Abstract
The human angiotensinogen (hAGT) gene contains an A/G polymorphism at −217, and frequency of −217A allele is increased in African-American hypertensive patients. The hAGT gene has seven polymorphic sites in the 1.2-kb region of its promoter, and variant −217A almost always occurs with −532T, −793A, and −1074T, whereas variant −217G almost always occurs with −532C, −793G, and −1074G. Since allele −6A is the predominant allele in African-Americans, the AGT gene can be subdivided into two main haplotypes, −6A:−217A (AA) and −6A:−217G (AG). To understand the role of these haplotypes on hAGT gene expression and on blood pressure regulation in an in vivo situation, we have generated double transgenic mice containing human renin gene and either AA or AG haplotype of the hAGT gene using knock-in strategy at the hypoxanthine phosphoribosyltransferase locus. We show here that 1) hAGT mRNA level is increased in the liver by 60% and in the kidney by 40%; and 2) plasma AGT level is increased by ∼40%, and plasma angiotensin II level is increased by ∼50% in male double transgenic mice containing AA haplotype of the hAGT gene compared with the AG haplotype. In addition, systolic blood pressure is increased by 8 mmHg in transgenic mice containing the AA haplotype compared with the AG haplotype. This is the first report to show the effect of polymorphisms in the promoter of a human gene on its transcription in an in vivo situation that ultimately leads to an increase in blood pressure.
Keywords: polymorphism, hypertension, hypoxanthine phosphoribosyltransferase
hypertension is a serious risk factor for myocardial infarction, heart failure, vascular disease, stroke, and renal failure. It is estimated that hypertension affects 50 million Americans, with a prevalence rate of 25–30% in the adult Caucasian population. The renin-angiotensin system plays an important role in the regulation of blood pressure (BP). The octapeptide, angiotensin II, is one of the most active vasopressor agents and is obtained by the successive proteolytic cleavage of a larger precursor molecule, angiotensinogen (AGT), by renin and angiotensin-converting enzyme. In experimental as well as clinical studies, angiotensin-converting enzyme inhibitors or angiotensin receptor blockers have proven effective in lowering BP.
The plasma concentration of AGT is close to the Michaelis constant of the enzymatic reaction between renin and AGT (13). For this reason, a rise in plasma AGT levels can lead to a parallel increase in the formation of angiotensin II that may ultimately result in hypertension. Previous studies have suggested a direct correlation between AGT and BP. These studies include 1) a highly significant relationship between plasma concentration of AGT and BP in human subjects (25); 2) higher plasma AGT levels in hypertensive subjects and in offspring of hypertensive parents compared with normotensive subjects (11, 26); and 3) elevation of BP in transgenic animals that overexpress AGT gene (19). In addition, Kim et al. (18) have introduced up to four copies of the AGT gene in mice, with each copy of the gene resulting in a successive increase in BP.
The incidence of hypertension and complications due to hypertension are even greater in the African-American population. Walker et al. (25) have shown a remarkably high correlation between plasma AGT concentration and elevated BP in this population. Bloem et al. (3) have also shown that 1) plasma AGT level is ∼19% higher in black children compared with white children; and 2) BP is normally higher and increases faster over time in black children compared with white children. Jeunemaitre et al. (17) have shown that M235T polymorphism of the AGT gene is linked with increased plasma AGT level and increased BP in Caucasian and Japanese subjects. The human angiotensinogen (hAGT) gene has an additional A/G polymorphism at −217 in the hAGT gene promoter, and our laboratory has shown that −217A allele of hAGT gene is associated with hypertension in African-American subjects (16). A recent study has supplemented our observation and shown that −217A allele is also associated with hypertension in the Chinese population (27). A recent paper by Pereira et al. (22) has performed meta-analysis on 26,818 subjects from 46 studies for association of four most studied polymorphisms of the AGT gene and hypertension. Statistically significant association with hypertension was identified for −217A/G polymorphism of the AGT gene (odds ratio: 1.37; 95% confidence interval: 1.17–1.59; P = 0.000006). This study supports our hypothesis about the role of −217 A allele of the AGT gene in hypertension and shows that this polymorphism may also play a role in hypertension in Caucasian population (22).
There are a total of seven polymorphic sites (A/G at −6, A/C at −20, A/G at −217, T/C at −532, T/C at −776, A/G at −793, and T/G at −1074) in 1.2-kb promoter of the hAGT gene. We have shown that variant −217A almost always occurs with variants −532T, −793A, and −1074T, and variant −217G always occurs with variants −532C, −793G, and −1074G (21). Since −6A is the predominant allele (frequency > 0.85) in the African-American population, hAGT gene may be subdivided into two major haplotypes containing −6A, −217A, −532T, −793A, and −1074T (AA haplotype); and −6A, −217G, −532C, −793G, and −1074G (AG haplotype). Although association and transient transfection studies have shown that hAGT gene containing −217A allele is associated with hypertension (15, 16), it is important to rigorously prove the transcriptional and physiological significance of a genetic polymorphism by in vivo experiments. We have, therefore, generated transgenic mice where 13.5-kb hAGT gene (consisting of all of the five exons, four introns, and 1.3 kb of the 5′-flanking region) containing either AA or AG haplotype was inserted at the hypoxanthine phosphoribosyl transferase (HPRT) locus, as described by Bronson et al. (5) (for simplicity, these mice are named as AA and AG haplotypes). Since hAGT is not cleaved by mouse renin, we also generated double transgenic mice containing human renin (hRen) gene (24) and either AA or AG haplotype of the hAGT gene. In this study, we show the physiological significance of AA and AG haplotypes of the hAGT gene on BP using transgenic mice.
