Abstract
Rats exposed to 3 h of restraint stress on each of 3 days (RRS) lose weight on the days of RRS and gain weight at the same rate as controls after stress ends, but do not return to the weight of controls. RRS rats also show an exaggerated endocrine response to subsequent novel stressors. Studies described here tested the effects of corticotropin-releasing factor receptor (CRFR) antagonism on RRS-induced weight loss, hypophagia, and corticosterone release during mild stress in the postrestraint period. Weight loss was not prevented by either peripheral or third-ventricle administration of a CRFR1 antagonist, antalarmin, before each restraint. Antalarmin did, however, allow recovery of body weight in the poststress period. Third-ventricle administration of a CRFR2 antagonist, antisauvagine 30, had no effect in RRS rats but caused sustained weight loss in control animals. Surprisingly, third-ventricle administration of the nonselective CRFR antagonist, astressin, caused hypophagia and reversible weight loss in control rats. It had no effect in RRS rats. None of the antagonists modified the corticosterone response to RRS or to mild stress in the post-RRS period, but antalarmin suppressed corticosterone during the period of restraint in Control rats. These results suggest that CRFR1 activation is required for the initiation of events that lead to a prolonged down-regulation of body weight in RRS rats. The sustained reduction in body weight is independent of the severity of hypophagia on the days of restraint and of RRS-induced corticosterone release.
Keywords: third ventricle, hypothalamic-pituitary adrenal axis, food intake, body weight
stress modifies the endocrine, sympathetic, metabolic, immunologic, and behavioral status of an individual, and these changes initiate the immediate reaction to stress, as well as facilitate a return to homeostasis once stress has ended. CRF and its homologues urocortin I (Ucn I), Ucn II, and Ucn III are the initiators of many of the physiological and behavioral responses to stress (28, 33, 44) through activation of CRF receptors (CRFR). CRFR can be divided into two major subtypes, CRFR1 and CRFR2, which are differentially expressed in sites throughout the brain and peripheral tissues (45). Studies with knockout mice and receptor antagonists show that different aspects of the stress response are dependent upon the type and location of the CRFR activated. Stimulation of the hypothalamic-pituitary-adrenal (HPA) axis, the immediate inhibition of food intake caused by stress (36), as well as initiation of behaviors indicative of anxiety, have been associated with activation of CRFR1 (4, 5, 42). CRF2 activation also has been associated with induction of anxiety-type behaviors (32, 47), with a delayed, but sustained, inhibition of food intake (12, 24, 31, 36), with the initiation of stress-coping behaviors (12) and with the regulation of activity of the hypothalamic-pituitary-adrenal axis during stress (5).
We have previously reported that when rats are subjected to 3 h of restraint on each of three consecutive days (repeated restraint stress, RRS), they lose weight on the days of restraint (20, 22). Following RRS, the stressed rats gain weight at the same rate as their controls, but they do not compensate for the stress-induced weight loss. This sustained reduction in body weight has been observed as many as 80 days after the end of RRS (21). The maintenance of a reduced body weight contrasts with weight loss that is induced by other interventions, such as food restriction, in which body weight is rapidly restored to control levels once the intervention that caused weight loss has been removed (19). Although the effects of RRS are enduring, RRS is an acute, rather than a chronic, stressor. RRS rats do not show the same increase in adrenal weight or decrease in thymus weight that is found in chronically stressed rats (1, 7, 21, 40). Chronic stress changes the diurnal pattern of corticosterone release, raising the morning nadir in rats (1), whereas the diurnal pattern of corticosterone release is not significantly different in RRS rats compared with controls once restraint has ended (18).
The initial stress-induced weight loss can be attributed to decreased food intake and increased energy expenditure on the days that the rats are restrained, although there is no sustained hypophagia (20) or increased thermogenesis once stress ends (21). Therefore, the change in body weight regulation of rats exposed to RRS appears to result from a cascade of events that is initiated during restraint, rather than from activation of stress-responsive pathways. It appears that body weight remains low because the rats fail to compensate for negative energy balance on the days of restraint, suggesting that RRS causes a functional change in a fundamental biological process that determines body mass. The objective of studies described here was to determine whether antagonism of specific subtypes of CRFR during restraint stress would prevent the long-term change in body weight of RRS rats. In the first experiment described here, a CRFR1-specific antagonist that is known to cross the blood-brain barrier was injected subcutaneously and would have blocked both peripheral and central receptors. In five subsequent experiments, the receptor antagonists were infused into the third ventricle. This site of infusion was chosen because it has been well established that mechanisms controlling both food intake and energy expenditure are coordinated, at least in part, by hypothalamic nuclei that express CRF receptors. These nuclei include the dorsomedial, arcuate, paraventricular, lateral, and ventromedial nuclei of the hypothalamus (6, 24, 35, 45). Although the brain stem also is an important site of integration for the control of energy balance, and there are nuclei that express CRFR (17, 45), we previously reported that infusion of CRFR antagonists into the fourth ventricle immediately before restraint stress did not modify RRS-induced weight loss or hypophagia (29).
