Abstract
The goal of this study was to test the hypothesis that NADPH oxidase contributes importantly to renal cortical oxidative stress and inflammation, as well as renal damage and dysfunction, and increases in arterial pressure. Fifty-four 7- to 8-wk-old Dahl salt-sensitive (S) or R/Rapp strain rats were maintained for 5 wk on a high sodium (8%) or high sodium + apocynin (1.5 mmol/l in drinking water). Arterial and venous catheters were implanted on day 21. By day 35 in the high-Na S rats, mRNA expression of renal cortical gp91phox, p22phox, p47phox, and p67phox NADPH subunits in S rats increased markedly, and treatment of high-Na S rats with the NADPH oxidase inhibitor apocynin resulted in significant decreases in mRNA expression of these NADPH oxidase subunits. At the same time, in apocynin-treated S rats 1) renal cortical GSH/GSSG ratio increased, 2) renal cortical O2•− release and NADPH oxidase activity decreased, and 3) renal glomerular and interstitial damage markedly fell. Apocynin also decreased renal cortical monocyte/macrophage infiltration, and apocynin, but not the xanthine oxidase inhibitor allopurinol, attenuated decreases in renal hemodynamics and lowered arterial pressure. These data suggest that NADPH oxidase plays an important role in causing renal cortical oxidative stress and inflammation, which lead to decreases in renal hemodynamics, renal cortical damage, and increases in arterial pressure.
Keywords: renal failure, apocynin, renal hemodynamics, oxidative stress, inflammation
salt-sensitive hypertension in humans and experimental models has been associated with progressive renal damage leading to end-stage renal disease. The Dahl salt-sensitive (S) rat is a good model of human salt-sensitive hypertension. The link between high blood pressure and renal damage may involve high renal oxidative stress and renal inflammation, and this has been shown in several studies in S rats (22, 37, 39, 41).
Oxidative stress can be caused by elevated levels of prooxidants such as NADPH oxidase, xanthine oxidase (XO), NO synthase, cyclooxygenase, and leakage of electrons during mitochondrial function. Also, a deficiency in antioxidants such as superoxide dismutase, catalase, or glutathione can cause oxidative stress. Increased general oxidative stress has been shown for the spontaneously hypertensive rat (SHR), the stroke-prone SHR, DOCA-salt hypertensive rats, and the S rat (4, 5, 8, 22, 23, 34, 44). Also, increased renal oxidative stress has been found in lead-induced hypertension (43) and coarctation of the aorta (2).
NADPH oxidase has been shown to be one of the most powerful prooxidants in both the vasculature and in the kidney. This oxidase is primarily made up of the membrane components gp91phox and p22phox and the cytosolic components p47phox, p67phox, and p40phox. Sprague-Dawley rats on a high-Na diet experienced increases in renal cortical gp91phox and p47phox subunits of mRNA expression (16). Renal medullary p22phox and p47phox protein were found to be elevated in the renal outer medulla in S rats compared with consomic, salt-resistant Dahl S-13BN rats (35). However, the role of renal cortical NADPH oxidase in S rat hypertension is not clear. The main goal of the present study was to test the hypothesis that renal cortical NADPH oxidase plays a role in increases in renal damage and arterial pressure and decreases in renal hemodynamics that occur in S rats subjected to a high-Na intake. A second goal was to initially assess the long-term role of XO in S rat hypertension. These goals were met in studies in S rats on either an 8% Na diet or an 0.3% Na diet over a 5-wk period with and without NADPH oxidase or XO inhibition. In the NADPH oxidase study, mRNA expression in renal cortical NADPH oxidase subunits due to either 5 wk of high- or normal-Na intake or to high- or normal-Na intake plus the NADPH oxidase inhibitor apocynin were measured, and we determined changes in renal cortical oxidative stress, renal hemodynamics, blood pressure, renal inflammation, and renal damage.
METHODS
Animal protocol and experimental measurements apocynin study.
Experiments were conducted over 5 wk on four groups of 7- to 8-wk-old S rats, Rapp strain (Harlan Sprague Dawley, Indianapolis, IN) with approval of the Institutional Animal Committee. Rats were randomly divided into four groups: S 8% Na (n = 11); S 8% Na+apocynin (Na+Apo; n = 9); S 0.3% Na (n = 7); S 0.3% Na+Apo (n = 7). Apo was administered in the drinking water at 1.5 mmol/l (4) starting 4 days before the 5-wk period. Catheters were implanted into the femoral artery and vein after 3 wk on the diets using isoflurane anesthesia (1%). Rats were allowed a 4-day recovery period after catheter surgery, and a 10-day period of collection of arterial pressure, heart rate, and renal hemodynamic data followed (23, 37, 39, 41). Glomerular filtration rate (GFR) and effective renal plasma flow (ERPF) were measured in conscious rats on day 34, as we have done before (22, 38). Briefly, a 4-h fasted plasma sample was taken, and iothalamate and aminohippurate concentrations were measured after a 12-h period of intravenous infusion of [125I]iothalamate and aminohippurate Na (3, 6).
To provide additional renal tissues for several assays, an additional 20 rats were subjected to the same diet regimen as the above groups for 5 wk, but without catheters. After 5 wk of the Na diets and before the kidney tissues were removed, arterial catheters were acutely inserted into the aorta under isoflurane anesthesia in all rats. Arterial pressures were determined in rats with chronic catheters and the 20 rats without chronic catheters to ensure that their pressures were not different. The kidneys were removed using isoflurane anesthesia, and the renal cortex was isolated and homogenized in appropriate buffers and protease inhibitors. Using a Berthold Autolumat Plus luminometer, basal chemiluminescence of renal cortical tissue was measured employing 5 μM lucigenin (bis-N-methylacridinium nitrate). Electron spin resonance methods have validated the accuracy of measuring oxygen free radical production with 5 μM lucigenin (19, 31). NADPH oxidase activity was determined by adding 100 μM NADPH to the kidney samples and measuring lucigenin chemiluminescence as above (14, 36). Protein amounts in renal tissues and urine were quantified with the Lowry assay and the Bradford assay, respectively.