MATERIALS AND METHODS
Generation of Targeting Vectors
The hAGT gene, containing either AA or AG haplotype, was amplified from a hypertensive subject containing AA haplotype and from a normotensive subject containing AG haplotype using oligonucleotide TATGCGGCCGCCCAGACA AGTGATTTTTGAGGAGT as the forward primer and ATTGCGGCCGCAGCATCACCTAAAACTTCAAAGGA as the reverse primer and subcloned as a Not1 fragment into a pMP8SKB HPRT vector (5). The forward primer starts at −1223 of the promoter, and the reverse primer starts 200 bp beyond the polyadenylation sites in the 3′-flanking region of the hAGT gene. Both of these primers contain Not1 restriction site at their 5′ ends to facilitate the cloning into pMP8SKB vector. PCR amplified fragments (LA-PCR kit version 2.1 from TAKARA) were analyzed by 0.8% agarose gel followed by their ligation into TOPO-XL vector from Invitrogen. Finally, the Not1 released fragments from TOPO-XL vector were ligated into Not1 digested pMP8SKB vector, generating pMP8SKB-AA-hAGT and pMP8SKB-AG-hAGT targeting vectors. The nucleotide sequence of the promoter, all of the exons, and 3′-flanking region were sequenced to confirm that they differ only in the promoter region and contained AA or AG haplotype.
Generation of Transgenic Mice
The above-mentioned recombinant vectors were linearized by Pvu1 digestion and introduced into BK4 embryonic stem (ES) cells by electroporation. The ES cells were then grown in selective hypoxanthine and thymidine (HAT) medium, and HAT-resistant colonies were isolated and expanded. DNA from different ES cells was amplified using oligonucleotide CCAGACAAGTGATTTTTGAGGAGTCCCTATCT as forward primer and AGCATCACCTAAAA CT TCAAAGGACTGCTAAG as reverse primer to detect the 13.5-kb hAGT gene. BK4 ES cells (containing either AA or AG haplotypes of the hAGT gene) were used to generate transgenic mice using standard protocol. The presence of hAGT gene was confirmed by PCR amplification of the tail DNAs of transgenic animals using hAGT gene-specific primers. The forward and reverse primers for the amplification of hAGT gene were CAGCAGTGAAACTCTGC and TTCAGTCATCACCGTGC, respectively, to produce a 342-bp amplification product. DNA from transgenic mice was further characterized by Southern hybridization. Briefly, ∼10 μg of DNA were treated with restriction enzyme BamH1, separated by agarose gel electrophoresis, transferred to nylon membrane, and probed with radioactive Rsa doub probe that hybridizes to a 250-bp sequence located in the third intron of the endogenous HPRT gene (5). The orientation of knock-in hAGT gene was determined by Southern analysis, as described previously (9). ES cells used to generate hAGT mice were from 129 mice; chimeras were bred to C57BL/6 mice and then successfully backcrossed to C57BL/6 for at least seven generations. All experiments involving animals were approved by the institutional internal review board of the New York Medical College.
Generation of Double Transgenic Mice
Female transgenic mice containing either AA or AG haplotype of the hAGT gene were crossed with male transgenic mice containing human renin (hRen) gene [P1 artificial chromosome (PAC)-hRen] developed by Sinn et al. (24). The genetic background of the PAC-hRen mice was originally B6SJL (C57BL/6J × SJL/JF2), but has since been backcrossed for at least five generations with C57BL/6J before breeding with hAGT mice. The double transgenic mice were genotyped for the hAGT and hRen genes by PCR amplification of the DNA isolated from their tails. The primers for the amplification of hAGT gene were the same as used in the analysis of single transgenic mice. The forward and reverse primers for the amplification of hRen gene were CTCTTCGATGCTTCGGATTC and TGGCAGAGTAG GGTGTTCCT, respectively, to produce a 250-bp product.
Quantitative RT-PCR of hAGT and Renin mRNA
Liver and kidney from 8-wk-old male transgenic mice containing either AA or AG haplotype of the hAGT gene were harvested following CO2 asphyxiation and stored in RNAlater (RNA stabilization solution, Ambion). RNA was isolated using GENTRA RNA isolation kit. Two micrograms of RNA were reverse transcribed into cDNA using Protoscript First Strand cDNA Synthesis Kit (New England Biolaboratories). Quantitative real-time RT-PCR was performed using SYBRgreen master mix and ABI PRISM 7900HT thermocycler (Applied Biosystems). SYBRgreen PCR master mix (catalog no. PA-012), primers for mouse and hAGT (catalog nos. PPM04219A, PPH01807A), mouse renin and hRen (catalog nos. PPM0748A, PPH07193A), and mouse GAPDH (catalog no. PPM02946A) genes were purchased from SuperArray Bioscience (MD). Following a 95°C incubation for 10 min, 40 cycles of PCR (95°C/15 s; 60°C/1 m) were then performed on an ABI Prism 7900HT Sequence Detection System using 3 μl of cDNA, 50 nM PCR primers, and 12.5 μl SYBRgreen PCR Master Mix in 25-μl reactions. Threshold cycles for three replicate reactions were determined using Sequence Detection System software (version 2.2.2), and relative transcript abundance was calculated following normalization with GAPDH. Primers used for the amplification of the RNA are hAGT specific and do not amplify endogenous mouse AGT mRNA.
