Abstract
Recent epidemiological and clinical studies indicate that the control of sleep-wake states may be an important factor in the regulation of energy metabolism. Leptin is a peripherally synthesized hormone that has critical signaling properties in the brain for the control of long-term energy homeostasis. In this study, we examined the hypothesis that leptin signaling exerts a role in sleep-wake regulation and that leptin may represent an important mechanistic link in the coordination of sleep-wake states and metabolism. Sleep-wake patterns were recorded in a genetic mouse model of obesity and diabetes, the db/db mouse, which harbors a mutation in a particular isoform of the leptin receptor (long form, LRb). We found that db/db mice exhibit a variety of alterations in sleep regulation, including an increase in overall sleep time, a dramatic increase in sleep fragmentation, attenuated diurnal rhythmicity in rapid eye movement sleep and non-rapid eye movement EEG delta power (a measure of sleep homeostatic drive), and a decrease in the compensatory response to acute (i.e., 6 h) sleep deprivation. The db/db mice also generated low amounts of locomotor activity and a reduction in the diurnal rhythm of activity. These results indicate that impaired leptin signaling has deleterious effects on the regulation of sleep amount, sleep architecture, and temporal consolidation of these arousal states. In summary, leptin may represent an important molecular component in the integration of sleep, circadian rhythms, and energy metabolism.
Keywords: sleep fragmentation, metabolic syndrome, energy metabolism, sleep homeostasis, sleep deprivation
obesity represents a major health epidemic in the United States and is continuing to increase in prevalence and severity in the adult, adolescent, and childhood age groups [National Health and Nutrition Examination Surveys; www.cdc.gov, (7)]. The causes of obesity and the complications that arise from it entail a combination of genetic, physiological, and behavioral factors. Interestingly, recent epidemiological and clinical studies indicate that the control of sleep-wake states may be an important factor in the regulation of energy metabolism (16, 28, 32–34). For example, numerous epidemiological studies have demonstrated a relationship between reduced sleep time and risk factors for obesity, diabetes, and cardiovascular disease (16, 22, 31, 33, 39). In humans, obesity is commonly associated with excessive daytime sleepiness, even in individuals without sleep-disordered breathing (6, 38). A few animal studies have shown changes in sleep-wake amount and architecture in genetic and diet-induced models of obesity (8, 14, 18, 19). These findings suggest that obesity may represent an independent risk factor for impaired sleep-wake regulation. If there is a link between sleep and obesity, then an important step will be to identify the nature of the physiological and molecular pathways that coordinate sleep-wake regulation and metabolic function.
One approach to elucidating the sleep-metabolism link has been to identify particular molecules that may coregulate sleep and energy metabolism. A primary hormone involved in glucose and lipid metabolism, as well as appetite regulation, is leptin (1). However, only a few studies have examined how leptin may affect sleep in animal models. For example, following the peripheral injection of a high-dose of leptin (1.3 mg/kg), rats increased slow-wave sleep and decreased rapid eye movement (REM) sleep (30). We have shown that peripheral leptin administration (1.3 mg/kg) reduces REM sleep time in mice (A. D. Laposky, unpublished observations). Two studies in leptin-resistant Zucker fatty rats (fa/fa) found increased sleep time and abnormal levels of sleep fragmentation; however, these studies had methodological limitations, including the lack of a proper control group (5) and a truncated recording period (21). Our laboratory has reported that the absence of leptin in mice, due to a mutation in the gene (ob) encoding leptin, results in elevated non-rapid eye movement (NREM) sleep time, increased sleep fragmentation, and other abnormalities in diurnal rhythms and homeostatic sleep drive (19). Because leptin is a critical factor in controlling long-term energy homeostasis and has the ability to modulate sleep-wake states, its role as a possible coregulatory factor between sleep and metabolic processes is of particular interest.
While the ob/ob mouse is an important model to examine leptin function, most cases of human obesity and diabetes do not involve leptin deficiency, but instead entail increased leptin production and resistance to its action. In mice, a diabetes (db) mutation was identified in the gene encoding an isoform (LRb) of the leptin receptor (2, 3, 12). Homozygous db/db mice produce high levels of leptin, but cannot respond properly to leptin signaling (35), reflecting a state of leptin resistance. In the present study, we performed a comprehensive phenotypic analysis of sleep-wake regulation in db/db mice and examined the diurnal rhythms of sleep and locomotor activity. The results show that db/db mice have notable changes in sleep time, sleep EEG waveforms, the diurnal rhythms of sleep and activity, as well as alterations in the compensatory response to sleep deprivation. The db/db mouse represents another important genetic model to investigate the physiological and molecular mechanisms linking sleep and energy metabolism.
MATERIALS AND METHODS
A group of 3- or 4-mo-old male B6-Leprdb (db/db, n = 13) mice and age-matched male wild-type (wt; n = 13) C57BL/6J controls were used for sleep-wake recordings. A separate group of db/db (n = 8) and wt (n = 8) mice were used for locomotor activity recordings. All mice were purchased from Jackson Laboratories (Bar Harbor, ME) and were housed in a light (12:12-h light-dark cycle, lights on 0800) and temperature (23–24°C)-controlled environment with free access to food (LabDiet, PMI Nutrition International, LLC, Brentwood, MO) (protein 18%, fat 6%, carbohydrate 55%, fiber 5%) and water. All procedures were in accordance with the policies and regulations outlined by the Animal Use and Care Committee at Northwestern University.
Sleep Recording and Data Analysis
Mice were surgically implanted with epidural electrodes for EEG recording and with bilateral nuchal muscle electrodes for electromyographic (EMG) recording, as previously detailed (19). Following a 14-day recovery period, db/db (n = 13) and wt (n = 13) mice were individually housed in sleep recording cages equipped with a wire tether/commutator system (Plastics One, Roanoke, VA) for the transmission of EEG/EMG signals. After 7 days of acclimation to the recording environment, EEG/EMG waveforms were collected for 48 h of baseline sleep-wake recording using standard amplification and filter settings (19). Animals were then sleep deprived for 6 h by gentle handling beginning 2 h after light onset (zeitgeber time, ZT 2). An uninterrupted recovery sleep period lasted for 16 h (ZT 8–ZT 24).
