Abstract
Chronic myocardial infarction (CMI) is associated with remodeling of the ventricle and evokes adaption in the cardiac neurohumoral control systems. To evaluate the remodeling of the intrinsic cardiac nervous system following myocardial infarction, the dorsal descending coronary artery was ligated in the guinea pig heart and the animals were allowed to recover for 7–9 wk. Thereafter, atrial neurons of the intrinsic cardiac plexus were isolated for electrophysiological and immunohistochemical analyses. Intracellular voltage recordings from intrinsic cardiac neurons demonstrated no significant changes in passive membrane properties or action potential configuration compared with age-matched controls and sham-operated animals. The intrinsic cardiac neurons from chronic infarcted hearts did demonstrate an increase in evoked action potential (AP) frequency (as determined by the number of APs produced with depolarizing stimuli) and an increase in responses to exogenously applied histamine compared with sham and age-matched controls. Conversely, pituitary adenylate cyclase-activating polypeptide (PACAP)-induced increases in intrinsic cardiac neuron-evoked AP frequency were similar between control and CMI animals. Immunohistochemical analysis demonstrated a threefold increase in percentage of neurons immunoreactive for neuronal nitric oxide synthase (NOS) in CMI animals compared with control and the additional expression of inducible NOS by some neurons, which was not evident in control animals. Finally, the density of mast cells within the intrinsic cardiac plexus was increased threefold in preparations from CMI animals. These results indicate that CMI induces a differential remodeling of intrinsic cardiac neurons and functional upregulation of neuronal responsiveness to specific neuromodulators.
Keywords: intrinsic cardiac nervous system, histamine, pituitary adenylate cyclase- activating polypeptide, mast cells, intracellular recording
remodeling of cardiac muscle in response to endogenous stressors, such as ischemia or pressure overload, has been demonstrated in numerous cellular systems (2, 7, 20, 21). These changes in muscle structure and biochemistry result in both compensatory and pathological alterations that ultimately result in cardiac dysfunction (16, 26). For example, previous studies have shown significant changes in the expression of nitric oxide synthase (NOS) isoforms within cardiac myocytes following myocardial infarction and reperfusion (7), as well as with chronic infarction (2). These changes are associated with alterations in myocyte contractility and regulation by sympathetic neurotransmitters (7). Although the alterations in cardiac myoctyes have been studied intensively postmyocardial infarction, there has been little attention given to potential changes in the intrinsic cardiac neurons, the regulatory system that functions as the final common pathway for neural control of the heart (1).
The intrinsic cardiac nervous system processes information from a variety of inputs, including sensory afferents, descending parasympathetic preganglionic fibers, sympathetic fibers, interneurons (local circuit neurons), and cardiac mast cells (1, 11, 22, 25). This convergence of information leads to integrative control of regional cardiac function. Alterations in subpopulations of these neurons, in response to injury, can result in significant changes in the neuronal regulation of regional cardiac function (1).
The present studies evaluated changes in physiological function and phenotype of atrial intrinsic cardiac neurons following a chronic myocardial infarction (CMI) of the left ventricle. Several different neuromodulators can modify neuronal excitability in these cells. For example, histamine (22), substance P (11), and pituitary adenylate cyclase-activating polypeptide (PACAP) (4, 28) can all increase evoked action potential (AP) frequency of these neurons. Within the guinea pig intrinsic cardiac nervous system, the primary source of histamine is from cardiac mast cells (22). Previous studies have shown that mast cell number can increase with heart disease (17) and that ischemic events can stimulate mast cell degranulation (3, 23). Therefore, we examined the histamine-induced responses of intrinsic cardiac neurons to determine whether CMI led to alterations in histamine sensitivity. The release of cytokines and other inflammatory mediators from mast cells also can lead to phenotypic alterations in expression of enzymes such as NOS and also may produce changes in neuronal responses. Accordingly, guinea pig intrinsic cardiac ganglia were examined for changes in mast cell density, NOS expression, and functional indexes of neuronal excitability.
Myocardial ischemia induces changes in autonomic control systems regulating regional cardiac function (1), with infarcted dogs exhibiting higher levels of parasympathetic activity showing reduced potential for sudden cardiac death during subsequent ischemic stress episodes (29). Our group (28) recently demonstrated that in vitro PACAP released during preganglionic firing may modulate neurotransmission within the guinea pig intrinsic cardiac ganglia. Moreover, almost all cholinergic preganglionic fibers terminating on guinea pig intrinsic cardiac neurons contain PACAP (4, 5). Therefore, we also determined whether CMI altered the functional neuronal responses to PACAP in guinea pig intrinsic cardiac ganglia.
MATERIAL AND METHODS
Animals.
Twenty 9-wk-old male Hartley guinea pigs (Charles River), weighing between 507 and 614 g, were used in these chronic studies. Nine animals of the same age and weight were used for time control (sham) surgeries in which the heart was visualized but not disturbed. Twenty additional age-matched male Hartley guinea pigs were used for controls. All procedures were approved by the Institutional Animal Care and Use Committees of East Tennessee State University and Ithaca College and were in accordance with the Guide for the Care and Use of Laboratory Animals (Washington, DC: National Academy, 1996).
Surgical preparation.
Guinea pigs were pretreated with atropine (0.1 mg/kg sc) and ketamine (80 mg/kg ip). Thereafter, anesthesia was induced with 3% isoflurane via an induction chamber (VetEquip, Pleasanton, CA). When guinea pigs were removed from the induction chamber, 2.5% isoflurane was delivered via a conical nose cone (VetEquip) until responses to hindlimb toe pinch stimuli had diminished. After endotracheal intubation, mechanical ventilation was initiated and maintained with a positive pressure ventilator (SAR-830/P ventilator; IITC, Woodland Hills, CA) using 100% O2. Anesthesia was maintained with isoflurane (1–3%). Core body temperature was maintained at 38.5°C with a circulating water heating pad. Buprenorphine (0.05 mg/kg sc) was administered preoperatively.
