Abstract
Dietary Na+ deprivation elicits a hormonal response to promote sodium conservation and a behavioral response to increase sodium ingestion. It has generally been accepted that the former occurs within 24 h after sodium deprivation, while the latter is delayed and may not appear until as much as 10 days later. Na+ deprivation of similar duration also decreases the sensitivity of the chorda tympani nerve (CT) to NaCl, suggesting that changes in CT responses are necessary for increased NaCl intake. However, previous work from our laboratory showed that licking responses to NaCl solutions increase after only 2 days of Na+ deprivation, suggesting rapidly occurring changes in response to NaCl taste. The present experiments examined the effects of 2 days of dietary Na+ deprivation on CT responses to NaCl and patterns of NaCl consumption and found that Na+-deficient rats licked significantly more during the first NaCl intake bout compared with control rats. CT responses to NaCl were reduced at all concentrations after brief Na+ deprivation compared with Na+-replete control rats and did not decrease further with prolonged (10 days) dietary Na+ deficiency. Moreover, amiloride, which suppressed CT responses to NaCl by ∼30% in control rats, had virtually no effect on CT responses in Na+-deprived rats. Thus, 2 days of Na+ deprivation is sufficient to alter patterns of ingestion of concentrated NaCl and to reduce gustatory responses to NaCl. Furthermore, changes in gustatory responses to NaCl during dietary Na+ deprivation may involve the amiloride-sensitive component of the CT.
Keywords: salt appetite, gustatory processing, chorda tympani nerve
sodium (Na+) is an essential mineral required in bodily processes including fluid balance, neural conduction, cell membrane transport, muscle contraction, enzymatic function, and blood pressure maintenance. Given its importance in all these functions, and given that sodium is not synthesized within the body, it is not surprising that body Na+ levels are very tightly regulated. In fact, changes in Na+ levels lead to numerous compensatory hormonal, physiological, and behavioral responses. For example, dietary Na+ deprivation activates the renin-angiotensin system, which in turn increases aldosterone secretion, thereby promoting Na+ conservation (10, 12). Na+ deprivation also results in the behavioral phenomenon known as salt appetite, which is characterized by an exaggeration of the natural preference for isotonic NaCl solutions and a willingness to ingest normally avoided, highly concentrated NaCl solutions, thereby replenishing body Na+ (8, 16, 28, 29, 34).
While 24 h of maintenance on a Na+-deficient diet is sufficient to activate hormonally mediated physiological responses, behavioral responses appear to be delayed, with 8–10 days of dietary Na+ deprivation necessary to increase intake of a hypertonic NaCl solution (12, 15, 22, 37). An explanation for the temporal disconnect between these two biologically relevant compensatory responses has been elusive. However, we recently found (15) that although 24-h intake of NaCl did not increase after 2 or 5 days of dietary Na+ deprivation, licking responses to NaCl solutions in short-term tests (10 s) increased after as little as 2 days of Na+ deprivation. These findings suggest that there are rapidly occurring changes in NaCl taste responses that intake tests, which typically last hours or days, lack the resolution to detect.
Obviously, changes in taste-mediated behavioral responses involve both the central and peripheral nervous systems; nonetheless, numerous studies of changes in NaCl taste responses during body Na+ imbalance have focused on the chorda tympani nerve (CT), one of the primary gustatory nerves that transmits NaCl information. Both adrenalectomy and Na+ depletion produced by furosemide, other experimental models of sodium appetite, reduced electrophysiological CT responses to NaCl (4, 23). Given that the firing rate of the CT is linearly related to NaCl concentration (18), the reduced responsiveness suggests a decrease in the intensity of the signal that corresponds to an increase in palatability or to a decrease in the aversive qualities of concentrated NaCl solutions (1, 11, 15). Reduced electrophysiological CT responses to NaCl also have been reported after 8- to 10-day maintenance on Na+-deficient chow (9, 11), a duration previously thought to be necessary to stimulate NaCl intake. However, in light of our recent study (15) showing that changes in behavioral taste responses occur after 2 days of dietary Na+ deprivation, we hypothesized that brief dietary Na+ deprivation is sufficient to decrease CT responses to NaCl and thereby alters behavioral responses to NaCl taste.
Accordingly, the first goal of these studies was to examine the effect of brief dietary Na+ deprivation on CT responses to NaCl. Previous research found that the reduction in CT responses following prolonged Na+ deprivation was attributable to a specific subpopulation of CT fibers (9, 11). Subsequent studies showed that these “Na+ specialists” are sensitive to amiloride, an epithelial Na+ channel (ENaC) blocker that partially inhibits both behavioral and electrophysiological responses to NaCl (3, 6, 14, 27). These observations suggest that an alteration of the amiloride-sensitive component of the CT may play a role in attenuated CT responses during dietary Na+ deprivation, as has been found in developmental studies (21). Thus, the second goal of these studies was to examine the effect of brief or prolonged dietary Na+ deprivation on CT responses to NaCl after lingual application of amiloride. Finally, we examined the pattern of hypertonic NaCl consumption after brief dietary Na+ deprivation to determine whether changes in NaCl taste responses had functional consequences in normally behaving rats.
EXPERIMENT 1
Previous studies showed blunted CT responses to NaCl in several models of sodium appetite, including prolonged dietary Na+ deprivation (4, 9, 11, 23). It has been proposed that these changes may contribute to increased sodium ingestion by decreasing the intensity of NaCl taste, particularly at high concentrations (1, 11, 15). To date, the effect of dietary Na+ deprivation for <8–10 days has not been assessed, primarily because previous behavioral studies suggested that an 8- to 10-day duration was necessary for the increase in NaCl ingestion. However, our recent study (15) strongly suggests that Na+ deprivation may cause changes in taste responses to NaCl more rapidly than was previously thought. Thus experiment 1 assessed CT sensitivity to a range of NaCl solutions after brief (2 days) dietary Na+ deprivation. In addition, to determine whether changes in CT responses to NaCl during brief or prolonged (10 days) Na+ deprivation involved the amiloride-sensitive component, we also recorded whole nerve CT responses to NaCl mixed with amiloride.