The maintenance of a reduced body weight is not the only chronic response found in RRS rats and mice. Although baseline serum corticosterone levels and the daily nadir of corticosterone release remain normal, RRS animals show an exaggerated secretion of glucocorticoids when challenged by a novel mild stressor in the post-stress period (18). This effect is not unique to RRS, as others have reported increased sensitivity to subsequent stressors following an acute stress. For example, rats exposed to one or three daily sessions of tail or foot shock give an exaggerated corticosterone response to the same stressor applied 10 days later (9, 37). In addition to enhanced endocrine sensitivity, mice exposed to RRS display increased anxiety-type behavior, as many as 20 days after the end of RRS (11). It is not known whether the long-term changes in body weight, HPA responsiveness, and anxiety caused by RRS are mediated by the same, or different, stress-related pathways. Therefore, in addition to testing the effects of CRFR antagonists on food intake and body weight of RRS rats, we also measured the endocrine response to a novel mild stress in rats 15, or more, days after they had been infused with receptor antagonists and exposed to restraint stress.
METHODS
All animals used in these studies were male Sprague-Dawley rats (Harlan Sprague Dawley, Indianapolis, IN) that weighed ∼275 g on arrival. Animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Georgia and met the guidelines described by the American Physiological Society (2). All rats were housed in humidity- (52%) and temperature-controlled (22.7°C) rooms with lights on for 12 h/day beginning at 7.00 A.M. and had free access to rat chow (LabDiet 5012, PMI Nutrition International, Brentwood, MO) and water unless stated otherwise.
Experiment 1: subcutaneous injection of antalarmin, an antagonist of CRFR1, before RRS.
In an initial study designed to test the importance of CRFR1 in mediating the changes in food intake, body weight, and/or the hyper-reactivity of the HPA axis in rats exposed to RRS, we tested rats that were injected 30 min before each restraint stress with 11.5 nmol/kg (5 mg/kg) NBI 27914, a specific CRFR1 antagonist. Results of a previous study indicated that this dose of NBI 27914 given subcutaneously before a 20-min period of restraint inhibited increases in anxiety-like behavior measured immediately after restraint (39). In this study, we found no effect of NBI 27914 on food intake or body weight of the rats. Fifteen days after the end of RRS, the rats were exposed to the novel mild stress of receiving an intraperitoneal injection of saline followed by placement in a new cage in a treatment room for 90 min. There were no differences in the adrenal response to this novel stress in rats that had been treated earlier in the study with vehicle or with NBI 27914. Others have reported that they also were unable to demonstrate biological activity of this antagonist in hamsters (38); therefore, this study tested a different CRFR1 antagonist, antalarmin, to determine whether the lack of effect in the previous study was because the NBI 27914 did not block the CRFR1, or because CRFR1 is not involved in the energetic and endocrine responses to RRS. The dose of antalarmin used in this study (20 mg/kg) has been reported to block ethanol self-administration in ethanol-dependent rats (16), whereas a higher dose of 30 mg/kg has been reported to cause acute toxicity (50).
Thirty-six rats, weighing ∼320 g, were adapted to the housing conditions, and then baseline food intake and body weight were recorded for 7 days. The rats were divided into four weight-matched groups and starting at 8:00 A.M. on each of the next three days (day 0–2), each rat received an intraperitoneal injection of 48 μmol/kg (20 mg/kg) antalarmin hydrochloride (ANT; Sigma Chemical, St. Louis, MO) in 10% DMSO, 90% PBS, or vehicle. Starting at 8:30 A.M., the rats within each treatment group were subjected to either 3 h of restraint stress or were nonrestrained controls. The four groups were ANT/Control, ANT/RRS, Veh/Control, and Veh/RRS. RRS rats were placed in Perspex restraining tubes (21.6 × 6.4 cm, Plas Labs, Lansing MI) in an experimental room for 3 h. Control rats were placed in shoebox cages in the same room without food or water during the 3 h of restraint stress. At the end of restraint, the rats were returned to their home cages. On the 2nd day of RRS, blood was collected by tail-bleeding from all rats immediately before injection (time 0) and at 15, 30, 60, 120, and 180 min after the onset of restraint for measurement of serum corticosterone concentration. Twenty-four days after the end of RRS (day 26), all of the rats were subjected to a novel mild stress of being housed in a test room in a shoebox cage containing wet bedding (500 g bedding, 750 ml water) for 2 h. Blood samples were collected before and 60 min after the start of mild stress. Wet bedding was used as the mild stressor because the nonrestrained control rats had already been subjected to intraperitoneal injections and placement in a novel room as part of the RRS protocol.
Experiment 2: third-ventricle infusion of antalarmin before repeated restraint.
In experiment 1, ANT/RRS rats lost weight on the days of restraint, but they returned to the weight of their nonstressed controls by the end of the experiment. In that experiment, the antagonist was given as a peripheral injection; therefore, this study tested whether inhibition of the sustained weight loss was due to antagonism of CRFR1 in areas accessed by a third-ventricle infusion of antagonist. Zorrilla et al. (48) reported that 5 μg antalarmin infused into the lateral ventricle prevented Ucn-induced consolidation of passive avoidance learning in rats. We halved the dose of antalarmin used by Zorrilla (48) because we were infusing into the third ventricle, which has a smaller volume than the lateral ventricle.
