Abstract
Transepithelial migration of polymorphonuclear neutrophils (PMN) plays a crucial role in inflammatory conditions of the intestine, such as inflammatory bowel diseases. Hypertonic saline (HS) exerts various inhibitory effects on PMN function. We hypothesized that HS could inhibit transepithelial migration of PMN and thereby prevent inflammatory events in experimental colitis. Isolated human PMN were treated with HS (40 mM), and their transmigration across a monolayer of T84 epithelial cells was induced by N-formyl-methionyl-leucyl-phenylalanine. Monolayer disruption was assessed by monitoring changes in transepithelial conductance in an Ussing chamber. Colitis in mice was induced by oral administration of dextran sulfate sodium (DSS). Animals were treated with 4 or 8 ml/kg of 7.5% saline intraperitoneally two times daily for 7 days. Controls received equivalent volumes of normal saline (NS, n = 6) or no intraperitoneal treatment (DSS, n = 12). The severity of inflammation was evaluated based on disease activity index and histology score. HS treatment of PMN in vitro significantly reduced cell migration and the disruption of T84 monolayers compared with untreated control cells (n = 5, P < 0.05). This effect of HS was dose dependent. HS treatment in vivo also reduced colitis-induced gut tissue damage, as indicated by an improved histology score compared with the NS and DSS groups. We conclude that HS inhibits transepithelial migration of PMN in vitro and gut tissue damage in vivo in a mouse model of colitis. Thus HS may have clinical value to reduce PMN-mediated intestinal damage.
Keywords: neutrophil activation, inflammatory bowel disease, hypertonic saline solution, inflammation, epithelium
although inflammatory diseases of the intestine are etiologically diverse, many of these diseases express similar histological features. Accumulation of neutrophils [polymorphonuclear neutrophils (PMN)] in the lamina propria is a major hallmark of the acute inflammatory response in disorders ranging from inflammatory bowel disease (IBD) to shock-induced gut dysfunction. In IBD, PMN also transmigrate from the lamina propria in the gut lumen. By measuring fecal excretion of leukocytes labeled with indium-111, migration of PMN from the circulation in the diseased intestine has been estimated to be increased by at least 10-fold in IBD patients (23). PMN are rapidly recruited from the bloodstream by cell adhesion to the vascular endothelium, transendothelial migration, and chemotaxis to the local inflammatory site. After their recruitment to such sites, PMN exert cytotoxic effects that can damage gut tissues via the release of cytotoxic mediators. A complex assortment of these cytotoxic agents, including reactive oxygen metabolites and proteolytic enzymes, is released during this inflammatory process and causes host tissue damage. In addition, PMN can further modulate such inflammatory processes by immunoregulatory functions they exert via the secretion of several important cytokines, including interleukin (IL)-1, IL-1 receptor antagonist, IL-6, IL-8, tumor necrosis factor-α, granulocyte colony-stimulating factor and granulocyte macrophage colony-stimulating factor (15).
The transepithelial migration of PMN in response to chemotactic stimuli depends on expression of integrins such as CD11b/18 and results in a disruption of epithelial barriers as demonstrated by transient decreases in transepithelial resistance in various in vitro epithelial models (17, 20). Several studies have shown that this decrease in epithelial barrier function is directly related to the number of transmigrating PMN. The integrity of the intestinal epithelium is crucial for preventing translocation of luminal pathogens in the lamina propria. Dysfunction of the epithelial barrier, therefore, could initiate an uncontrolled mucosal immune response. Thus the transepithelial migration of PMN, associated with a decrease in epithelial resistance, is likely an important step in the inflammatory cascade associated with IBD. Similar processes may be responsible for gut barrier dysfunction in hemorrhagic shock where hypoxemia causes the impairment of epithelial barrier function that is followed by translocation of luminal contents and stimulation of subepithelial immunocytes (9). Recent evidence suggests that cytotoxic mediators from the gut carried in the mesenteric lymphatics mediate secondary tissue injury (e.g., in the lungs) in hemorrhagic shock conditions (16).
Different strategies have been developed to inhibit intestinal inflammatory responses in attempts to treat IBD. These strategies range from the use of anti-inflammatory drugs to immune modulating substances. However, the efficacy of these treatments is limited, and some are associated with considerable side effects. Hence, additional treatment strategies for IBD are needed. Recently, there has been renewed interest in the use of hyperosmolar resuscitation fluids in shock therapy. Infusion of hypertonic saline (HS) results in a rapidly increased intravascular blood volume due to osmotically induced fluid shifts from the extravascular compartments to the vascular bed (28). Hypertonic fluids have been shown to benefit patients with hypovolemic shock and head injury (2, 25, 29). Moreover, HS also modulates PMN function (reviewed in Ref. 26). Treatment of isolated PMN with HS attenuates several cytotoxic responses, including the release of proteolytic enzymes and reactive oxygen metabolites or upregulated β2-integrin expression induced by N-formyl-methionyl-leucyl-phenylalanine (fMLP) (6, 12, 27). In a dorsal skinfold chamber model, postischemic leukocyte rolling and sticking were significantly reduced when animals were treated with HS (18). Recent studies have shown that the underlying mechanisms involve the release of cellular ATP, formation of extracellular adenosine, and the stimulation of A2a adenosine receptors that increase intracellular cAMP and thereby block PMN activation (4, 5, 19).