Methods
Subjects.
Male Sprague-Dawley rats (Charles River Laboratories) weighing 350–600 g at the start of the experiment were individually housed in a temperature-controlled (72°F) room and maintained on a 12:12-h light-dark cycle with lights on at 0700. Separate groups of rats were given access to ad libitum deionized water (dH2O) and maintained either on standard laboratory rodent chow (Purina no. 5001; control, n = 10) or on Na+-deficient chow (0.01% Na+; Harlan Teklad Sodium Deficient Diet TD 90228, Madison, WI) for 2 (NaD2, n = 7) or 10 (NaD10, n = 7) days before electrophysiological recording. All procedures were approved by the Florida State University Animal Care and Use Committee.
Procedure.
Whole nerve electrophysiological recordings were obtained from the CT branch of the facial nerve in urethane-anesthetized (1.5 g/kg body wt) rats with methods described previously (25, 31). Briefly, after the trachea was cannulated, the rat was placed in a nontraumatic head holder and the right CT branch was exposed by a mandibular approach. The CT was transected proximally, the perineurium was removed, and the distal portion of the cut nerve was placed on a tungsten wire electrode. A silver wire indifferent electrode was placed in muscle tissue near the base of the nerve, allowing differential amplification (×10,000) of action potentials.
Protocol.
The tongue was slightly extended and held in place with a small suture on the ventral surface. Taste stimuli made from reagent-grade chemicals dissolved in dH2O were delivered across the anterior portion of the tongue at a constant flow rate with a custom-designed computer-controlled delivery system and software (R. Henderson, Florida State University) as described previously (5, 31).
A range of NaCl concentrations (75, 150, 300, 450, 600 mM) was followed by the same NaCl concentrations mixed in the ENaC blocker amiloride hydrochloride (100 μM; Sigma, St. Louis, MO; NaCl + amiloride); both 30 mM quinine hydrochloride (QHCl) and 600 mM NaCl were presented at the beginning and end of each protocol to verify the viability of the nerve. If the response to NaCl at the end of the protocol was <85% of the initial NaCl response, the data from the recording were not included in the analysis. All taste stimuli were delivered for 10 s at 50 μl/s. Sixty-second dH2O rinses (50 μl/s) preceded and followed each taste stimulus to ensure that neural activity returned to stable baseline levels.
Amplified nerve activity was integrated with a root mean square calculation and a time constant of 150 ms. All data were stored on videotape for off-line analysis with a GW Instrument 15-μs data acquisition board and custom data analysis software. Baseline neural activity, recorded during the dH2O rinses that preceded each stimulus and averaged over 10 s, was used to calculate the area under the curve (AUC) for the integrated response to each stimulus, which also was averaged over 10 s. To control for individual differences among preparations, each response was normalized to the response to 30 mM QHCl, which was used as the standard stimulus based on our previous study (31) that showed that amiloride does not affect CT responsiveness to QHCl.
Statistical analyses.
All data are presented as group means ± SE. Amiloride suppression of the CT response to NaCl (%) at each concentration was calculated for each rat as [(normalized NaCl response − normalized NaCl + amiloride response)/normalized NaCl response] × 100.
One-way analysis of variance (ANOVA) was used to compare baseline activity as well as the average responses to QHCl. Normalized responses to NaCl (AUC) with and without amiloride were compared with a three-way (diet × drug × concentration) repeated-measures (RM) ANOVA (Statistica, Statsoft, Tulsa, OK). Amiloride suppression at each concentration was compared with a two-way (diet × concentration) RM ANOVA. Pairwise comparisons of statistically significant (P < 0.05) main effects or interactions were evaluated with Newman-Keuls tests.
Results
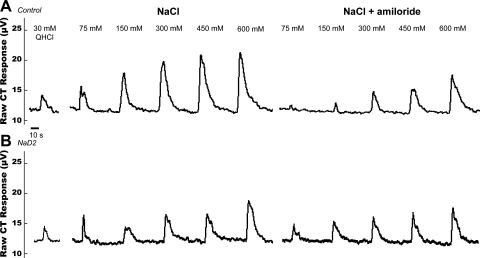
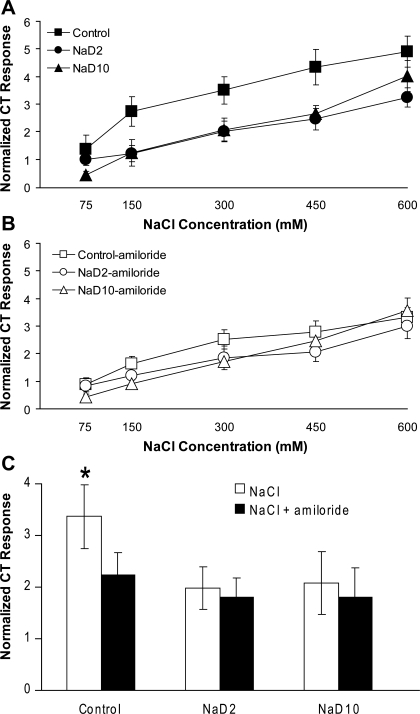
Representative traces of electrophysiological CT activity (μV) are shown in Fig. 1. Figure 2 shows normalized CT responses (AUC) to NaCl (Fig. 2A) and to NaCl + amiloride (Fig. 2B) at each concentration for control, NaD2, and NaD10 rats. CT responses depended on the main effect of NaCl concentration [F(4,84) = 94.22, P < 0.01] and of drug [F(1,21) = 14.17, P < 0.01]. There also was a significant diet × drug interaction [F(2,21) = 5.26, P < 0.01]. Post hoc analyses of this interaction revealed that, independent of concentration, CT responses to NaCl alone in control rats were significantly greater than those in all other conditions (Fig. 2C, P < 0.01). There were no differences in CT responses to NaCl + amiloride regardless of diet. CT responses to NaCl alone in NaD2 and NaD10 rats were similar, and, in contrast to the suppression of CT responses to NaCl by amiloride in the control group, amiloride had no effect on CT responses to NaCl in either Na+-deficient group.