Animal identification.
With the use of a 12-gauge needle, an AVID microchip (AVID MicroChip I.D. Systems, Folsom, LA) was injected subcutaneously into the interscapular space. A MiniTracker (AVID MicroChip I.D. Systems) scanner was then passed over the implant site to detect the unique identification number for each animal.
Induction of chronic myocardial infarction.
A thoracotomy was performed in the left fourth intercostal space, and the pericardium was opened to expose the heart. A 4-0 monofilament polypropylene suture on a curved, tapered needle was passed around the ventral descending coronary artery just distal to its first diagonal branch and tied. Because of collateral flow, a second suture was placed and tied ∼1 cm distal to the first suture. The incision was closed in multiple layers. Thereafter, residual air was withdrawn from the thoracic cavity via a chest tube, and the chest tube was removed. Postoperatively, analgesic therapy (buprenorphine, 0.05 mg/kg sc) was administered as needed at 4- to 6-h intervals for 24 h and as needed thereafter. Sutures were removed 10–14 days after surgery. The animal was allowed to recover for 4 wk before transfer to Ithaca College (Ithaca, NY) for terminal studies. Two animals died from surgical complications either during or within 1 h after surgery and consequently were excluded from the final analysis.
Terminal experiments.
Guinea pigs (14–18 wk of age, 800–1,000 g at termination) were euthanized by CO2 inhalation and exsanguination. The heart was removed, weighed, and placed into ice-cold Krebs-Ringer solution (mM: 121 NaCl, 5.9 KCl, 2.5 CaCl2, 1.2 MgCl2, 1.2 NaH2PO4, 25 NaHCO3, and 8 glucose, aerated with 95% O2-5% CO2 for a pH of 7.4). The cardiac plexus, located in the epicardium of the atria, was dissected as previously described (11). Briefly, the region studied, which is located primarily in the wall of the left atria underlying the area of the coronary sinus, was exposed by opening the atria and removing the overlying muscle and connective tissue. The tissue was pinned to a Sylgard-lined 60-mm petri dish and continuously superfused (6–8 ml/min) with 35–37°C Krebs-Ringer. Histamine (Sigma; 10−4 M in Krebs solution) and PACAP 1-27 (American Peptides; 50 μM in Krebs solution) were applied by local pressure ejection (6–9 lb./in.2; Picospritzer, General Valve) through small-tip-diameter (5–10 μm) glass micropipettes positioned 50–100 μm from the individual neuron. For multiple tests of responses in the same cell, the cells were allowed to wash (via the circulating Krebs solution) for several minutes between applications.
Analysis of ventricle size and infarct size.
After both atria were removed, the ventricles were fixed in 4% paraformaldehyde for 12–24 h at 4°C and then placed in 70% ethanol at 4°C until processing. Subsequently, the ventricles were weighed and cut into 2-mm-thick slices, parallel to the atrioventricular groove. Areas of infarction (scar tissue) and outlines of the left ventricular (LV) and right ventricular (RV) chambers at each level were traced onto plastic overlays. These areas were then measured using computer-assisted planimetry (Image Research). RV and LV areas and infarct area were calculated by multiplying each area by tissue thickness, and their products were summed and expressed (in cm3; mean ± SD) for both ventricles. Infarct area is expressed as a percentage of LV area.
Electrophysiological methods.
Intracellular voltage recordings from intracardiac neurons were obtained with an AxoClamp 2B amplifier (Axon Instruments) from cells impaled with 2 M KCl-filled microelectrodes (40–80 MΩ). Data were collected, digitized, and analyzed using pCLAMP 8.2 (Axon Instruments). Individual neurons were used for an experiment if the membrane potential was −40 mV or below and they produced APs with an overshoot of at least 20 mV. The neurons were identified as putative parasympathetic postganglionic neurons by their morphology (∼30 μm in diameter) and their basic electrical properties relative to previous studies (9, 11).
Single APs were stimulated by positive current injection (0.1–0.7 nA, 5 ms), averaged (5–6 individual recordings), and analyzed to determine the amplitude and duration of the afterhyperpolarizing potential (AHP). Input resistance (IR) was determined by negative current injection (0.1–0.6 nA, 1 s). The voltage change was measured at 800 ms, and the slope of the current injection vs. voltage was calculated to determine IR for each cell. Neuronal excitability was monitored by observing the response to a series of long depolarizing current pulses (0.1–0.6 nA, 500 ms). Neurons were categorized as phasic (1–3 APs at the onset of depolarizing stimuli only) or tonic (multiple APs throughout depolarizing stimuli). The AP frequency vs. stimulus amplitude was determined to assess relative changes in excitability.
For each cell, following characterization of the basic electrophysiological properties, induced changes in evoked AP frequency were assessed immediately following 1–2 s of application of either histamine (100 μM) or PACAP (50 μm). Each was applied by local pressure injection, immediately adjacent (∼50–100 μm) to the recorded neuron, and doses were chosen that produce maximal or near-maximal responses (12, 28). Induced changes in AP frequency vs. stimulus amplitude were determined to assess relative drug-induced changes in excitability for both phasic and tonic intrinsic cardiac neurons derived from control animals compared with animals with CMI.
Immunohistochemistry.