This work suggests that HS-treatment may reduce PMN-associated tissue damage in IBD. We therefore sought to determine if treatment of isolated PMN with HS could inhibit disruption of epithelial barrier function and gut tissue damage in a mouse model of colitis.
MATERIALS AND METHODS
Isolation of PMN.
Heparinized blood was obtained from healthy donors. After sedimentation in 5% dextran T500, red blood cells were discarded, and PMN were isolated from the remaining fraction using gradient centrifugation with 55 and 73% Percoll. The resulting PMN preparation was washed two times with Hanks’ balanced salt solution (HBSS). Cell viability was >98%, as determined by trypan blue dye exclusion. After isolation, cells were resuspended in HBSS and adjusted to a concentration of 107 cells/ml. For transmigration studies, PMN were incubated with different concentrations of HS (ranging from 5 to 40 mM). After 20 min, cells were washed and resuspended in HBSS.
T84 cell monolayers.
T84 human colonic epithelial cells (passages 23–28) were seeded on 0.6-cm2 Millipore polycarbonate filters and grown in Dulbecco's modified Eagle's medium-F-12 medium supplemented with 5% newborn calf serum until they formed confluent monolayers with resistances >1,500 Ω·cm2, using methods that have been described previously (1).
Electrophysiological studies of PMN transepithelial migration.
Plates containing confluent T84 monolayers on filter supports were placed horizontally on a heating block at 37°C, and spontaneous potential differences generated across the cell monolayers were negated by the application of short-circuit current via agar bridges. Isolated PMN (400 μl of a cell suspension containing 1 × 107 cells/ml) were carefully placed on the apical side of the monolayer. To establish a chemotactic gradient, fMLP (10−6 M) was added to the basolateral compartment. Changes in monolayer conductance (the inverse measure of transepithelial resistance) associated with PMN transmigration were then monitored for 70 min.
Fluorescence-activated cell sorter analysis.
CD11b expression was analyzed using flow cytometry and single color staining. Briefly, PMN (0.5 × 106 cells) were incubated with human serum (10 μl/100 μl cell suspension) to block nonspecific binding. The cells were then incubated with a monoclonal mouse anti-CD11b antibody (Serotec, Oxford, UK). After two washings, PMN were incubated with a phycoerythrin-labeled rabbit anti-mouse IgG antibody (Accurate, Westbury, NY). Cells were washed, and fluorescence was analyzed and reported as the mean channel fluorescence using a FACScalibur (BD Biosciences, San Jose, CA). An isotype-matched mouse IgG antibody (Accurate) served as the negative control. All steps were performed at 4°C.
Induction of colitis.
Male Balb/c mice weighing 18–20 g were used for induction of colitis. All studies received approval from the University of California San Diego Committee on Investigations Involving Animal Subjects. Animals were housed in a room maintained at 22°C and kept on standard laboratory pellet food. Mice were treated ad libitum with 5% dextran sulfate sodium (DSS), molecular weight 40,000 (ICN Biomedicals, Aurora, OH), in the drinking water for 7 days. The treatment groups received 4 or 8 ml/kg of 7.5% saline intraperitoneally two times daily. One control group was injected with comparable volumes of normal saline (NS), whereas another control group received no intraperitoneal treatment. Disease was determined according to a disease activity index (DAI), which reflects weight loss, stool consistency, and positive hemoccult or rectal bleeding (7). The DAI was calculated daily. After 7 days, the mice were killed, and colonic tissue was obtained for histological grading. Colonic tissue samples were fixed in formalin, embedded in paraffin, and stained with hematoxylin and eosin. Histological grading was performed by an experienced pathologist (Wolf) who was blinded to the treatment modalities. A score from 0 to 4 was used, reflecting epithelial architecture and subepithelial leukocyte infiltration (Table 1).
Table 1.