Fig. 1.
Chorda tympani nerve (CT) whole nerve integrated, rectified activity (μV) in response to lingual application of quinine hydrochloride (QHCl), NaCl (75, 150, 300, 450, 600 mM), and NaCl mixed with amiloride. A: representative trace from a control rat. B: representative trace from a 2-day Na+-deprived (NaD2) rat.
Fig. 2.
Normalized CT whole nerve responses to a range of NaCl concentrations (A), to a range of NaCl concentrations mixed with amiloride (B), and averaged across concentrations (C) in control, NaD2, and 10-day Na+-deprived (NaD10) rats. Independent of concentration, mean CT responses to NaCl alone in control rats, relative to 30 mM QHCl response, were significantly greater than all other conditions (*P < 0.01).
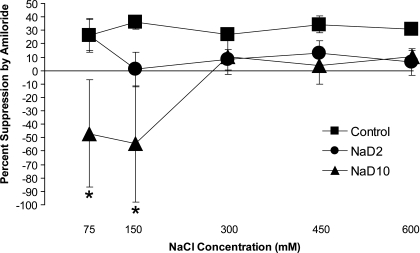
Figure 3 shows the percent suppression of CT responses to NaCl by amiloride. There was a significant diet × concentration interaction [F(8,84) = 2.61, P < 0.05]. Post hoc analyses revealed that amiloride suppression of CT responses to 75 mM and 150 mM NaCl was significantly less in NaD10 rats than in control and NaD2 rats (Fig. 3, P < 0.05). Clearly, there was substantial variability in the percent suppression, especially at lower NaCl concentrations. Nonetheless, the effect of amiloride also depended on diet [F(2,21) = 4.47, P < 0.05], with overall percent suppression significantly less in NaD10 rats than in control rats (30.9%).
Fig. 3.
Percent suppression by amiloride of CT responses to a range of NaCl concentrations in control, NaD2, and NaD10 rats. At 75 and 150 mM NaCl, amiloride suppressed CT responses significantly less in NaD10 rats compared with control and NaD2 rats (*P < 0.05).
Importantly, there were no differences among groups in raw CT activity (μV) during baseline (control = 14.31 ± 0.59, NaD2 = 14.30 ± 0.75, NaD10 = 16.01 ± 0.83; P = 0.19) or in CT responses to 10-s application of the 30 mM QHCl standard stimulus (AUC; control = 2.87 ± 0.50, NaD2 = 3.95 ± 0.84, NaD10 = 3.11 ± 0.23; P = 0.39).
Discussion
Experiment 1 revealed profound differences in CT responses to NaCl between Na+-replete and Na+-deprived animals. After brief or prolonged Na+ deprivation, CT responses to all concentrations of NaCl were reduced compared with control responses (Fig. 2). Given that the CT is highly sensitive to NaCl (11, 31) and, more specifically, that the firing rate of the CT is directly proportional to the stimulus intensity, the reduced responsiveness of the CT to NaCl during Na+ deprivation suggests a decrease in the intensity of NaCl taste at all concentrations. Furthermore, amiloride suppressed CT responses to NaCl in control rats but had less effect on CT responses to NaCl in Na+-deprived rats. These observations suggest that the amiloride-sensitive component of NaCl taste may be involved in the reduction of CT responses during Na+ deprivation. That is, the decreased CT responses may be attributable to a rapidly occurring reduction in amiloride-sensitive Na+ channels. Hence, these results support the hypothesis that brief Na+ deprivation decreases CT responses to NaCl, and we believe that the decrease in NaCl taste intensity may reduce the aversive qualities of NaCl solutions during Na+ deprivation (1, 15).
EXPERIMENT 2
While it has generally been accepted that increased NaCl intake requires 8–10 days of dietary Na+ deprivation, we recently used very short (10 s) tests and showed that taste responses to NaCl changed with as little as 2 days of dietary Na+ deprivation (15). Interestingly, prolonging the dietary exposure to Na+-deficient chow for up to 10 days did not further alter behavioral responses—the increase in licking was maximal after 2 days. These findings, in conjunction with the results of experiment 1, suggest more rapidly occurring and long-term changes in CT-mediated behavioral taste responses than have previously been suspected. However, it is unknown whether such changes are detectable only in rats that have been trained to lick during restricted access to NaCl solutions in very short tests or whether changes in taste responses also exhibit a subtle influence on NaCl consumption by rats during more naturalistic, longer tests after 2 days of Na+ deprivation. Numerous studies, including those examining NaCl taste responses in adrenalectomized rats (7), clearly demonstrate the advantages of examining the pattern of ingestive behavior, because such analysis shows, not only the amount of solution consumed, but also finer details of when and “how” the rat consumes each fluid (13, 36). Thus experiment 2 investigated the pattern of concentrated NaCl ingestion by Na+-deprived and Na+-replete rats over 24 h.
Methods
Subjects.
Male Sprague-Dawley rats, weighing 345–450 g at the start of the study, were individually housed in modified plastic cages with a metal attachment on the back of the cage to hold two drinking bottles. Rats had ad libitum access to standard laboratory rodent chow and dH2O, except as noted.