Cardiac ganglion preparations were fixed in Zamboni's (2% paraformaldehyde, 0.1% picric acid) overnight at 4°C. The tissue was then washed in phosphate-buffered saline (PBS) and blocked with 0.3% Triton X-100 and 4% donkey serum before incubation with the primary antibodies for 12–18 h at 4°C. Preparations were then washed and incubated with secondary antibodies for 90 min at room temperature. Antibodies used included mouse anti-microtubule-associated protein 2 (MAP2; 1:500; Sigma), rabbit anti-neuronal NOS (nNOS; 1:500; Cayman), mouse anti-inducible NOS (iNOS; 1:100; Transduction Labs), goat anti-choline acetyltransferase (ChAT; 1:100; Chemicon), and rabbit anti-histamine (1:500; Accurate), donkey anti-mouse biotin (1:500), streptavidin-AMCA (1:500), donkey anti-rabbit rhodamine (1:500), donkey anti-goat FITC (1:500), and donkey anti-mouse FITC (1:500; all from Jackson ImmunoResearch).
The percentage of nNOS cells was determined by counting the total number of either MAP2 or ChAT-immunoreactive cells and then determining the number of nNOS-immunoreactive cells. This method controlled for the variability in the total number of neurons per preparation, which can vary significantly (18). The percentage of iNOS cells was determined using the same procedure.
Mast cell density was determined by histamine immunoreactivity. Previous studies have demonstrated that histamine labels mast cells in these tissues (22). The numbers of histamine-immunoreactive cells were determined in three separate fields (∼100 mm2 each) on each preparation. The average number of mast cells as a function of area (mm2) was determined for each sample.
Statistical analysis.
Values are means ± SE. Statistical significance was determined using Student's t-test or ANOVA, with a P value <0.05 considered significant. Best-fit lines for the frequency curves were generated using either a linear or exponential relationship, which provided estimates of the adjusted R2 values.
RESULTS
Analysis of myocardial infarction.
To assess the impact of the CMI on the animals, we determined the weight of the heart and lungs as a percentage of body weight at the termination of the experiment (Table 1). There was a significant increase in heart weight in the CMI animals compared with sham-operated controls. In addition, there was an increase in the LV size in CMI animals compared with controls but no corresponding change in RV volume. Histological analysis of the infarcts showed an average infarct size of 5.62% of LV in the CMI group. Lung weights, both wet and dry, were similar between groups.
Table 1.
Analysis of infarct size, heart size, and lung weight in sham-operated and CMI animals
| Sham | CMI | |
|---|---|---|
| Number of samples | 7 | 7 |
| Age at termination, wk | 18.4±1.6 | 15.8±0.9 |
| Postoperative recovery, wk | 9.4±1.6 | 6.7±0.9 |
| LV volume, cm3 | 1.02±0.18 | 1.33±0.11# |
| RV volume, cm3 | 0.18±0.06 | 0.23±0.06 |
| Infarct size, % | 0 | 5.62±2.64# |
| Heart weight, %body wt | 0.66±0.09 | 0.74±0.04* |
| Wet lung weight, %body wt | 0.51±0.05 | 0.48±0.05 |
| Dry lung weight, %body wt) | 0.09±0.01 | 0.10±0.01 |
Values are means ± SD. CMI, chronic myocardial infarction; LV, left ventricular; RV, right ventricular. Infarct size is expressed as a percentage of LV volume.
P < 0.05 vs. sham.
P < 0.002 vs. sham.
Resting membrane properties.
The guinea pig cardiac plexus consists of multiple cell types, including parasympathetic postganglionic neurons and interneurons. For these studies, the results were taken from cells that can be generally classified as parasympathetic postganglionic on the basis of their approximate size (30 μm) and their membrane properties (9, 11). Intracellular recordings were obtained from 194 neurons from 49 different animals (20 controls, 9 sham, and 20 CMI). The average resting membrane potentials were not different in controls, shams, or CMI animals (see Table 2). Resting membrane input resistance likewise was the same in control, sham, and CMI neurons. Single APs were evoked with brief positive current injections (0.1–0.6 nA, 5 ms) and analyzed to determine AHP amplitudes and durations. There was no significant change in either AHP amplitudes or duration with CMI (Table 2).
Table 2.
Basic membrane properties of intrinsic cardiac neurons derived from age-matched controls, time-matched surgical controls (sham), and CMI models
| RMP, mV | IR, MΩ | AHP Amplitude, mV | AHP Duration, ms | n | |
|---|---|---|---|---|---|
| Control | −45.8±1.4 | 72.4±10.8 | 14.3±0.5 | 241.3±7.6 | 50 |
| Sham | −50.0±1.3 | 60.0±4.7 | 15.7±0.4 | 246.0±9.0 | 26 |
| CMI | −45.4±0.8 | 60.4±3.9 | 15.2±0.3 | 243.2±7.1 | 118 |
Values are means ± SD. RMP, resting membrane potential; IR, input resistance; AHP, afterhyperpolarization. Data from phasic and tonic neurons for each group were pooled.
Measured AP frequency.
Guinea pig intracardiac neurons are typically phasic in their firing properties; that is, the majority of neurons will fire APs only at the onset of a depolarizing pulse (Fig. 1, top). A smaller percentage of neurons are tonic in nature, firing repetitively during a prolonged depolarizing pulse. In control animals, resting neuronal excitability was tested in a total of 46 neurons, using positive current step injections (0.1–0.6 nA, 500 ms). Of these, 35 neurons were phasic in nature and 11 neurons showed tonic activity. A total of 22 neurons were tested in surgical time control (sham) animals, and of these, 17 were phasic and 5 were tonic. In CMI animals, 77 neurons were tested. Of these, 53 were phasic and 24 neurons were tonic, showing no significant change (P > 0.05 by χ2 test) in the relative number of phasic or tonic neurons in the CMI animals compared with controls.
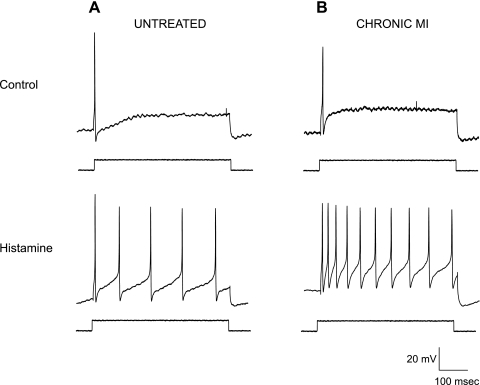
Fig. 1.