Scoring system used to grade histological severity of colitis in mice treated with DSS
| 0 | 1 | 2 | 3 | 4 | |
|---|---|---|---|---|---|
| A) Epithelium | Intact | Erosion | Ulcer | Multiple ulcers | Segmental necrosis |
| B) Density of leukocyte infiltration | None | Slight | Marked | Strong | Massive |
| C) Depth of infiltration | None | Subepithelial | Confined to the lamina propria | Muscularis involved | Serosa involved |
DSS, dextran sulfate sodium. *An overall score was calculated as follows: (A + B + C)/3.
Statistical analyses.
All data are expressed as means ± SE for a series of n experiments. Student's t-test or ANOVA with post hoc analysis (least-significant difference test) was used to compare mean values as appropriate; P values <0.05 were considered to be significant.
RESULTS
Treatment of PMN with HS inhibits the disruption of T84 intestinal epithelial cell monolayers.
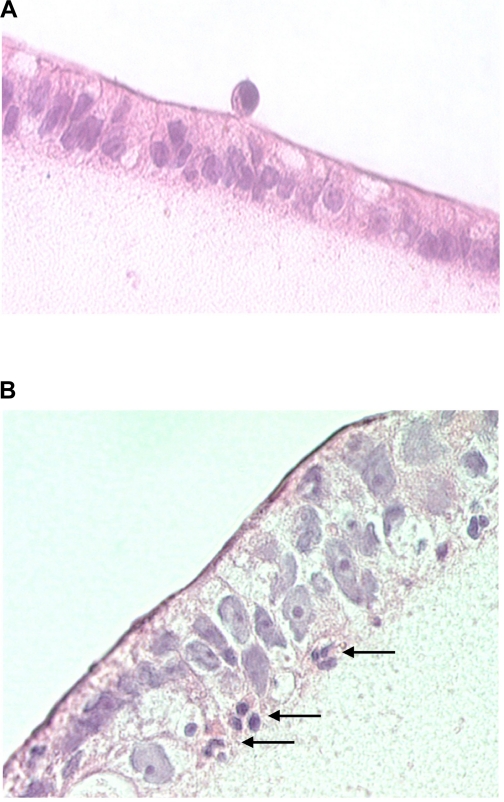
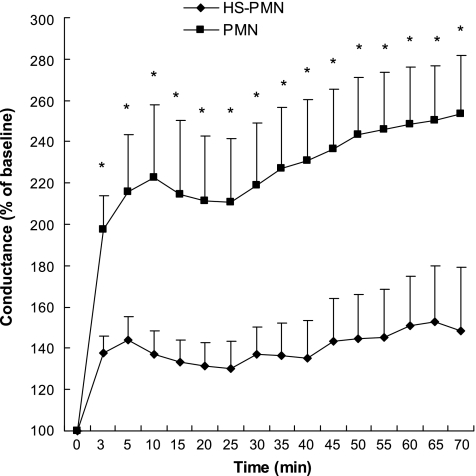
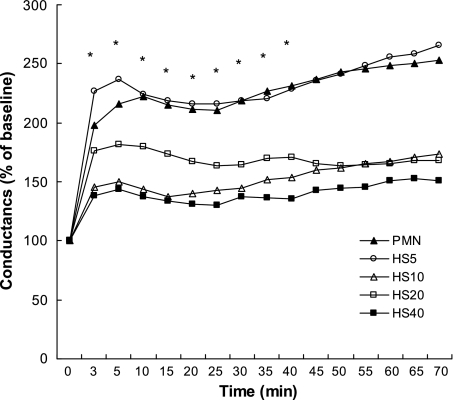
Figure 1 shows representative micrographs of T84 cell monolayers before and after PMN transmigration, resulting in a disruption of the continuity of the epithelial cell lining by interposed PMN. As shown in Fig. 2, the conductance of T84 monolayers essentially doubled 3 min after adding PMN (4 × 106) apically and fMLP (10−6 M) basolaterally. The conductance continued to increase over 70 min, suggesting ongoing damage to the epithelial monolayer by transmigrating PMN. However, pretreatment of PMN with HS (to increase the extracellular tonicity by 40 mM) for 20 min before addition of the PMN to T84 monolayers significantly inhibited damage to the T84 epithelial cell monolayers. This effect of HS was concentration dependent over a range of 5–40 mM (Fig. 3), which represents clinically feasible levels of hypertonicity. These data combined with previous reports that HS can block PMN chemotaxis (12) suggest that decreased PMN transmigration is responsible for the decrease in T84 cell damage.
Fig. 1.
Histological sections of T84 cell monolayers incubated with neutrophils [polymorphonuclear neutrophils (PMN)]. In A, PMN were placed on the apical side of T84 monolayers. B: appearance 30 min after adding N-formyl-methionyl-leucyl-phenylalanine (fMLP) to the basolateral side. Marked epithelial transmigration of PMN is apparent (arrows).
