge 2001). Activation of transcription also requires the recruitment of a large coactivator complex composed of p160 coactivators including GRIP1 and SRC-1 and the histone acetyltransferases p300/CREB-binding protein and pCAF (Webb et al 2003). While the DNA binding domain mediates ERE recognition, the mediation of coactivator recruitment occurs via distinct activation functions (AF) located in the N-terminal domain (AF-1) and the ligand binding domain (AF-2) (Figure 2). Coactivators are tissue-specific and there is some evidence that ERα and ERβ differ in their requirements for coactivators in a cell and tissue dependent manner (Smith and O’Malley 2004).
Figure 2.
Schematic representation of the modular structures of ERα and ERβ The modular structure of the two estrogen receptors (ERα and ERβ) illustrating several distinct functional domains. The numbers within the ERα domains represent the percentage homology between the two receptors. There is little homology between activation function-1 (AF-1) in the N-terminal domain, whereas the core sequences of AF-2 in the ligand binding domain (LBD) are identical. The numbers above each receptor represent the amino acid position of each boundary. The DNA binding domain (DBD) shows the greatest degree of homology.
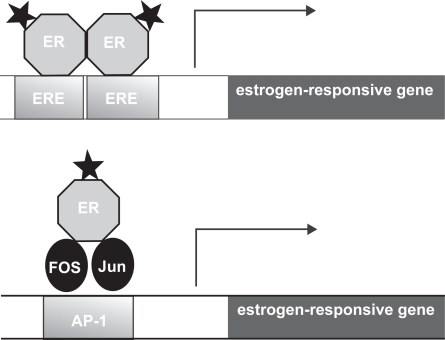
In addition to the well characterized mode of action via EREs, estrogen receptors may also interact with other transcription factors bound to their cognate DNA binding sites through protein-protein interactions. For example, both ERα and ERβ enhance the transcription of genes that contain AP-1 sites, the cognate binding site for the Jun/Fos complex (Paech et al 1997) (see Figure 3). ERα and ERβ can also enhance the transcription of genes that contain binding sites for other transcription factors (Elgort et al 1996). While the precise mechanism of ER action at AP-1 sites is still unclear, studies suggest that there are striking differences from ER action at EREs (Webb et al 1999).
Figure 3.
Models of estrogen action at a classical estrogen response element (ERE) and an ER-dependent AP-1 response element. The stars represent the ligand bound to the estrogen receptor (ER). In the classical mode of action activated ERs bind as dimers to the ERE which is comprised of 2 inverted hexanucleotide repeats, and activates gene transcription. In a different mode of action, the ligand activated ER can mediate gene transcription via an AP-1 enhancer element that requires the AP-1 transcription factors Fos and Jun for transcriptional activation. The difference between the actions of the SERMs tamoxifen and raloxifene are due to their ability/inability to activate these pathways. Tamoxifen inhibits the transcription of genes regulated by the classical ERE, but activates the transcription of genes regulated by an AP-1 element. In contrast raloxifene does not have the ability to activate AP-1. Therefore there are numerous combinations by which SERMS can modulate the estrogen receptor in a tissue-specific manner.
Since ERα and ERβ are often co-localized within many tissues, it was assumed that they would exert their effects on different target genes. However, there is evidence that ERα and ERβ can form both homodimers and heterodimers and that ERα homodimers and ERα/ERβ heterodimers are formed in preference to ERβ homodimers (Cowley et al 1997). It has been proposed that in a cell where both estrogen receptors are present, the overall estrogen responsiveness may be determined by the ERα:ERβ ratio (Stenn and Paus 2001). Furthermore, where ERs are binding to AP-1 sites, binding of ERα or ERβ homodimers can have opposing effects on gene transcription (Paech et al 1997; Kushner et al 2000). Thus, this creates the possibility that ERα and ERβ can work antagonistically or synergistically (Matthews and Gustafsson 2003).
Selective estrogen receptor modulators (SERMs)
Clinically, estrogens are widely used in the form of both oral contraceptives and HRT. Although they are highly effective both as a contraceptive and managing menopausal symptoms in women, nevertheless the use of estrogens has also been implicated as a risk factor in breast and uterine cancer (Rossouw et al 2002).
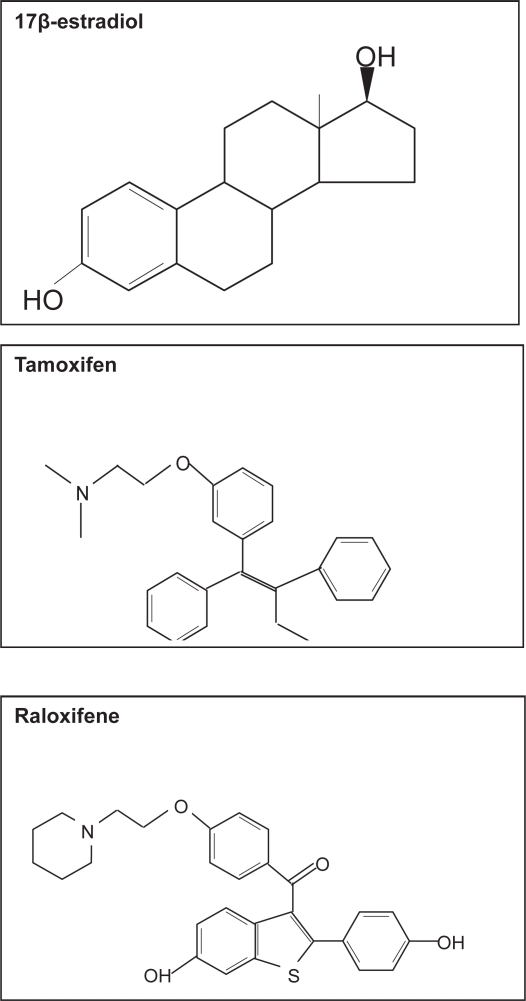
Tamoxifen is a non-steroidal triphenylethylene (Figure 4), which is widely used in the management of women with hormonally responsive early and metastatic breast cancer, since it acts as an antagonist of estrogen action in breast tissue (Cataliotti et al 2006). Although tamoxifen blocks estrogen action in breast cancer cells, conversely it stimulates proliferation of uterine cells by acting as an estrogen agonist, thereby resulting in an increase in the risk of endometrial cancer by three fold (Stygar et al 2003).
Figure 4.
Molecular structure of 17β-estradiol, tamoxifene and raloxifene.
The study of the selective biological effects of tamoxifen in different tissues has led to the embodiment of the concept of selective estrogen receptor modulators or SERMs. SERMs bind to estrogen receptors and can act as either estrogen agonists or antagonists depending on the target tissue. Current SERMs generally act as agonists in liver, in bone by inhibiting bone resorption, and on the cardiovascular system by reducing the markers of cardiovascular risk such as LDL. They are often antagonists in tissues such as breast and brain, but exhibit a mixed response in the uterus (Jordan 1998; Diel 2002). The undesirable uterine side-effects of tamoxifen have provided the drive for the development of the “ideal” SERM.