RNA isolation and cDNA synthesis.
Frozen kidney was pulverized in a mortar and pestle under liquid nitrogen. The powdered tissue was added to TRI Reagent (Molecular Research Center) in a 50-ml conical centrifuge tube and homogenized using a Polytron homogenizer on low speed. The sample was centrifuged at 12,000 g for 10 min at 4°C to remove large molecular-weight DNA and insoluble structural proteins, and the supernatant was processed to isolate RNA. Aliquots of 5 μg of RNA isolate were treated with DNase I (DNA-free RNA kit; Zymo Research). The concentration of nucleic acid was assessed using a UV spectrophotometer (SmartSpec 3000; Bio-Rad Laboratories). RNA quality was assessed by A260-to-A280 ratio and by electrophoresis of 0.9–1.0 μg aliquot on a 1.2% agarose gel using 1× TBE buffer, with ethidium bromide staining. RNA was judged to be intact if the sample lane showed prominent discrete bands for 18S and 28S rRNA with no smearing. DNase-treated RNA was then used as a template for cDNA synthesis (iScript cDNA synthesis kit; Bio-Rad Laboratories) following the manufacturer's protocol. The cDNA samples were diluted 1:10 with nuclease-free water before being used as a template for real-time RT-PCR.
Real-time RT-PCR.
Real-time RT-PCR assays were performed using iQ SYBR Green Supermix (Bio-Rad Laboratories) on an iCycler iQ Real-Time PCR Detection System (Bio-Rad Laboratories). Specific oligonucleotide primers for gp91phox, p22phox, p47phox, and p67phox were used for PCR amplifications. Undiluted (−)RT products were used as templates in negative control reactions to check for genomic DNA contamination within the cDNA. Other negative control reactions included NT reactions (which contained SYBR Green Supermix, forward and reverse primers, and nuclease-free water) as well as a blank (which contained SYBR Green Supermix and nuclease-free water). Relative fold expression of mRNA was quantified by using the 2−ΔΔCt mathematical model (7, 20).
Measurement of renal glutathiones and renal monocytes/macrophages.
Reduced (GSH) and oxidized glutathione (GSSG) were determined using the fluorescent detection of dansyl derivatives using HPLC according to the method of Jones (15) as we have done before (40, 41). Renal monocytes/macrophages from tissues collected at 5 wk of the various diets were measured by indirect immunoperoxidase methodology (26) using ED-1, a monoclonal antibody to monocytes/macrophages (Chemicon).
Analysis of glomerular and tubulointerstitial injury.
Kidney sections were examined for necrotic and sclerotic glomeruli at a ×200 magnification using PAS-hematoxylin/eosin stains (22, 37). Tubulointerstitial renal injury was determined using a Masson trichrome-stained kidney section from each rat. Briefly, this injury was measured as the area of interstitial tissue with increased amounts of blue staining, dilated cast-containing tubules, or tubules showing acute injury divided by the area of nonglomerular and nonvascular cortex.
Allopurinol study.
Arterial and venous catheters were implanted as above in three 8% Na diet rats and three 8% Na+allopurinol (Allo) rats to block XO, and studies were run over a 5-wk period. Allo was administered in the drinking water at ∼10 mg·kg−1·day−1 starting 4 days before the 5-wk period began, and this dose of Allo has been shown to totally inactivate renal XO (17). Mean arterial pressure was measured continually over the last 10 days of the experiment, and GFR was measured on day 34 as above.
Statistical analysis.
Comparisons of data from S rats on high or low sodium with and without apocynin treatment were performed using ANOVA followed by a Fisher least significant difference test for post hoc analysis. Differences were considered to be statistically significant if P < 0.05. All data are expressed as means ± SE.
RESULTS
Renal cortical NAD(P)H oxidase subunit responses to high-Na+Apo.
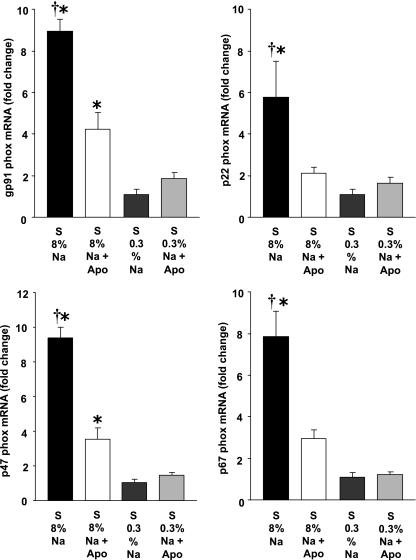
Figure 1 shows that S rats on high-Na-intake experienced significant increases in renal cortical gp91phox, p22phox, p47phox, and p67phox mRNA compared with 0.3% Na rats. Apo treatment in high-Na rats resulted in marked decreases in all subunits of NAD(P)H oxidase. Therefore, transcription of NAD(P)H oxidase is importantly affected by Na intake and by Apo treatment.
Fig. 1.