Histamine-induced increases in excitability of intrinsic cardiac neurons increase postmyocardial infarction. Indexes of neuronal excitability were determined by monitoring voltage responses to positive current injections (indicated rectangular pulse, 0.3 nA, 500 ms). Action potential (AP) frequency was examined in neurons from control (untreated; A) preparations and tissue derived from chronic myocardial infarction (CMI; B) animals. Before application of exogenous neuromodulators (top), both cells showed a single AP with prolonged depolarization (phasic neuron). After a 1-s application of histamine (bottom), both neurons showed an increase in AP frequency, with the CMI cell showing a greater increase in firing frequency. Resting membrane potentials: untreated, −48 mV; CMI, −45 mV.
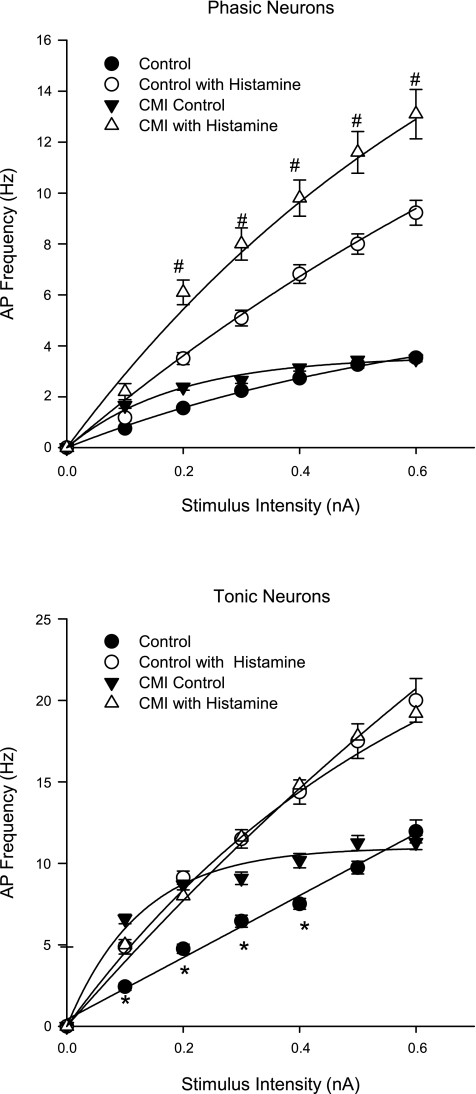
CMI differentially remodeled atrial intrinsic cardiac neuron excitability as assessed by frequency of evoked AP generation in response to positive current injections. Whereas phasic neurons exhibited similar increases in AP generation to imposed depolarizing current injections in both CMI and control tissues (Fig. 2, top), tonic neurons from CMI animals demonstrated increased AP generation at lower (0.1–0.4 nA) stimulus intensities (Fig. 2, bottom). Moreover, for tonic neurons, the frequency vs. stimulus curve shifted from linear to exponential, with R2 > 0.98 and 0.99, respectively. This suggests that there is an underlying change in the modulation of excitability in these cells.
Fig. 2.
Functional upregulation of histaminergic responses on AP frequency preferentially affect phasic intrinsic cardiac neurons postmyocardial infarction. The frequency of APs (mean ± SE) was determined at increasing stimulus intensities (500-ms duration) in atrial intrinsic cardiac neurons derived from control and CMI models before and after histamine application. Because there were no significant differences in evoked AP frequency between untreated animals and surgical time controls (shams), these data were pooled. Neurons were classified as either phasic (top) or tonic (bottom) on the basis of their ability to fire multiple APs with a prolonged stimulus. In untreated conditions, CMI differentially enhanced AP generation in tonic neurons to depolarizing current injections, with no induced change in phasic neurons. After histamine application, phasic neurons derived from CMI models exhibited increased AP frequency to depolarizing current injections compared with controls, an effect not seen in tonic neurons. In all cases except for untreated tonic neurons, the frequency vs. stimulus curve was best fit with an exponential curve, with R2 > 0.99. For the control tonic neurons, the relationship between frequency and stimulus was a linear fit with an R2 of 0.98. Statistical significance was evaluated at each stimulus intensity for a given treatment (i.e., control untreated vs. CMI untreated) by t-test. *P < 0.05, untreated control vs. untreated CMI. #P < 0.05, histamine-treated control vs. histamine-treated CMI.
Neuromodulator responses.
Evoked AP frequency was measured before experiments, immediately following application of histamine, and after tissue was washed for 30 s or more. Control neurons showed a significant increase in the number of APs fired with increasing stimulus amplitudes (Figs. 1 and 2) following histamine application. The frequency of APs produced by either phasic or tonic neurons from surgical time control (sham) animals was not different from that of control animals in either the presence or absence of histamine, and these data were pooled. Phasic neurons from CMI animals demonstrated a significant increase in the frequency of APs produced following histamine application compared with age-matched controls (Fig. 2, top). However, tonic neurons in CMI tissues exhibited no such enhanced histamine response (Fig. 2, bottom).