Western Blotting
Plasma samples were collected by cardiac puncture from mice immediately after CO2 asphyxiation. Approximately 250 μl of plasma were collected from each mouse by centrifuging 500 μl of blood sample at 12,000 rpm for 15 min at 4°C. Equal volumes of plasma samples from transgenic mice were fractionated by SDS-PAGE (10% polyacrylamide) in duplicate and transferred to Immobilon-P transfer membrane (Millipore) to detect hAGT protein. Membranes were blocked in 10% nonfat dry milk (Bio-Rad), and one membrane was immunoblotted with 1:1,500 dilution of commercially available monoclonal antibodies for hAGT (catalog no. H00000183-M01, Abnova, Taiwan), while the other was incubated with 1:10,000 dilution of mouse albumin antibody (Bethyl Laboratories; catalog no. A90134P). Immune complexes were detected by horseradish peroxidase-conjugated antimouse IgG (catalog no. I1904-25C, US Biologicals) using SuperSignal West Pico chemiluminisence assay (Pierce Chemical, Rockford, IL), according to the manufacturer's protocol. Densitometric analysis of protein bands was performed by Quantity One quantitation software from Bio-Rad (Bio Rad Laboratories).
Immunohistochemistry.
Mice were perfused with normal saline. Liver and kidney were excised in phosphate-buffered saline (PBS) and fixed in 4% paraformaldehyde-PBS for 3 h at room temperature, kept overnight at 4°C in 30% sucrose solution, and then embedded in optimum cutting temperature compound (Sakura Finetek, Torrence, CA). Croystat sections (8-μm thickness) were taken on slides and treated with blocking solution (5% goat serum in PBS with 0.2% Triton X-100) for 1 h at room temperature. Sections were then incubated overnight at 4°C with mouse anti-human primary antibody for AGT (Abnova) followed by washes with PBS (4× for 10 min each) and incubation (2 h) with secondary Cy3-conjugated goat anti-mouse antibody (Abnova) at room temperature. Slides were finally washed with PBS (4× for 10 min each) and mounted. Immunofluorescence was visualized using a Zeiss Axioplan-2 fluorescent microscope. Images were captured and analyzed using AxioVision 2 multichannel image processing software (Zeiss, Gottingum, Germany).
Plasma angiotensin II level.
Plasma angiotensin II levels were determined by ELISA assay. Equal amount of plasma from control (nontransgenic) and double transgenic mice containing either AA or AG haplotype of the hAGT gene were passed through phenylsilylsilica extraction columns (ALPCO Diagnostics, Salem, NH). Reversed-phase extraction method was applied to extract the plasma as per the manufacturer's protocol. Peptide enzyme immunoassay for angiotensin II was performed in extracted plasma samples using EIA kit (Peninsula Laboratories), as per the manufacturer's protocol. Plasma angiotensin II levels were calculated, as described by the manufacturer using standard curve.
Physiology
All mice were fed standard mice chow and had access to water ad libitum. BP was measured in the conscious state by two different procedures.
Automated tail cuff.
Before the study was initiated, at least 3 days of training sessions were performed so that mice became accustomed to the tail cuff procedure (Columbus Instruments, model NIBP-8). After stabilization of the BP, 10–15 tail cuff measurements were made around 1 PM. BP was measured for 3 consecutive days, and animals were then treated with angiotensin receptor blocker, losartan (Sigma Aldrich) (30 mg·kg−1·day−1), in drinking water. The BP was then measured by tail-cuff method after 24 h of losartan treatment.
Telemetry.
Radiotelemetric system from Data Science International (St. Paul, MN) was used for this procedure. Briefly, mice were anesthetized with ketamine and xylazine (90 and 10 mg/kg, respectively), and the left carotid artery was isolated. The tip of the telemetric catheter (model TA11PA-C10) was then inserted into the carotid artery and advanced into the aortic arch, with the telemetric device main body positioned into a subcutaneous pocket into the right flank. After 1 wk of recovery from the surgical procedure, BP readings were recorded every 10 min using Data-Science instrument, as described previously (6). Mean BP values were calculated for every hour from the values taken over 4 days.
Statistical Analysis
Values are expressed as means ± SE. Differences between group means were determined by a two-factor ANOVA followed by a Newman-Keuls post hoc analysis (NCSS 2007); P < 0.05 was considered significant (NCSS, Kaysville, UT). Statistically significant results are marked by an asterisk (P < 0.05).
RESULTS
Double Transgenic Mice
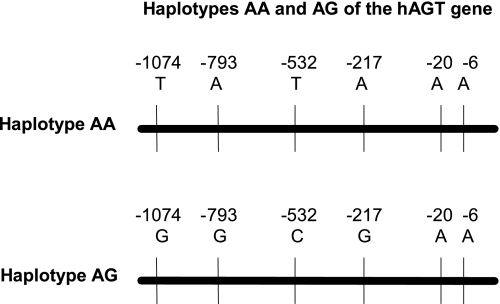
To generate transgenic mice containing AA and AG haplotypes of the hAGT gene, we amplified 13.5-kb genomic DNA from one hypertensive subject that had AA haplotype and from one normotensive subject that had AG haplotype in the AGT gene promoter. The forward primer starts at −1223 of the promoter, and the reverse primer starts 200 bp beyond the polyadenylation sites in the 3′-flanking region of the hAGT gene. This DNA contains all of the exons and introns of the hAGT gene. These amplified fragments were then ligated in TOPO-XL vector from Invitrogen. The nucleotide sequence of the cloned fragments showed that AA haplotype contains −6A, −20A, −217A, −532T, −793A, and −1074T, whereas AG haplotype contains −6A, −20A, −217G, −532C, −793G, and −1074G (Fig. 1).