Effects of apocynin (Apo) and Na diet on the mRNA expression of NADPH oxidase subunits in Dahl salt-sensitive (S) rats in 8% Na (n = 9), 8% Na+Apo (n = 8), 0.3% Na (n = 6–7), and 0.3% Na+Apo groups (n = 6). †P < 0.05 when comparing 8% Na with 8% Na+Apo group. *P < 0.05 compared with 0.3% Na alone group.
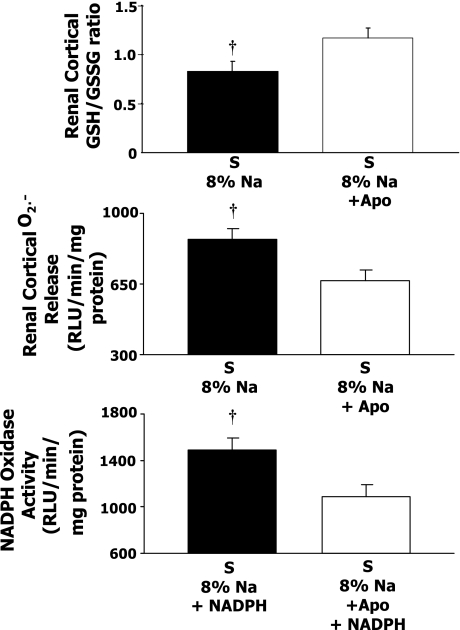
Renal cortical GSH/GSSG and renal cortical O2•− release responses to high-Na+Apo
Figure 2 shows that S rats on long-term Apo treatment and high-Na intake had a significantly higher GSH-to-GSSG ratio in the renal cortex compared with high-Na S rats. This ratio has been shown to be a highly reliable index of oxidative stress with higher values, indicating less oxidative stress (15). Figure 2 also shows that renal cortical O2•− release was lower in high-Na S rats on Apo compared with high-Na S rats. Figure 2, bottom, shows that Apo treatment caused a lower NADPH oxidase activity in renal cortical samples.
Fig. 2.
Reduced-to-oxidized glutathione ratio (GSH/GSSG) in renal cortical tissue (8% Na, n = 9; 8% Na+Apo, n = 12), and renal cortical superoxide release and renal cortical NADPH oxidase activity in 8% Na (n = 13) and 8% Na+Apo (n = 9) in Dahl S rats. †P < 0.05 when comparing 8% Na with 8% Na+Apo group.
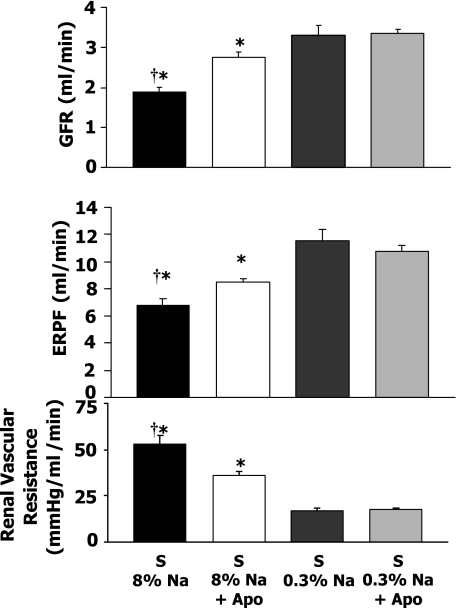
GFR and renal plasma flow responses to high-Na+Apo.
Figure 3 shows that an 8% Na intake in S rats caused a significantly lower GFR (1.9 ± 0.1 ml/min) compared with the S 0.3% Na rats (3.3 ± 0.3 ml/min). The Apo treatment of 8% Na rats resulted in a significantly higher GFR (2.8 ± 0.1 ml/min compared with the 8% Na rats). Therefore, Apo treatment prevented a decrease in GFR in S rats on high-Na intake. High-Na intake in S rats caused a significant decrease in effective renal plasma flow (ERPF) to 6.3 ± 0.5 ml/min compared with 0.3% Na S rats (12.6 ± 0.8 ml/min), and ERPF in Apo-treated rats was 8.5 ± 0.3 ml/min. Renal vascular resistance averaged 53.2 ± 4.5 mmHg·ml−1·min−1 in high-Na S rats and 36.0 ± 2.1 mmHg·ml−1·min−1 in high-Na S rats treated with Apo (P < 0.05). Apo treatment in S rats on a 0.3% Na diet caused no significant change in GFR, ERPF, or renal vascular resistance compared with 0.3% Na rats.
Fig. 3.
Glomerular filtration rate (GFR), renal plasma flow (ERPF), and renal vascular resistance responses in Dahl S rats in 8% Na (n = 9), 8% Na+Apo (n = 8), 0.3% Na (n = 7), and 0.3% Na+Apo (n = 7) groups. †P < 0.05 when comparing 8% Na with 8% Na+Apo group. *P < 0.05 compared with 0.3% Na alone group.
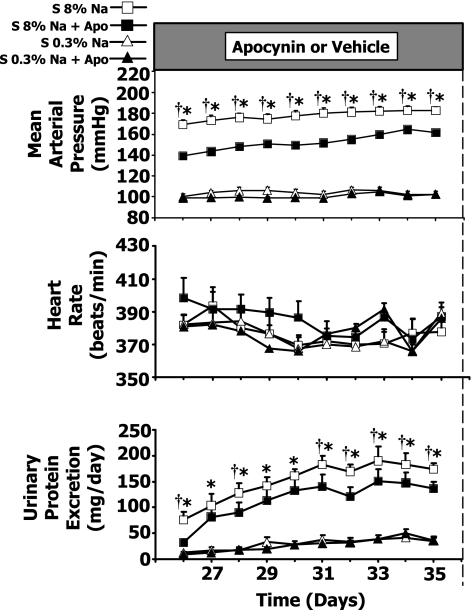
Mean arterial pressure, heart rate, and urinary protein excretion responses to high-Na+Apo.