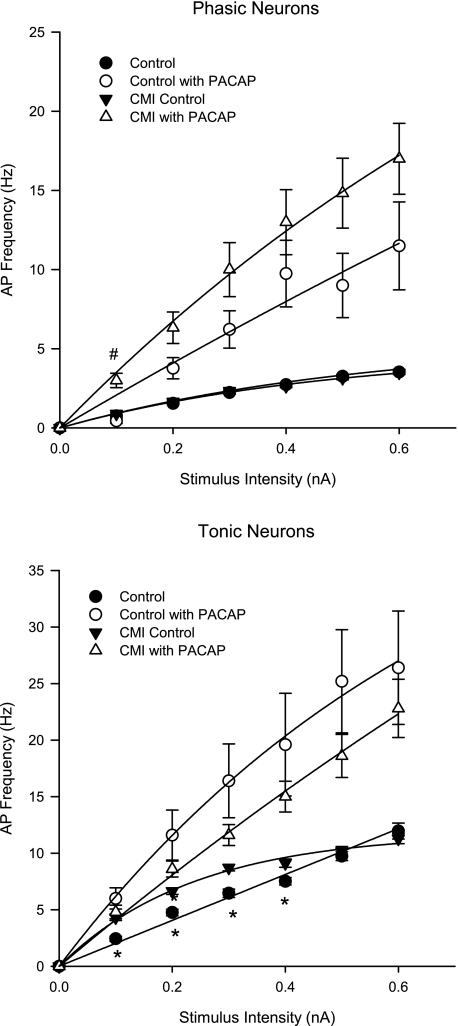
AP frequency was also measured in the same preparations before experiments, immediately following application of PACAP, and after tissues was washed for 30 s or more. Whereas the CMI-induced increase in excitability in tonic neurons to depolarizing pulses was still evident in the untreated condition (Fig. 3, bottom), as with histamine exposure, there was no difference in the increase in evoked AP frequency induced by PACAP in tonic cells in CMI compared with control (Fig. 3, bottom). For phasic neurons derived from CMI animals, in marked contrast to the augmented response to histamine (Fig. 2, top), a significant difference in the PACAP response was seen only at the lowest stimulus intensity (0.1 nA; Fig. 3, top).
Fig. 3.
CMI does not modify the pituitary adenylate cyclase-activating polypeptide (PACAP)-induced increase in AP frequency of intrinsic cardiac neurons. The frequency of APs was determined at increasing stimulus intensities (500 ms) in atrial intrinsic cardiac neurons derived from control and CMI animal models before and after PACAP application. Neurons were classified as either phasic (top) or tonic (bottom) on the basis of their ability to fire multiple APs with a prolonged stimulus. There is no significant difference in the increase in evoked AP frequency induced by PACAP in tonic cells between control and CMI-derived neurons. In phasic neurons, a significant difference in the PACAP-evoked response between groups was seen only at the lowest stimulus intensity (0.1 nA). *P < 0.05, untreated control vs. CMI. #P < 0.05, PACAP-treated control vs. PACAP-treated CMI.
Immunohistochemical analysis.
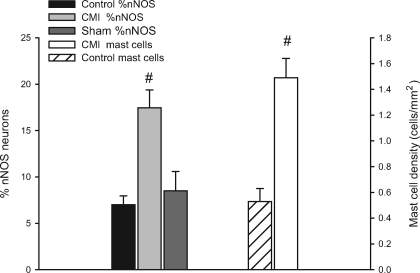
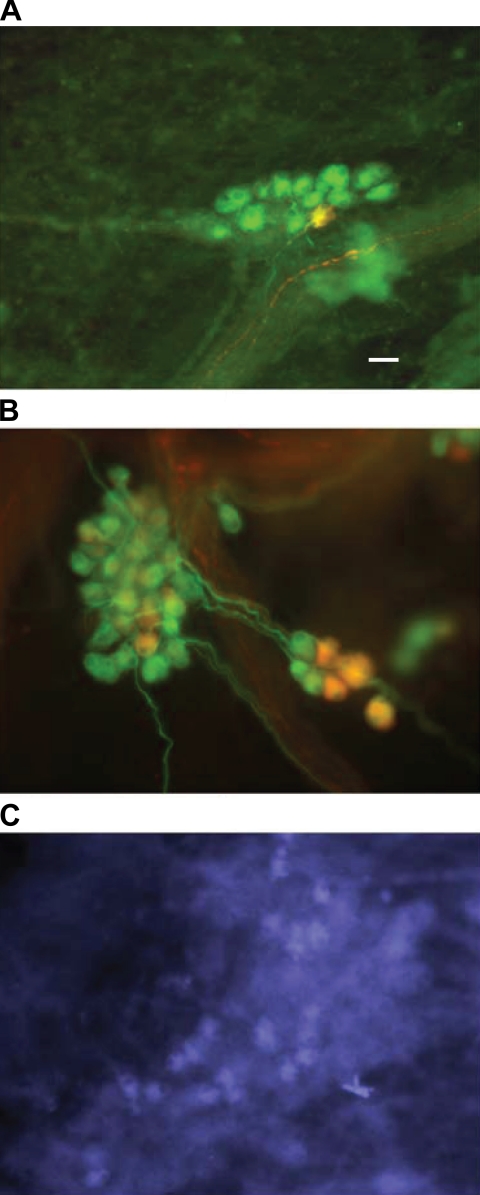
Immunohistochemistry was used to detect changes in neuronal phenotype following CMI. More specifically, previous studies showed that NOS levels are altered in cardiac myocytes and vascular tissues with heart disease (7). For this study, we focused on expression of nNOS and iNOS. A small percentage of intracardiac neurons normally express nNOS (18), and similarly, in the current study, both age-matched controls and surgery time controls demonstrated a similar percentage of nNOS expression of ∼7% (see Fig. 6). Examination of cardiac ganglia from CMI animals demonstrated a threefold increase in the percentage of nNOS-immunoreactive neurons (Fig. 6), normalized to labeling with antibodies targeted to either ChAT or MAP2. Preparations were also labeled with antibodies to iNOS. In control preparations, there was no evidence for iNOS-immunoreactive neurons (data not shown). However, in all preparations derived from CMI animals evaluated (n = 4), iNOS-immunoreactive neurons were observed (Fig. 4).