More recently a new SERM, raloxifene has been introduced (Figure 4). Raloxifene is derived from a benzothiophene series of anti-estrogens and is now approved for the prevention and treatment of osteoporosis in post-menopausal women (reviewed in Neven and Vergotta 2001). While raloxifene exhibits antiestrogen activity in the breast, in contrast to tamoxifen, it lacks uterotrophic activity and thus signifies an improved agonist/antagonist profile (Stygar et al 2003).
Since the three-dimensional structure of tamoxifen and raloxifene differ to 17β-estradiol (Figure 4) binding of these ligands to the estrogen receptor in breast tissue results in a conformational change that blocks AF-2 activity. This results in differences in their ability to recruit essential co-activators, thus eliminating the estrogen effect (Sporn 2004). Although tamoxifen inhibits the transcription of genes that are regulated by a classical ERE, tamoxifen activates the AP-1 target gene in uterine cells (Kushner 2000) in an AF-independent manner. Raloxifene, however, does not have the ability to activate AP-1 which would account for the differences seen in their effects on the uterus. This gives numerous combinations by which SERMS can modulate estrogen receptors in a tissue-specific manner. Understanding why SERMs exhibit different estrogen activities will help to identity new SERMs with more favourable profiles.
Since hypoestrogenism has detrimental effects on many other tissues including the skeleton, the CNS and the cardiovascular system, many consider the ideal SERM to have estrogenic activity in bone, the cardiovascular system, vagina and CNS, yet have antiestrogenic activity in the breast and uterus. However, since neither tamoxifen nor raloxifene possess this spectrum of properties, there is clearly a market for the development of specific SERMs to address these issues.
SERMs and skin biology
Despite the well documented effects of estrogen on skin physiology and aging, there is very limited data on the effect of SERMs on the skin. One of the adverse effects of tamoxifen include hot flashes and vaginal dryness (reviewed in Neven and Vergotta 2001), but there have been no studies to determine effects on skin thickness, collagen content, elastic fibers or the formation of wrinkles. A histopathological assessment of rat skin following subcutaneous administration of tamoxifen observed that tamoxifen treatment resulted in the appearance of abnormal hair follicles, epidermal atrophy and increased dermal fibrosis, particularly around the hair follicles (Inaloz et al 2002). There have been reports of tamoxifen treatment causing diffuse thinning of the hair with moderate receding of the frontal hair line (Ayoub et al 1997) and the development of alopecia on the crown, which was reversed when treatment was stopped (Gateley and Bundred 1997). Although alopecia is reported on the data and patient information sheet for proprietary tamoxifen, alopecia is not reported in the datasheets for the generic form of tamoxifen (Gateley and Bundred 1997).
We have recently reported that tamoxifen alone has no effect on human hair shaft elongation in organ culture, suggesting it is not an estrogen agonist (Nelson 2006). However, a 10-fold excess of tamoxifen incubated in combination with 17β-estradiol eliminated the inhibitory effect of 17β-estradiol, suggesting that tamoxifen acts as an antagonist of estrogen in the female scalp hair follicle (Nelson 2006).
Notwithstanding, there have been some studies that suggest tamoxifen may be useful in the treatment of keloids. Tamoxifen has been shown to inhibit the proliferation of fibroblasts cultured from keloid biopsies, and to inhibit their contraction (Hu et al 1998). Keloid dermal fibroblasts have also been shown to secrete higher levels of TGF-β1 than fetal dermal fibroblasts, which can be counteracted by incubation with tamoxifen (Mikulec et al 2001).
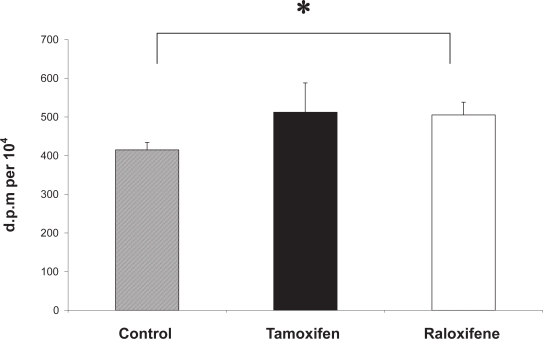
Together these provide evidence that tamoxifen may improve wound healing and improve dermal scarring. Cell migration is a key element of the wound healing process and a recent study has shown that estrogen and tamoxifen induce cytoskeleton remodeling and migration in endometrial cancer cells (Acconcia et al 2006). Another fundamental aspect of cutaneous wound healing is an increase in dermal fibroblast proliferation, and recent studies in our laboratory have shown that monolayers of human dermal fibroblasts that have been mechanically wounded in vitro demonstrate a significantly increased rate of both migration and proliferation in response to 17β-estradiol (Stevenson et al 2005). Furthermore, we have recently demonstrated that the proliferative activity of mechanically wounded cultured human dermal fibroblasts is significantly increased in the presence of tamoxifen and raloxifene (Figure 5), supporting the application of SERMs as potential therapeutic agents to improve wound healing.
Figure 5.
Tamoxifen and raloxifene increase the DNA synthesis of mechanically wounded human dermal fibroblasts in vitro. A 3H-thymidine assay was used to measure DNA synthesis in wounded dermal fibroblasts (n = 3) in the presence of 100 nM tamoxifen (black bar) and 100 nM raloxifene (white bar) and vehicle control (0.0001% absolute ethanol (hatched bar). All assays were performed in triplicate dishes. Data are presented as patient mean +/– SEM. * p < 0.05.
Other groups using cultured human dermal fibroblasts have shown that the levels of expression of ERα and ERβ can be modulated by 17β-estradiol, tamoxifen and raloxifene (Haczynski et al 2004). Another study has shown that raloxfine has a stronger positive stimulatory effect on collagen biosynthesis than 17β-estradiol (Surazynski et al 2003) and that in contrast to estradiol, raloxifene inhibits the expression of MMP-9. Tamoxifen has been used in clinical trials for patients with melanoma since the late 1970s. Initially it showed promise as a single agent and then was combined with chemotherapy in 1984 (Rusthoven 1998). Since then, a number of phase II clinical trials have combined tamoxifen with different chemotherapeutic agents with overall response rates ranging from 8% to 60%. However, treatment of melanoma with tamoxifen still remains controversial, since it is still unclear whether the strength of evidence from the randomized trials outweighs the combined evidence from numerous nonrandomized trials (Rusthoven 1998).
In vitro, tamoxifen has also been shown to inhibit the proliferation of a melanoma cell line (SK-Mel–28) (Lama et al 1999). Cell attachment to plastic and invasion through fibronection in vitro of the highly metastatic cutaneous melanoma cell line (A375-SM) is also reduced in the presence of tamoxifen (Dewhurst et al 1997). Others have shown that tamoxifen can induce cell death in malignant melanoma cell lines and this cytoxicity may be due to inactivation of the insulin-like growth factor receptor (IGF-1R) (Kanter-Lewensohn et al 1999). Clearly, malignant melanomas and the derived cell lines may be heterogeneous and the actions of estrogens in these tissues is worthy of further study.