For data analysis, EEG and EMG recordings were divided into 10-s epochs and scored via visual inspection as either wake, NREM, or REM sleep, using previously described criteria (19). The distribution of sleep/wake parameters was quantified by determining sleep and wake amounts for the individual 12-h light and 12-h dark periods, as well as for individual 4-h intervals across the light-dark (LD) periods. The diurnal distribution of sleep-wake states was calculated as the amount of each state occurring in the 12-h light phase as a percentage of 24-h sleep time (light %). Sleep architecture was determined by the number of arousals from sleep (NREM or REM sleep interrupted by a 10-s epoch of wakefulness), stage shifts (number of transitions between 10-s epochs of wake, NREM, and REM), sleep/wake bouts (at least two consecutive epochs, 20 s, of wake, NREM, or REM) and the average duration (minutes) of sleep/wake bouts. For quantitative spectral analysis of the EEG, the following frequency bins were used: delta (1–4 Hz), theta I (4–8 Hz), theta II (6–10 Hz), sigma (11–15 Hz), and higher-frequency (15–35) ranges. Because absolute power density values can show substantial interindividual variability, the power values were normalized and expressed as a percentage of the individual 24-h mean.
Locomotor activity monitoring.
At 12 wk of age, 6 male db/db (51.3 ± 1.5 g) and 8 age-matched wt mice (26.5 ± 0.7 g) were individually housed in cages with wall-mounted infrared sensors. After 2 wk of acclimation to the recording environment, activity patterns were recorded (ClockLab, Actimetrics, Evanston, IL). For each day of recording, activity counts were accumulated over the 12-h light, 12-h dark, and overall 24-h periods. Activity counts were averaged over 5 days of recording in each db/db and wt mouse to reduce variability within the samples.
Basic metabolic phenotype assessment.
Measurements of body weight, food intake, and plasma glucose, insulin, and leptin levels were taken in a subset of wt (n = 8) and db/db (n = 8) mice 1 wk after the completion of sleep-wake testing. Body weight was taken at light onset (ZT0). Food intake was determined by manually weighing food just before ZT0 and just before dark onset (ZT12). At ZT0 and ZT12 (same day), blood was collected (tail nick, 80 μl) for glucose, insulin, and leptin measurements. Plasma glucose concentrations were determined using an automatic glucose monitor (Precision QIU System, Medisense, Bedford, MA). Insulin and leptin samples were analyzed using commercially available ELISA kits (Crystal Chem, Elk Grove, IL), as detailed in previous publications (17, 36).
Statistical analysis.
Repeated-measures ANOVA was used to make genotype (db/db vs. wt), time (12-h light vs. 12-h dark phases, or 4-h intervals), and genotype × time comparisons. In a few instances, t-tests were performed to make simple between- and within-genotype comparisons. Significance levels were set at P < 0.05 in all analyses. Statistics were performed using Statistica (StatSoft, Tulsa, OK).
RESULTS
Baseline Sleep/Wake Architecture
Sleep-wake amount.
Total sleep time was increased in db/db (+56 min) compared with wt mice [genotype, F(1,24) = 5.9, P < 0.05], due to a genotype difference in the 12-h dark phase [genotype × time, F(1,24) = 7.0, P < 0.01] (Table 1). Overall, NREM sleep was elevated in db/db mice (+65 min) [genotype, F(1,24) = 8.4, P < 0.01] due to increases in the dark phase [genotype × time, F(1,24) = 4.7, P < .05]. In contrast, db/db mice had reduced total REM sleep (−9 min) [genotype, F(1,24) = 6.2, P < .05], due to a combined decrease in the light phase and increase in the dark phase [genotype × time, F(1,24) = 13.5, P < 0.001].
Table 1.
Sleep-wake amounts
| wt | db/db | P | |
|---|---|---|---|
| TST | |||
| Light | 475±10 | 476±9 | |
| Dark | 222±11 | 277±12 | † |
| 24-h | 697±15 | 753±16 | * |
| NREM | |||
| Light | 425±8 | 438±8 | |
| Dark | 207±11 | 260±11 | † |
| 24-h | 633±14 | 698±16 | † |
| REM | |||
| Light | 49±3 | 37±2 | † |
| Dark | 14±1 | 17±1 | * |
| 24-h | 63±3 | 54±3 | * |
Amount of total sleep (TST), non-rapid eye movement (NREM) sleep, and rapid eye movement (REM) sleep in wild-type (wt, n =13) and (db/db, n =13) mice. Sleep amounts (minutes ± SE) were accumulated over the total 24-h recording period, and for the individual 12-h light and 12-h dark periods. Group differences for each time block (
P < 0.05,
P < 0.01).