Fig. 6.
CMI remodels intrinsic cardiac neuron phenotype. Phenotypic changes in nNOS expression and mast cell density were quantified in 6 preparations for each condition. The percentage of nNOS-immunoreactive cells was determined by counting the total number of MAP2-positive cells and expressing the number of nNOS cells as a percentage in time-matched control preparations (solid bar), surgical time controls (sham; dark shaded bar), and CMI tissues (light shaded bar). The mast cell density was determined by counting the number of histamine-immunoreactive cells as a function of area (mm2) in control (hatched bar) and CMI tissues (open bar). CMI resulted in a significant increase in both the percentage of nNOS cells and the density of mast cells. #P < 0.05.
Fig. 4.
Nitric oxide synthase (NOS) immunoreactivity in cardiac ganglia from control and CMI animals. Atrial intrinsic cardiac ganglia derived from control (A) and CMI (B) animals were fixed and labeled with antibodies to neuronal NOS [rabbit anti-nNOS, 1:500; and donkey anti-rabbit rhodamine (Rh), 1:500] and microtubule-associated protein 2 (mouse anti-MAP2, 1:500; and donkey anti-mouse FITC, 1:500). Merged images of the FITC and Rh immunofluorescence are shown. The percentage of nNOS-immunoreactive neurons in the untreated preparations was between 6 and 8%. The percentage of nNOS-immunoreactive neurons was significantly greater in the tissue from the CMI animals. Image in C shows inducible NOS (iNOS)-immunoreactive cells (mouse anti-iNOS, 1:100; donkey anti-mouse biotin, 1:500; and streptavidin-AMCA, 1:500) in a cardiac ganglion from a CMI animal. Scale bar, 30 μm.
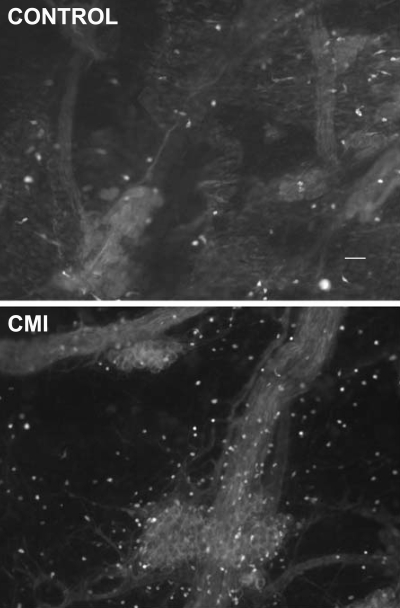
Since previous studies showed an increase in cardiac mast cells with disease (17), we evaluated whether there was a corresponding change in mast cell density within the atrial elements of the intrinsic cardiac ganglionated plexus in the CMI animals. Whole mounts of cardiac ganglia were labeled with a histamine antibody, and the number of histamine-immunoreactive mast cells as a function of area (mm2) was determined in both control and CMI preparations (Fig. 5 and 6). There was a threefold increase in the density of mast cells within the cardiac ganglion in the CMI tissues.
Fig. 5.
Histamine immunoreactivity/mast cell density increases in atrial intrinsic cardiac ganglia postmyocardial infarction. Cardiac ganglia from control and CMI animals were labeled with an antibody to histamine (rabbit anti-histamine, 1:500; and donkey anti-rabbit FITC, 1:500), which is localized in cardiac mast cells. The density of mast cells was increased in the atrial ganglia tissues derived from CMI animals compared with control animals. These atrial tissues are not in the perfusion bed of the infarcted tissue. Scale bar, 50 μm.
DISCUSSION
CMI of the guinea pig heart leads to significant and differential changes in the physiological responses of intracardiac neurons, as well as induced changes in NOS expression within the intrinsic cardiac neurons. These results demonstrate that, in addition to the previously described remodeling of cardiac myocytes postmyocardial infarction, there is also a significant remodeling of the neuronal components on the intrinsic cardiac nervous system. These neuronal changes are induced in elements of the intrinsic cardiac nervous system whose perfusion was not compromised by infarction localized to the ventricular myocardium.
In the current study, CMI of the left ventricle was associated with evidence of cardiac hypertrophy, as demonstrated by a differential increase in LV volume and an increase in relative heart weight. However, given the extensive collateral coronary circulation in the guinea pig, the infarctions were relatively small (5.62% of LV mass). Moreover, lung weight was equivalent among all three groups (control, sham, and CMI). Together, these data indicate that the CMI-stressed guinea pig heart had not progressed into overt heart failure. Although this was a relatively small disruption in LV function, it was associated with significant physiological changes in the intrinsic cardiac nervous system.
CMI differentially impacts the functional responses of subpopulations of intrinsic cardiac neurons. The neurons evaluated in the current study are primarily parasympathetic postganglionic, based on their approximate size (30 μm) and their membrane properties (9, 11). They are further defined as phasic or tonic neurons, based on the AP response to imposed intracellular depolarization (9, 11). Overall, the basal membrane properties of both types of atrial intrinsic cardiac neurons showed minimal induced changes associated with chronic infarction. However, the AP frequency measured in tonic neurons differentially increased with CMI. The output of these cells to low-intensity stimuli was significantly increased over that of intrinsic cardiac neurons derived from age-matched controls. Further studies are needed to determine the ionic mechanisms leading to these changes and the overall functional implications for the neurons.
Cardiac disease is known to stimulate a variety of inflammatory signals, including a proliferation of mast cells and an increase in the production and release of various cytokines (3, 20). These signals in turn stimulate changes within the cardiac tissue, leading to long-term changes in the expression or activity of different proteins. In ventricular muscle, these signals can result in an increase in nNOS expression in ventricular myocytes (2, 7). Similarly, the increase in cytokines such as TNF-α can lead to the expression of the calcium-independent inducible form of NOS (3, 21).