Procedure.
To ensure that the NaCl solution would not be novel during testing, rats were given 2 days of ad libitum access to 0.5 M NaCl and dH2O. Intakes were recorded after 24 h and 48 h, the NaCl solution then was removed, and rats were given either Na+-deficient chow (NaD, n = 10) or regular chow (control, n = 10). After 2 days on the diets, chow was removed from the cages and rats were given access to 0.5 M NaCl and dH2O.
Licking was recorded continuously for 24 h and analyzed with a custom computer software program (R. Henderson; Florida State University). Each time the rat's tongue contacted a drinking spout, a circuit was completed that was recorded by the computer as a lick. The computer recorded the number of licks from each drinking spout in 30-s bins. A licking bout was defined by the following parameters: 10 licks in 1 min to start a bout, 10 licks in 1 min to continue a bout, and 5 min without licking to end a bout (36, 38). For each solution, the total number of bouts, the duration of each bout, the number of licks per bout, and the interbout intervals were recorded. Total intakes of 0.5 M NaCl and dH2O were recorded after 24 h.
Statistical analyses.
Intakes of 0.5 M NaCl and dH2O before maintenance on the diets were averaged over the 2 days and compared with those after 2 days of maintenance on the diets with a three-way [diet × time (before or after Na+ deprivation) × solution] RM ANOVA.
Independent t-tests were used to compare the total number of NaCl intake bouts, the total number of water intake bouts, and the interval between the first and second NaCl bouts.
Data from the individual NaCl bouts were analyzed with a two-way (diet × bout) RM ANOVA. Preliminary examination of these data revealed that NaD rats consumed NaCl primarily during the first NaCl bout. Therefore, we calculated the average number of licks in and the average duration of the remaining NaCl bouts for each rat. These values were compared with the number of licks in and duration of the first NaCl bout with a two-way (diet × bout) RM ANOVA.
The rate of ingestion of 0.5 M NaCl during the first drinking bout was calculated for each rat for both the first 30 s and the last 30 s of the bout by dividing the number of licks by 30. Ingestion rates at the beginning and end of the first NaCl bout were compared with a two-way (diet × time) RM ANOVA. Due to technical problems, data from two control rats and three NaD rats were not included in this analysis.
Finally, a two-way (diet × solution) RM ANOVA was performed to compare the number of licks of dH2O and NaCl during the first drinking bout. Pairwise comparisons of statistically significant (P < 0.05) main effects or interactions were evaluated with Newman-Keuls tests.
Results
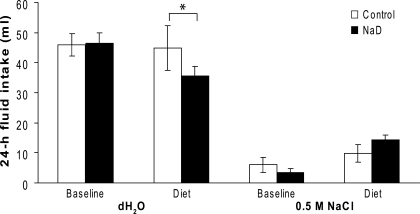
Figure 4 shows 24-h intakes of 0.5 M NaCl and dH2O during baseline and after 2 days of maintenance on the Na+-deficient diet or the control diet. The water intake was significantly greater than the NaCl solution intake for both diet groups [F(1,18) = 85.77, P < 0.01]. There was also a significant diet × time × solution interaction [F(1,18) = 11.20, P < 0.01]. Post hoc analyses of the interaction revealed no differences between the groups in intake of 0.5 M NaCl or dH2O during baseline. There were no differences in the amount of 0.5 M NaCl consumed after maintenance on diets; however, NaD rats drank significantly less dH2O than control rats during that period (P < 0.01).
Fig. 4.
Mean 24-h baseline and test diet intake (ml) of 0.5 M NaCl and deionized water (dH2O) by control and 2-day Na+-deprived (NaD) rats. Control rats had a significantly higher test dH2O intake than NaD rats (*P < 0.01). Control and NaD rats drank comparable amounts of NaCl solution during baseline and test and comparable amounts of dH2O during baseline conditions.
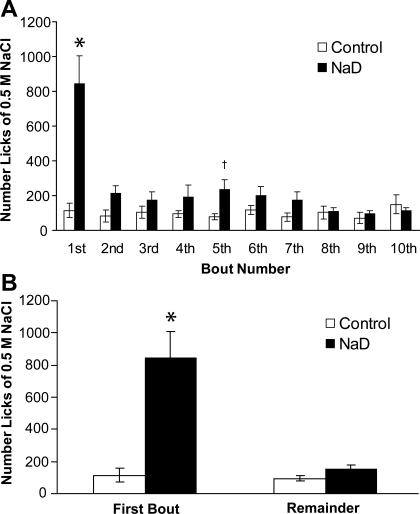
There were no differences between control and NaD groups in the total number of NaCl bouts (14.9 ± 3.3, 14.2 ± 1.6, respectively; P = 0.85) or water bouts (17.8 ± 1.9, 16.5 ± 2.2, respectively; P = 0.66). In addition, there was no difference in the interval (min) between the first and second NaCl bouts in control and NaD rats (81.6 ± 38.3, 90.5 ± 31.2, respectively; P = 0.86). Nonetheless, there were differences in licking patterns that were not apparent in 24-h intakes. Figure 5A shows the number of licks by NaD and control rats during the first 10 bouts of 0.5 M NaCl. NaD rats licked significantly more during the first and fifth NaCl bouts than control rats (P < 0.01, 0.05, respectively), but licking between the two groups was comparable in the other bouts. The number of licks by control rats did not differ for any of their bouts; in contrast, NaD rats licked more during the first bout (P < 0.01) than during any other bout.
Fig. 5.