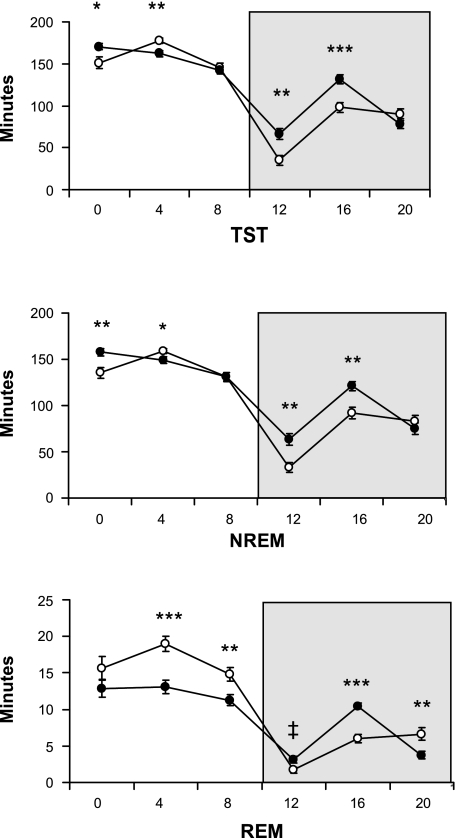
Figure 1 depicts the distribution of sleep-wake states in 4-h intervals across the LD cycle to show more specifically where genotype differences occurred in total sleep time [genotype, F(1,24) = 5.9, P < 0.05; genotype × time, F(5,120) = 7.7, P < 0.001], NREM sleep [genotype, F(1,24) = 8.4, P < 0.001; genotype × time, F(5,120) = 6.8, P < 0.001], and REM sleep [genotype, F(1,24) = 6.2, P < 0.05; genotype × time, F(5,120) = 9.9, P < 0.001].
Fig. 1.
Amount (minutes ± SE) of total sleep time (TST), non-rapid eye movement sleep (NREM), and rapid eye movement sleep (REM) in 4-h intervals across the 12:12-h light-dark cycle. There are a number of time points in which wild-type (wt; n = 13, ○) and db/db (n = 13, •) differ in the light and dark (shaded background) phases. Time of day is depicted as zeitgeber time (ZT, x-axis label); ZT0, onset of the light phase; ZT12, onset of the dark phase. Genotype differences at respective time intervals (*P < 0.05, **P < 0.01, ***P < 0.001, and ‡P = 0.07).
Sleep consolidation and bout analysis.
In db/db mice, sleep architecture was disrupted with increased arousals from sleep [genotype, F(1,24) = 10.0, P < 0.01] and more stage shifts [genotype, F(1,24) = 21.2, P < 0.001] (Table 2). The number of wake bouts [genotype, F(1,24) = 27.2, P < 0.001] and NREM bouts [genotype, F(1,24) = 22.3, P < 0.001] was elevated in db/db mice, whereas the average duration of individual wake bouts [genotype, F(1,24) = 16.1, P < 0.001] and NREM bouts [genotype, F(1,24) = 11.5, P < 0.01; genotype × time, F(1,24) = 4.5, P < 0.05] was shorter vs. wt mice. For REM sleep, the number of bouts was decreased in the light phase and increased in the dark phase [genotype × time, F(1,24) = 10.1, P < 0.01].
Table 2.
Sleep-wake fragmentation and bout analysis
| wt | db/db | P | |
|---|---|---|---|
| Stage Shifts | |||
| Light | 329.3±15.4 | 430.5±26.9 | § |
| Dark | 201.3±18.1 | 272.9±11.5 | § |
| 24 h | 530.6±29.2 | 703.4±24.6 | § |
| Arousals | |||
| Light | 85.4±5.1 | 109.1±4.7 | † |
| Dark | 48.1±5.1 | 59.6±2.8 | ‡ |
| 24 h | 132.4±8.8 | 167.6±6.1 | † |
| No. W bouts | |||
| Light | 61.1±2.9 | 91.3±4.7 | § |
| Dark | 48.1±4.2 | 70.3±3.9 | § |
| 24 h | 108.8±6.1 | 161.2±7.6 | § |
| No. NREM bouts | |||
| Light | 140.3±6.2 | 186.1±5.6 | § |
| Dark | 87.3±8.1 | 119.4±5.5 | † |
| 24 h | 227.1±12.3 | 305.0±9.9 | § |
| No. REM bouts | |||
| Light | 36.5±2.2 | 29.2±1.8 | * |
| Dark | 11.2±1.0 | 14.5±0.8 | * |
| 24 h | 47.6±2.1 | 43.7±2.1 | |
| Dur. W bouts | |||
| Light | 3.9±0.3 | 2.5±0.1 | § |
| Dark | 11.6±1.3 | 6.5±0.5 | † |
| 24 h | 7.0±0.5 | 4.2±0.2 | § |
| Dur. NREM bouts | |||
| Light | 3.1±0.1 | 2.4±0.1 | § |
| Dark | 2.5±0.2 | 2.2±0.1 | |
| 24 h | 2.9±0.1 | 2.3±0.1 | § |
| Dur. REM bouts | |||
| Light | 1.4±0.04 | 1.3±0.05 | |
| Dark | 1.3±0.07 | 1.2±0.04 | |
| 24 h | 1.3±0.04 | 1.2±0.04 |
Amount of sleep fragmentation was quantified in wild-type (wt, n =13) and db/db (n =13) mice across the 24-h recording period and for individual 12-h-light and 12-h-dark periods. Groups were compared for stage shifts, brief arousals from sleep, the number (No.) of sleep and wake bouts and the average duration (Dur., minutes) of individual sleep and wake bouts. Values represent group means ± SE. Genotype comparisons (
P < 0.07,
P < 0.05,
P < 0.01,
P < 0.001).
The diurnal rhythm of sleep was less robust in db/db mice, indicated by a smaller proportion of total NREM sleep [wt, 67.3 ± 1.2% vs. db/db, 62.9 ± 1.1%; t(24) = 2.7, P < 0.05] and total REM sleep [wt, 77.0 ± 2.0% vs. db/db, 67.6 ± 1.7%; t(24) = 3.5, P < 0.01], occurring in the light phase (Table 1 and Fig. 1).
EEG spectral power.