In the CMI animals, a significant increase in the density of cardiac mast cells was observed within the atrial elements of the cardiac ganglionated plexus, as well as an increase in the number of neurons expressing nNOS and the de novo expression of iNOS by some neurons. Previous studies have suggested that NO can increase vagal tone through a presynaptic mechanism to increase acetylcholine release from preganglionic terminals (8). Although the specific functions of NO in the cardiac plexus have not been fully characterized, it is possible that the upregulation of NOS within the parasympathetic postganglionic neurons may serve, in part, as a protective function to reduce sympathetic stimulation of the heart and increase the effectiveness of the parasympathetic pathways. The possible link between the upregulation of NOS and the increase in mast cell density requires further investigation.
Mast cells, when stimulated, release several bioactive compounds (e.g., histamine, prostaglandins, serine proteases, and leukotrienes) into the cardiac interstitial space that impact on cardiac myocytes, structural elements of the heart, and the cardiac nervous system (16, 22, 26). Previous work from our laboratory (22) demonstrated that histamine normally produces an increase in evoked AP frequency in guinea pig intracardiac neurons. In animals with CMI, the increases in AP frequency observed with histamine were differentially enhanced in phasic neurons, which represent the majority of the cells recorded in vitro. Prior studies have shown that the histamine-induced increase in AP frequency is dependent on an influx of extracellular Ca2+ through voltage-gated Ca2+ channels (12). One of the changes that has been observed in other cells in response to ischemia is a change in Ca2+ channels. In hippocampal neurons, ischemia produces an increase in NO production that is associated with an increase in Ca2+ entry via L-type Ca2+ channels (14, 27). Similarly, in cardiac myocytes, myocardial infarction induces the expression of T-type Ca2+ channels (30). Increases in Ca2+ entry through voltage-gated Ca2+ channels in intracardiac neurons in response to CMI could explain the increase in histamine responses observed in these studies.
PACAP, applied exogenously (4) or released endogenously by stimulation of preganglionic parasympathetic neurons (28), contributes to the generation of the spontaneous excitatory postsynaptic potentials and can modulate excitability of guinea pig atrial intrinsic cardiac neurons. However, the ionic mechanism responsible for the PACAP-induced increase in AP generation is different from the histamine-induced mechanism. The PACAP- induced change appears to be due, in large part, to modulation of the hyperpolarization-activated (Ih) current (19). Conversely, the histamine-induced response does not involve Ih (12). The facilitatory effect of PACAP is maintained in the neurons derived from animals with CMI, but in contradistinction to the histamine response, it does not functionally upregulate in either phasic or tonic neurons. This supports the hypothesis that the upregulation in the histamine response is specific to the ionic mechanisms that underlie this pathway.
Perspectives and Significance
The observed changes in physiological properties of the intracardiac neurons, combined with potential enhancement of parasympathetic signaling due to increased NOS levels, would suggest that one consequence of remodeling would be an overall increase in parasympathetic tone within the heart. Endogenous release of histamine may contribute to facilitation of ganglionic neurotransmission within the intrinsic cardiac nervous system postmyocardial infarction. Whether this change would be beneficial to the physiological function of the heart or whether it might contribute to the development of cardiac dysfunction requires further investigation. However, multiple studies, both clinical and basic, indicate the cardioprotective effects of minimizing sympathoexcitation and/or maximizing parasympathetic activity postmyocardial infarction (1, 15, 29). Moreover, recent studies have indicated the therapeutic relevance of targeting and stabilizing information processing within the peripheral aspects of the cardiac nervous system (10) and thereby reducing the cardiac damage induced by ischemic heart disease (6, 24).
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants R15 HL60619 (to J. C. Hardwick) and HL71830 (to J. L. Ardell).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Armour JA Cardiac neuronal hierarchy in health and disease. Am J Physiol Regul Integr Comp Physiol 287: R262–R271, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Bendall JK, Damy T, Ratajczak P, Loyer X, Monceau V, Marty I, Milliez P, Robidel E, Marotte F, Samuel J, Heymes C. Role of myocardial neuronal nitric oxide synthase-derived nitric oxide in β-adrenergic hyporesponsiveness after myocardial infarction-induced heart failure in rat. Circulation 110: 2368–2375, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Bidri M, Féger F, Varadaradjalou S, Hamouda NB, Guillosson JJ, Arock M. Mast cells as a source and target for nitric oxide. Int Immunopharmacol 1: 1543–1558, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Braas KM, May V, Harakall SA, Hardwick JC, Parsons RL. Pituitary adenylate cyclase-activating polypeptide expression and modulation of neuronal excitability in guinea pig cardiac ganglia. J Neurosci 18: 9766–9779, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calupca MA, Vizzard MA, Parsons RL. Origin of pituitary adenylate cyclase-activating polypeptide (PACAP)-immunoreactive fibers innervating guinea pig parasympathetic ganglia. J Comp Neurol 423: 26–39, 2000. [PubMed] [Google Scholar]