A: mean number of licks to 0.5 M NaCl during the first 10 bouts by control and 2-day NaD rats. NaD rats had significantly greater licks during the first and fifth NaCl bouts compared with control rats (*P < 0.01, †P < 0.05). There was no difference between NaD and control rats in number of licks in any other NaCl bouts. B: mean NaCl ingestion (licks) during first NaCl bout vs. all remaining NaCl bouts by control and 2-day NaD rats. Compared with control rats, NaD rats licked more to NaCl in the first bout (*P < 0.01); however, NaD and control rats had comparable numbers of licks in the remaining NaCl bouts.
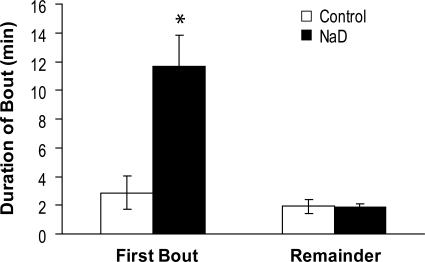
Figure 5B shows the number of licks by NaD and control rats during the first 0.5 M NaCl bout and the remaining bouts. There was a significant effect of diet [F(1,18) = 20.36, P < 0.01] and a diet × bout interaction [F(1,18) = 16.78, P < 0.01]. NaD rats licked significantly more during the first NaCl bout than control rats (P < 0.01), but licking by the two groups was comparable in the remaining NaCl bouts. Similarly, there was a diet × bout interaction in the duration of NaCl intake bouts [Fig. 6; F(1,18) = 15.33, P < 0.01]. Compared with control rats, NaD rats licked significantly longer during the first NaCl bout (P < 0.01), while remaining bouts were of comparable duration.
Fig. 6.
Mean duration of first NaCl bout vs. remaining NaCl bouts by control and 2-day NaD rats. NaD rats licked significantly longer in the first NaCl bout than control rats (*P < 0.01). NaD and control rats licked for comparable durations in the remaining NaCl bouts.
Analyses of ingestion rate during the first NaCl bout revealed a significant effect of diet [F(1, 13) = 11.55, P < 0.01] and of time [F(1,13) = 7.37, P < 0.05] and a diet × time interaction [F(1, 13) = 5.45, P < 0.05]. Post hoc analyses of the interaction revealed that NaD rats had a higher mean ingestion rate (licks/s) for NaCl at the beginning of the bout than control rats (3.5 ± 0.6 and 1.2 ± 0.3, respectively; P < 0.01). However, at the end of the bout, there was no difference in the mean ingestion rate (licks/s) between NaD rats and control rats (1.3 ± 0.5 and 1.0 ± 0.2, respectively; P = 0.91).
Analyses of number of licks in the first dH2O and NaCl drinking bouts by NaD and control rats revealed a significant effect of diet [F(1,18) = 17.06, P < 0.01] and of solution [F(1,18) = 7.49, P < 0.05] and a diet × solution interaction [F(1,18) = 11.73, P < 0.01]. Post hoc analyses of the interaction revealed that the number of licks by NaD rats during the first NaCl bout was significantly greater than the number during the first dH2O bout (P < 0.01). In contrast, the number of licks by control rats during the first NaCl bout were not different from those during the first dH2O bout. There was no difference in the mean number of licks during the first dH2O bout between control and NaD rats (185.3 ± 59.6 and 202.6 ± 81.3, respectively; P = 0.91).
Discussion
Although it has generally been accepted that 8–10 days of Na+ deprivation is necessary to increase intake of a concentrated NaCl solution (15, 22, 37), our laboratory recently found that in very short tests changes in licking may occur after as little as 2 days. The results of experiment 2 show that 2 days of dietary Na+ deprivation also was sufficient to alter patterns of ingestion of concentrated NaCl during longer tests. Similar to previous studies, mean 24-h intakes of a 0.5 M NaCl solution did not differ between Na+-deficient rats and Na+-replete control rats (Fig. 4; Refs. 15, 37); however, there were striking differences in licking patterns between the groups.
There was an exaggerated first licking bout to 0.5 M NaCl by Na+-deficient animals, with the number of licks during the first NaCl bout more than four times greater (Fig. 5) and the duration of the first NaCl bout nearly four times longer (Fig. 6) than those of Na+-replete control animals. During the first NaCl bout, Na+-deprived rats initially ingested NaCl at a substantially faster rate than control rats, although the rate decreased by the end of the first bout to a rate not different from that of control rats. Na+-deprived rats began licking immediately after the NaCl solution was presented and continued to lick until they had consumed ∼4 ml of 0.5 M NaCl, significantly more fluid than during their first water intake bout. In fact, control and NaD groups drank similar amounts of dH2O during the first bout, which rules out the possibility that NaD rats were simply licking more overall. Rather, the exaggerated first NaCl licking bout following Na+ deprivation may represent a rapid behavioral change that occurs in an attempt to replete body Na+ levels.
GENERAL DISCUSSION
Together, the results of experiment 1 and experiment 2 show a decrease in CT responses to NaCl that occurred after as little as 2 days of dietary Na+ deficiency and persisted during 10 days of Na+ deficiency. This change involves a decrease in the amiloride-sensitive component of the CT response and appears to have important consequences for NaCl taste that may underlie the compensatory increase in ingestion of concentrated NaCl solutions, the behavioral archetype of sodium appetite.
The findings of experiment 1 showing decreased CT responses after 2 and 10 days of dietary Na+ deprivation parallel those from developmental studies in which it was reported that CT responses to NaCl in rats that were maintained on a Na+-deficient diet throughout gestation until 28–90 days after birth were reduced compared with rats reared on normal dietary Na+ (21, 31). Moreover, the present study replicated the results of previous work by Contreras and Frank (11) showing that 10 days of dietary Na+ deprivation reduced CT responses to NaCl, and extended that study by showing that such changes occur with as little as 2 days of Na+ deprivation. In addition, we report for the first time that amiloride did not affect CT responses to NaCl in adult rats maintained for 2 or 10 days on Na+-deficient chow. In the present study, lingual application of amiloride suppressed CT responses to NaCl by 31% in control rats as expected (19). Interestingly, the amiloride effect in control rats reduced CT responses to NaCl to levels comparable to those in Na+-deprived rats without amiloride; in contrast, amiloride had very little effect on CT responses in NaD2 or NaD10 rats (Figs. 2 and 3).