Fig. 2.
Hypertonic saline (HS) pretreatment of PMNs inhibits increases in epithelial conductance (G) associated with PMN transmigration. Isolated neutrophils (4 × 106 cells) were incubated with HBSS (PMN) or HS (HS-PMN, 40 mM hypertonicity) for 20 min and then washed with HBSS and carefully placed on the apical side of confluent T84 monolayers. A chemotactic gradient was established by adding fMLP (10−6 M) to the basolateral compartment. Changes in monolayer conductance were then monitored for 70 min. Data are expressed as a percentage of baseline conductance. *Significant differences between cells in the presence vs. absence of HS. *P < 0.05 by t-test, means ± SE, n = 5.
Fig. 3.
The inhibitory effect of HS on PMN transmigration is dose dependent. Neutrophils were incubated with different doses of HS (5, 10, 20, and 40 mM beyond isotonicity) or HBSS (PMN alone). *Time points where the responses were significantly different among the groups. *P < 0.05 vs. PMN alone by ANOVA, means for n = 3–5. Error bars have been omitted for clarity.
Inhibition of PMN transmigration by HS is not caused by downregulation of CD11b.
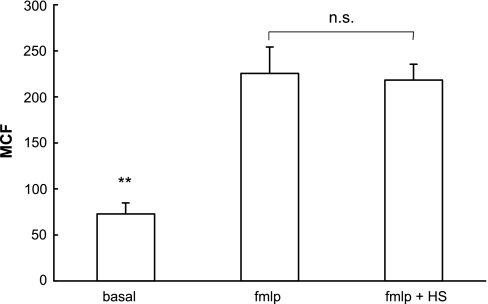
To investigate whether the inhibitory effect of HS on PMN transepithelial migration was due to downregulation of the cell-surface expression of CD11b, we stimulated PMN with fMLP at the same concentration used in transmigration studies (i.e., 10−6 M) in the absence or presence of HS treatment and determined CD11b expression by fluorescence-activated cell sorter analysis. fMLP stimulation resulted in a significant increase in CD11b expression compared with unstimulated PMN. This effect, however, was not attenuated by either simultaneous treatment of PMN with fMLP and HS (Fig. 4), or by pretreatment of PMN with 40 mM HS before addition of fMLP (data not shown). These data suggest that HS inhibits epithelial transmigration by affecting PMN responses other than CD11b expression. The results also indicate that the effect of HS on PMN function documented above is likely not a reflection of nonspecific cytotoxicity.
Fig. 4.
The ability of HS to inhibit responses associated with PMN transmigration is not caused by downregulation of CD11b. Isolated PMN were incubated in HS (40 mM hypertonicity) or HBSS and stimulated with fMLP (10−6 M) for 20 min. CD11b expression on unstimulated cells (PMN) was compared with that on fMLP-stimulated PMN (fMLP) or PMN coincubated with fMLP and HS (fMLP + HS), as determined by fluorescence-activated cell sorter (FACS) analysis. MCF, mean channel fluorescence. *Significant differences between both fMLP-treated groups. **P < 0.01 by ANOVA, means ± SE, n = 5.
HS treatment improves the clinical and histological activity of DSS colitis in Balb/c mice.
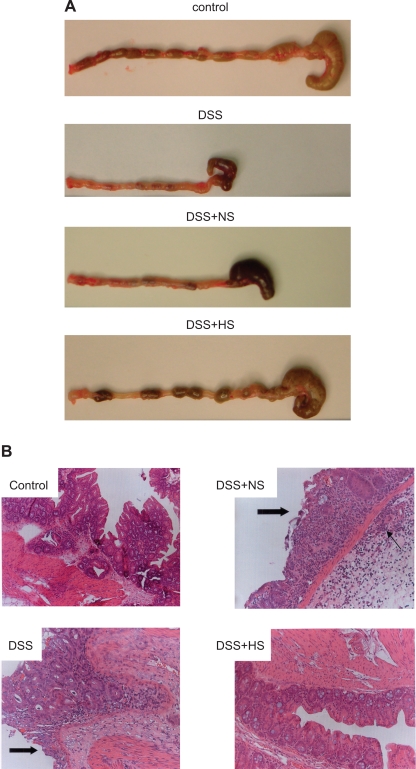
To determine whether the effects of HS on PMN-epithelial interactions observed in vitro were paralleled in an in vivo setting, we studied the effect of HS treatment in a well-established murine colitis model. As expected, administration of DSS to mice for 7 days caused severe colitis (Fig. 5) accompanied by an increase in the DAI (Fig. 6).
Fig. 5.