Potential SERMs in development
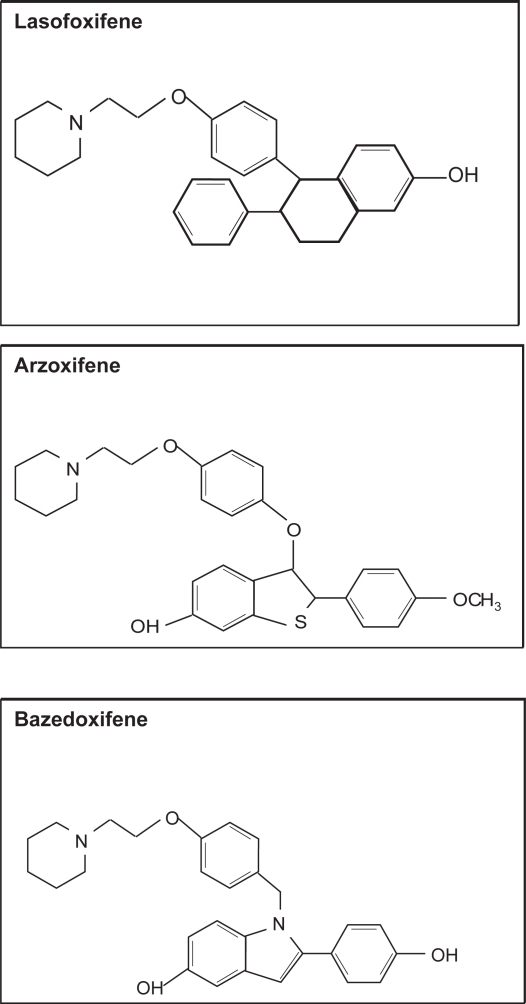
Recently much emphasis has been placed on developing SERMS, which have tissue specific estrogen actions that can separate the positive biological effects from the undesirable ones. Several SERM trials are underway but are being evaluated in terms of traditional outcomes such as bone mineral density, breast and endometrial cancer, and cardiovascular risk. A number of compounds are currently undergoing Phase III clinical trials (http://www.clinicaltrials.gov). These include lasofoxifene (Pfizer), arzoxifene (Lilly) and bazedoxifene (Wyeth) (see Figure 6).
Figure 6.
Molecular structure of SERMs in phase III clinical trials.
The safety and effectiveness of lasofoxifene in reducing new spinal fractures in women with osteoporosis is currently being investigated; (PEARL: Postmenopausal Evaluation And Risk-Reduction with Lasofoxifene). This study is expected to enroll 7500 women and the primary outcomes will investigate new radiographic vertebral fractures at 3 years, while the secondary outcomes will compare clinical fractures, non-vertebral fractures, bone mineral density, breast cancer, cardiovascular events, and gynecological safety events at 3 years.
Eli Lilly and Company is sponsoring a study of another SERM, arzoxifene, in a phase III clinical trial to determine its effects on bone mass and the uterus of postmenopausal women. This study will determine the effects of arzoxifene on bone mineral density at the spine and hip as well as the effects on the uterus. Secondary outcomes will include the effects of arzoxifene on blood tests that measure changes in bone rebuilding in postmenopausal women with low bone density; also the effects on blood lipids and other blood markers of heart disease and effects on breast density will be determined.
A third SERM in phase III clinical trial sponsored by Wyeth is bazedoxifene. The purpose of this study is to determine whether bazedoxifene/conjugated estrogens combinations are effective in the treatment of moderate to severe vasomotor symptoms associated with menopause and whether they are effective in the treatment of vaginal atrophy. The effectiveness of bazedoxifene/conjugated estrogens combinations for the prevention of endometrial hyperplasia and osteoporosis in postmenopausal women is also under investigation. However, none of these studies have been designed to assess the effects of these SERMs in the skin and it’s appendages in the women studied.
Conclusion
Evident changes associated with skin aging are thinning of the skin, increased dryness and reduced vascularity. The protective function of the skin can become impaired and aging is also associated with impaired wound healing, hair loss and skin cancer. Clearly the skin is an important estrogen target tissue, yet we still do not fully understand the molecular processes involved and the mechanisms by which estrogens and related compounds regulate skin function and delay skin aging. The widespread and constant expression of estrogen receptors in human skin may provide protection for the skin and its appendages, which are continuously exposed to oxidative damage due to UVB radiation.
However, the mechanisms of estrogen signaling are complex and intricate, involving multiple signaling pathways. There are at least three different receptors that may act independently, synergistically, or have opposing actions. Gene transcription also requires specialized co-activators, which are tissue and cell specific. A class of compounds that demonstrate estrogenic activity in some tissues conversely act as estrogen antagonists in other tissues. This has led to the embodiment of the term selective estrogen receptor modulators (SERMs). With the development of new generation SERMs to prevent osteoporosis and reduce the incidence of cardiovascular disease in postmenopausal women, it is important to understand their effects on the skin and the hair follicle.
Undesirable effects on the skin may lead to chronic wound healing or an increased incidence of skin cancer; in addition women find the adverse occurrence of hair loss very distressing. Conversely, there may be SERMs currently in use or in development that may act as estrogen agonists in human skin and the hair follicle and may potentially provide a useful therapy for clinical application to delay the aging manifestations of the skin. However, research into the potential use of SERMs as a therapy for symptoms of skin aging appears to be very much in its infancy.
In order to understand more comprehensively the mechanism of estrogen action on skin aging, we need to improve our understanding of the complex interactions of estrogen signaling pathways. In addition to SERMs, new advances in the development of selective ligands for ERα and ERβ, and activators of nongenotrophic estrogen-like signaling (ANGELS) may also provide a basis for further interventions in pathological processes that involve the impairment of estrogen action in human skin. This may have important implications for therapies for skin aging, hair growth, skin cancer and wound healing.
Acknowledgments
We are indebted to the following for their help and encouragement relating to various aspects of this article, Professor David Sharpe, Dr Louisa Nelson and Dr Ian Laing.