These results are consistent with those from developmental studies showing reduced amiloride suppression of CT responses to NaCl in rats reared on a Na+-deficient diet (21, 31) and have two important implications. First, brief dietary Na+ deprivation may be sufficient to produce changes in CT responses in adult rats without the necessity of Na+ deficiency during some “window” in development. Second, changes in peripheral gustatory nerve responses to NaCl during Na+ imbalance may be mediated by a rapid and long-lasting decrease in the amiloride-sensitive component of CT responses to NaCl (20, 21). We believe that decreased input from the amiloride-sensitive component of the CT corresponds to decreased NaCl taste intensity that reduces the aversive qualities of concentrated NaCl solutions. Observations that amiloride increased licking to concentrated NaCl solutions by Na+-replete rats (3) are consistent with this idea. On the other hand, the same investigators also found that amiloride decreased licking to concentrated or dilute NaCl solutions by Na+-depleted rats (3), and it seems counterintuitive that a further reduction in aversive NaCl taste properties would decrease licking. However, if the number or sensitivity of isolated ENaC is decreased, as suggested by the present findings that dietary Na+ deficiency attenuates the amiloride suppression of CT responses to NaCl, further decreasing—or even eliminating—their contribution to NaCl taste transduction with amiloride may diminish NaCl taste properties to the point that these solutions no longer are identifiable, or desirable, to Na+-deprived rats.
Given that many taste receptor cells innervated by the CT contain ENaC that are highly responsive to amiloride (24), the present findings also suggest that changes in CT responses during Na+ deprivation may be attributable to a decrease in the number or sensitivity of ENaC on the tongue (23), and a number of mechanisms may underlie this decrease during dietary Na+ deprivation. For example, the lack of Na+ stimulation during dietary Na+ deprivation may alter the receptors and lead to a reduction in amiloride sensitivity. However, we believe this is not a likely explanation, because taste receptors are bathed in low levels of salivary Na+ even during dietary Na+ deprivation (2). Moreover, stimulation with NaCl throughout our nerve recording sessions did not increase CT responses, as might be predicted if the stimulation of these receptors by ambient NaCl determined their activity. Another possibility is that decreased plasma Na+ levels contribute to the decreased CT response. Consistent with this idea, adrenalectomized rats have both reduced CT responses to NaCl (23) and lower plasma Na+ levels (22) compared with intact animals. However, rats that were Na+ deprived during development and then switched to a Na+-replete diet for 1, 5, or 10 days had increased plasma Na+ levels compared with control rats, while rats fed the Na+-deficient diet continually during development or as adults had plasma Na+ levels similar to those of control rats (21). Although we cannot rule out changes due to a subtle and short-lived decrease in plasma Na+ levels, this mechanism seems unlikely to account for increased Na+ intake and the prolonged reduction of CT nerve responses to NaCl during Na+ deficiency.
As an alternative, we suggest that a hormonal mechanism may underlie both increased NaCl intake and decreased CT nerve responses to NaCl. One prime candidate in this regard is the Na+-conserving hormone aldosterone. Aldosterone alters the amiloride sensitivity of CT responses to NaCl (20), and peripheral administration of deoxycorticosterone acetate, a synthetic aldosterone precursor, elicits a sodium appetite in normal rats (see, e.g., Ref. 32). Moreover, the retention of Na+ evident within the first 24 h of Na+ deprivation (12) suggests that increased aldosterone occurs in a time frame appropriate for the observed changes in both CT and behavioral responses to NaCl during dietary Na+ deprivation. However, aldosterone has been shown to increase ENaC expression in kidney (26). Moreover, despite their lack of aldosterone, adrenalectomized rats have decreased CT responses to NaCl (23) and a large and persistent increase in NaCl intake (33). On the other hand, the NaCl intake by adrenalectomized rats is accompanied by increased circulating levels of angiotensin II (35). Although at present it is not known whether angiotensin II affects CT activity, peripheral administration of angiotensin II rapidly induces salt appetite in rats (see, e.g., Ref. 17). Thus angiotensin II may be another hormonal candidate for the increased salt intake and decreased CT responses to NaCl during dietary Na+ deprivation.
Regardless of the mechanism, decreased CT activity was paralleled by changes in behavioral responses as evidenced in experiment 2. More specifically, 2 days of maintenance on a Na+-deficient diet was sufficient to increase the number of licks during the first NaCl bout and to increase the duration of that first bout. These observations suggest that physiological and behavioral changes during Na+ deprivation may be more closely linked temporally than previously was thought. Moreover, since 2 days of dietary Na+ deprivation was sufficient to alter both licking patterns and CT responses to NaCl, the shift appears to occur within 48 h. We believe that the increased need for Na+ during deprivation leads to changes in gustatory signaling that may allow NaCl to taste more palatable or less aversive than under normal conditions (1, 15) and, consequently, to result in the appropriate behavioral response to Na+ deprivation-increased NaCl intake.