Effect of HS or normal saline (NS) treatment on macroscopic and histological appearance of dextran sulfate sodium (DSS)-induced colitis in mice. Treatment with 7.5% HS (4 and 8 ml/kg), bid ip, but not with NS resulted in a marked reduction of inflammatory tissue damage in DSS mice. Healthy mice served as controls. As shown in A, after 7 days of DSS treatment, the colon was hyperemic with gross blood in the cecum and a lack of formed stool pellets. In HS-treated DSS mice, only mild hyperemia and no gross bleeding was observed. Stool pellets were seen in the colonic lumen of HS-treated mice and controls. Colons from mice treated with DSS + NS, on the other hand, still showed evidence of inflammation and poorly formed stools. B: corresponding histological appearances. In colonic sections of mice receiving DSS alone, severe epithelial damage (block arrow) and dense PMN infiltration was seen (note infiltrating PMN in the submucosa). A similar appearance was seen in NS-treated mice. In HS-treated mice, only slight inflammation was seen (hematoxylin and eosin staining).
Fig. 6.
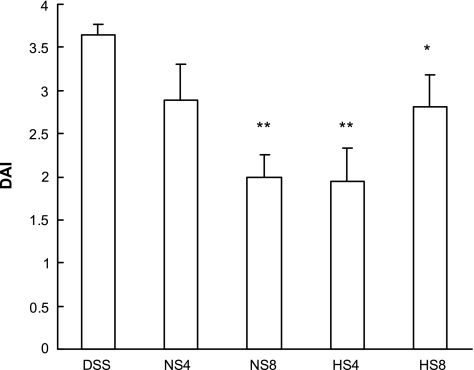
Effect of HS and NS on disease activity index (DAI) in mice with DSS-induced colitis. Clinical activity in DSS colitis was improved by treatment with 4 ml/kg (HS4, n = 6) and 8 ml/kg hypertonic saline (HS8, n = 10) but also by fluid replacement therapy with 8 ml/kg normal saline (NS8, n = 10), compared with DSS mice without further treatment (DSS, n = 12). Statistical analysis was done by ANOVA. *P < 0.05 and **P < 0.01 compared with DSS alone. Values are means ± SE.
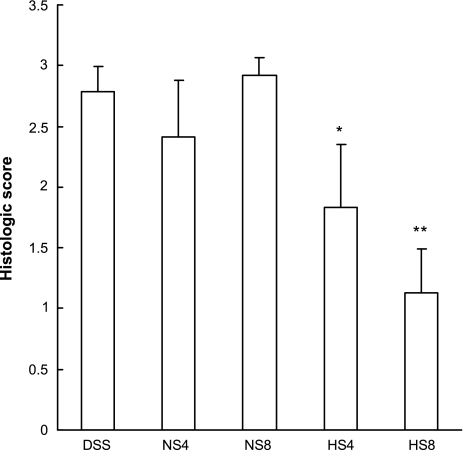
In keeping with our findings with HS in vitro, treatment with 7.5% HS at a dose of 4 or 8 ml/kg resulted in a significant reduction in intestinal tissue damage. This protective effect was evident macroscopically (Fig. 5A). Histological grading of tissue injury revealed that, in animals receiving DSS in combination with HS or NS, only HS had a significant beneficial effect at both concentrations (Fig. 7). These data suggest that the ability of HS to diminish PMN transmigration and perhaps other PMN functions may ameliorate their deleterious effect on epithelial function. Similarly, while treatment with 7.5% HS at a dose of 4 ml/kg resulted in a significant reduction in DAI, such effects were not seen with administration of 4 ml/kg NS. However, when higher volumes (8 ml/kg) of HS or NS were administered, the clinical benefit of NS was superior to that of HS. This implies that at least a part of the overall ability to reduce clinical signs of disease may relate to improved hydration of the animals and/or that toxic side effects may occur at the higher dose of HS.
Fig. 7.
Effect of HS and NS on histological scores in mice with DSS-induced colitis. The histological score in DSS colitis was improved by treatment with 4 ml/kg HS (HS4, n = 6) and 8 ml/kg HS (HS8, n = 10), but not with 4 ml/kg NS (NS4, n = 6) or 8 ml/kg NS (NS8, n = 10). Statistical analysis was done by ANOVA. *P < 0.05 and **P < 0.0001 vs. DSS mice without further treatment (DSS, n = 12). Values represent means ± SE.