Figure 4 shows that Apo treatment in high-Na S rats resulted in only moderate decreases in arterial pressure. The arterial pressures are illustrated for the last 10 days of the 5-wk experiment, and had a value of 183 ± 3 mmHg in the high-Na S rats compared with 162 ± 4 mmHg in the high-Na+Apo rats on day 35 (P < 0.05). Heart rate did not change significantly in any group. Figure 4, bottom, shows that the high-Na S rats had elevated values of urinary protein excretion compared with 0.3% Na S rats and the high-Na S+Apo rats throughout the last 10 days of the experiment except on days 27, 29, and 30 in the high-Na+Apo group. Apo treatment thus prevented renal damage in high-Na S rats.
Fig. 4.
Mean arterial pressure, heart rate, and urinary protein excretion responses in Dahl S rats in 8% Na (n = 11), 8% Na+Apo (n = 9), 0.3% Na (n = 7), and 0.3% Na+Apo (n = 7) groups. †P < 0.05 when comparing 8% Na with 8% Na+Apo group. *P < 0.05 compared with 0.3% Na alone group.
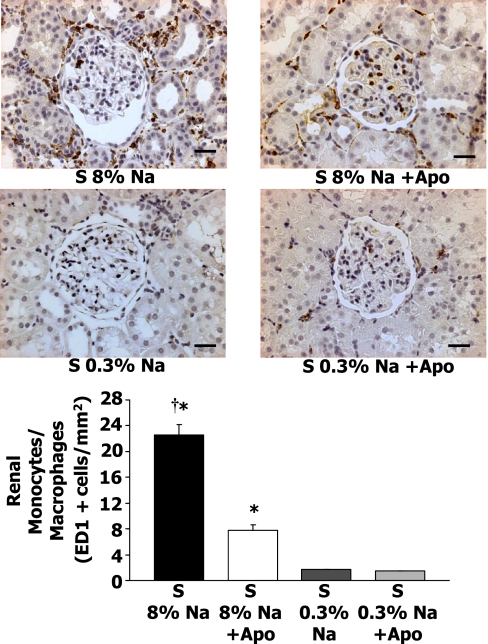
Renal monocyte/macrophage responses to high-Na+Apo.
Figure 5 presents representative renal micrographs of the four groups of rats with ED-1+cells (monocytes/macrophages). Figure 5, bottom, shows the average renal ED-1+cells and indicates that renal monocyte/macrophages reached a value of 22.6 ± 1.6 cells/mm2 in 8% Na S rats compared with 1.8 ± 0.03 cells/mm2 in 0.3% Na S rats (P < 0.05). Apo treatment of high-Na S rats resulted in a significantly lower value of 7.8 ± 0.8 ED-1+cells/mm2.
Fig. 5.
Top: representative ED-1+cells (monocytes/macrophages) in the renal cortex of 8% Na or 0.3% Na Dahl S rats with and without Apo treatment. Kidneys were removed after 5 wk of the Na diet. Renal infiltration of monocytes/macrophages decreased in Dahl S rats treated with Apo. Bottom: average renal cortical monocyte/macrophage infiltration in Dahl S rats with 8% Na (n = 12), S 8% Na+Apo (n = 7), Dahl S rats with 0.3% Na (n = 7), and S 0.3% Na + Apo (n = 7) groups. †P < 0.05 when comparing 8% Na with 8% Na+Apo group. *P < 0.05 compared with 0.3% Na alone group. Scale bar = 30 μM.
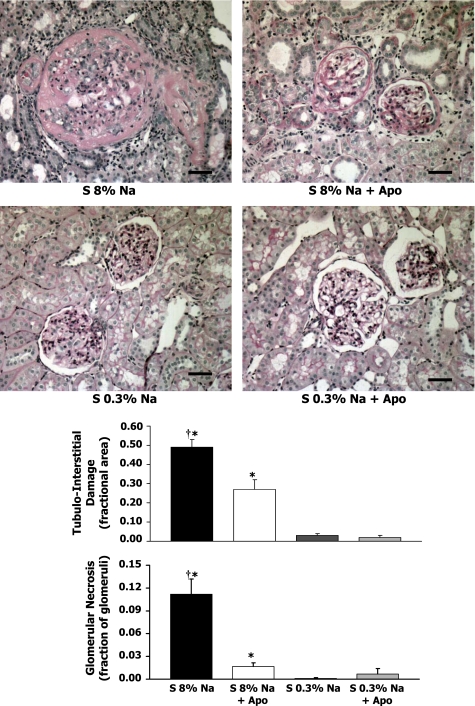
Histological analyses of kidneys with or without Apo.
Figure 6 shows representative kidney sections for each group of rats. In the 8% Na rats (Fig. 6, top left), the kidneys exhibited fibrinoid arterial and arteriolar necrosis and fibrinoid glomerular necrosis. Some glomeruli at all levels of the cortex showed global or segmental glomerular sclerosis. In the 8% Na+Apo rats (Fig. 5, top right), the kidneys had a significantly smaller number of glomeruli that showed fibrinoid glomerular and arteriolar necrosis. Tubulointerstitial injury in 8% Na+Apo rats, which was much less common than in the high-Na rats, contained some areas with dilated tubules surrounded by increased amounts of interstitial connective tissue. In the 0.3% Na and 0.3% Na+Apo groups (Fig. 5, bottom), the kidneys had a normal appearance with infrequent sclerotic glomeruli in the subcapsular cortex.