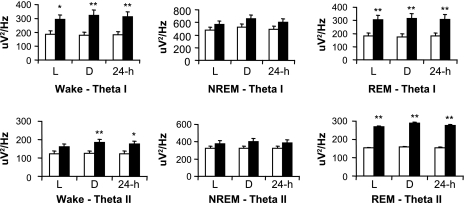
In the waking state, db/db mice had higher levels of Theta I [genotype, F(1,24) = 8.8, P < 0.01] and Theta II [genotype, F(1,24) = 4.9, P < 0.05; genotype × time, F(1,24) = 17.1, P < 0.001] power compared with wt mice (Fig. 2), whereas no genotype differences occurred in the other frequency bands (data not shown). During REM sleep, Theta I [genotype, F(1,24) = 9.6, P < 0.01] and Theta II [genotype, F(1,24) = 9.2, P < 0.01] were elevated in db/db mice (Fig. 2). No genotype differences occurred for any frequency band during NREM sleep.
Fig. 2.
EEG spectral analysis was performed during wakefulness (wake, left), NREM (middle), and REM (right), as well as for the 12-h light and 12-h dark phases. Absolute EEG power (μV2/Hz ± SE) in the theta I (4–8 Hz) and theta II (6–10 Hz) frequency bands is presented. There is a significant increase in theta I and theta II power in db/db mice vs. wt animals during wakefulness and REM sleep. Genotype differences (*P < 0.05, **P < 0.01).
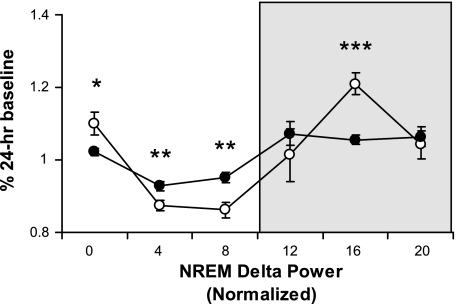
The diurnal rhythm of homeostatic sleep drive was quantified by examining the distribution of NREM delta power across the LD cycle (Fig. 3). Db/db mice showed an attenuated NREM delta power rhythm [genotype × time, F(1,120) = 4.1, P < 0.01] with lower levels at light onset, less of a decline across the light phase, and less of an increase during the dark phase vs. wt mice.
Fig. 3.
NREM delta (1–4 Hz) power (means ± SE) was determined for each 4-h interval across the 12:12-h light-dark cycle and normalized as a percentage of the 24-h baseline value. Genotype differences between wild-type (○) and db/db (•) mice in both the light and dark (shaded background) phases (*P < 0.05, **P < 0.01, ***P < 0.001). These data indicate that db/db mice have an attenuated diurnal rhythm of sleep drive across the L:D cycle.
Recovery From 6-h Sleep Deprivation
Baseline vs. recovery (within-genotype comparisons).
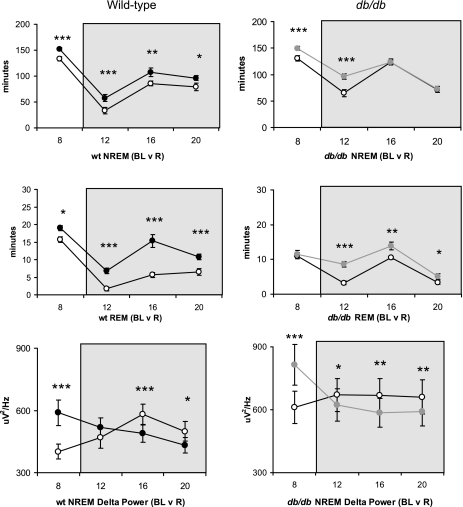
The response to sleep deprivation was assessed within each genotype by comparing recovery sleep to a corresponding period of baseline sleep (Fig. 4). In wt mice, NREM [recovery, F(1,10) = 63.1, P < 0.001] and REM [recovery, F(1,10) = 78.4, P < 0.001] sleep time was significantly elevated throughout the recovery period compared with baseline. NREM delta power was increased at the beginning of the recovery opportunity, then decreased to below baseline levels during the dark phase [recovery × time, F(3,30) = 14.8, P < 0.001].
Fig. 4.
The recovery response from sleep deprivation is graphed separately for wild-type (left) and db/db (right) mice. Animals were sleep deprived for 6-h between ZT2 and ZT8 (zeitgeber time). The recovery period is depicted in 4-h intervals and consists of the last 4-h of the light phase (ZT8–Z12) and the immediate 12-h dark period (shaded background). For the wild-type group, baseline (○) is compared with recovery (•) for NREM sleep (minutes, top), REM sleep (minutes, middle), and absolute NREM delta power (μV2/Hz, bottom). Data represent group means ± SE at each time interval. Similar comparisons were made for baseline (○) vs. recovery (gray circles) in db/db mice. Within-genotype differences at respective time points (*P < 0.05, **P < 0.01, ***P < 0.001).
In db/db mice, the rebound in NREM [recovery, F(1,10) = 34.5, P < 0.001; recovery × time, F(3,30) = 9.6, P < 0.001] and REM [recovery, F(1,10) = 29.8 P < 0.01; recovery × time, F(3,30) = 6.7, P < 0.001] sleep was time dependent (Fig. 4). Db/db mice showed an increase in NREM delta power in the light phase and a decrease to below baseline levels in the dark [recovery × time, F(3,30) = 35.2, P < 0.001].
Recovery sleep: percentage change from baseline (between-genotype comparisons).
Because there were differences in baseline sleep between wt and db/db, particularly in the dark phase, genotype comparisons were made on normalized (% change from baseline) recovery values, rather than on absolute values of recovery sleep. The magnitudes of NREM and REM recovery were significantly reduced in db/db vs. wt mice (Table 3); however, the magnitude of NREM EEG delta power rebound was similar between genotypes.
Table 3.