DISCUSSION
Intestinal inflammation underlies a diverse group of digestive diseases that are often accompanied by migration of neutrophils in the intestinal lumen. HS, on the other hand, has recently emerged as a simple, inexpensive, and largely nontoxic means to modulate PMN function. In the current study, we examined whether HS could alter the interactions of PMN with intestinal epithelial cells and whether this might have beneficial consequences in the setting of colitis. In fact, in addition to other inhibitory effects of HS on PMN function described previously, we could demonstrate a HS-mediated inhibition of PMN transmigration across epithelial monolayers in vitro. Rosengren et al. (21) demonstrated in a Boyden chamber system that hyperosmolar shrinking decreases PMN migration toward fMLP (21). Similarly, Junger et al. (12) showed that HS reduces PMN chemotaxis induced by zymosan-activated serum. However, and significantly, pretreatment with HS was also able to abrogate inflammatory responses and tissue injury in a mouse model of colitis.
Osmotic stress is associated with a rearrangement of various components of the cytoskeleton. DiCiano et al. provided evidence for a hypertonicity-induced accumulation of F-actin, cortactin, and increased myosin light chain phosphorylation (10, 11). Newly polymerized F-actin localizes predominantly under the cell membrane in the form of a thick ring that persists as long as hypertonicity is maintained. The authors speculate that this mechanism could offer mechanical protection against the deleterious effects of cell shrinkage. On the other hand, this response to HS results in “immobilization” and impaired migratory functioning of PMN. The ability of HS to modulate transepithelial migration of PMN could also be due to altered expression of cell-surface adhesion molecules. However, in contrast to the findings of Thiel et al. (27), CD11b expression was not inhibited by HS in our in vitro setting. The apparent discrepancy between our findings and the prior work is possibly attributable to the relatively high concentration of the chemoattractant (i.e., fMLP 10−6 M) that was used here. Nevertheless, the findings underscore the fact that HS likely has multifaceted effects on PMN that impair their ability to participate in inflammatory responses.
Because transmigration of PMN is considered as a crucial event in the pathophysiology of IBD, the inhibition of PMN transmigration suggested that HS might also exert beneficial effects in the setting of intestinal inflammation. Accordingly, we were able to demonstrate, in an in vivo model of murine colitis, that HS pretreatment indeed results in a significant reduction of various indexes of gut tissue damage. Our findings demonstrate that HS exerts potent anti-inflammatory effects, resulting in decreased infiltration of PMN in the intestinal mucosa. At the lower dose, these effects of HS were paralleled by clinical signs of disease improvements.
In clinical trials investigating the efficacy of HS as a resuscitation fluid for the treatment of hemorrhagic shock, HS is typically administered intravenously as a single dose of 4 ml/kg of 7.5% NaCl solutions. In an attempt to obtain maximal anti-inflammatory effects, we tested this and a higher dose of HS in the studies presented here. Repeated peritoneal administration of HS at a dose of 4 ml/kg proved to be both clinically and histologically superior to an equivalent volume of NS. NS administered at this dose had no significant effects on either DAI or the histological score used to assess gut tissue inflammation and damage. However, at a dose of 8 ml/kg (a dose comparable to 1,120 ml/day in a 70-kg adult), the effect of HS on DAI was similar to that of NS, even though HS but not NS at this dose clearly prevented histological signs of inflammation and gut tissue injury. This may suggest that, if the osmotic load exceeds a certain amount, adverse effects may occur that counterbalance the protection provided by the ability of HS to prevent PMN infiltration and damage of gut tissues. Such adverse effects may be related to metabolic side effects like hyperchloremic acidosis. As shown by Scheingraber et al. (24), infusion of 30 ml·kg−1·h−1 (total amount 70 ml/kg) of 0.9% saline leads to metabolic acidosis, associated with hyperchloremia. This dose is comparable to 8.4 ml/kg of HS and therefore approximates the dose administered in our high-dose group. Hyperosmolarity could also lead to an increased uptake of DSS and thereby aggravated colitis. Because most cell membranes are freely permeable to water, an increase in extracellular osmolarity may result in a shift of water from the intracellular to the extracellular compartment, leading to activation of the osmoregulatory system. Changes in osmolarity as small as 2–3% increase water uptake in adults (8). Likewise, it was demonstrated that subcutaneous injections of HS result in robust water drinking by mice (22). In addition, increased diuresis was reported after the administration of hypertonic solutions (28). Therefore, in the HS group, a higher consumption of DSS provided through the drinking water may have occurred. Conversely, fluid replacement by NS may have reduced the intake of DSS-water. Thus a potential methodical drawback of the DSS colitis model used in our studies may have led to greater amounts of DSS ingested in the HS groups. Despite this, however, we found reduced PMN infiltration and tissue damage in the HS groups compared with all other groups. These findings along with the considerations described above suggest that grading of the clinical signs of disease with DAI may not fully correspond with that actual gut tissue injury inflicted by PMN.