Fifty-six male Sprague-Dawley rats, housed as described in experiment 1, were fitted with a 26-gauge guide cannula (Plastics One, Roanoke, VA) aimed at the third ventricle using stereotaxic techniques and coordinates (anteroposterior −2.8 mm, lateral 0.0 mm, ventral −8.3 mm relative to bregma) based on the Paxinos and Watson rat brain atlas (30). The cannula was anchored to the skull with jeweler's screws and dental acrylic cement. Rats were allowed 7 days to recover from surgery, and then cannula placement was verified by infusion of 20 ng ANG II in 2 μl of sterile saline. Rats that drank water within 2 min of infusion were included in the experiment. All infusions were administered with Hamilton syringes controlled by an infusion pump (PHD 2000 Infusion pump, Harvard Apparatus, Holliston, MA). Rats were given 2 days to recover from placement verification before the experiment started.
After measurement of baseline food intakes and body weights for 6 days, rats were subjected to RRS, as described above. They were divided into four weight-matched groups (n = 14): vehicle control (Veh/Control), vehicle restraint (Veh/RRS), antalarmin control (ANT/Control), and antalarmin restraint (ANT/RRS). Thirty minutes before the start of restraint on each day of RRS rats received an intracerebroventricular infusion of 2 μl DMSO (Veh/Control and Veh/RRS) or 6 nmol (2.5 μg) ANT dissolved in 2 μl DMSO (Ant/Control or Ant/RRS) over 2 min and returned to their home cages until the initiation of restraint. On the 2nd day of RRS, blood was collected by tail bleeding from all rats immediately before infusion (time 0) and 60 min after the start of restraint for measurement of serum corticosterone concentration. After RRS, food intakes and body weights were recorded for 12 days. Twelve days after RRS (day 15), rats in each treatment group were divided into two subgroups; control or MS (n = 7). MS rats were exposed to the MS of an intraperitoneal injection of saline and placement in a novel cage and room, as described above, while control rats remained in their home cages. Blood was collected immediately before MS (time 0) and at 30, 60, and 90 min after MS for measurement of serum corticosterone concentration.
Experiment 3: third ventricle infusion of antisauvagine-30, a CRFR2-specific antagonist, before repeated restraint.
Activation of CRFR2 has been reported to play a role in the mediation of stress-induced hypophagia in rats (13, 24) and also to dampen stress-induced responses caused by CRFR1 receptors (12); therefore, this study tested whether antagonism of CRFR2 immediately before restraint would modify the effect of RRS on food intake, body weight, and the subsequent endocrine response to a novel mild stress. A dose-response study was performed with third-ventricle infusions of 0.3 to 3 nmol of the selective CRFR2 antagonist, antisauvagine-30 (ASV) (34). The high dose (3 nmol = 13.6 μg) was the highest concentration that we could get into solution and represented a dose similar to one that has been reported to prevent burn-induced hypermetabolism in rats (10 μg) (10) but was greater than a lateral ventricle dose (5 μg) that partially reversed an inhibition of food intake during the first hour after restraint stress in rats (36).
Sixty-three male Sprague-Dawley rats were housed as described in the previous experiments. Guide cannulas were implanted in the third ventricle, and placement was verified 1 wk later. Baseline food intakes and body weights were recorded for 7 days, as described above. The rats were divided into seven weight-matched groups (n = 9): Veh/Control, Veh/RRS, 3.0 nmol ASV/control (ASV/Control), 3.0 nmol ASV/RRS, 1.5 nmol ASV/RRS, 0.75 nmol ASV/RRS, and 0.3 nmol ASV/RRS. Fifteen minutes before the start of restraint on each day of RRS, rats received an intracerebroventricular infusion of 2 μl sterile saline (Veh/Control and Veh/RRS) or ASV (Sigma Aldrich) dissolved in 2 μl of sterile saline over 2 min. Blood was collected by tail-bleeding before infusion (time 0) and 60 min after the beginning of restraint on the 2nd day of RRS for measurement of serum corticosterone concentration. Following RRS, food intakes and body weights were recorded for a further 13 days (day 16). On day 15, all of the rats were exposed to the mild stress of an intraperitoneal injection of 2 ml of sterile saline and placement in a novel cage in a treatment room. Tail blood samples were collected for measurement of serum corticosterone at 0, 30, 60, and 90 min after the start of MS.
Experiment 4: third-ventricle infusion of astressin, a nonspecific CRFR antagonist, before repeated restraint.
The results of the previous experiments indicated that third-ventricle infusion of a CRFR1 antagonist, but not a CRFR2 antagonist, before restraint stress prevented the sustained reduction in body weight in RRS rats. This experiment tested whether antagonism of all CRFR during RRS would block the poststress hyperreactivity of the HPA axis in addition to stress-induced weight loss. This was a dose-response study, testing four different concentrations of astressin (AST). The highest dose used in this study (6 nmol = 21.4 μg) was much greater than that delivered to the lateral ventricle to prevent social stress-induced anxiety behavior (1 μg) or CRF-induced anxiety behavior (5 μg) in rats (41).