Fig. 1.
Different haplotypes of the human angiotensinogen (hAGT) gene. The AGT gene can be separated in two haplotypes (AA and AG) based on nucleotide A at −6 and either nucleotide A or G at −217. Nucleotide −217A almost always occurs with −532T, −793A, and −1074T, whereas nucleotide −217G almost always occurs with −532C, −793G, and −1074G in the hAGT gene promoter.
To target the hAGT gene in a single copy upstream of the HPRT locus, we subcloned 13.5-kb hAGT gene with either AA or AG haplotype in pMP8SKB vector. These vectors were then electroporated in HAT-sensitive BK4 cells. These cells represent an HPRT-deficient E14Tg2a ES cell line. The targeting vectors with hAGT gene haplotypes has homology to 5′ and 3′ of HPRT to assist in recombination process. These vectors also contain a part of promoter and exon regions of HPRT gene that are missing in the BK4 cells. After electroporation, ES cells were grown in selective HAT medium, and HAT-resistant colonies were isolated and expanded. DNA from different ES cells was amplified to detect 13.5-kb hAGT gene. Four targeted BK4 clones containing either AA or AG haplotypes of the hAGT gene were expanded and used to create chimeric mice by blastocyst injection. The resulting chimeric male mice with the highest degree of BK4 cell contribution (as judged by agouti coat color of the offspring) were allowed to mate with C57BL/6J mice. Female mice from this cross were positive for the hAGT gene expression, as determined by PCR (since BK4 ES cells are male cells and HPRRT locus is located on the X chromosome, only female offspring from the first cross could receive the hAGT gene). The presence of hAGT gene in transgenic mice was further confirmed by PCR and by Southern hybridization. This analysis also confirmed that each line of the transgenic mice had only one copy of the hAGT gene (data not shown).
Female transgenic mice containing either AA or AG haplotype of the hAGT gene were crossed with male transgenic mice containing hRen gene (PAC-hRen) (24). In PAC-hRen mice, the hRen gene is inserted in the mouse genome in the context of 160 kb of human sequence, as part of a PAC. Expression of the renin gene has been shown to be strictly in the kidney, and its expression is tightly regulated in response to physiological mediators in these transgenic mice. The reason to generate double transgenic mice is due to strict species specificity of the renin-AGT reaction. The double transgenic mice were genotyped for the hAGT and hRen genes by PCR amplification of the DNA isolated from their tails.
AGT mRNA is increased in the liver and kidney of transgenic mice containing AA haplotype of the hAGT gene compared with the AG haplotype.
Since liver and kidney are the most important sites for the expression of AGT gene, we examined the effect of AA and AG haplotypes on the hAGT gene on its expression in these tissues of transgenic mice. Since hAGT gene is located at the HPRT locus on the X chromosome in these mice, its expression can be affected by random X inactivation. Therefore, we analyzed male transgenic mice separately. hAGT mRNA level in the liver and kidney of transgenic mice was analyzed by quantitative real-time RT-PCR using ABI PRISM 7900HT thermocycler (Applied Biosystems). Threshold cycle values for three replicate reactions were determined using Sequence Detection System software (version 2.2.2) and relative transcript abundance of hAGT calculated following normalization with mouse GAPDH. The quantitation of hAGT mRNA level in AA haplotype was calculated using RNA level in AG haplotype as one. Results of these experiments show that AGT mRNA level in the liver (Fig. 2A) and kidney (Fig. 2B) of transgenic mice containing AA haplotype is increased by 1.62- and 1.45-fold, respectively, compared with the transgenic mice containing AG haplotype (P < 0.05).
Fig. 2.
hAGT mRNA level in the liver (A) and kidney (B) of transgenic mice containing AA and AG haplotype of the hAGT gene. RNA from the liver and kidney of transgenic mice containing either AA or AG haplotype of the hAGT gene was analyzed by QRT-PCR using ABI system. A: liver from AA haplotype had 1.62-fold increased hAGT mRNA compared with AG haplotype. B: kidney from AA haplotype had 1.45-fold increased hAGT mRNA compared with AG haplotype. Values are means ± SE; n = 6. *P < 0.05.
Plasma and tissue AGT level is increased in male transgenic mice containing AA haplotype of the hAGT gene compared with the AG haplotype.
We next determined whether 1) hAGT protein in transgenic mice is correctly processed in the liver and secreted into plasma; and 2) plasma and tissue hAGT level is increased in transgenic mice containing AA haplotype of the hAGT gene compared with the AG haplotype. We, therefore, determined AGT level in plasma of double transgenic animals containing either AA or AG haplotype of the hAGT gene and hRen gene essentially as described (8). Equal volume of plasma from male transgenic animals containing either AA or AG haplotype of the hAGT gene was analyzed by Western blot assay along with human plasma (Fig. 3A). Results were normalized against mouse albumin (Fig. 3B). Quantitation of protein level from three experiments (Fig. 3C) showed that AGT level is increased ∼40–50% in the plasma of transgenic mice containing AA haplotype compared with the AG haplotype (P ≤ 0.05). Electrophoretic mobility of hAGT protein in transgenic mice was similar to that of AGT in human plasma. We also confirmed the overexpression of hAGT protein in liver and kidney of AA haplotype compared with AG haplotype with immunocytochemistry (Fig. 4). In liver (Fig. 4, A–D), the hAGT expression was diffused compared with the kidney (Fig. 4, E–H), where it is expressed more prominently in the proximal tubules. Sections from nontransgenic mice showed much reduced signal (Fig. 4, C and G), whereas no signal was observed when sections were not treated with the primary antibody (Fig. 4, D and H).