In clinical studies addressing hypovolemic conditions, HS has been administered intravenously (14). In this study, we chose the intraperitoneal route for administration of HS or NS for practical reasons. It has been shown that intraperitoneal HS infusion causes an increase in plasma osmolarity comparable to that obtained through the intravenous route (3). Similarly, in emergency settings when rapid intravenous access cannot be obtained (e.g., in combat casualties) the intraosseous route has been suggested, and entry of HS in the vascular space at a rate comparable to that achieved with HS administration via the intravenous route has been demonstrated (13).
It is likely that HS administration may affect the functions of other cell populations, including endothelial and epithelial cells. Interestingly, hyperosmolar activation of the Na+-K+-2Cl− cotransporter (NKCC) was demonstrated in vascular endothelial cells (25). We observed similar effects in T84 epithelial cells (data not shown). Because chloride secretion is markedly downregulated in the setting of colonic inflammation, and may contribute to a loss of barrier function, an upregulation of NKCC activity by HS could help to restore epithelial secretory function (23, 41).
In summary, the data presented here demonstrate for the first time inhibitory effects of HS on PMN transmigration across model epithelia in vitro and on PMN-induced gut tissue inflammation in vivo. The underlying mechanisms may be related to suppressive effects of HS on PMN chemotaxis or other PMN functions, including superoxide generation and release of granule contents (5, 6, 17, 26). Our findings support the idea that hypertonic fluids exert inhibitory effects on PMN function that may be beneficial in inflammatory conditions of the intestine, such as IBD, or in patients whose intestinal integrity has been compromised by shock/trauma. Additional work will be required to evaluate if HS can be useful as an adjunctive therapy with conventional pharmacological agents such as steroids to control IBD flares.
GRANTS
This work was supported by a research grant of the Austrian Science Fund to W. Tillinger and grants from the National Institute of Health to K. E. Barrett (DK-28305) and to W. G. Junger (GM-60475).
Acknowledgments
We thank Glenda Wheeler for administrative assistance and Andrea Ableidinger for graphical assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Barrett KE, Huott PA, Shah SS, Dharmsathaphorn K, Wasserman SI. Differing effects of apical and basolateral adenosine on the colonic epithalial cell line T84. Am J Physiol Cell Physiol 256: C197–C203, 1989. [DOI] [PubMed] [Google Scholar]
- 2.Battison C, Andrews PJ, Graham C, Petty T. Randomized, controlled trial on the effect of a 20% mannitol and a 7.5% saline/6% dextran solution on increased intracranial pressure after brain injury. Crit Care Med 33: 196–202, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Caston-Balderrama A, Nijland MJM, McDonald TJ, Ross MG. Central Fos expression in fetal and adult sheep after intraperitoneal hypertonic saline. Am J Physiol Heart Circ Physiol 276: H725–H735, 1999. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, Hashiguchi N, Yip L, Junger WG. Hypertonic saline enhances neutrophil elastase release and degranulation through activation of P2 and A3 receptors. Am J Physiol Cell Physiol 290: C1051–C1059, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Shukla A, Namiki S, Insel PA, Junger WG. A putative osmoreceptor system that controls neutrophil function through the release of ATP, its conversion to adenosine, and activation of A2 adenosine and P2 receptors. J Leukocyte Biol 76: 245–253, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Ciesla DJ, Moore EE, Zallen G, Biffl WL, Silliman CC. Hypertonic saline attenuation of polymorphonuclear neutrophil cytotoxicity: timing is everything. J Trauma 48: 388–95, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Cooper HS, Murthy SNS, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest 69: 238–249, 1993. [PubMed] [Google Scholar]
- 8.Davison JM, Gilmore EA, Durr J, Robertson GL, Lindheimer MD. Altered osmotic thresholds for vasopressin secretion and thirst in human pregnancy. Am J Physiol Renal Fluid Electrolyte Physiol 246: F105–F109, 1984. [DOI] [PubMed] [Google Scholar]