Sixty three rats were fitted with third-ventricle cannulas, as described above. Following recovery from cannula placement verification, baseline daily food intakes and body weights were recorded for 7 days, and then the rats were divided into seven weight-matched groups. Fifteen minutes before the start of restraint on the days of RRS, each rat received a third-ventricle infusion of 2 μl saline (Veh/Control, Veh/RRS) or of AST (0.75, 1.5, 3.0, or 6 nmol; Sigma Aldrich). All doses of AST were tested in RRS rats, but only saline and the highest dose of AST were tested in nonstressed controls (AST/Control). On the 2nd day of RRS, blood was collected by tail bleeding from all rats immediately before infusion (time 0) and 60 min after the start of restraint for measurement of serum corticosterone concentration. On day 15, all of the rats were exposed to the MS of saline injection and placement in a novel cage in a treatment room, as described above. Blood was collected immediately before MS (time 0) and at 30, 60, and 90 min after the start of MS for measurement of serum corticosterone concentration.
Experiment 5: third-ventricle infusion of a single, high dose of astressin before repeated restraint.
Surprisingly, the nonspecific antagonist did not block either the RRS-induced inhibition of food intake or the sustained weight loss in stressed rats in experiment 4. Therefore, study was repeated with the highest dose of antagonist to confirm the results of the dose-response experiment, and the time interval between infusion of the antagonist and the start of restraint was extended to ensure effective blockade of receptors before the onset of stress.
Fifty-six rats were fitted with third-ventricle cannulas and housed as described in Experiment 1. Following recovery from cannula placement verification, baseline daily food intakes and body weights were recorded for 7 days. The rats were divided into four weight-matched groups (n = 14): Veh/Control, Veh/RRS, AST/Control, and AST/RRS. Thirty minutes before the start of restraint on each day of restraint, rats received an intracerebroventricular infusion of 2 μl sterile saline (Veh/Control and Veh/RRS) or 6 nmol astressin dissolved in 2 μl saline (AST/Control or AST/RRS). On the 2nd day of RRS, blood was collected by tail bleeding from all rats immediately before infusion (time 0) and 60 min after the start of restraint for measurement of serum corticosterone concentration. Following RRS, food intakes and body weights were recorded for 15 days.
Experiment 6: effect of CRF on food intake in rats infused with astressin, a nonselective CRFR antagonist.
The results of experiments 4 and 5 indicated that while selective antagonism of CRFR1 prevented sustained weight loss in RRS rats, nonselective antagonism of both CRFR subtypes with AST had no effect on RRS-induced weight loss. Therefore, this study was performed as a test of the effect of AST on a behavior that was independent of RRS. We tested whether 6 nmol AST would prevent the hypophagia caused by third-ventricle administration of CRF.
Sixteen rats were fitted with third-ventricle guide cannulas, and 2 days after verification of cannula placement, all of the rats were fasted overnight. The next morning, rats were divided into four weight-matched groups (n = 4): astressin CRF (AST/CRF), astressin control (AST/Control), vehicle CRF (Veh/CRF), and vehicle control (Veh/Control). AST groups received an intracerebroventricular infusion of 6 nmol astressin in 2 μl sterile saline and vehicle groups (Veh/CRF and Veh/Control) received 2 μl sterile saline. Thirty minutes after the first infusion, each rat received an intracerebroventricular infusion of 3 μg CRF (Sigma Aldrich) in 2 μl sterile saline (AST/CRF and Veh/CRF) or 2 μl sterile saline (AST/Control and Veh/Control). Food intake was recorded 1, 2, 4, 6, and 24 h after infusion. Three days after the first set of infusions, rats were retested, but the groups were rotated, so that each rat was exposed to astressin and CRF only one time.
Statistical analysis.
Food intake, change in body weight, and MS serum corticosterone values were compared using repeated-measures ANOVA (Statistica, StatSoft, Tulsa OK). Average food intake from the 3 days before RRS and blood collected at time 0 for measures of serum corticosterone concentration were used as covariates in the appropriate analyses. Two-way ANOVA was used to analyze RRS corticosterone data in studies in which there were four treatment groups in a balanced design. Duncan's multiple-range test was used for post hoc comparisons between groups. Differences were considered significant at P < 0.05.
RESULTS
Experiment 1: peripheral injection of antalarmin.