Fig. 6.
Top: representative renal histology (all ×200 magnification) in the renal cortex of Dahl S rats with 8% Na (n = 12), S 8% Na+Apo (n = 7), Dahl S rats with 0.3% Na (n = 7), and S 0.3% Na+Apo (n = 7) groups. Bottom: average tubulointerstitial damage (fractional area) and glomerular necrosis (fraction of glomeruli). †P < 0.05 when comparing 8% Na with 8% Na+Apo group. *P < 0.05 compared with 0.3% Na alone group. Scale bar = 30 μM.
Average glomerular and tubulointerstitial damage responses to high-Na+Apo.
Figure 6 shows, tubulointerstitial damage averaged 0.49 ± 0.04 (fractional area) in the high-Na S group, and this area decreased significantly in high-Na S rats on Apo treatment to 0.27 ± 0.05. Figure 6 also shows that the average glomerular necrosis was markedly increased in the high-Na S rats compared with either the S high-Na+Apo rats or S 0.3% Na groups. The Apo treatment decreased the fraction of glomerular necrosis in the S rats on a high-Na intake by 85%.
Mean arterial pressure, GFR, and renal plasma flow responses to high-Na+Allo.
Allo was given in the drinking water of three 8% Na S rats and three 8% Na+Allo S rats for 5 wk to inhibit XO. There were no significant changes in the average mean arterial pressure over the last 10 days of the experiment (183 ± 1 mmHg in 8% Na and 182 ± 1 mmHg in 8% Na+Allo). There were also no significant changes in GFR (2.0 ± 0.2 ml/min in 8% Na and 2.0 ± 0.4 ml/min in 8% Na+Allo). Therefore, we found no evidence for a protective effect of Allo for the rise in arterial pressure or the decrease in GFR experienced in high-Na S rats.
DISCUSSION
There were several new findings in this study. First, renal cortical mRNA levels of renal cortical gp91phox, p22phox, p47phox, and p67phox NADPH subunits in Dahl S rats were significantly upregulated by the 5-wk high-Na diet, and treatment of high-Na S rats but not low-Na S rats with Apo resulted in much lower values of NADPH subunit mRNA expression. A second novel finding was that in Apo-treated S rats, the renal cortical GSH-to-GSSG ratio increased, the renal cortical O2•− release decreased, and NADPH oxidase activity in the renal cortex was lower. A third new finding was that in high-Na S rats that received Apo, but not in rats that received Allo, GFR and renal plasma flow were higher, and renal vascular resistance was lower. A fourth novel finding was that long-term arterial pressure was lower, and renal cortical inflammation and renal glomerular and interstitial damage were lower in Apo-treated S rats on high-Na intake.
A previous study in DOCA salt-hypertensive rats reported that aortic p22phox mRNA was elevated in the DOCA rats compared with sham rats, and systemic Apo treatment in DOCA rats resulted in lower values (but was not statistically significant) of p22phox mRNA (4). Apo also reduced systolic pressure in DOCA rats (4). Systemic Apo prevented hypertension in aldosterone-infused rats, and aortic mRNA for p22phox decreased (24). Systemic Apo also prevented dexamethasone-induced hypertension in rats (12), and thick ascending loop of Henle superoxide production was inhibited by Apo (11). Adrenocorticotropic hormone-induced hypertension was prevented or reversed by systemic Apo but not Allo (46). The above studies agree with the present study in that Apo reduced blood pressure and NADPH subunit mRNA expression. However, the above studies mainly focused on blood pressure regulation, and NADPH oxidase subunit mRNA was measured only in nonrenal vessels. In a recent elegant study, infusion of Apo into the renal medulla reduced arterial pressure in Dahl S rats (35). Also, in this study, levels of p22phox and p47phox protein in the renal outer medulla were lower in the salt-resistant SS-13BN rats compared with the salt-sensitive SS rats (35), and the data support an important role of NADPH oxidase in the renal medulla in Dahl S rat hypertension. In contrast, our study demonstrates an important renal cortical role of NADPH oxidase in Dahl S rat hypertension and the associated oxidative stress and inflammation in the renal cortex and the resulting renal damage and dysfunction.
In the present study, on day 35 mean arterial pressure was 21 mmHg lower in the high-Na rats treated with Apo compared with high-Na alone rats. Other investigators have also measured blood pressure during Apo administration. Taylor et al. (35) reported a decrease in mean arterial pressure of 18 mmHg in Dahl S rats after 13 days of Apo into the renal medulla. Beswick et al. (4) found that the systolic blood pressure of DOCA-salt rats was 54 mmHg lower in Apo-treated rats. The pressure decrease in the present study is similar to that in the study by Taylor et al. (35), which indicates that the increase in blood pressure in Dahl S rats is less dependent on NADPH oxidase than in models such as DOCA-salt hypertension. However, in spite of the moderate decrease in blood pressure in the present study, the amount of renal damage and dysfunction was markedly lower during Apo treatment, indicating that Apo has a protective role in hypertension-induced kidney damage.
Two studies previously done in our laboratory indicate that Dahl R rats subjected to high-Na intake maintain blood pressure and renal cortical superoxide production at low-Na control values (23, 40). Also, renal damage and proteinuria were unchanged when comparing low- and high-Na R rats. We do not have direct measurements of NADPH oxidase activity, but the lack of change of renal cortical superoxide production during high Na (23) suggests that NADPH oxidase activity would be unchanged.