Recovery sleep (% change from baseline)
| Wt | db/db | P | |
|---|---|---|---|
| NREM | |||
| 4-h Light | +15±2.7 | +15.1±3.0 | |
| 12-h Dark | +34±7.0 | +13.6±3.4 | * |
| 16-h Total | +25±3.8 | +13.6±2.3 | * |
| REM | |||
| 4-h Light | +25±9.0 | +3.5±7.8 | * |
| 12-h Dark | +159±29.6 | +62.0±10.2 | † |
| 16-h Total | +79±9.4 | +38.0±6.9 | † |
| TST | |||
| 4-h Light | +15.3±2.4 | +15.3±2.4 | |
| 12-h Dark | +40.4±6.9 | +16.4±3.3 | † |
| 16-h Total | +29.3±3.5 | +15.2±2.3 | † |
| NDP | |||
| 4-h Light | +44.5±5.2 | +34.3±3.1 | |
| 12-h Dark | −13.1±2.6 | −11.9±1.9 | |
| 16-h Total | +5.4±2.7 | +1.7±1.6 |
The magnitude of recovery sleep following acute (i.e., 6-h) sleep deprivation was determined individually for wild-type and db/db mice. The amount of NREM and REM sleep (minutes), as well as NREM EEG delta (1–4 Hz) power (NDP) was calculated as a percentage change from respective baseline levels. The recovery period was divided into time blocks corresponding to the last 4 h of the light phase, the 12-h dark period, and the total 16-h recovery period. The magnitude of sleep recovery (percentage change from baseline (%) ± SE) was compared between genotypes at each respective time block (
P < 0.05,
P < 0.01). Sleep recovery time is consistently reduced in magnitude in db/db vs. wt mice.
Basic Metabolic Phenotype Assessments
The db/db mice exhibited a significant increase in body weight, glucose, insulin, leptin, and food intake levels during both the light and dark periods compared with wt mice (Table 4). Overall, these data confirm that db/db animals were obese and hyperphagic, as well as leptin and insulin resistant at the time of sleep-wake recording.
Table 4.
Basic metabolic phenotype in wt and db/db mice
| wt | db/db | P | |
|---|---|---|---|
| BW, g | |||
| am | 28.7±0.8 | 50.8±1.1 | ‡ |
| pm | |||
| Glucose, ml/dl | |||
| am | 120.3±4.6 | 205.9±31.7 | * |
| pm | 145.3±3.2 | 420.5±35.7 | ‡ |
| Insulin, ng/ml | |||
| am | 1.4±0.4 | 29.7±1.7 | ‡ |
| pm | 3.7±1.0 | 37.5±1.9 | ‡ |
| Leptin, ng/ml | |||
| am | 3.0±0.5 | 64.4±4.6 | ‡ |
| pm | 4.0±0.5 | 47.2±3.4 | ‡ |
| Food intake, g | |||
| am | 1.4±0.2 | 1.5±0.3 | |
| pm | 3.9±0.4 | 6.0±0.4 | † |
Basic metabolic phenotype in adult (3–4 mo) wt (n = 8) and db/db (n = 8) mice. Data were collected at lights on (am) and lights off (pm). Values are expressed as means ± SE values for body weight (ZT 0) and glucose (ml/dl), insulin (ng/ml), leptin (ng/ml), and food intake (grams) (ZT0 and ZT12) were compared between genotypes (
P < 0.05,
P < 0.01,
P < 0.001). These data confirm that db/db mice were obese, hyperphagic, insulin resistant, and hyperleptinemic at the time of sleep-wake recording.
Locomotor Activity Levels and Diurnal Rhythms in Wild-Type and db/db Mice
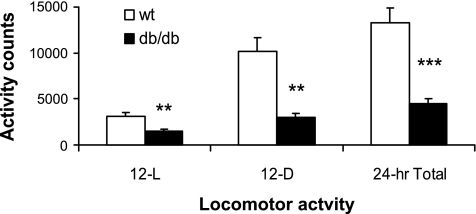
Db/db mice produced significantly less activity counts compared with wt mice [genotype, F(1,11) = 77.3, P < 0.001] (Fig. 5). Furthermore, db/db mice generated a smaller percentage of 24-h activity during the 12-h dark period [wt, 79 ± 2.8% vs. db/db, 66.0 ± 2.3%; t(11) = 3.71, P < 0.01].
Fig. 5.
Locomotor activity was measured in wild-type (wt, n = 8, open bars) and db/db mice (n = 6, closed bars) by infrared sensor beam breaks (y-axis, activity counts) across the 12-h light (12-L), 12-h dark (12-D), and total 24-h period (x-axis). Data represent group means ± SE for activity counts. Db/db mice have significantly less activity across the entire day vs. wt mice (**P < 0.01, ***P < 0.001). The db/db mice have an attenuated diurnal rhythm in activity with less of a variation across the 12-L and 12-D periods compared with wt animals (see text for statistics).
DISCUSSION
An accumulation of epidemiological and human clinical studies has shown that voluntary or experimental daily sleep restriction may be an important risk factor associated with obesity, diabetes, and cardiovascular disease (16, 32, 33). While sleep loss may place the individual on a trajectory for developing cardiometabolic disease, there may also be unique and independent effects of altered metabolic processes (e.g., obesity) on sleep-wake patterns. For example, obese individuals commonly exhibit excessive daytime sleepiness (EDS), even in the absence of sleep-disordered breathing, indicating the obesity may represent an independent risk factor for sleep-wake disturbance (6, 38). An important aspect of elucidating the relationship between sleep and energy metabolism is to carefully define the sleep phenotype in obese subjects and to identify key molecular and genetic mechanisms that may serve a common coregulatory function that link sleep and metabolic processes. In this study, we examined sleep-wake regulation in the adult db/db mouse, a genetic model of leptin resistance resulting in obesity and accompanying symptoms of metabolic dysregulation (13, 35, 40).