Abbreviations
- ERβ:
estrogen receptor beta;
- ERα:
estrogen receptor alpha;
- HSP:
heat shock protein;
- ERE:
estrogen response elements;
- DHEA:
dehydroepiandrosterone;
- 17 β -HSD:
17β-hydroxysteroid dehydrogenase;
- SERM:
selective estrogen receptor modulators;
- ANGELS:
activators of nongenotrophic estrogen-like signaling;
- HRT:
hormone replacement therapy;
- MMP:
matrix metalloproteinase;
- ECM:
extracellular matrix
References
- Acconcia F, Barnes CJ, Kumar R. Estrogen and tamoxifen induce cytoskeletal remodeling and migration in endometrial cancer cells. Endocrinology. 2006;147:1203–12. doi: 10.1210/en.2005-1293. [DOI] [PubMed] [Google Scholar]
- Affinito P, Palomba S, Sorrentino C, et al. Effects of postmenopausal hypoestrogenism on skin collagen. Maturitas. 1999;33:239–47. doi: 10.1016/s0378-5122(99)00077-8. [DOI] [PubMed] [Google Scholar]
- Aronica SM, Kraus WL, Katzenellenbogen BS. Estrogen action via the cAMP signaling pathway: stimulation of adenylate cyclase and cAMP-regulated gene transcription. Proc Natl Acad Sci USA. 1994;91:8517–21. doi: 10.1073/pnas.91.18.8517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora S, Veves A, Caballaro AE, et al. Estrogen improves endothelial function. J Vasc Surg. 1998;27:1141–6. doi: 10.1016/s0741-5214(98)70016-3. discussion 1147. [DOI] [PubMed] [Google Scholar]
- Ashcroft GS, Ashworth JJ. Potential role of estrogens in wound healing. Am. J. Clin. Dermatol. 2003;4:737–43. doi: 10.2165/00128071-200304110-00002. [DOI] [PubMed] [Google Scholar]
- Ashcroft GS, Horan MA, Ferguson MWJ. The effects of aging on cutaneous wound-healing in mammals. J Anat. 1995;187:1–26. [PMC free article] [PubMed] [Google Scholar]
- Ashcroft GS, Greenwell-Wild T, Horan MA, et al. Topical estrogen accelerates cutaneous wound healing in aged humans associated with an altered inflammatory response. Am J Pathol. 1999;155:1137–46. doi: 10.1016/S0002-9440(10)65217-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft GS, Horan MA, Herrick SE, et al. Age-related differences in the temporal and spatial regulation of matrix metalloproteinases (MMPs) in normal skin and acute cutaneous wounds of healthy humans. Cell Tissue Res. 1997;290:581–91. doi: 10.1007/s004410050963. [DOI] [PubMed] [Google Scholar]
- Ayoub J-P M, Valero V, Hortobagyi GN. Tamoxifen–induced female androgenetic alopecia in a patient with breast cancer. Annals of Internal Medicine. 1997;126:745–6. doi: 10.7326/0003-4819-126-9-199705010-00033. [DOI] [PubMed] [Google Scholar]
- Azzi L, El-Alfy M, Martel C, et al. Gender differences in mouse skin morphology and specific effects of sex steroids and dehydroepiandrosterone. J Invest Dermatol. 2005;124:22–7. doi: 10.1111/j.0022-202X.2004.23545.x. [DOI] [PubMed] [Google Scholar]
- Baumann L. A dermatologist’s opinion on hormone therapy and skin aging. Fertil Steril. 2005;84:289–90. doi: 10.1016/j.fertnstert.2005.03.032. discussion 295. [DOI] [PubMed] [Google Scholar]
- Beas F, Vargas L, Spada RP, et al. Pseudoprecocious puberty in infants caused by a dermal ointment containing estrogens. J Pediatr. 1969;75:127–30. doi: 10.1016/s0022-3476(69)80113-7. [DOI] [PubMed] [Google Scholar]
- Benten WPM, Stephan C, Lieberherr M, et al. Estradiol signalling via sequestrable surface receptors. Endocrinol. 2001;142:1669–77. doi: 10.1210/endo.142.4.8094. [DOI] [PubMed] [Google Scholar]
- Bidmon HJ, Pitts JD, Solomon HF, et al. Estradiol distribution and penetration in rat skin after topical application, studied by high resolution autoradiography. Histochemistry. 1990;95:43–54. doi: 10.1007/BF00737227. [DOI] [PubMed] [Google Scholar]
- Boyd AS, Morris LF, Phillips CM, et al. Psoriasis and pregnancy: hormone and immune system interaction. Int J Dermatol. 1996;35:169–72. doi: 10.1111/j.1365-4362.1996.tb01632.x. [DOI] [PubMed] [Google Scholar]
- Brincat M, Versi E, Moniz CF, et al. Skin collagen changes in post-menopausal women receiving different regimens of estrogen therapy. Obstet Gynecol. 1987;70:123–7. [PubMed] [Google Scholar]
- Brincat M, Moniz CJ, Studd JW, et al. Long-term effects of the menopause and sex hormones on skin thickness. Br J Obstet Gynaecol. 1985;92:256–9. doi: 10.1111/j.1471-0528.1985.tb01091.x. [DOI] [PubMed] [Google Scholar]
- Brincat MP. Hormone replacement therapy and the skin. Maturitas. 2000;35:107–17. doi: 10.1016/s0378-5122(00)00097-9. [DOI] [PubMed] [Google Scholar]
- Brincat MP, Baron YM, Galea R. Estrogens and the skin. Climacteric. 2005;8:110–23. doi: 10.1080/13697130500118100. [DOI] [PubMed] [Google Scholar]
- Buzdar J, Douma N, Davidson R, et al. Brady Phase III, multicenter, double-blind, randomized study of letrozole, an aromatase inhibitor, for advanced breast cancer versus megestrol acetate. Journal of Clinical Oncology. 2001;19:3357–66. doi: 10.1200/JCO.2001.19.14.3357. [DOI] [PubMed] [Google Scholar]
- Callens A, Vaillant L, Lecomte P, et al. Does hormonal skin aging exist? A study of the influence of different hormone therapy regimens on the skin of postmenopausal women using non-invasive measurement techniques. Dermatology. 1996;193:289–94. doi: 10.1159/000246272. [DOI] [PubMed] [Google Scholar]
- Castelo–Branco C, Duran M, Gonzalez-Merlo J. Skin collagen changes related to age and hormone replacement therapy. Maturitas. 1992;15:113–19. doi: 10.1016/0378-5122(92)90245-y. [DOI] [PubMed] [Google Scholar]
- Castelo-Branco C, Figueras F, Martinez de Osaba MJ, et al. Facial wrinkling in postmenopausal women. Effects of smoking status and hormone replacement therapy. Maturitas. 1998;29:75–86. doi: 10.1016/s0378-5122(97)00087-x. [DOI] [PubMed] [Google Scholar]
- Cataliotti L, Buzdar AU, Noguchi S, et al. Comparison of anastrozole versus tamoxifen as preoperative therapy in postmenopausal women with hormone receptor-positive breast cancer: the Pre-Operative “Arimidex” Compared to Tamoxifen (PROACT) trial. Cancer. 2006;106:2095–103. doi: 10.1002/cncr.21872. [DOI] [PubMed] [Google Scholar]