Rats injected with ANT before each restraint ate less and lost more weight on the days of restraint than Veh/RRS rats (Fig. 1, A and C). Intake of ANT/Control rats was reduced to the same level as that of Veh/RRS rats and was inhibited further in ANT/RRS rats (RRS: P < 0.004, ANT: P < 0.001, RRS × ANT: NS). The intake of Veh/RRS rats remained lower than that of their controls for 7 days after the end of RRS, but food intakes of ANT/RRS rats were the same as those of their controls 2 days after the end of RRS. The total amount of food consumed during the experimental period was lower in the RRS rats than their controls (Fig. 1B : P < 0.001), but there was no effect of ANT on intake of either the Control or RRS rats. Both ANT/Control and ANT/RRS rats gradually regained weight after RRS ended. ANT/Controls weighed the same as Veh/Controls by day 17 and ANT/RRS rats weighed the same as ANT/Controls by day 21 and more than Veh/RRS rats from day 18. Veh/RRS rats weighed less than their controls from day 1 to the end of the experiment (Fig. 1C: RRS: P < 0.001, ANT: NS, RRS × Day: P < 0.001, ANT × Day: P < 0.001).
Fig. 1.
Average food intake for 3 days before, during, and after repeated restraint stress (RRS; A), total food intake during the experimental period (B), change in body weight during the experimental period (C), and serum corticosterone measured on the 2nd day of restraint (D) in experiment 1 for rats that received peripheral injections of antalarmin or vehicle before each restraint. Data are expressed as means ± SE for 9 rats. *Significant difference between control and RRS groups. #Significant difference between antalarmin hydrochloride (ANT)/RRS and Veh/RRS rats. $Significant difference between Veh/RRS and Veh/Control rats. δSignificant difference between ANT-treated and Veh-treated rats. A,B,CAveraged food intakes for the days of RRS or for total food intake that do not share a common letter superscript are significantly different at P < 0.05.
Corticosterone was higher in restrained rats than controls on the 2nd day of restraint, and there was no effect of ANT on the response to restraint, but prestress (time 0) corticosterone was significantly higher in rats that had been injected with ANT the day before (Fig. 1D: ANT: P < 0.05, RRS: P < 0.0001, Time P < 0.001 ANT × RRS: NS, ANT × Time: P < 0.001, RRS × Time: P < 0.03). On day 26, serum corticosterone was higher during MS in rats that had been exposed to RRS than their controls, but there was no effect of ANT treatment on this response (data not shown).
Experiment 2: third-ventricle infusion of antalarmin.
The average food intake of all of the rats in experiment 2 was lower on the days of restraint than during the baseline period, presumably because of the third-ventricle infusions. There was an overall inhibitory effect of RRS on food intake, but there was no independent effect of ANT (Fig. 2A : ANT: NS, RRS: P < 0.008, ANT × RRS: NS). There were no statistically significant differences between the total amounts of food consumed by the different groups of rats during the 16-day experimental period (Fig. 2B). Both Veh/RRS and ANT/RRS rats lost weight on the days of restraint, and Veh/RRS rats weighed less than Veh/Control rats from the 2nd day of restraint until the end of the experiment. In contrast, ANT/RRS rats weighed less than their controls only from day 2 to day 5 of the experiment (Fig. 2C; RRS: P < 0.005, Day: P < 0.001, RRS × Day: P < 0.001). The ANT/RRS rats weighed more than Veh/RRS rats from day 9 until the end of the experiment, and by day 12, ANT/RRS rats weighed the same as ANT/Control rats.
Fig. 2.
Average food intake for 3 days before, during and after RRS (A), total food intake during the experimental period (B), change in body weight during the experimental period (C), and serum corticosterone measured on the 2nd day of restraint (D) in experiment 2 for rats that received third-ventricle injections of antalarmin or vehicle before each restraint. Data are expressed as means ± SE for 14 rats. $Significant difference between vehicle (Veh)/RRS and Veh/Control rats. %Significant differences between ANT/RRS and ANT/Control animals (P < 0.05). #Significant difference between ANT/RRS and Veh/RRS animals (P < 0.05). A,B,CValues for food intake on the days of RRS or corticosterone values at the 60-min time point that do not share a common letter superscript are significantly different at P < 0.001.
Serum corticosterone concentration was significantly higher in RRS rats than Controls 60 min after the start of restraint on the 2nd day of RRS (Fig. 2D: ANT: P < 0.012, RRS: P < 0.001, ANT × RRS: 0.97). Corticosterone concentration was also higher in Control rats 60 min after the start of restraint than at time 0 but was higher in Veh/Control than ANT/Control rats. On day 15, there was no effect of either previous exposure to RRS or ANT infusion during RRS on the corticosterone response to MS (data not shown).
Experiment 3: third-ventricle infusion of antisauvagine-30.