Apo is believed to inhibit NADPH oxidase activity by impeding the movement of p47phox from the cytosol to the membrane (45). We submit new information in the Dahl S rat in the present study, which shows that renal cortical mRNA levels of gp91phox, p22phox, p47phox, and p67phox NADPH subunits were markedly lower in Apo-treated high-Na S rats. This result confirms the decrease in aortic p22phox mRNA by Apo in aldosterone-infused rats (24). The decreased NADPH subunit transcription plus a possible impediment in the translocation of p47phox helps to explain why NADPH oxidase activity decreased. Although Apo could have a direct effect on reducing phox subunit transcription, a more likely mechanism is that Apo caused a reduction in invading leukocytes and thus decreased the NADPH oxidase subunits contained in the leukocytes. This is supported by the lack of effect of Apo on phox subunits in the 0.3% rats.
The mRNA of NADPH oxidase subunits decreased a greater percentage than did the NADPH oxidase activity during Apo treatment. A possible reason is that Nox4 does not require activation by any cytosolic subunits of NADPH oxidase and thus is unaffected by Apo (30). Therefore, Nox4 could continue to produce basal amounts of superoxide, which are unaffected by Apo, and this helps to explain the attenuated drop in NADPH oxidase activity.
Apo has been traditionally used to inhibit NADPH oxidase, but to be effective it has to be converted to an active dimer by myeloperoxidase (MPO) or other peroxidases (10, 42). Studies on HEK-293 cells, which do not express MPO, showed that Apo did not decrease NADPH oxidase activation or decrease superoxide formation (10), but under these conditions could scavenge reactive oxygen species. However, this should not be an important concern in the intact kidneys of Dahl S rats, which we and others have shown to have an abundance of leukocytes (21, 27, 39). Leukocytes in the kidney have been shown to have high concentrations of MPO needed to oxidize Apo into an active dimer (32), which would allow Apo to inhibit NADPH oxidase activity within the leukocytes. This is the most likely mechanism by which Apo decreased this oxidase activity in the present experiment. Astern et al. (1) have shown that MPO released by neutrophil degradation was internalized into endothelial cells facilitated by cytokeratin 1. This presents a possibility, although unproven, that MPO could enter some of the cells in kidney tissue and activate Apo. That Apo was activated in the present study is evidenced by the significant decrease in renal cortical NADPH oxidase activity in S rats treated with Apo. This suggests that Apo may be an effective tool for inactivating NADPH oxidase in renal tissue, and, in addition, renal damage was markedly reduced by Apo.
Progressive declines in GFR and renal plasma flow are evident in end-stage renal disease, and these declines in GFR and renal plasma flow as well as renal damage were apparent in high-Na S rats in the present study. Treatment of high-Na S rats with Apo improved GFR and renal plasma flow but not in low-Na S rats, and renal damage was markedly reduced in high-Na+Apo rats. A previous study in S rats on a high-Na diet also showed decreases in GFR and renal plasma flow, as well as marked proteinuria and renal damage (33), and studies in our laboratory showed that general antioxidants prevent declines in renal hemodynamics and renal damage (37, 40, 41). The present study clearly shows that inhibiting the increase in NADPH oxidase activity prevented much of the renal damage, especially in the renal cortex, and this was accomplished in spite of only a 20 mmHg decrease in arterial pressure.
Figure 6 shows that glomerular damage was prevented in high-Na+Apo S rats. In these rats renal tubulointerstitial damage was reduced by one half, which is similar to the change seen in high-Na S rats with vitamin C and E treatment (21) but less than the 83% improvement seen in high-Na S rats treated with N-acetylcysteine (41). Proteinuria in high-Na S rats was significantly decreased in high-Na S rats in the present study, but the magnitude of proteinuria decrease was smaller than that seen when using a general antioxidant (37, 41). Since urine protein excretion is affected by both the permeability of the glomerular membrane and the reabsorption by the tubules (9), perhaps the high remaining tubulointerstitial damage in Apo-treated rats suggests that protein reabsorption in these damaged tubules was poor. This tubulointerstitial damage, with its poor protein reabsorption, plus the remaining high blood pressure would cause elevated proteinuria in Apo rats.
In addition to increasing renal angiotensin II content, infiltration of immune cells can lead to renal damage. Indeed, renal cortical macrophages were at very high levels in high-Na S rats in this study. We showed that Apo treatment markedly reduced the number of renal macrophages in high-Na rats. We have previously seen renal macrophage invasion in high-Na S rats (39, 41), and treatment with either anti-inflammatory compounds or antioxidants reduced macrophage infiltration and the associated renal damage (39). Reduction of oxidative stress in the present study by specifically inhibiting NADPH oxidase also effectively inhibited macrophage infiltration into the kidney and also reduced the renal damage, suggesting that NADPH oxidase plays an important role in end-stage renal damage. Reduction in macrophage infiltration may have decreased intrarenal angiotensin II (28), which could have in turn decreased mRNA transcription of NADPH oxidase subunits.
Perspectives
Oxidative stress and inflammation have been shown to play a major role in many forms of experimental hypertension. NADPH oxidase is an important contributor to oxidative stress with effects on renal inflammation, blood pressure, renal hemodynamics, and renal damage. Oxidative stress studies in humans are scarce, but the results thus far indicate an important role of reactive oxygen species in human hypertension (18, 25, 29). Antihypertensive therapy in these patients decreased free radical production and lipid peroxides to normal (25). A recent study has shown that renal monocytes/macrophages were significantly increased in African-American and white hypertensive patients, and these immune cells were localized to areas of cortical fibrosis and glomerular obsolescence (13). The beneficial role of NADPH inhibition in the current study indicates that an increase in oxidative stress and inflammation could be contributory mechanisms in human hypertension and the renal damage associated with hypertension.