- Clifton VL, Crompton R, Read MA, et al. Microvascular effects of corticotropin-releasing hormone in human skin vary in relation to estrogen concentration during the menstrual cycle. J Endocrinol. 2005;186:69–76. doi: 10.1677/joe.1.06030. [DOI] [PubMed] [Google Scholar]
- Conrad F, Ohnemus U, Bodo E, et al. Estrogens and human scalp hair growth-still more questions than answers. J Invest Dermatol. 2004;122:840–2. doi: 10.1111/j.0022-202X.2004.22344.x. [DOI] [PubMed] [Google Scholar]
- Conrad F, Paus R. Estrogens and the hair follicle. J German Soc Dermatol. 2004;2:412–23. doi: 10.1046/j.1439-0353.2004.04037.x. [DOI] [PubMed] [Google Scholar]
- Cowley SM, Hoare S, Mosselman S, et al. Estrogen receptors alpha and beta form heterodimers on DNA. J Biol Chem. 1997;272:19858–62. doi: 10.1074/jbc.272.32.19858. [DOI] [PubMed] [Google Scholar]
- Creidi P, Faivre B, Agache P, et al. Effect of a conjugated oestrogen (Premarin) cream on ageing facial skin. A comparative study with a placebo cream. Maturitas. 1994;19:211–23. doi: 10.1016/0378-5122(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Dewhurst LO, Gee JW, Rennie IG, et al. Tamoxifen, 17beta-oestradiol and the calmodulin antagonist J8 inhibit human melanoma cell invasion through fibronectin. Br J Cancer. 1997;75:860–8. doi: 10.1038/bjc.1997.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diel P. Tissue-specific estrogenic response and molecular mechanisms. Toxicol Lett. 2002;127:217–24. doi: 10.1016/s0378-4274(01)00503-3. [DOI] [PubMed] [Google Scholar]
- Draelos ZD. Topical and oral estrogens revisited for antiaging purposes. Fertil Steril. 2005;84:291–2. doi: 10.1016/j.fertnstert.2005.03.033. discussion 295. [DOI] [PubMed] [Google Scholar]
- Dunn LB, Damesyn M, Moore AA, et al. Does estrogen prevent skin aging? Results from the First National Health and Nutrition Examination Survey (NHANES I) Arch Dermatol. 1997;133:339–42. doi: 10.1001/archderm.133.3.339. [DOI] [PubMed] [Google Scholar]
- Dunna SF, Finlay AY. Psoriasis: improvement during and worsening after pregnancy. Br J Dermatol. 1989;120:584. doi: 10.1111/j.1365-2133.1989.tb01338.x. [DOI] [PubMed] [Google Scholar]
- Durvasula R, Ahmed SM, Vashisht A, et al. Hormone replacement therapy and malignant melanoma: to prescribe or not to prescribe? Climacteric. 2002;5:197–200. [PubMed] [Google Scholar]
- Eisenbeiss C, Welzel J, Schmeller W. The influence of female sex hormones on skin thickness: evaluation using 20 MHz sonography. Br J Dermatol. 1998;139:462–7. doi: 10.1046/j.1365-2133.1998.02410.x. [DOI] [PubMed] [Google Scholar]
- Elgort MG, Zou A, Marschke KB, et al. Estrogen and estrogen receptor antagonists stimulate transcription from the human retinoic acid receptor-alpha 1 promoter via a novel sequence. Mol Endocrinol. 1996;10:477–87. doi: 10.1210/mend.10.5.8732679. [DOI] [PubMed] [Google Scholar]
- Enmark E, Pelto-Huikko M, Grandien K, et al. Human estrogen receptor beta-gene structure, chromosomal localization, and expression pattern. J Clin Endocrinol Metab. 1997;82:4258–65. doi: 10.1210/jcem.82.12.4470. [DOI] [PubMed] [Google Scholar]
- Gateley CA, Bundred NJ. Alopecia and breast disease. British Medical Journal. 1997;314:481. doi: 10.1136/bmj.314.7079.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giguere V, Tremblay A, Tremblay GB. Estrogen receptor beta: re-evaluation of estrogen and antiestrogen signalling. Steroids. 1998;63:335–9. doi: 10.1016/s0039-128x(98)00024-5. [DOI] [PubMed] [Google Scholar]
- Grady D, Herrington D, Bittner V, et al. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II) JAMA. 2002;288:49–57. doi: 10.1001/jama.288.1.49. [DOI] [PubMed] [Google Scholar]
- Gustafsson JA. Estrogen receptor beta – a new dimension in estrogen mechanism of action. J Endocrinol. 1999;163:379–83. doi: 10.1677/joe.0.1630379. [DOI] [PubMed] [Google Scholar]
- Haapasaari KM, Raudaskoski T, Kallioinen M, et al. Systemic therapy with estrogen or estrogen with progestin has no effect on skin collagen in postmenopausal women. Maturitas. 1997;27:153–62. doi: 10.1016/s0378-5122(97)01128-6. [DOI] [PubMed] [Google Scholar]
- Haczynski J, Tarkowski R, Jarzabek K, et al. Human cultured skin fibroblasts express estrogen receptor alpha and beta. Int J Mol Med. 2002;10:149–53. [PubMed] [Google Scholar]
- Haczynski J, Tarkowski R, Jarzabek K, et al. Differential effects of estradiol, raloxifene and tamoxifen on estrogen receptor expression in cultured human skin fibroblasts. Int J Mol Med. 2004;13:903–8. [PubMed] [Google Scholar]
- Hall GK, Phillips TJ. Skin and hormone therapy. Clin Obstet Gynecol. 2004;47:437–49. doi: 10.1097/00003081-200406000-00020. [DOI] [PubMed] [Google Scholar]
- Hasselquist MB, Goldberg N, Schroeter A, et al. Isolation and characterization of the estrogen receptor in human skin. J Clin Endocrinol Metab. 1980;50:76–82. doi: 10.1210/jcem-50-1-76. [DOI] [PubMed] [Google Scholar]
- Henneman DH. Effect of estrogen on in vivo and in vitro collagen biosynthesis and maturation in old and young female guinea pigs. Endocrinology. 1968;83:678–90. doi: 10.1210/endo-83-4-678. [DOI] [PubMed] [Google Scholar]
- Henry F, Pierard–Franchimont C, Cauwenbergh G, et al. Age-related changes in facial skin contours and rheology. J Am Geriatr Soc. 1997;45:220–2. doi: 10.1111/j.1532-5415.1997.tb04512.x. [DOI] [PubMed] [Google Scholar]
- Holzer G, Riegler E, Honigsmann H, et al. Effects and side-effects of 2% progesterone cream on the skin of peri- and postmenopausal women: results from a double-blind, vehicle-controlled, randomized study. Br J Dermatol. 2005;153:626–34. doi: 10.1111/j.1365-2133.2005.06685.x. [DOI] [PubMed] [Google Scholar]
- Hosokawa M, Ishii M, Inoue K, et al. Estrogen induces different responses in dermal and lung fibroblasts: special reference to collagen. Connect Tissue Res. 1981;9:115–20. doi: 10.3109/03008208109160249. [DOI] [PubMed] [Google Scholar]