- 6.Cardinal R, Ardell JL, Linderoth B, Vermeulen M, Foreman RD, Armour JA. Spinal cord activation differentially modulates ischemic electrical responses to different stressors in canine ventricles. Auton Neurosci 111: 37–47, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Casadei B The emerging role of neuronal nitric oxide synthase in the regulation of myocardial function. Exp Physiol 91: 943–955, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Choate JK, Danson EJF, Morris JF, Paterson DJ. Peripheral vagal control of heart rate is impaired in neuronal NOS knockout mice. Am J Physiol Heart Circ Physiol 281: H2310–H2317, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Edwards FR, Hirst GDS, Klemm MF, Steele PA. Different types of ganglion cell in the cardiac plexus of guinea-pigs. J Physiol 486: 453–471, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foreman RD, Linderoth B, Ardell JL, Barron KW, Chandler MJ, Hull SS, TerHorst GJ, DeJongste MJL, Armour JA. Modulation of intrinsic cardiac neural activity by spinal cord stimulation: implications for its therapeutic use in angina pectoris. Cardiovasc Res 47: 367–375, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Hardwick JC, Mawe GM, Parsons RL. Evidence for afferent fiber innervation of parasympathetic neurons of the guinea-pig cardiac ganglion. J Auton Nerv Syst 53: 1966–174, 1995. [DOI] [PubMed] [Google Scholar]
- 12.Hardwick JC, Kotarski AF, Powers MJ. Ionic mechanisms of histamine-induced responses in guinea pig intracardiac neurons. Am J Physiol Regul Integr Comp Physiol 290: R241–R250, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Jelson GS, DeMasi GM, Sager KL, Hardwick JC. Modulation of guinea pig intrinsic cardiac neurons by prostaglandins. Am J Physiol Regul Integr Comp Physiol 285: R682–R689, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Jian k, Chen J, Cao X, Zhu XH, Fung ML, Gao TM. Nitric oxide modulation of voltage-gated calcium currents by S-nitrosylation and cGMP pathway in cultured rat hippocampal neurons. Biochem Biophys Res Commun 359: 481–485, 2007. [DOI] [PubMed] [Google Scholar]
- 15.La Rovere MT, Bersano Gnemmi C, Specchia M, G, Schwartz PJ. Exercise-induced increase in baroreflex sensitivity predicts improved prognosis after myocardial infarction. Circulation 106: 945–949, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Levick SP, Gardner JD, Holland M, Hauer-Jensen M, Janicki JS, Brower GL. Protection again adverse remodeling secondary to chronic volume overload in mast cell deficient rats. J Mol Cell Cardiol 45: 56–61, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marone G, deCresenzo G, Adt M, Patella V, Arbustini E, Genovese A. Immunological characterization and functional importance of human heart mast cells. Immunopharmacology 31: 1–18, 1995. [DOI] [PubMed] [Google Scholar]
- 18.Mawe GM, Talmage EK, Lee KP, Parsons RL. Expression of choline acetyltransferase immunoreactivity in guinea pig cardiac ganglia. Cell Tissue Res 285: 281–286, 1996. [DOI] [PubMed] [Google Scholar]
- 19.Merriam LA, Barstow KL, Parsons RL. Pituitary adenylate cyclase-activating polypeptide enhances the hyperpolarization-activated nonselective cationic conductance, Ih, in dissociated guinea pig intracardiac neurons. Regul Pept 123: 123–33, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Moro C, Jouan M, Rakotovao A, Toufektsian M, Ormezzano O, Nagy N, Tosaki A, de Leiris J, Boucher F. Delayed expression of cytokines after reperfused myocardial infarction: possible trigger for cardiac dysfunction and ventricular remodeling. Am J Physiol Heart Circ Physiol 293: H3014–H3019, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Ono K, Matsumori A, Shioi T, Furukawa Y, Sasayama S. Cytokine gene expression after myocardial infarction in rat hearts: possible implication in left ventricular remodeling. Circulation 98: 149–156, 1998. [DOI] [PubMed] [Google Scholar]
- 22.Powers MJ, Peterson BA, Hardwick JC. Regulation of parasympathetic neurons by mast cells and histamine in the guinea pig heart. Auton Neurosci 87: 37–45, 2001. [DOI] [PubMed] [Google Scholar]
- 23.Singh M, Saini HK. Resident cardiac mast cells and ischemia reperfusion injury. J Cardiovasc Pharmacol Ther 8: 135–148, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Southerland EM, Milhorn DM, Foreman RD, Linderoth B, DeJongste MJL, Armour JA, Subramanian V, Singh M, Singh K, Ardell JL. Pre-emptive, but not reactive, spinal cord stimulation mitigates transient ischemia induced myocardial infarction via cardiac adrenergic neurons. Am J Physiol Heart Circ Physiol 292: H311–H317, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Steele PA, Gibbons IL, Morris JL, Mayer B. Multiple populations of neuropeptide-containing neurons in the guinea-pig heart. Neuroscience 62: 241–250, 1994. [DOI] [PubMed] [Google Scholar]
- 26.Steward JA, Wei CC, Brower GL, Rynders PE, Hankes GH, Dillon AR, Lucchesi PA, Janicki JS, Dell'Italia LJ. Cardiac mast cell- and chymase-mediated matrix metalloproteinase activity and left ventricular remodeling in mitral regurgitation in the dog. J Mol Cell Cardiol 35: 311–319, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Tjong YW, Jian K, Li M, Chen M, Gao TM, Fung ML. Elevated endogenous nitric oxide increases Ca2+ flux via L-type Ca2+ channels by S-nitrosylation in rat hippocampal neurons during severe hypoxia and in vitro ischemia. Free Radic Biol Med 42: 52–63, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Tompkins JD, Ardell JL, Hoover DB, Parsons RL. Neurally released pituitary adenylate cyclase-activating polypeptide enhances guinea pig intrinsic cardiac neurone excitability. J Physiol 582: 87–93, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vanoli E, Adamson PB, Foreman RD, Schwartz PJ. Prediction of unexpected death among healthy dogs by a novel marker of autonomic neural activity. Heart Rhythm 5: 300–305, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Yasui K, Niwa N, Takemura H, Opthol T, Muto T, Horiba M, Shimizu A, Lee JK, Honjo H, Kamiya K, Kodama I. Pathophysiological significance of T-type Ca2+ channels: expression of T-type Ca2+ channels in fetal and diseased hearts. J Pharmacol Sci 99: 205–210, 2005. [DOI] [PubMed] [Google Scholar]