Interestingly, except for the first NaCl bout, NaD and control rats were similar in the number of licks in and duration of NaCl bouts. This novel observation may explain the inability to detect changes in longer (hours to days) intake tests, but it also raises the question of why the patterns of NaCl ingestion do not differ between groups after the first bout. In other words, why did NaD rats stop drinking the concentrated NaCl solution? One possibility is that after the first NaCl bout the NaD rats consumed sufficient NaCl (∼4 ml) to replete body Na+. This explanation seems plausible, because NaD rats in the present study consumed 1.98 meq of Na+ during the first drinking bout, an amount in excess of urinary Na+ loss during the first 2 days of Na+ deprivation (see, e.g., Ref. 12). Alternatively, Na+ repletion also may involve a hormonal mechanism that affects CT responses to NaCl, though in the opposite direction of that during Na+ deficiency. In any case, the observation that remaining NaCl bouts were comparable between NaD rats and control rats suggests a reversion to “basal” CT activity levels that also occurs rapidly, likely even more rapidly than the reduction during Na+ deprivation. In fact, the decrease in the rate of ingestion by NaD rats during the first bout suggests the intriguing possibility that such a reversal may even occur within the first bout. Admittedly, muscle fatigue due to the prolonged bout also may contribute to the slowing rate of ingestion; accordingly, ongoing studies are investigating these issues.
In summary, we found that decreased CT responses to NaCl occurred after as little as 2 days of dietary Na+ deprivation, and this change involves the amiloride-sensitive component of the CT response. Furthermore, decreased CT responses may underlie increased NaCl intake because, contrary to the previous belief that increased NaCl intake requires 8–10 days of dietary Na+ deprivation, we found changes in behavioral taste responses in normally behaving rats after only 2 days of Na+ deprivation. These rapid behavioral changes did not persist after a single bout of NaCl ingestion, and consequently mean 24-h intakes of 0.5 M NaCl did not differ between Na+-deficient and Na+-replete control rats, suggesting that repletion of body Na+ may restore basal gustatory responses.
Perspectives and Significance
In 1936, Richter's pioneering study (33) showed that rats that were maintained on a salt-free diet after adrenalectomy lived only 11 days, whereas access to NaCl substantially increased the life span, clearly demonstrating the necessity of Na+ for survival. Thirty years later, Nachman and Valentino (30) reported that in adrenalectomized rats the stimulation produced by consuming NaCl inhibited subsequent NaCl intake, but the same amount of NaCl infused directly into the stomach did not. These observations strongly suggest that taste is of critical importance in NaCl ingestion and in the satiation of NaCl appetite during pathophysiological Na+ deficiency. The present studies suggest that taste also plays a primary role in identifying and consuming Na+ (including concentrations that may be aversive under normal circumstances) during more physiological conditions of Na+ need. Our findings of rapid changes in gustatory and behavioral responses associated with dietary Na+ deprivation have important implications. Decreased CT responses to NaCl after dietary Na+ deprivation suggest that the peripheral nervous system interprets external sensory information differently during Na+ deprivation. This altered gustatory information, in turn, permits the behavioral responses that ultimately promote fluid and electrolyte homeostasis. Clearly, rapidly occurring changes in gustatory signaling may take place at other levels of the neuroaxis, including central areas such as the nucleus of the solitary tract or the parabrachial nucleus (8, 29, 35, 38). However, the present data demonstrate that the peripheral gustatory system changes during conditions of Na+ need. These changes allow the rat to adapt to physiological challenges by facilitating the detection and ingestion of Na+, and thereby increase the likelihood of survival.
GRANTS
This research was supported by National Institutes of Health Grants DC-04785 (R. J. Contreras), DC-06360 (K. S. Curtis), and T-32-NS-07437 (J. M. Garcia).
Acknowledgments
The authors thank Dr. David Dietz for assistance with the behavioral testing.
Portions of these data were presented in preliminary form at the 27th and 28th annual meetings of the Association for Chemoreception Sciences in Sarasota, FL as well as the 35th annual meeting of the Society for Neuroscience.
Present address of K. S. Curtis: Oklahoma State University Center for Health Sciences, Tulsa, Oklahoma.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bare J The specific hunger for sodium chloride in normal and adrenalectomized white rats. J Comp Physiol Psychol 42: 242–253, 1949. [DOI] [PubMed] [Google Scholar]
- 2.Beidler LM Saliva: its functions and disorders. In: Handbook of Olfaction and Gustation: Neurological Disease and Therapy, edited by Doty RL. New York: Dekker, 1995, vol. 32, p. 503–519.
- 3.Bernstein IL, Hennessy CJ. Amiloride-sensitive sodium channels and expression of sodium appetite in rats. Am J Physiol Regul Integr Comp Physiol 253: R371–R374, 1987. [DOI] [PubMed] [Google Scholar]
- 4.Bernstein IL, Taylor EM. Amiloride sensitivity of the chorda tympani response to sodium chloride in sodium-depleted Wistar rats. Behav Neurosci 106: 722–725, 1992. [DOI] [PubMed] [Google Scholar]
- 5.Breza J, Curtis KS, Contreras RJ. Temperature modulates taste responsiveness and stimulates gustatory neurons in the rat geniculate ganglion. J Neurophysiol 95: 674–685, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Brot MD, Watson CH, Bernstein IL. Amiloride-sensitive signals and NaCl preference and appetite: a lick-rate analysis. Am J Physiol Regul Integr Comp Physiol 279: R1403–R1411, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Bykowski MR, Smith JC, Stricker EM. Regulation of NaCl solution intake and gastric emptying in adrenalectomized rats. Physiol Behav 92: 781–789, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carr WJ The effect of adrenalectomy upon the NaCl taste threshold in rat. J Comp Physiol Psychol 45: 377–380, 1952. [DOI] [PubMed] [Google Scholar]
- 9.Contreras RJ Changes in gustatory nerve discharges with sodium deficiency: a single unit analysis. Brain Res 121: 373–378, 1977. [DOI] [PubMed] [Google Scholar]
- 10.Contreras RJ Gustatory mechanisms of a specific appetite. In: Neural Mechanisms in Taste, edited by Cagan RH. Boca Raton, FL: CRC, 1989, p. 119–145.