- Hu D, Hughes MA, Cherry GW. Topical tamoxifen – a potential therapeutic regime in treating excessive dermal scarring? Br J Plast Surg. 1998;51:462–9. doi: 10.1054/bjps.1997.0100. [DOI] [PubMed] [Google Scholar]
- Inaloz HS, Deveci E, Inaloz SS, et al. The effects of tamoxifen on rat skin. Eur J Gynaecol Oncol. 2002;23:50–2. [PubMed] [Google Scholar]
- Jordan VC. Designer estrogens. Sci Am. 1998;279:60–7. doi: 10.1038/scientificamerican1098-60. [DOI] [PubMed] [Google Scholar]
- Kanda N, Watanabe S. 17beta-estradiol inhibits oxidative stress-induced apoptosis in keratinocytes by promoting Bcl-2 expression. J Invest Dermatol. 2003a;121:1500–9. doi: 10.1111/j.1523-1747.2003.12617.x. [DOI] [PubMed] [Google Scholar]
- Kanda N, Watanabe S. 17beta-estradiol inhibits the production of interferon-induced protein of 10 kDa by human keratinocytes. J Invest Dermatol. 2003b;120:411–19. doi: 10.1046/j.1523-1747.2003.12066.x. [DOI] [PubMed] [Google Scholar]
- Kanda N, Watanabe S. 17beta-estradiol stimulates the growth of human keratinocytes by inducing cyclin D2 expression. J Invest Dermatol. 2004;123:319–28. doi: 10.1111/j.0022-202X.2004.12645.x. [DOI] [PubMed] [Google Scholar]
- Kanter-Lewensohn L, Girnita L, Girnita A, et al. Tamoxifen-induced cell death in malignant melanoma cells: possible involvement of the insulin-like growth factor-1 (IGF-1) pathway. Mol Cell Endocrinol. 2000;165:131–7. doi: 10.1016/s0303-7207(00)00253-7. [DOI] [PubMed] [Google Scholar]
- Klinge CM. Estrogen receptor interaction with estrogen response elements. Nucleic Acids Res. 2001;29:2905–19. doi: 10.1093/nar/29.14.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kousteni S, et al. Reversal of bone loss in mice by nongenotropic signaling of sex steroids. Science. 2002;298:843–6. doi: 10.1126/science.1074935. [DOI] [PubMed] [Google Scholar]
- Kousteni S, Han L, Chen JR, et al. Kinase-mediated regulation of common transcription factors accounts for the bone-protective effects of sex steroids. J Clin Invest. 2003;111:1651–64. doi: 10.1172/JCI17261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroumpouzos G, Cohen LM. Dermatoses of pregnancy. J Am Acad Dermatol. 2001;45:1–19. doi: 10.1067/mjd.2001.114595. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Carlsson B, Grandien K, et al. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863–70. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- Kushner PJ, Agard DA, Greene GL, et al. Estrogen receptor pathways to AP-1. Steroid Biochem Mol Biol. 2000;74:311–17. doi: 10.1016/s0960-0760(00)00108-4. [DOI] [PubMed] [Google Scholar]
- Labrie F. Adrenal androgens and intracrinology. Semin Reprod Med. 2004;22:299–309. doi: 10.1055/s-2004-861547. [DOI] [PubMed] [Google Scholar]
- Lama G, Angelucci C, Bruzzese N, et al. Sensitivity of human melanoma cells to oestrogens, tamoxifen and quercetin: is there any relationship with type I and II oestrogen binding site expression? Melanoma Res. 1998;8:313–22. doi: 10.1097/00008390-199808000-00004. [DOI] [PubMed] [Google Scholar]
- Levin ER. Cellular functions of plasma membrane estrogen receptors. Steroids. 2002;67:471–5. doi: 10.1016/s0039-128x(01)00179-9. [DOI] [PubMed] [Google Scholar]
- Lieberherr M, Grosse B, Kachkache M, et al. Cell signaling and estrogens in female rat osteoblasts: a possible involvement of unconventional nonnuclear receptors. J Bone Mineral Res. 1993;8:1365–76. doi: 10.1002/jbmr.5650081111. [DOI] [PubMed] [Google Scholar]
- Lynfield YL. Effect of pregnancy on the human hair cycle. J Invest Dermatol. 1960;35:323–7. doi: 10.1038/jid.1960.54. [DOI] [PubMed] [Google Scholar]
- Maheux R, Naud F, Rioux M, et al. A randomized, double-blind, placebo-controlled study on the effect of conjugated estrogens on skin thickness. Am J Obstet Gynecol. 1994;170:642–9. doi: 10.1016/s0002-9378(94)70242-x. [DOI] [PubMed] [Google Scholar]
- Marino M, Acconcia F, Bresciani F, et al. Distinct nongenomic signal transduction pathways controlled by 17beta-estradiol regulate DNA synthesis and cyclin D(1) gene transcription in HepG2 cells. Mol Biol Cell. 2002;13:3720–9. doi: 10.1091/mbc.E02-03-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews J, Gustafsson JA. Estrogen signaling: a subtle balance between ER alpha and ER beta. Mol Interv. 2003;3:281–92. doi: 10.1124/mi.3.5.281. [DOI] [PubMed] [Google Scholar]
- Mikulec AA, Hanasono MM, Lum J, et al. Effect of tamoxifen on transforming growth factor beta1 production by keloid and fetal fibroblasts. Arch Facial Plast Surg. 2001;3:111–14. doi: 10.1001/archfaci.3.2.111. [DOI] [PubMed] [Google Scholar]
- Miller JG, Mac Neil S. Gender and cutaneous melanoma. Br J Dermatol. 1997;136:657–65. [PubMed] [Google Scholar]
- Moalli PA, Talarico LC, Sung VW, et al. Impact of menopause on collagen subtypes in the arcus tendineous fasciae pelvis. Am J Obstet Gynecol. 2004;190:620–7. doi: 10.1016/j.ajog.2003.08.040. [DOI] [PubMed] [Google Scholar]
- Moverare S, Lindberg MK, Faergemann J, et al. Estrogen receptor alpha, but not estrogen receptor beta, is involved in the regulation of the hair follicle cycling as well as the thickness of epidermis in male mice. J Invest Dermatol. 2002;119:1053–8. doi: 10.1046/j.1523-1747.2002.00637.x. [DOI] [PubMed] [Google Scholar]
- Nadal A, Ropero AB, Fuentes E, et al. The plasma membrane estrogen receptor: nuclear or unclear? Trends in Pharm Sci. 2001;22:597–9. doi: 10.1016/s0165-6147(00)01846-0. [DOI] [PubMed] [Google Scholar]
- Naftolin F. Prevention during the menopause is critical for good health: skin studies support protracted hormone therapy. Fertil Steril. 2005;84:293–4. doi: 10.1016/j.fertnstert.2005.04.018. discussion 295. [DOI] [PubMed] [Google Scholar]