Fig. 3.
hAGT level in plasma of double transgenic mice. Plasma from double transgenic mice containing either AA or AG haplotype of hAGT gene and human renin (hRen) gene was analyzed by mouse albumin and for AGT level, as described in the text. A: Western blot using hAGT antibody. B: Western blot using mouse albumin antibody. Lanes CON (control), AG, and AA denote plasma from human and transgenic animals containing AG and AA haplotype of the hAGT gene. Position of molecular weight markers is shown on the right side. C: quantitation of AGT protein in transgenic animals containing AG and AA haplotype of the hAGT gene. AGT protein level in transgenic mice containing AA haplotype of the hAGT gene was calculated by assuming protein level in transgenic mice containing AG haplotype as one. Values are means ± SE. *P < 0.05.
Fig. 4.
Immunohistochemical staining of hAGT in liver and kidney of double transgenic mice using mouse anti-hAGT antibody. A–C: expression of hAGT in sections from the liver of transgenic mice containing AA haplotype, AG haplotype, and nontransgenic mice (C57), respectively. D: expression in liver of transgenic mice containing AA haplotype but without treatment with primary antibody. E–H: corresponding results in kidney sections.
Plasma angiotensin II level is increased in male transgenic mice containing AA haplotype of the hAGT gene compared with the AG haplotype.
We next examined the effect of AA and AG haplotypes of the hAGT gene on plasma angiotensin II level in double transgenic mice. Plasma was collected from control (nontransgenic), and male double transgenic mice containing either AA or AG haplotype of the hAGT gene and plasma angiotensin II level was analyzed by ELISA. Results of this experiment (Fig. 5) showed that plasma angiotensin II level in transgenic mice containing AG haplotype was increased by ∼40% and in transgenic mice containing AA haplotype was increased by 80% compared with the control animals (P ≤ 0.05). Thus plasma angiotensin II level was increased by ∼50% in double transgenic mice containing AA haplotype of the hAGT gene compared with the AG haplotype. Plasma angiotensin II level in single transgenic mice containing either hAGT or hRen gene was the same as that for control C57 mice (data not shown).
Fig. 5.
Plasma angiotensin II level. Plasma angiotensin II level is shown in nontransgenic CON and double transgenic mice containing either AG or AA haplotype of the hAGT gene. Values are means ± SE; n = 6. *P < 0.05.
Mouse renin and hRen level is decreased in double transgenic mice containing hRen and either AA or AG haplotype of the hAGT gene.
Since it is known that increased amount of AGT downregulates renin expression, we were interested in examining the effect of AA and AG haplotypes of the hAGT gene on mouse renin and hRen gene expression in double transgenic mice. For this purpose, we analyzed the mRNA level of endogenous mouse renin and hRen gene in the kidney of transgenic mice by QRT-PCR. Results of this experiment showed that mouse renin mRNA level in transgenic mice containing AG and AA haplotype of the hAGT gene was ∼60 and 40%, respectively, compared with nontransgenic control mice (Fig. 6A). On the other hand, the hRen mRNA level in transgenic mice containing AG and AA haplotype of the hAGT gene was ∼70 and 60%, respectively, compared with that in control PAC-hRen mice (Fig. 6B). This suggested that, although mouse renin as well as hRen gene expression is downregulated by overexpression of the hAGT gene in transgenic mice, this effect is more pronounced in transgenic mice containing AA haplotype compared with the AG haplotype.
Fig. 6.
Expression of mouse and hRen mRNA in kidney of double transgenic mice containing AG and AA haplotype of the hAGT gene. The mouse and human kidney mRNA level in double transgenic animals was determined by QRT-PCR, as described in materials and methods. A: mouse renin mRNA level in kidney of transgenic animals containing AG or AA haplotype is shown by assuming renin level in the kidney of C57BL6 mice as one (marked CON in the figure). B: hRen mRNA level in kidney of transgenic animals containing AG or AA haplotype is shown by assuming renin level in the kidney of PAC (P1 artificial chromosome)-hRen mice as one (marked CON in the figure). Values are means ± SE; n = 6. *P < 0.05.
BP is increased in male transgenic mice containing AA haplotype of the hAGT gene compared with the AG haplotype.
We next examined the effect of AA and AG haplotypes of the hAGT gene on BP in transgenic mice. We first measured the BP in control nontransgenic mice and transgenic mice containing either AA or AG haplotype of the hAGT gene and hRen gene by tail-cuff method during the daytime. BP was measured by an automated tail cuff method in the conscious state. Systolic BP in six male transgenic mice (3 mo old) was measured for 3 consecutive days, and mean values were calculated. Results of this experiment (Fig. 7C) showed that mean BP of control nontransgenic mice was 111 mmHg, of AG transgenic mice was 117 mmHg, and of AA transgenic mice was 125 mmHg (n = 6 for different groups). Statistical analysis showed that these values differ significantly with each other (P < 0.05). Results of these experiments suggested that 1) male transgenic animals with AA haplotype of the hAGT gene have increased BP compared with the AG haplotype; and 2) transgenic animals with AG haplotype of the hAGT gene have increased BP compared with the nontransgenic control animals. BP in single transgenic mice containing hAGT gene or hRen gene was the same as in control C57 mice (data not shown).