- 11.Contreras RJ, Frank M. Sodium deprivation alters neural responses to gustatory stimuli. J Gen Physiol 73: 569–94, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Contreras RJ, Hatton GI. Gustatory adaptation as an explanation for dietary-induced sodium appetite. Physiol Behav 15: 569–576, 1975. [Google Scholar]
- 13.Contreras RJ, Smith JC. NaCl concentration alters temporal patterns of drinking and eating by rats. Chem Senses 15: 295–310, 1990. [Google Scholar]
- 14.Contreras RJ, Studley JL. Amiloride alters lick rate responses to NaCl and KCl in rats. Chem Senses 19: 219–229, 1994. [DOI] [PubMed] [Google Scholar]
- 15.Curtis KS, Krause EG, Contreras RJ. Altered NaCl taste responses precede increased NaCl ingestion during Na+ deprivation. Physiol Behav 72: 743–749, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Epstein AN, Stellar E. The control of salt preference in the adrenalectomized rat. J Comp Physiol Psychol 48: 167–172, 1955. [DOI] [PubMed] [Google Scholar]
- 17.Fitts DA, Thunhorst RL. Rapid elicitation of salt appetite by an intravenous infusion of angiotensin II in rats. Am J Physiol Regul Integr Comp Physiol 270: R1092–R1098, 1996. [DOI] [PubMed] [Google Scholar]
- 18.Frank M An analysis of hamster afferent taste nerve response functions. J Gen Physiol 61: 588–618, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heck GL, Mierson S, DeSimone JA. Salt taste transduction occurs through an amiloride-sensitive sodium transport pathway. Science 223: 403–405, 1984. [DOI] [PubMed] [Google Scholar]
- 20.Herness MS Aldosterone increases the amiloride-sensitivity of the rat gustatory neural response to NaCl. Comp Biochem Physiol Comp Physiol 103: 269–273, 1992. [DOI] [PubMed] [Google Scholar]
- 21.Hill DL Susceptibility of the developing rat gustatory system to the physiological effects of dietary sodium deprivation. J Physiol 393: 413–424, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jalowiec JE, Stricker EM. Sodium appetite in adrenalectomized rats following dietary sodium deprivation. J Comp Physiol Psychol 82: 66–77, 1973. [DOI] [PubMed] [Google Scholar]
- 23.Kosten T, Contreras RJ. Adrenalectomy reduces peripheral neural responses to gustatory stimuli in the rat. Behav Neurosci 99: 734–741, 1985. [DOI] [PubMed] [Google Scholar]
- 24.Lin W, Finger TE, Rossier BC, Kinnamon SC. Epithelial Na+ channel subunits in rat taste cells: localization and regulation by aldosterone. J Comp Neurol 405: 406–420, 1999. [DOI] [PubMed] [Google Scholar]
- 25.Lundy RF, Contreras RJ. Temperature and amiloride alter taste nerve responses to Na+, K+, and NH4+ salts in rats. Brain Res 744: 309–317, 1997. [DOI] [PubMed] [Google Scholar]
- 26.Masilamani S, Kim GH, Mitchell C, Wade JB, Knepper MA. Aldosterone-mediated regulation of ENaC alpha, beta, and gamma subunit proteins in rat kidney. J Clin Invest 104: R19–R23, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCutcheon NB Sodium deficient rats are unmotivated by sodium chloride solutions mixed with the sodium channel blocker amiloride. Behav Neurosci 105: 764–766, 1991. [DOI] [PubMed] [Google Scholar]
- 28.Morrison GR, Young JC. Taste control over sodium intake in sodium deficient rats. Physiol Behav 8: 29–32, 1972. [DOI] [PubMed] [Google Scholar]
- 29.Nachman M Taste preferences for sodium salts by adrenalectomized rats. J Comp Physiol Psychol 55: 1124–1129, 1962. [DOI] [PubMed] [Google Scholar]
- 30.Nachman M, Valentino DA. Roles of taste and postingestional factors in the satiation of sodium appetite in rats. J Comp Physiol Psychol 62: 280–283, 1966. [DOI] [PubMed] [Google Scholar]
- 31.Pittman DW, Contreras RJ. Rearing on basal or high dietary NaCl modifies chorda tympani nerve responses in rats. Physiol Behav 77: 277–289, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Rice KK, Richter CP. Increased sodium chloride and water intake of normal rats treated with desoxycorticosterone acetate. Endocrinology 33: 106–115, 1943. [Google Scholar]
- 33.Richter CP Increased salt appetite in adrenalectomized rats. Am J Physiol 115: 155–161, 1936. [Google Scholar]
- 34.Richter CP Salt taste thresholds for normal and adrenalectomized rats. Endocrinology 24: 367–371, 1939. [Google Scholar]
- 35.Sakai RR, Epstein AN. Dependence of adrenalectomy-induced sodium appetite on the action of angiotensin II in the brain of the rat. Behav Neurosci 104: 167–176, 1990. [DOI] [PubMed] [Google Scholar]
- 36.Smith JC Microstructure of the rat's intake of food, sucrose and saccharin in 24-hour tests. Neurosci Biobehav Rev 24: 199–212, 2000. [DOI] [PubMed] [Google Scholar]
- 37.Stricker EM, Thiels E, Verbalis JG. Sodium appetite in rats after prolonged dietary sodium deprivation: a sexually dimorphic phenomenon. Am J Physiol Regul Integr Comp Physiol 260: R1082–R1088, 1991. [DOI] [PubMed] [Google Scholar]
- 38.Warwick ZS, Synowski J, Rice RD, Smart AB. Independent effects of diet palatability and fat content on bout size and daily intake in rats. Physiol Behav 80: 253–258, 2003. [DOI] [PubMed] [Google Scholar]