- 9.Deitch EA Multiple organ failure: pathophysiology and potential future therapy. Ann Surg 216: 117–134, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Ciano C, Nie Z, Szaszi K, Lewis A, Uruno T, Zhan X, Rotstein OD, Mak A, Kapus A. Osmotic stress-induced remodeling of the cortical cytoskeleton. Am J Physiol Cell Physiol 283: C850–C865, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Di Ciano-Oliveira C, Sirkomany G, Szaszi K, Arthur WT, Masszi A, Peterson M, Rotstein OD, Kapus A. Hyperosmotic stress activates Rho: differential involvement in Rho kinase-dependent MLC phosphorylation and NKCC activation. Am J Physiol Cell Physiol 285: C555–C566, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Junger WG, Hoyt DB, Davis RE, Herdon-Remelius C, Namiki S, Junger H, Loomis W, Altman A. Hypertonicity regulates the function of human neutrophils by modulating chemoattractant receptor signaling and activating mitogen-activated protein kinase p38. J Clin Invest 101: 2768–2779, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kramer GC, Walsh JC, Hands RD, Perron PR, Gunther RA, Mertens S, Holcroft JW, Blaisdell FW. Resuscitation of hemorrhage with intraosseous infusion of hypertonic saline/dextran. Braz J Med Biol Res 22: 283–286, 1989. [PubMed] [Google Scholar]
- 14.Kreimeier U, Mesmer K. Small-volume resuscitation: from experimental evidence to clinical routine. Advantages and disadvantages of hypertonic solutions. Acta Anaesthesiol Scand 46: 625–638, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Lloyd AR, Oppenheim JJ. Poly's lament: the neglected role of the polymorphonuclear neutrophil in the afferent limb of the immune response. Immunol Today 13: 169–172, 1992. [DOI] [PubMed] [Google Scholar]
- 16.Magnotti LJ, Upperman JS, Xu DZ, Lu Q, Deitch EA. Gut-derived mesenteric lymph but not portal blood increases endothelial cell permeability and promotes lung injury after hemorrhagic shock. Ann Surg 228: 518–527, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nash S, Stafford J, Madara JL. Effects of polymorphonuclear leukocyte transmigration on the barrier function of cultured intestinal epithelial monolayers. J Clin Invest 80: 1104–1113, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nolte D, Bayer M, Lehr HA, Becker M, Krombach F, Kreimeier U, Messmer K. Attenuation of postischemic microvascular disturbances in striated muscle by hyperosmolar saline dextran. Am J Physiol Heart Circ Physiol 263: H1411–H1416, 1992. [DOI] [PubMed] [Google Scholar]
- 19.Orlic T, Loomis WH, Shreve A, Namiki S, Junger WG. Hypertonicity increases cAMP in PMN and blocks oxidative burst by PKA-dependent and -independent mechanisms. Am J Physiol Cell Physiol 282: C1261–C1269, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Parkos CA, Delp C, Arnaout MA, Madara JL. Neutrophil migration across a cultured intestinal epithelium. Dependence on a CD11b/CD18-mediated event and enhanced efficiency in physiological direction. J Clin Invest 88: 1605–1612, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosengren S, Henson PM, Worthen GS. Migration-associated volume changes in neutrophils facilitate the migratory process in vitro. Am J Physiol Cell Physiol 267: C1623–C1632, 1994. [DOI] [PubMed] [Google Scholar]
- 22.Rowland NE, Fregly MJ. Characteristics of thirst and sodium appetite in mice (Mus musculus). Behav Neurosci 102: 969–974, 1988. [DOI] [PubMed] [Google Scholar]
- 23.Saverymuttu SH, Peters AM, Lavender JP, Pepys MB, Hodgson HJ, Chadwick VS. Quantitative faecal indium-111 labelled leucocyte excretion in assessment of disease activity in Crohn's disease. Gastroenterology 85: 1333–1339, 1983. [PubMed] [Google Scholar]
- 24.Scheingraber S, Rehm M, Sehmisch C, Finsterer U. Rapid saline infusion produces hyperchloremic acidosis in patients undergoing gynecologic surgery. Anesthesiology 90: 1265–1270, 1999. [DOI] [PubMed] [Google Scholar]
- 25.Shi HP, Deitch EA, Da Xu Z, Lu Q, Hauser CJ. Hypertonic saline improves intestinal mucosa barrier function and lung injury after trauma-hemorrhagic shock. Shock 17: 496–501, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Shukla A, Hashiguchi N, Chen Y, Coimbra R, Hoyt DB, Junger WG. Osmotic regulation of cell function and possible clinical applications. Shock 21: 391–400, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Thiel M, Buessecker F, Eberhardt K, Chouker A, Setzer F, Kreimeier U, Arfors KE, Peter K, Messmer K. Effects of hypertonic saline on expression of human polymorphonuclear leukocyte adhesion molecules. J Leukoc Biol 70: 261–273, 2001. [PubMed] [Google Scholar]
- 28.Tollofsrud S, Tonnessen T, Skraastad O, Noddeland H. Hypertonic saline and dextran in normovolaemic and hypovolaemic healthy volunteers increases interstitial and intravascular fluid volumes. Acta Anaestesiol Scand 42: 145–153, 1998. [DOI] [PubMed] [Google Scholar]
- 29.Vassar MJ, Perry CA, Holcroft JW. Prehospital resuscitation of hypotensive trauma patients with 7.5% NaCl vs 75% NaCl with added dextran: a controlled trial. J Trauma 34: 622–632, 1993. [PubMed] [Google Scholar]