GRANT
This research was supported by National Heart, Lung, and Blood Institute Grant HL-51971.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Astern JM, Pendergraft WF III, Falk RJ, Jennette JC, Schmaier AH, Mahdi F, Preston GA. Myeloperoxidase interacts with endothelial cell-surface cytokeratin 1 and modulates bradykinin production by the plasma Kallikrein-Kinin system. Am J Pathol 171: 349–360, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barton CH, Ni Z, Vaziri ND. Enhanced nitric oxide inactivation in aortic coarctation-induced hypertension. Kidney Int 60: 1083–1087, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Berber EY, Farber SJ, Earle DP Jr. Comparison of the constant infusion and urine collection techniques for the measurement of renal function. J Clin Invest 27: 710–719, 1948. [PubMed] [Google Scholar]
- 4.Beswick RA, Dorrance AM, Leite R, Webb RC. NADH/NADPH oxidase and enhanced superoxide production in the mineralocorticoid hypertensive rat. Hypertension 38: 1107–1111, 2001. [DOI] [PubMed] [Google Scholar]
- 5.Beswick RA, Zhang H, Marable D, Catravas JD, Hill WD, Webb RC. Long-term antioxidant administration attenuates mineralocorticoid hypertension and renal inflammatory response. Hypertension 37: 781–786, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Brands MW, Lee WF, Keen HL, Alonso-Galicia M, Zappe DH, Hall JE. Cardiac output and renal function during insulin hypertension in Sprague-Dawley rats. Am J Physiol Regul Integr Comp Physiol 271: R276–R281, 1996. [DOI] [PubMed] [Google Scholar]
- 7.Chalothorn D, Clayton JA, Zhang H, Pomp D, Faber JE. Collateral density, remodeling, and VEGF-A expression differ widely between mouse strains. Physiol Genomics 30: 179–191, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Chen X, Touyz RM, Park JB, Schiffrin EL. Antioxidant effects of vitamins C and E are associated with altered activation of vascular NADPH oxidase and superoxide dismutase in stroke- prone SHR. Hypertension 38: 606–611, 2001. [DOI] [PubMed] [Google Scholar]
- 9.D'Amico G, Bazzi C. Pathophysiology of proteinuria. Kidney Int 63: 809–825, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schroder K, Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension 51: 211–217, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Hong NJ, Garvin JL. Flow increases superoxide production by NADPH oxidase via activation of Na-K-2Cl cotransport and mechanical stress in thick ascending limbs. Am J Physiol Renal Physiol 292: F993–F998, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Hu L, Zhang Y, Lim PS, Miao Y, Tan C, McKenzie KU, Schyvens CG, Whitworth JA. Apocynin but not l-arginine prevents and reverses dexamethasone-induced hypertension in the rat. Am J Hypertens 19: 413–418, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Hughson MD, Gobe GC, Hoy WE, Manning RD Jr, Douglas-Denton R, Bertram JF. Associations of glomerular number and birth weight with clinicopathological features of African Americans and whites. Am J Kidney Dis 2008. [DOI] [PubMed]
- 14.Iliescu R, Cucchiarelli VE, Yanes LL, Iles JW, Reckelhoff JF. Impact of androgen-induced oxidative stress on hypertension in male SHR. Am J Physiol Regul Integr Comp Physiol 292: R731–R735, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Jones DP Redox potential of GSH/GSSG couple: assay and biological significance. Methods Enzymol 348: 93–112, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Kitiyakara C, Chabrashvili T, Chen Y, Blau J, Karber A, Aslam S, Welch WJ, Wilcox CS. Salt intake, oxidative stress, and renal expression of NADPH oxidase and superoxide dismutase. J Am Soc Nephrol 14: 2775–2782, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Laakso J, Mervaala E, Himberg JJ, Teravainen TL, Karppanen H, Vapaatalo H, Lapatto R. Increased kidney xanthine oxidoreductase activity in salt-induced experimental hypertension. Hypertension 32: 902–906, 1998. [DOI] [PubMed] [Google Scholar]
- 18.Lacy F, O'Connor DT, Schmid-Schonbein GW. Plasma hydrogen peroxide production in hypertensives and normotensive subjects at genetic risk of hypertension. J Hypertens 16: 291–303, 1998. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Zhu H, Kuppusamy P, Roubaud V, Zweier JL, Trush MA. Validation of lucigenin (bis-N-methylacridinium) as a chemilumigenic probe for detecting superoxide anion radical production by enzymatic and cellular systems. J Biol Chem 273: 2015–2023, 1998. [DOI] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 25: 402–408, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Mattson DL, James L, Berdan EA, Meister CJ. Immune suppression attenuates hypertension and renal disease in the Dahl salt-sensitive rat. Hypertension 48: 149–156, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Meng S, Cason GW, Gannon AWRL, Manning RD Jr. Oxidative stress in Dahl salt-sensitive hypertension. Hypertension 41: 1346–1352, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Meng S, Roberts LJ, Cason GW, Curry TS, Manning RD Jr. Superoxide dismutase and oxidative stress in Dahl salt-sensitive and -resistant rats. Am J Physiol Regul Integr Comp Physiol 283: R732–R738, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Park YM, Park MY, Suh YL, Park JB. NAD(P)H oxidase inhibitor prevents blood pressure elevation and cardiovascular hypertrophy in aldosterone-infused rats. Biochem