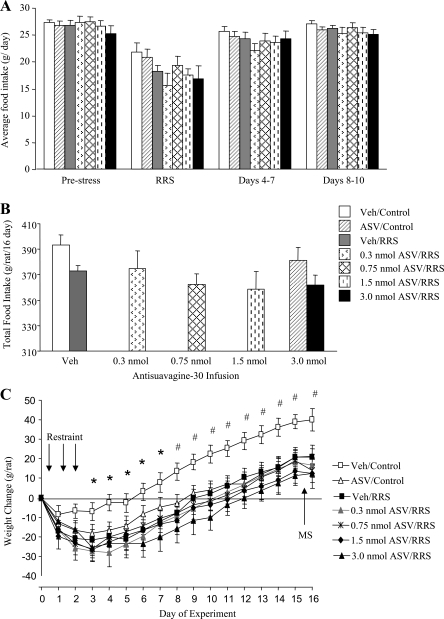
In this dose-response study, food intake was inhibited for all groups on the days of restraint. When all treatment groups were considered in the analysis, there was no significant difference within any of the time intervals considered. When only the rats receiving infusions of saline or 3 nmol ASV were considered in a two-way ANOVA (RRS: P < 0.02, ASV: NS, RRS × Day: NS), then rats in the 3 nmol ASV/RRS group ate less than the Veh/Control and ASV/Control groups on the days of restraint and ate less than Veh/Controls during the 3 days after restraint had ended. This difference between the two groups was also present when food intake during the entire experimental period was considered (P < 0.01), but there was no significant effect of RRS or of ASV on total food intake if all groups were included in the analysis (Fig. 3B). All of the rats lost some weight on the days of restraint stress (Fig. 3C; RRS: P < 0.007, Day: P < 0.001, RRS × Day: NS). Veh/Control rats weighed more than all of the RRS rats by the end of the 3 days of restraint and from day 8, five days after the end of restraint, Veh/Control rats weighed more than all other groups, including ASV/Controls. Serum corticosterone was increased after 60 min of RRS on the 2nd day of restraint. This effect was not significant if all of the groups were included in the analysis, but it was significant if the analysis were limited to the Veh-infused and 3.0 nmol ASV-infused rats. There was no effect of ASV on serum corticosterone either before or during RRS (data not shown). There was no effect of either RRS or ASV on corticosterone concentrations measured during MS on day 15 (data not shown).
Fig. 3.
Average food intake for three days before, during, and after RRS (A), total food intake during the experimental period (B), and change in body weight during the experimental period (C) for rats that received third-ventricle infusions of antisauvagine-30 or vehicle before each restraint in experiment 3. Data are means ± SE for 9 rats. *Significant differences between RRS and control animals. #Significant difference in weight change of Veh/Control rats compared with all other groups.
Experiment 4: third-ventricle astressin dose response study.
Food intake of all of the RRS rats in the dose-response study was less than that of controls groups on the 3 days of RRS (Treatment: P < 0.019, Time: P < 0.0001, Interaction: P < 0.001), but there were no differences between the RRS groups or between the two control groups (Fig. 4A). There were no differences in food intakes of any of the groups in the poststress period, and there was no effect of treatment on the total amount of food consumed during the 16-day experimental period (Fig. 4B). Because of the unbalanced design, the previous analysis was performed as a one-way ANOVA. When only Veh and 6 nmol AST groups were compared by two-way ANOVA, there was a significant effect of RRS (P < 0.0001) on food intake on the days of stress and on total intake during the experimental period (P < 0.03), but there was no effect of AST. All rats lost some weight on the days of RRS, presumably due to the third-ventricle infusions, but the RRS rats lost significantly more weight than controls. All groups of RRS rats weighed less than the Veh/Control and AST/Control groups on all days of the experimental period except days 1, 2, 12, and 16, which was the day after MS and the last day of the experiment. There was no significant effect of AST on weight loss in either RRS or control rats (Fig. 4C: Treatment P < 0.0001, Day: P < 0.0001, Interaction: NS).
Fig. 4.
Average food intake for 3 days before, during, and after RRS (A), total food intake during the experimental period (B), change in body weight during the experimental period (C), and serum corticosterone measured on the 2nd day of restraint (D) in rats that received third-ventricle infusions of different doses astressin (AST) or vehicle before each restraint in experiment 4. Data are expressed as means ± SE for 9 rats. *Significant differences between Control and RRS animals. A,BValues for averaged food intake on the days of restraint or for corticosterone 60 min after the start of restraint that do not share a common letter superscript are significantly different.
Serum corticosterone was higher 60 min after the start of restraint on the 2nd day of restraint for all rats (Fig. 4D: Treatment: NS, Time: P < 0.001, Treatment × Time: P < 0.01). At 60 min, serum corticosterone was higher in all RRS rats than in Veh/Controls. Corticosterone in AST/Controls was not different from that in any other group. On day 15, MS increased serum corticosterone in all rats, and there was no significant effect of RRS or of AST on area under the curve for any of the groups (data not shown).
Experiment 5: third-ventricle high-dose astressin.
This experiment confirmed the outcome of the dose-response study. Average food intake was lower during the 3 days of RRS than before stress for all groups except Veh/Control rats (Fig. 5A : AST: P < 0.001, RRS: P < 0.05, AST × RRS: NS). The food intake of all of the rats returned to baseline in the 3 days immediately following RRS, and there was no effect of RRS or of AST on total intake during the 16-day experimental period (Fig. 5B). Both groups of restrained rats lost more weight than controls on the days of RRS, and they maintained a lower weight than controls in the poststress period (Fig. 5C: AST: NS, RRS: P < 0.001, Day: P < 0.001, RRS × Day: P < 0.001). Infusion of AST immediately before restraint had no effect on the change in body weight of RRS rats. AST/Control rats weighed significantly less than Veh/Control rats, but more than either group of RRS rats on days 2–4 (P < 0.05). RRS significantly increased serum corticosterone concentration, and AST had no effect on corticosterone in either control or RRS rats (data not shown: AST: NS, RRS: P < 0.004, AST × RRS: NS). Sixty minutes after the start of mild stress, serum corticosterone was higher in rats that had been subjected to RRS than in those that had been Controls (Fig. 5).