The db/db mice exhibited a variety of alterations in baseline sleep-wake architecture, including an increase in 24-h NREM sleep time and a decrease in overall REM sleep (Table 1 and Fig. 1). In addition to changes in sleep amount, sleep fragmentation was abnormally elevated in db/db animals (Table 2). Db/db mice awakened and fell asleep more frequently than wt mice and were unable to sustain individual sleep-wake bouts for a normal length of time. The diurnal rhythms of sleep-wake states, locomotor activity, and NREM EEG delta power (a quantitative measure of sleep intensity and homeostatic sleep drive) were attenuated in db/db mice (Figs. 1, 3, and 5). Following acute (6-h) sleep deprivation, both wt and db/db mice exhibited a similar compensatory response in NREM delta power (Fig. 4); however, the magnitude of NREM and REM rebound was smaller in db/db mice (Table 3), indicating partial impairment in recovering lost sleep time. In combination, the data indicate that db/db mice exhibit fundamental alterations in sleep-wake regulation under both baseline conditions and following acute sleep loss.
On the basis of epidemiological and clinical data, a plausible hypothesis is that obese animals will exhibit reduced amounts of sleep time, consistent with the findings that sleep loss can precede the onset of metabolic perturbation, even in otherwise healthy young adults (16, 31, 32). Another hypothesis is that obese animals will have increased sleep time, consistent with the findings of increased EDS in obese humans (6, 38). One conceptual model is that these may be independent processes; insufficient sleep contributes to the manifestation of cardiometabolic perturbations, and alterations in metabolism have independent effects on sleep-wake regulatory processes. Because few studies have quantified EEG/EMG-defined sleep-wake patterns in obesity, it is not definitive whether increased EDS in obesity is the result of alterations in sleep time, impaired sleep architecture (e.g., sleep fragmentation), or a combination of factors. Our data clearly indicate that adult db/db mice, which exhibit a fully manifested obesity and leptin-resistant phenotype, generate significantly increased amounts of NREM sleep, as well as abnormal levels of sleep fragmentation (frequent arousals from sleep and increased transitions between sleep-wake states). It is possible that db/db mice attempt to compensate for impaired sleep consolidation by increasing NREM sleep time. However, because the extra sleep is characterized by fragmented NREM episodes, it is unlikely that this extra sleep time is restorative in nature and indicates that these mice may be in a state of chronic sleep impairment. The extra sleep obtained by db/db mice occurs predominantly during the dark phase, and mimics the EDS that is common in obesity, as well as sleep disorders that disrupt sleep architecture, such as obstructive sleep apnea, periodic leg movement disorder, and narcolepsy (24, 37). The increase in sleep fragmentation may also account for the altered diurnal rhythm of homeostatic sleep drive in db/db mice; they may be unable to accumulate and dissipate sleep drive normally due to the frequent disruption of sleep and wake bouts. The attenuated rhythm in NREM delta power indicates that db/db mice maintain a more constant level of sleep pressure across the 12:12-h light-dark cycle, whereas wt animals consolidate their sleep pressure and release to more defined periods of the light-dark cycle. In combination, these data indicate that the db/db mouse may represent a valuable animal model to investigate the consequences of sleep fragmentation on arousal system function, as well as to establish sleep fragmentation as a potentially important factor contributing to metabolic dysregulation.
Sleep-wake patterns have previously been characterized in obese leptin-deficient, ob/ob, mice and in a murine model of diet-induced obesity (DIO) (8, 14, 19). Perhaps, not surprisingly, sleep regulation is quite similar in db/db and ob/ob animals. Ob/ob mice exhibited increased NREM sleep time in the dark phase of about the same magnitude as db/db mice (∼1 h increase), increased sleep fragmentation, attenuated diurnal rhythms in REM and NREM delta power, a trend for decreased REM sleep time, and reduced sleep rebound in response to acute sleep deprivation (19). The major physiological difference between db/db and ob/ob mice is leptin resistance due to a mutation in the gene encoding the long (LRb) receptor isoform vs. leptin deficiency due to a mutation in the gene encoding the production of leptin, respectively (2, 40). On the basis of the similarity of the sleep phenotypes in these genetic models, it is likely that the effects of leptin on sleep-wake regulation are mediated through the LRb receptor rather than being a consequence of impaired leptin signaling in general, or through one of the other leptin receptor isoforms (23). Indeed, the majority of central nervous system functions of leptin, in particular, energy regulation, are known to occur via the LRb receptor (23).
Replication of the sleep phenotype in db/db mice provides a framework to investigate specific areas in the central nervous system and particular metabolic pathways, involving leptin signaling that may mediate the direct effects of leptin on sleep and the integration of sleep and energy metabolism. For example, Coppari and colleagues (4) performed an eloquent study to isolate the role of leptin signaling in the arcuate nucleus on glucose regulation and locomotor activity levels in mice. Therefore, an interesting working hypothesis is that sleep and energy homeostasis is integrated through leptin-mediated mechanisms in the arcuate nucleus. Indeed, there are other regions of the hypothalamus and brain stem, where the LRb exists, that have roles in metabolism and sleep (9, 20). The pursuit of anatomical localization, possibly though transgenic approaches to LRb expression, may enhance knowledge regarding leptin's potential independent role in sleep-wake regulation, as well as the pathways by which this hormone coordinates sleep and energy metabolism.
Another obesity model, DIO, in mice results in notable increases in NREM sleep time, similar to the db/db and ob/ob sleep phenotypes (8, 14, 18). On the other hand, there is no indication of reduced REM sleep time, attenuated diurnal sleep-wake rhythms, alterations in EEG spectral power, or impairments in recovery from acute sleep deprivation in DIO mice (8, 14, 18). These results represent clear differences from the db/db and ob/ob models. In our data set, DIO resulted in elevated sleep fragmentation (18), whereas NREM consolidation was normal in DIO mice in studies by Jenkins et al. (14) and Guan et al. (8). This discrepancy could be due to methodological differences in how sleep bouts were defined between the studies. This is an important point that needs further clarification, particularly in the context of determining whether increased sleep time in DIO is related to the degree of sleep fragmentation, or is mediated by some other independent effect of the diet or obesity. Guan et al. (8) recently demonstrated the normalization in the sleep-wake patterns of DIO mice after weight loss, although levels of leptin and insulin were not measured to determine whether the sleep changes were related to changes in metabolic hormones. We have collected preliminary data indicating that leptin replacement in ob/ob mice results in improved sleep-wake patterns (e.g., normalizes sleep time and reduces sleep fragmentation); however, further analysis is required to determine whether these changes are directly related to weight loss, or to other physiological or molecular signaling pathways mediated by leptin (29).