Fig. 7.
Blood pressure in double transgenic mice and effect of losartan. A: 24-h blood pressure of CON and double transgenic mice containing AA and AG haplotype of hAGT gene and hRen gene was measured by telemetry for 4 days. Each bar represents mean from 6 male animals taken over 4 days. B: blood pressure of control and double transgenic mice during daytime (6 AM to 6 PM), nighttime (6 PM to 6 AM), and whole day (24 h) period, as determined by telemetry. C: daytime blood pressure of CON and double transgenic mice containing AA and AG haplotypes of the hAGT gene and hRen gene in the presence (+) and absence (−) of losartan, as determined by tail cuff method. Values are means ± SE; n = 6. *P < 0.05.
To determine whether ANG II is acting through AT1 receptors, we used losartan as AT1 receptor blocker and determined the BP by the tail-cuff method. Results from Fig. 7C suggest that losartan reduces the systolic BP in both AA and AG haplotypes to or below the nontransgenic baseline level and contribute effectively in maintaining the BP response in double transgenic mice.
However, since BP can be measured only during the daytime by the tail-cuff method, and since it is known that BP increases during nighttime in the mice, we also measured 24-h BP in our transgenic mice by telemetry. Control (nontransgenic) and double transgenic mice containing either AA or AG haplotype of the hAGT gene and hRen gene (3-mo-old male mice) were surgically instrumented with telemetric probes. After 1 wk of recovery from the surgical procedure, BP readings were recorded every 10 min using Data-Science instrument. Systolic BP was measured over a period of 4 days, and mean values for daytime (6 AM to 6 PM), nighttime (6 PM to 6 AM), as well as average for the whole day (24 h) were calculated. Results of this experiment showed that, although BP of transgenic mice containing AA haplotype of the hAGT gene compared with the AG haplotype was increased during the daytime (as observed by tail-cuff method), this difference was more pronounced during the nighttime (Fig. 7B). Thus BP of AA transgenic animals was increased by ∼7 ± 2 mmHg compared with the AG haplotype during the daytime, but it was increased by 11 ± 2 mmHg during the nighttime. A graph showing the mean BP values over a 24-h period of the AA and AG haplotype of transgenic mice (number of animals = 6, days of recording = 4) is shown in Fig. 6A. Modulation of BP due to circadian rhythm is clear from this graph. It is clear from these data that BP is increased in double transgenic mice containing AA haplotype of the hAGT gene compared with the AG haplotype, and this difference is more pronounced during the nighttime.
DISCUSSION
Nucleotide sequence of hAGT gene promoter containing −217A has homology with the consensus CCAAT/enhancer binding protein (C/EBP) binding site, as well as glucocorticoid-receptor binding site (glucocorticoid response element). C/EBP family of transcription factors plays an important role in the expression of a gene in hepatocytes (2, 12, 20) and adipocytes (7), two cell types in which AGT gene is primarily expressed. In addition, C/EBP-β and -δ are involved in IL-6 and TNF-induced expression of a gene (1). Previous studies have shown that inflammation (4) and IL-6 (23) play an important role in transcriptional regulation of the AGT gene. Our gel shift data have shown that an oligonucleotide with nucleoside A at −217 binds more strongly to glucocorticoid receptor and C/EBP family of transcription factors. In addition, reporter constructs containing hAGT gene promoter with nucleoside A at −217 have increased basal and IL-6-induced promoter activity in human liver cells, primary human hepatocytes, and adipocytes (15, 16, 21). Increased promoter activity of reporter constructs containing −217A in the hAGT gene promoter has also been reported recently by Dickson et al. (10). In view of these observations, we have generated a new animal model system, which allowed us to test the physiological and transcriptional effect of two different haplotypes containing either −217A or −217G of the hAGT gene in an in vivo situation. We amplified 13.5-kb hAGT gene containing either AA or AG haplotype using genomic DNA from two subjects. The amplified fragments contained all of the exons, introns, and 1.3 kb of the promoter region of the hAGT gene. Previous studies have shown that knock-in of the 13.5-kb hAGT gene at the HPRT locus leads to the expression of this gene in a tissue- and sex-specific manner in transgenic mice (9). The identical genetic environment in both genetic background and transgene location allowed us to directly compare the effect of two haplotypes on transcriptional and physiological activity of the hAGT gene in an in vivo situation.
Results of our experiments show that hAGT mRNA level is increased in the liver and kidney of male transgenic mice containing AA haplotype of the hAGT gene compared with the AG haplotype. In addition, AGT protein level is increased in the plasma of male transgenic mice containing AA haplotype compared with the AG haplotype. Our studies have also shown that plasma angiotensin II level is increased in double transgenic mice, and this effect is more pronounced in mice containing AA haplotype of the hAGT gene compared with the transgenic mice containing AG haplotype. In addition, we have shown that mouse renin and hRen gene expression is downregulated in the kidney of double transgenic mice compared with the control animals.