- Neifeld JP. Endocrinology of melanoma. Semin Surg Oncol. 1996;12:402–6. doi: 10.1002/(SICI)1098-2388(199611/12)12:6<402::AID-SSU5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Nelson L, Messenger D, Karoo AG, et al. 17beta-eatradiol but not 17alpha-estradiol inhibits human hair growth in whole follicle organ culture. J. Invest. Dermatol. 2003;121:821a. [Google Scholar]
- Nelson LD.2006The role of oestrogen in skinPhD ThesisBradford, UK: School of Life Sciences, University of Bradford [Google Scholar]
- Nelson LR, Bulun SE. Estrogen production and action. J Am Acad Dermatol. 2001;45:S116–24. doi: 10.1067/mjd.2001.117432. [DOI] [PubMed] [Google Scholar]
- Neven P, Vergote I. Tamoxifen, screening and new oestrogen receptor modulators. Best Pract Res Clin Obstet Gynaecol. 2001;15:365–80. doi: 10.1053/beog.2001.0182. [DOI] [PubMed] [Google Scholar]
- t et al. 2006The hair follicle as an estrogen target and source Endocr Rev July28[Epub ahead of print]. [DOI] [PubMed]
- Paech K, Webb P, Kuiper GG, et al. Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites. Science. 1997;277:1508–10. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- Pappas TC, Gametchu B, Watson CS. Membrane estrogen receptors identified by multiple antibody labelling and impeded-ligand binding. FASEB J. 1995;9:404–10. doi: 10.1096/fasebj.9.5.7896011. [DOI] [PubMed] [Google Scholar]
- Parker CR, Mixon RL, Brissie RM, et al. Ageing alters zonation in the adrenal cortex of men. J Clin Endocrinol Metab. 1997;82:3898–901. doi: 10.1210/jcem.82.11.4507. [DOI] [PubMed] [Google Scholar]
- Patriarca MT, Goldman KZ, Dos Santos JM, et al. Effects of topical estradiol on the facial skin collagen of postmenopausal women under oral hormone therapy: a pilot study. Eur J Obstet Gynecol Reprod Biol. 2007;130:202–5. doi: 10.1016/j.ejogrb.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endor Rev. 2004;25:947–70. doi: 10.1210/er.2003-0030. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Demircay Z, Sahu M. Hormonal effects on skin aging. Clin. Geriatr Med. 2001;17:661–72. doi: 10.1016/s0749-0690(05)70092-6. [DOI] [PubMed] [Google Scholar]
- Pierard-Franchimont C, Letawe C, Goffin V, et al. Skin water-holding capacity and transdermal estrogen therapy for menopause: a pilot study. Maturitas. 1995;22:151–4. doi: 10.1016/0378-5122(95)00924-a. [DOI] [PubMed] [Google Scholar]
- Pierard-Franchimont C, Cornil F, Dehavay J, et al. Climacteric skin ageing of the face – a prospective longitudinal comparative trial on the effect of oral hormone replacement therapy. Maturitas. 1999;32:87–93. doi: 10.1016/s0378-5122(99)00019-5. [DOI] [PubMed] [Google Scholar]
- Punnonen R. Effect of castration and peroral estrogen therapy on the skin. Acta Obstet Gynecol Scand Suppl. 1972;21:3–44. [PubMed] [Google Scholar]
- Punnonen R, Lovgren T, Kouvonen I. Demonstration of estrogen receptors in the skin. J Endocrinol Invest. 1980;3:217–21. doi: 10.1007/BF03348266. [DOI] [PubMed] [Google Scholar]
- Punnonen R, Vaajalahti P, Teisala K. Local oestriol treatment improves the structure of elastic fibers in the skin of postmenopausal women. Ann Chir Gynaecol Suppl. 1987;202:39–41. [PubMed] [Google Scholar]
- Raychaudhuri SP, Navare T, Gross J, et al. Clinical course of psoriasis during pregnancy. Int J Dermatol. 2003;42:518–20. doi: 10.1046/j.1365-4362.2003.01760.x. [DOI] [PubMed] [Google Scholar]
- Razandi M, Pedram A, Greene Gl, et al. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ERalpha and ERbeta expressed in Chinese hamster ovary cells. Mol Endocrinol. 1999;13:307–19. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
- Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Russel KS, Haynes MP, Sinha D, et al. Human vascular endothelial cells contain membrane binding sites for estradiol, which mediate rapid intracellular signalling. Proc Natl Acad Sci USA. 2000;97:5930–5. doi: 10.1073/pnas.97.11.5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusthoven JJ. The evidence for tamoxifen and chemotherapy as treatment for metastatic melanoma. Eur J Cancer. 1998;34(Suppl 3):S31–6. doi: 10.1016/s0959-8049(97)10162-9. [DOI] [PubMed] [Google Scholar]
- Sator PG, Schmidt JB, Rabe T, et al. Skin aging and sex hormones in women – clinical perspectives for intervention by hormone replacement therapy. Exp Dermatol. 2004;13(Suppl 4):36–40. doi: 10.1111/j.1600-0625.2004.00259.x. [DOI] [PubMed] [Google Scholar]
- Sator PG, Schmidt JB, Sator MO, et al. The influence of hormone replacement therapy on skin ageing: a pilot study. Maturitas. 2001;39:43–55. doi: 10.1016/s0378-5122(00)00225-5. [DOI] [PubMed] [Google Scholar]
- Sauerbronn AV, Fonseca AM, Bagnoli VR, et al. The effects of systemic hormonal replacement therapy on the skin of postmenopausal women. Int J Gynaecol Obstet. 2000;68:35–41. doi: 10.1016/s0020-7292(99)00166-6. [DOI] [PubMed] [Google Scholar]
- Savvas M, Bishop J, Laurent G, et al. Type III collagen content in the skin of postmenopausal women receiving oestradiol and testosterone implants. Br J Obstet Gynaecol. 1993;100:154–6. doi: 10.1111/j.1471-0528.1993.tb15212.x. [DOI] [PubMed] [Google Scholar]
- Schmidt JB, Binder M, Demschik G, et al. Treatment of skin aging with topical estrogens. Int J Dermatol. 1996;35:669–74. doi: 10.1111/j.1365-4362.1996.tb03701.x. [DOI] [PubMed] [Google Scholar]
- Shah MG, Maibach HI. Estrogen and skin: an overview. Am J Clin Dermatol. 2001;2:143–50. doi: 10.2165/00128071-200102030-00003. [DOI] [PubMed] [Google Scholar]
- Shaul PW. Rapid activation of endothelial nitric oxide synthase by estrogen. Steroids. 1999;64:28–34. doi: 10.1016/s0039-128x(98)00105-6. [DOI] [PubMed] [Google Scholar]
- Simpson ER. Genetic mutations resulting in estrogen insufficiency in the male. Mol Cell Endocrinol. 1998;145:55–9. doi: 10.1016/s0303-7207(98)00169-5. [DOI] [PubMed] [Google Scholar]
- Simpson D, Curran MP, Perry CM. Letrozole: a review of its use in postmenopausal women with breast cancer. Drugs. 2004;64:1213–30. doi: 10.2165/00003495-200464110-00006. [DOI] [PubMed] [Google Scholar]