A consequence of obesity that may impact sleep time and sleep architecture is impaired respiration. While there is no indication that db/db or ob/ob mice have upper airway obstruction (i.e., obstructive sleep apnea), it has been demonstrated that ob/ob mice exhibit reduced baseline ventilation and impaired hypercapnic ventilatory response during both wakefulness and sleep (15, 26). Therefore, a notable feature of sleep in obese mice, increased sleep fragmentation and EDS, could result from the inability to regulate respiration and blood gas levels normally (25, 27). Future studies are needed to determine whether an improvement in ventilation following leptin administration plays a role in the improvements in sleep-wake patterns. An important topic in clinical research is to differentiate the role of intermittent hypoxia vs. sleep fragmentation as effectors of cardiometabolic disease in sleep-disordered breathing. Comparative studies using genetic and environmental models of obesity may represent an important approach in these investigations.
While we did not measure body temperature in db/db mice, we previously reported that ob/ob mice have lower body temperature and have smaller changes in body temperature between sleep-wake state transitions compared with control animals (19); hypothermia is well characterized db/db mice. It is possible that thermoregulatory deficits underlie some of the sleep alterations in db/db mice, including sleep time and fragmentation, as well as decreased locomotor activity. Intriguingly, in mammals that hibernate, sleep appears to be an important transition state in and out of hibernation (11). In states of torpor, NREM sleep predominates, and as body temperature continues to decrease, REM sleep is suppressed (11), similar to the sleep phenotype in db/db and ob/ob mice. Furthermore, both leptin resistance and torpor represent states in which the organism is programmed to conserve energy. While the extent to which sleep in db/db mice could reflect a torpor-like state is unclear, the conceptual parallel raises interesting questions regarding the coordination of energy metabolism and sleep-wake regulation.
It is also interesting to note that wake and REM EEG theta (4–10 Hz) power were increased in db/db animals. Theta oscillations in the hippocampus have been shown to be important for the induction of long-term potentiation (LTP) and long-term depression (LTD), neurophysiological processes involved in synaptic plasticity and memory consolidation (10). The leptin receptor is expressed in various regions of the hippocampus, and leptin-resistant fa/fa rats and db/db mice exhibit impairments in LTP and LTD, as well as on behavioral tests of memory performance (10). On the basis of reports that leptin administration can enhance LTP, LTD, and cognitive performance in rodents (10), it has become an interesting molecular target in the link between sleep regulation and cognition. Our finding of increased theta power during wakefulness and REM sleep in the db/db mouse is intriguing, although the significance for memory-related function remains to be determined.
Perspectives and Significance
Sleep, energy metabolism, and circadian systems have evolved together over millions of years to optimize the coordination of the organism with its environment, as well as to maintain internal coordination among multiple physiological and molecular processes. However, it has been only in recent years that the extent to which these systems are coordinated with one another at the mechanistic level and the implications that these links have to clinical medicine and human health have come to be appreciated. For example, recent discoveries that chronic partial sleep restriction and circadian desynchronization at the behavioral, as well as molecular level, may represent significant risk factors for obesity, diabetes, metabolic syndrome, and cardiovascular disease have created paradigm shifts in thinking within these basic research and clinical fields. The incidence and severity of obesity and cardiovascular disease are continuing to increase, particularly in industrialized societies where sleep time and normal circadian patterns of behavior are often sacrificed due to work, family, and social demands. Continued research is necessary to identify the key molecular and genetic mechanisms that may serve common coregulatory functions that link sleep, circadian, and metabolic processes. The sleep and circadian fields represent critical research arenas to contribute to the understanding of major health epidemics and to make important contributions relevant to the development of future preventive and treatment solutions to improve human health.
GRANTS
This work was supported by National Institutes of Health Grants P01AG11412 (to F. W. Turek and J. Bass) and R01HL075020 (to J. Bass).