Biophys Res Commun 313: 812–817, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Prabha PS, Das UN, Koratkar R, Sagar PS, Ramesh G. Free radical generation, lipid peroxidation and essential fatty acids in uncontrolled essential hypertension. Prostaglandins Leukot Essent Fatty Acids 41: 27–33, 1990. [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez-Iturbe B, Pons H, Quiroz Y, Gordon K, Rincon J, Chavez M, Parra G, Herrera-Acosta J, Gomez-Garre D, Largo R, Egido J, Johnson RJ. Mycophenolate mofetil prevents salt-sensitive hypertension resulting from angiotensin II exposure. Kidney Int 59: 2222–2232, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez-Iturbe B, Vaziri ND, Herrera-Acosta J, Johnson RJ. Oxidative stress, renal infiltration of immune cells, and salt-sensitive hypertension: all for one and one for all. Am J Physiol Renal Physiol 286: F606–F616, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez-Iturbe B, Vaziri ND, Herrera-Acosta J, Johnson RJ. Oxidative stress, renal infiltration of immune cells, and salt-sensitive hypertension: all for one and one for all. Am J Physiol Renal Physiol 286: F606–F616, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Russo C, Olivieri O, Girelli D, Faccini G, Zenari ML, Lombardi S, Corrocher R. Anti-oxidant status and lipid peroxidation in patients with essential hypertension. J Hypertens 16: 1267–1271, 1998. [DOI] [PubMed] [Google Scholar]
- 30.Serrander L, Cartier L, Bedard K, Banfi B, Lardy B, Plastre O, Sienkiewicz A, Forro L, Schlegel W, Krause KH. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem J 406: 105–114, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skatchkov MP, Sperling D, Hink U, Mulsch A, Harrison DG, Sindermann I, Meinertz T, Munzel T. Validation of lucigenin as a chemiluminescent probe to monitor vascular superoxide as well as basal vascular nitric oxide production. Biochem Biophys Res Commun 254: 319–324, 1999. [DOI] [PubMed] [Google Scholar]
- 32.Son TG, Zou Y, Yu BP, Lee J, Chung HY. Aging effect on myeloperoxidase in rat kidney and its modulation by calorie restriction. Free Radic Res 39: 283–289, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Sterzel RB, Luft FC, Gao Y, Schnermann J, Briggs JP, Ganten D, Waldherr R, Schnabel E, Kriz W. Renal disease and the development of hypertension in salt-sensitive Dahl rats. Kidney Int 33: 1119–1129, 1988. [DOI] [PubMed] [Google Scholar]
- 34.Swei A, Lacy F, Delano FA, Parks DA, Schmid-Schonbein GW. A mechanism of oxygen free radical production in the Dahl hypertensive rat. Microcirculation 6: 179–187, 1999. [PubMed] [Google Scholar]
- 35.Taylor NE, Glocka P, Liang M, Cowley AW Jr. NADPH oxidase in the renal medulla causes oxidative stress and contributes to salt-sensitive hypertension in Dahl S rats. Hypertension 47: 692–698, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Taylor NE, Maier KG, Roman RJ, Cowley AW Jr. NO synthase uncoupling in the kidney of Dahl S rats: role of dihydrobiopterin. Hypertension 48: 1066–1071, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Tian N, Thrasher KD, Gundy PD, Hughson MD, Manning RD Jr. Antioxidant treatment prevents renal damage and dysfunction and reduces arterial pressure in salt-sensitive hypertension. Hypertension 45: 934–939, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Tian N, Gannon A, Khalil R, Manning RD Jr. Mechanisms of salt-sensitive hypertension: Role of medullary inducible nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol 284: R372–R379, 2003. [DOI] [PubMed] [Google Scholar]
- 39.Tian N, Gu JW, Jordan S, Rose RA, Hughson MD, Manning RD Jr. Immune suppression prevents renal damage and dysfunction and reduces arterial pressure in salt-sensitive hypertension. Am J Physiol Heart Circ Physiol 292: H1018–H1025, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Tian N, Moore RS, Braddy S, Rose RA, Gu JW, Hughson MD, Manning RD Jr. Interactions between oxidative stress and inflammation in salt-sensitive hypertension. Am J Physiol Heart Circ Physiol 293: H3388–H3395, 2007. [DOI] [PubMed] [Google Scholar]
- 41.Tian N, Rose RA, Jordan S, Dwyer TM, Hughson MD, Manning RD Jr. N-acetylcysteine improves renal dysfunction, ameliorates kidney damage and decreases blood pressure in salt-sensitive hypertension. J Hypertens 24: 2263–2270, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Touyz RM Apocynin, NADPH oxidase, and vascular cells. A complex matter. Hypertension 51: 172–174, 2008. [DOI] [PubMed] [Google Scholar]
- 43.Vaziri ND, Liang K, Ding Y. Increased nitric oxide inactivation by reactive oxygen species in lead-induced hypertension. Kidney Int 56: 1492–1498, 1999. [DOI] [PubMed] [Google Scholar]
- 44.Welch WJ, Tojo A, Wilcox CS. Roles of NO and oxygen radicals in tubuloglomerular feedback in SHR. Am J Physiol Renal Physiol 278: F769–F776, 2000. [DOI] [PubMed] [Google Scholar]
- 45.Ximenes VF, Kanegae MP, Rissato SR, Galhiane MS. The oxidation of apocynin catalyzed by myeloperoxidase: proposal for NADPH oxidase inhibition. Arch Biochem Biophys 457: 134–141, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Y, Chan MM, Andrews MC, Mori TA, Croft KD, McKenzie KU, Schyvens CG, Whitworth JA. Apocynin but not allopurinol prevents and reverses adrenocorticotropic hormone-induced hypertension in the rat. Am J Hypertens 18: 910–916, 2005. [DOI] [PubMed] [Google Scholar]