Male double transgenic mice containing hREN gene and either AA or AG haplotype of the hAGT gene were then used to analyze the BP. Results of this experiment show that systolic BP is increased by ∼7 mmHg in double transgenic mice containing AA haplotype of the hAGT gene compared with transgenic mice containing AG haplotype of the hAGT gene, as measured by the tail cuff method. Since we can measure BP only during the daytime by the tail cuff method, we also measured BP by telemetry. Surprisingly, our results show that BP is further increased in transgenic mice containing AA haplotype of the hAGT gene during the nighttime. Thus, whereas BP increased ∼7 mmHg during daytime (6 AM to 6 PM), it increased by 11 mmHg during the nighttime (6 PM to 6 AM) in double transgenic mice containing AA haplotype compared with the transgenic mice containing AG haplotype of the hAGT gene. Administration of angiotensin receptor blocker, losartan, effectively reduced the BP in transgenic mice containing either AA or AG haplotype of the hAGT gene to control values, suggesting that hAGT gene is increasing BP in these animals mainly through angiotensin receptor type I.
Cvetkovic et al. (8) have used this strategy to analyze the effect of haplotypes containing A/G polymorphism at the −6 position in the promoter of hAGT gene and M235T polymorphism in the coding region on its transcriptional regulation and physiological function. Although association studies have shown that −6A allele is associated with hypertension in the Caucasian and Japanese population, and reporter constructs containing −6A have increased promoter activity on transient transfections in human liver cells (14), Cvetkovic et al. (8) found neither increased expression of the hAGT gene, nor an increase in the BP in transgenic mice containing −6A/235Thr haplotype compared with the transgenic mice containing −6G/235Met haplotype. Thus our results on BP of transgenic mice containing AA or AG haplotype of the hAGT gene differ from those of Cvetkovic et al. It could be due to the fact that we have analyzed the physiological effect of a different haplotype of the hAGT gene in transgenic mice.
In conclusion, we generated double transgenic mice containing hRen gene and either AA or AG haplotype of the hAGT gene. Male transgenic mice containing AA haplotype of the hAGT gene have 1) increased hAGT mRNA levels in the liver and kidney; 2) increased hAGT protein level in the liver and kidney; and 3) increased plasma AGT and angiotensin II levels, compared with the AG haplotype. In addition, transgenic animals containing AA haplotype of the hAGT gene have increased BP during daytime, as well as nighttime. These studies suggest that polymorphisms in the promoter of hAGT gene affect the transcription of this gene that ultimately modulate the BP in transgenic mice. Since our previous studies have suggested that −217 A/G polymorphism affects the binding of glucocorticoid receptor and C/EBP family of transcription factors, it is possible that increased binding of these transcription factors may be responsible for increased transcription of the hAGT gene containing AA haplotype.
Perspectives and Significance
The AGT gene is expressed in a number of tissues (such as liver, kidney, fat, heart, and brain), and its expression is regulated by a number of factors (such as inflammation, IL-6, glucocorticoids, estrogen, and androgen). The availability of transgenic animals, described in this paper, will help us understand the role of AA and AG haplotypes on transcription of the hAGT gene in a tissue- and sex-specific manner by different agents, which was not possible so far. It will also be important in the future to knock out the endogenous mouse AGT gene and examine the physiological effect of AA and AG haplotypes of the hAGT gene in an in vivo situation. Since hypertension is a multigenic disease, these transgenic animals may also be useful to analyze the epistatic effect of AGT and other genes involved in hypertension.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants (HL081752 and HL66296) to A. Kumar.
Acknowledgments
We are extremely thankful to Dr. Curt Sigmund for providing hPac-REN transgenic mice, Dr. Sarah Bronson for providing the Rsa doub probe, and to Dr. Carl Thompson for help in statistical analysis.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Alam T, An MR, Mifflin RC, Hsieh CC, Ge X, Papaconstantinou J. Trans-activation of the alpha 1-acid glycoprotein gene acute phase responsive element by multiple isoforms of C/EBP and glucocorticoid receptor. J Biol Chem 268: 15681–15688, 1993. [PubMed] [Google Scholar]
- 2.Baumann H, Morella KK, Campos SP, Cao Z, Jahreis GP. Role of CAAT-enhancer binding protein isoforms in the cytokine regulation of acute-phase plasma protein genes. J Biol Chem 267: 19744–19751, 1992. [PubMed] [Google Scholar]
- 3.Bloem LJ, Foroud TM, Ambrosius WT, Hanna MP, Tewksbury DA, Pratt JH. Association of the angiotensinogen gene to serum angiotensinogen in blacks and whites. Hypertension 29: 1078–1082, 1997. [DOI] [PubMed] [Google Scholar]
- 4.Brasier AR, Tate JE, Ron D, Habener JF. Multiple cis-acting DNA regulatory elements mediate hepatic angiotensinogen gene expression. Mol Endocrinol 3: 1022–1034, 1989. [DOI] [PubMed] [Google Scholar]