- Sinclair R. Hair structure and function In Sinclair R, Banfiel C, Dawber R Handbook of diseases of the hair and scalp. New York: Blackwell, Oxford Press; 1999. [Google Scholar]
- Smith QT, Allison DJ. Studies on the uterus, skin and femur of rats treated with 17-beta-oestradiol benzoate for one to twenty-one days. Acta Endocrinol (Copenh) 1966;53:598–610. doi: 10.1530/acta.0.0530598. [DOI] [PubMed] [Google Scholar]
- Smith CL, O’Malley BW. Coregulator functions: a key to understanding tissue specificity of selective receptor modulators. Endocr Rev. 2004;25:45–71. doi: 10.1210/er.2003-0023. [DOI] [PubMed] [Google Scholar]
- Sobel H, Lee KD, Hewlett MJ. Effect of estrogen on acid glycosaminoglycans in skin of mice. Biochem Biophys Acta. 1965;101:225–9. doi: 10.1016/0926-6534(65)90056-4. [DOI] [PubMed] [Google Scholar]
- Son ED, Lee JY, Lee S, et al. Topical application of 17beta-estradiol increases extracellular matrix protein synthesis by stimulating tgf-Beta signaling in aged human skin in vivo. J Invest Dermatol. 2005;124:1149–61. doi: 10.1111/j.0022-202X.2005.23736.x. [DOI] [PubMed] [Google Scholar]
- Sporn MB, Dowsett SA, Mershon J, et al. Role of raloxifene in breast cancer prevebtion: clinical evidence and potential mechanisms of action. Clin Ther. 2004;26:830–40. doi: 10.1016/s0149-2918(04)90127-0. [DOI] [PubMed] [Google Scholar]
- Stampfer MJ, Colditz GA, Willett WC, et al. Postmenopausal estrogen therapy and cardiovascular disease. Ten-year follow-up from the nurses‘ health study. N Engl J Med. 1991;325:756–62. doi: 10.1056/NEJM199109123251102. [DOI] [PubMed] [Google Scholar]
- Stenn KS, Paus R. Controls of hair follicle cycling. Physiol Rev. 2001;81:449–94. doi: 10.1152/physrev.2001.81.1.449. [DOI] [PubMed] [Google Scholar]
- Stevenson S, Nelson LD, Huq S, et al. 2005Oestrogens and wound healing: migration, proliferation and secretion of paracrine factors by human dermal fibroblasts in vitroSoc for Endocrinol Annual Meeting. URL: http://www.endocrine-abstracts.org10P80
- Stumpf WE, Sar M, Joshi SG. Estrogen target cells in the skin. Experientia. 1974;30:196–8. doi: 10.1007/BF01927732. [DOI] [PubMed] [Google Scholar]
- Stygar D, Muravitskaya N, Eriksson B, et al. Effects of SERM (selective estrogen receptor modulator) treatment on growth and proliferation in the rat uterus. Reprod Biol Endocrinol. 2003;1:40. doi: 10.1186/1477-7827-1-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumino H, Ichikawa S, Abe M, et al. Effects of aging and postmenopausal hypoestrogenism on skin elasticity and bone mineral density in Japanese women. Endocr J. 2004;51:159–64. doi: 10.1507/endocrj.51.159. [DOI] [PubMed] [Google Scholar]
- Surazynski A, Jarzabek K, Haczynsk J, et al. Differential effects of estradiol and raloxifene on collagen biosynthesis in cultured human skin fibroblasts. Int J Mol Med. 2003;12:803–9. [PubMed] [Google Scholar]
- Thornton MJ. The biological actions of estrogens on skin. Exp Dermatol. 2002;11:487–502. doi: 10.1034/j.1600-0625.2002.110601.x. [DOI] [PubMed] [Google Scholar]
- Thornton MJ. Oestrogen functions in skin and skin appendages. Expert Opin Ther Targets. 2005;9:617–29. doi: 10.1517/14728222.9.3.617. [DOI] [PubMed] [Google Scholar]
- Thornton MJ, Taylor AH, Mulligan K, et al. Estrogen receptor beta (ERβ) is the predominant estrogen receptor in human scalp. Exp Dermatol. 2003;12:181–90. doi: 10.1034/j.1600-0625.2003.120209.x. [DOI] [PubMed] [Google Scholar]
- Tsukahara K, Moriwaki S, Ohuchi A, et al. Ovariectomy accelerates photoaging of rat skin. Photochem Photobiol. 2001;73:525–31. doi: 10.1562/0031-8655(2001)073<0525:oapors>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Tsukahara K, Nakagawa H, Moriwaki S, et al. Ovariectomy is sufficient to accelerate spontaneous skin ageing and to stimulate ultraviolet irradiation-induced photoageing of murine skin. Br J Dermatol. 2004;151:984–94. doi: 10.1111/j.1365-2133.2004.06203.x. [DOI] [PubMed] [Google Scholar]
- Urano R, Sakabe K, Seiki K, et al. Female sex hormone stimulates cultured human keratinocyte proliferation and its RNA- and protein-synthetic activities. J Dermatol Sci. 1995;9:176–84. doi: 10.1016/0923-1811(94)00375-o. [DOI] [PubMed] [Google Scholar]
- Varila E, Rantala I, Oikarinen A, et al. The effect of topical oestradiol on skin collagen of postmenopausal women. Br J Obstet Gynaecol. 1995;102:985–9. doi: 10.1111/j.1471-0528.1995.tb10906.x. [DOI] [PubMed] [Google Scholar]
- Verdier-Sevrain S, Bonte F, Gilchrest B. Biology of estrogens in skin: implications for skin aging. Exp Dermatol. 2006;15:83–94. doi: 10.1111/j.1600-0625.2005.00377.x. [DOI] [PubMed] [Google Scholar]
- Verdier-Sevrain S, Yaar M, Cantatore J, et al. Estradiol induces proliferation of keratinocytes via a receptor mediated mechanism. Faseb J. 2004;18:1252–4. doi: 10.1096/fj.03-1088fje. [DOI] [PubMed] [Google Scholar]
- Wade TR, Wade SL, Jones HE. Skin changes and diseases associated with pregnancy. Obstet Gynecol. 1978;52:233–42. [PubMed] [Google Scholar]
- Webb P, Nguyen P, Valentine C, et al. The estrogen receptor enhances AP-1 activity by two distinct mechanisms with different requirements for receptor transactivation functions. Mol Endocrinol. 1999;13:1672–85. doi: 10.1210/mend.13.10.0357. [DOI] [PubMed] [Google Scholar]
- Webb P, Nguyen P, Kushner PJ. Differential SERM effects on corepressor binding dictate ERα activity in vivo. J Biol Chem. 2003;278:6912–20. doi: 10.1074/jbc.M208501200. [DOI] [PubMed] [Google Scholar]
- Weinstock MA. Epidemiologic investigation of nonmelanoma skin cancer mortality: the Rhode Island Follow-Back Study. J Invest Dermatol. 1994;102:6S–9S. doi: 10.1111/1523-1747.ep12385735. [DOI] [PubMed] [Google Scholar]
- Wolff EF, Narayan D, Taylor HS. Long-term effects of hormone therapy on skin rigidity and wrinkles. Fertil Steril. 2005;84:285–8. doi: 10.1016/j.fertnstert.2004.12.062. [DOI] [PubMed] [Google Scholar]