Acknowledgments
We would like to thank Ms. Carmela Estrada for her technical expertise in performing hormone analyses.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Benoit SC, Clegg DJ, Seeley RJ, Woods SC. Insulin and leptin as adiposity signals. Recent Prog Horm Res 59: 267–285, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, Lakey ND, Culpepper J, Moore KJ, Breitbart RE, Duyk GM, Tepper RI, Morgenstern JP. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell 84: 491–495, 1996. [DOI] [PubMed] [Google Scholar]
- 3.Chua SC, Chung WK, Wu-Peng XS, Zhang Y, Liu SM, Tartaglia L, and Leibel RL. Phenotypes of mouse diabetes and rat fatty due to mutations in the OB (leptin) receptor. Science 271: 994–996, 1996. [DOI] [PubMed] [Google Scholar]
- 4.Coppari R, Ichinose M, Lee CE, Pullen AE, Kenny CD, McGovern RA, Tang V, Liu SM, Ludwig T, Chua SC Jr, Lowell BB, and Elmquist JK. The hypothalamic arcuate nucleus: a key site for mediating leptin's effects on glucose homeostasis and locomotor activity. Cell Metab 1: 63–72, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Danguir J Sleep patterns in the genetically obese Zucker rat: effect of acarbose treatment. Am J Physiol Regul Integr Comp Physiol 256: R281–R283, 1989. [DOI] [PubMed] [Google Scholar]
- 6.Dixon JB, Dixon ME, Anderson ML, Schachter L, O'Brien PE. Daytime sleepiness in the obese: not as simple as obstructive sleep apnea. Obesity 15: 2504–2511, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Ford ES Prevalence of the metabolic syndrome in U.S. populations. Endocrinol Metab Clinics N Am 33: 333–350, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Guan Z, Vgontzas AN, Bixler EO, Fang J. Sleep is increased by weight gain and decreased by weight loss in mice. Sleep 31: 627–633, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hakansson ML, Brown H, Ghilardi N, Skoda RC, Meister B. Leptin receptor immunoreactivity in chemically defined target neurons of the hypothalamus. J Neurosci 18: 559–572, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harvey J, Solovyova N, Irving A. Leptin and its role in hippocampal synaptic plasticity. Prog Lipid Res 45: 369–378, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heller CH, Ruby NF. Sleep and circadian rhythms in mammalian torpor. Ann Rev Physiol 66: 275–289, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Hummel KP, Dickie MM, Coleman DL. Diabetes, a new mutation in the mouse. Science 153: 1127–1128, 1966. [DOI] [PubMed] [Google Scholar]
- 13.Ingalls AM, Dickie MM, Snell GD. Obese, a new mutation in the house mouse. J Hered 41: 317–318, 1950. [DOI] [PubMed] [Google Scholar]
- 14.Jenkins JB, Omori T, Guan Z, Vgontzas AN, Bixler EO, Fang J. Sleep is increased in mice with obesity induced by high-fat food. Physiol Behav 87: 255–262, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Kessler R, Chaouat A, Schinkewitch P, Faller M, Casel S, Krieger J, Weitzenblum E. The obesity-hypoventilation syndrome revisited: a prospective study of 34 consecutive cases. Chest 120: 369–376, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med Rev 11: 163–178, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, Kobayashi Y, Turek FW, Bass J. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab 6: 414–421, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Laposky AD, Arbruzova J, Shelton J, Bass J, Turek FW. High-fat diet induces changes in sleep-wake patterns in mice (Abstract). Sleep 29: A38, 2006. [Google Scholar]
- 19.Laposky AD, Shelton J, Bass J, Dugovic C, Perrino N, Turek FW. Altered sleep regulation in leptin-deficient mice. Am J Physiol Regul Integr Comp Physiol 290: R894–R903, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Leshan RL, Bjornholm M, Munzberg H, Myers MG Jr. Leptin receptor signaling and action in the central nervous system. Obesity 14 Suppl 5: 208S–212S, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Megirian D, Dmochowski J, Farkas GA. Mechanism controlling sleep organization of the obese Zucker rats. J Appl Physiol 84: 253–256, 1998. [DOI] [PubMed] [Google Scholar]
- 22.Meier-Ewert HK, Ridker PM, Rifai N, Regan MM, Price NJ, Dinges DF, Mullington JM. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol 43: 678–683, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Myers MG Leptin receptor signaling and the regulation of mammalian physiology. Recent Prog Horm Res 59: 287–304, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Nishino S Clinical and neurobiological aspects of narcolepsy. Sleep Med 8: 373–399, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Donnell CP, Schaub CD, Haines AS, Berkowitz DE, Tankersley CG, Schwartz AR, Smith PL. Leptin prevents respiratory depression in obesity. Am J Respir Crit Care Med 159: 1477–1484, 1999. [DOI] [PubMed] [Google Scholar]
- 26.O'Donnell CP, Tankersley CG, Polotsky VP, Schwartz AR, Smith PL. Leptin, obesity, and respiratory function. Respir Physiol 119: 163–170, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Polotsky VY, Rubin AE, Balbir A, Dean T, Smith PL, Schwartz AR, O'Donnell CP. Intermittent hypoxia causes REM sleep deficits and decreases EEG delta power in NREM sleep in the C57BL/6J mouse. Sleep Med 7: 7–16, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Schultes B, Schmid S, Peters A, Born J, Fehm HL. Sleep loss and the development of diabetes: a review of current evidence. Exp Clin Endocrinol Diabetes 113: 563–567, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Shelton J, Perrino NM, Dugovic C, Bass J, Turek FW, Laposky AD. Sleep alterations in ob/ob mice are reversed with chronic leptin repletion (Abstract). Sleep 28: A94, 2005. [Google Scholar]
- 30.Sinton CM, Fitch TE, Gershenfeld HK. The effects of leptin on REM sleep and slow wave delta in rats are reversed by food deprivation. J Sleep Res 8: 197–203, 1999. [DOI] [PubMed] [Google Scholar]
- 31.Spiegel K, Leproult R, L'Hermite-Baleriaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab 89: 5762–5771, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet 354: 1435–1439, 1999. [DOI] [PubMed] [Google Scholar]
- 33.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med 141: 846–850, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci USA 105: 1044–1049, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tschop M, Heiman ML. Rodent obesity models: an overview. Exp Clin Endocrinol Diabetes 109: 307–319, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J. Obesity and metabolic syndrome in circadian Clock mutant mice. Science 308: 1043–1045, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vaugn BV, D'Cruz OF. Cardinal manifestations of sleep disorders. In: Principles and Practice of Sleep Medicine, 4th ed., edited by Kryger MH, Roth T, Dement, W. C. Philadelphia: Elsevier Saunders, 2005, p. 594–601.
- 38.Vgontzas AN, Bixler EO, Tan TL, Kantner D, Martin LF, Kales A. Obesity without sleep apnea is associated with daytime sleepiness. Arch Intern Med 158: 1333–1337, 1998. [DOI] [PubMed] [Google Scholar]
- 39.Vgontzas AN, Zoumakis E, Bixler EO, Lin HM, Follett H, Kales A, Chrousos GP. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab 89: 2119–2126, 2004. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature 372: 425–432, 1994. [DOI] [PubMed] [Google Scholar]