Fig. 5.
Average food intake for 3 days before, during, and after RRS (A), total food intake during the experimental period (B), change in body weight during the experimental period (C), and serum corticosterone measured during MS on day 15, 12 days after the end of restraint (D) in rats that received third-ventricle infusions of a high dose of astressin or vehicle before each restraint in experiment 5. *Significant differences between Control and RRS animals (P < 0.03). φSignificant difference between AST/Control and all other groups. A,BAveraged food intake values on the days of restraint that do not share a common letter superscript are significantly different.
Experiment 6: effect of CRF on food intake in rats infused with astressin.
Pretreatment with AST partially prevented CRF-induced inhibition of food intake. This effect was significant for cumulative food intake at both 1 and 2 h (Fig. 6A : AST: NS, CRF: P < 0.001, AST × CRF: P < 0.02) after infusion. There was no difference in cumulative food intakes of the four groups of rats measured 4, 6 or 24 h after CRF infusion. When food intake during each time interval was considered, CRF inhibited food intake only during the first hour after infusion. Four hours after infusion both groups of CRF-infused rats ate more than their controls to compensate for the earlier hypophagia (Fig. 6B). There was no effect of antagonist or CRF infusion on 24-h weight gain (data not shown).
Fig. 6.
Effect of 3 μg of CRF on cumulative (A) or intervalled (B) food intake of rats infused in the third ventricle with either AST or Veh 30 min before CRF infusion in experiment 6. Data are expressed as means ± SE for 8 rats. Superscript letters indicate significant (P < 0.03) differences between groups at a specific time point.
DISCUSSION
The objective of experiments described here was to determine whether specific antagonism of either CRFR1 or CRFR2 would attenuate stress-induced weight loss or the sustained changes in body weight and in reactivity of the HPA axis that are observed in rats that have been exposed to RRS. The results demonstrate that activation of CRFR1 on the days of restraint is required for the long-term downregulation of body weight, but not for the initial weight loss that occurs on the days of restraint. In both experiments 1 and 2, ANT/RRS rats gradually regained weight in the poststress period until they weighed the same as nonstressed controls. The rate of recovery of body weight was faster in the rats that received the central infusion of ANT, even though the amount of weight lost on the days of restraint was similar for the two groups of RRS rats. It is possible that peripherally administered ANT initiated different metabolic and endocrine responses than the more localized delivery of the antagonist into the third ventricle and that the slow recovery in peripherally injected rats reflected compensation for these unidentified changes. The results from these two experiments imply that CRFR1 located in areas that are accessed by infusion of an antagonist into the third ventricle are responsible for the initiation of events that lead to the sustained reduction in body weight of RRS rats. The results of experiments 1 and 2 also suggest that hypophagia on the days of RRS contributes to the amount of weight that is lost on the days of restraint but does not impact the long-term downregulation of body weight in RRS rats. In experiment 1, peripheral administration of astressin exaggerated hypophagia and weight loss in RRS rats on the days of restraint, but the AST/RRS rats returned to the weight of their controls by the end of the experiment. In experiment 2, there was no difference in the food intake of Veh/RRS and AST/RRS rats on the days of restraint, but the AST/RRS rats subsequently returned to control body weight, whereas the Veh/RRS rats did not. The lack of association between food intake and body weight gain of RRS rats suggests an important role for energy expenditure in determining body weight. We have previously shown that energy expenditure of rats exposed to RRS is increased during the period of restraint, but that it returns to control levels once the animals come out of the restraining tubes (21). Further studies are needed to determine whether energy expenditure of AST-infused rats is inhibited in the poststress period.
The effects of stress on food intake are mediated by both CRFR1 and CRFR2, but along different time frames. Studies with knockout mice have shown that Ucn I, a ligand for both CRFR1 and CRFR2 (46), inhibits food intake for 10 h in wild-type mice, but only for 6 h in CRFR2-deficient mice (12), whereas there is no effect of Ucn I on food intake of CRFR1-deficient mice until 90 min after the injection (8). Similarly, lateral ventricle infusion of a CRFR1-specific receptor antagonist (CRA1000) prevents restraint-induced hypophagia during the hour immediately following restraint, but even high doses of ASV have only a small stimulatory effect on the food intake of restrained rats during the same time period (36). Thus, CRFR1 mediate the immediate inhibition of food intake by stress in a response that lasts for only 1 or 2 h, whereas CRFR2 mediate changes in food intake that start later and last longer. In the studies described here, we measured daily intake; therefore, we did not expect to find any effect of specific CRFR1 antagonism on food intake. Surprisingly, the results of experiment 1, in which rats received peripheral injections of ANT, clearly demonstrated that CRFR1 activity is required to maintain normal food intake. All rats injected with ANT had a lower food intake than their respective saline-injected groups and lost weight on the days that they were injected, indicating that a tonic level of CRFR1 activation is required to support food intake even in nonstress conditions. RRS reduced the intake of ANT-treated rats even further. This additive inhibitory effect of stress and antagonism of CRFR1 on food intake suggests that different populations of receptors were responsible for the individual responses. The data from our study do not differentiate between changes in food intake caused by central vs. peripheral CRFR1, but there are relatively low levels of CRFR1 expression in peripheral tissues (14), making it likely that the effect was caused by antagonism of central receptors. Because we did not find any effect of ANT on food intake in either Control or RRS rats in experiment 2, the receptors responsible for non-stress-related hypophagia do not appear to have been located in areas accessed by antagonist infusion into the third ventricle. It is well established that stress inhibits motility of the gastrointestinal tract (25), making it unlikely that hypophagia caused by the receptor antagonist was secondary to changes in gut motility.