- 5.Bronson SK, Plaehn EG, Kluckman KD, Hagaman JR, Maeda N, Smithies O. Single-copy transgenic mice with chosen-site integration. Proc Natl Acad Sci USA 93: 9067–9072, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butz GM, Davisson RL. Long-term telemetric measurement of cardiovascular parameters in awake mice: a physiological genomics tool. Physiol Genomics 5: 89–97, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Cao Z, Umek RM, McKnight SL. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev 5: 1538–1552, 1991. [DOI] [PubMed] [Google Scholar]
- 8.Cvetkovic B, Keen HL, Zhang X, Davis D, Yang B, Sigmund CD. Physiological significance of two common haplotypes of human angiotensinogen using gene targeting in the mouse. Physiol Genomics 11: 253–262, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Cvetkovic B, Yang B, Williamson RA, Sigmund CD. Appropriate tissue- and cell-specific expression of a single copy human angiotensinogen transgene specifically targeted upstream of the HPRT locus by homologous recombination. J Biol Chem 275: 1073–1078, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Dickson ME, Zimmerman MB, Rahmouni K, Sigmund CD. The −20 and −217 promoter variants dominate differential angiotensinogen haplotype regulation in angiotensinogen-expressing cells. Hypertension 49: 631–639, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Fasola AF, Martz BL, Helmer OM. Renin activity during supine exercise in normotensives and hypertensives. J Appl Physiol 21: 1709–1712, 1966. [DOI] [PubMed] [Google Scholar]
- 12.Friedman AD, Landschulz WH, McKnight SL. CCAAT/enhancer binding protein activates the promoter of the serum albumin gene in cultured hepatoma cells. Genes Dev 3: 1314–1322, 1989. [DOI] [PubMed] [Google Scholar]
- 13.Gould AB, Green D. Kinetics of the human renin and human substrate reaction. Cardiovasc Res 5: 86–89, 1971. [DOI] [PubMed] [Google Scholar]
- 14.Inoue I, Nakajima T, Williams CS, Quackenbush J, Puryear R, Powers M, Cheng T, Ludwig EH, Sharma AM, Hata A, Jeunemaitre X, Lalouel JM. A nucleotide substitution in the promoter of human angiotensinogen is associated with essential hypertension and affects basal transcription in vitro. J Clin Invest 99: 1786–1797, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jain S, Li Y, Patil S, Kumar A. A single-nucleotide polymorphism in human angiotensinogen gene is associated with essential hypertension and affects glucocorticoid induced promoter activity. J Mol Med 83: 121–131, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Jain S, Tang X, Narayanan CS, Agarwal Y, Peterson SM, Brown CD, Ott J, Kumar A. Angiotensinogen gene polymorphism at −217 affects basal promoter activity and is associated with hypertension in African-Americans. J Biol Chem 277: 36889–36896, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Jeunemaitre X, Soubrier F, Kotelevtsev YV, Lifton RP, Williams CS, Charru A, Hunt SC, Hopkins PN, Williams RR, Lalouel JM, et al. Molecular basis of human hypertension: role of angiotensinogen. Cell 71: 169–180, 1992. [DOI] [PubMed] [Google Scholar]
- 18.Kim HS, Krege JH, Kluckman KD, Hagaman JR, Hodgin JB, Best CF, Jennette JC, Coffman TM, Maeda N, Smithies O. Genetic control of blood pressure and the angiotensinogen locus. Proc Natl Acad Sci USA 92: 2735–2739, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kimura S, Mullins JJ, Bunnemann B, Metzger R, Hilgenfeldt U, Zimmermann F, Jacob H, Fuxe K, Ganten D, Kaling M. High blood pressure in transgenic mice carrying the rat angiotensinogen gene. EMBO J 11: 821–827, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Landschulz WH, Johnson PF, McKnight SL. The DNA binding domain of the rat liver nuclear protein C/EBP is bipartite. Science 243: 1681–1688, 1989. [DOI] [PubMed] [Google Scholar]
- 21.Li Y, Jain S, Patil S, Kumar A. A haplotype of angiotensinogen gene that is associated with essential hypertension increases its promoter activity in adipocytes. Vascul Pharmacol 44: 29–33, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Pereira TV, Nunes AC, Rudnicki M, Yamada Y, Pereira AC, Krieger JE. Meta-analysis of the association of 4 angiotensinogen polymorphisms with essential hypertension: a role beyond M235T? Hypertension 51: 778–783, 2008. [DOI] [PubMed] [Google Scholar]
- 23.Sherman CT, Brasier AR. Role of signal transducers and activators of transcription 1 and 3 in inducible regulation of the human angiotensinogen gene by interleukin-6. Mol Endocrinol 15: 441–457, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Sinn PL, Davis DR, Sigmund CD. Highly regulated cell type-restricted expression of human renin in mice containing 140- or 160-kilobase pair P1 phage artificial chromosome transgenes. J Biol Chem 274: 35785–35793, 1999. [DOI] [PubMed] [Google Scholar]
- 25.Walker WG, Whelton PK, Saito H, Russell RP, Hermann J. Relation between blood pressure and renin, renin substrate, angiotensin II, aldosterone and urinary sodium and potassium in 574 ambulatory subjects. Hypertension 1: 287–291, 1979. [DOI] [PubMed] [Google Scholar]
- 26.Watt GC, Harrap SB, Foy CJ, Holton DW, Edwards HV, Davidson HR, Connor JM, Lever AF, Fraser R. Abnormalities of glucocorticoid metabolism and the renin-angiotensin system: a four-corners approach to the identification of genetic determinants of blood pressure. J Hypertens 10: 473–482, 1992. [DOI] [PubMed] [Google Scholar]
- 27.Wu SJ, Chiang FT, Chen WJ, Liu PH, Hsu KL, Hwang JJ, Lai LP, Lin JL, Tseng CD, Tseng YZ. Three single-nucleotide polymorphisms of the angiotensinogen gene and susceptibility to hypertension: single locus genotype vs. haplotype analysis. Physiol Genomics 17: 79–86, 2004. [DOI] [PubMed] [Google Scholar]