In experiment 3, we did not find any effect of third-ventricle infusion of ASV on the hypophagia of RRS rats on the days of restraint. This was unexpected because Sekino et al. (36) reported that a lateral ventricle infusion of 5 μg (∼1 nmol) of ASV immediately before 1 h of restraint stress partially reversed the inhibition of food intake measured 1 h after the end of restraint in rats. Similarly, Cullen et al. (13) reported that infusion of the CRFR ligands CRF or Ucn for 2 wk into the lateral ventricle of rats caused a dose-dependent inhibition of food intake and weight gain that was fully reversed by ASV in the Ucn-infused rats and partially reversed by ASV in the CRF-infused rats. In contrast to these studies, de Groote et al. (15) found that acute lateral ventricle infusions of Ucn III, a specific CRFR2 agonist, increased food intake for several hours, possibly as a nonspecific result of behavioral activation. This increased intake was prevented by pretreatment with 5 μg ASV. There are a number of reasons that may explain why our study had a different outcome from those described above. The first is that ASV would have reached different nuclei following a third ventricle infusion than following a lateral ventricle infusion. The second is that rats in our studies were restrained for 3 h, which may have resulted in different levels of activation of stress-responsive sites compared with 1 h of restraint. In vitro studies have shown ASV to be stable for 4 h at 22°C (23); therefore, a third possibility is that the ASV prevented hypophagia during the first hour after restraint ended, but not during the beginning of the dark cycle when food intake of control and RRS rats continues to be different (22). A final possible explanation for the failure to find an effect of ASV on food intake would be if the time interval between antagonist infusion and the start of restraint were too short for effective blockade of the CRFR2 receptors; however, de Groote et al. (15) demonstrated that 15 min is adequate for development of an antagonist effect with lateral ventricle infusions of ASV. An additional argument against the time between antagonist infusion and initiation of restraint being too short is that the pattern of weight loss in AST/control rats was similar to that of RRS rats and significantly different from that of Veh/Control rats, indicating a specific effect of ASV in these animals. The weight loss in ASV/control rats was unexpected and was not associated with a change in food intake. This suggests that energy expenditure was increased in these animals, which would be consistent with a previous report of increased sympathetic tone in CRFR2-knockout mice (3). Although ASV promoted weight loss in control rats, it did not exaggerate weight loss RRS rats. It is thought that CRFR2 attenuates some of the stress responses that are initiated by CRFR1. Therefore, it is possible that antagonism of CRFR2 in control animals allowed for a relative increase in CRFR1 activity that resembled that caused by RRS, whereas the level of activation of CRFR1 in RRS rats overwhelmed any moderating effects of CRFR2.
Although the results of experiments 1 and 2 clearly show that antagonism of CRFR1 during stress prevented the sustained reduction in body weight of RRS rats, there was no effect of the nonspecific CRFR antagonist AST in experiments 4 and 5. Experiment 6 provided independent evidence of efficacy of the antagonist because AST blocked the short-term inhibition of food intake produced by infusion of CRF into the third ventricle. The binding affinity of AST for CRFR1 is about 10-fold lower than that of ANT (49) and about the same as that of ASV for CRFR2 (49). Equivalent molar concentrations of AST and ANT were used in the studies described here; therefore, it is possible that the lack of effect of AST was due to incomplete blockade of CRFR1 receptors. In vitro studies have shown that AST dissociates from CRFR1 receptors faster than the agonist Ucn I (33); therefore, it also is possible that the AST was not effectively blocking CRFR1 receptors for the entire time that stress-related pathways were activated in RRS rats. The molar concentration of AST used in experiments 4 and 5 was greater than that of ASV used in experiment 3, and some aspects of the pattern of response in ANT/Control rats was similar to that of ASV/Control rats. Both groups of rats lost weight on the days of infusion, but the ANT/Control rats gradually returned to the weight of Veh/Control rats in the poststress period, whereas ASV/Control rats did not. The recovery of weight in AST/Controls was similar to that of ANT/RRS rats, which implies that the dose of AST used in these studies was adequate for blockade of CRFR1 activity in Control rats but may not have been fully effective in the RRS rats.
It is well established that stress-induced activation of the HPA axis is mediated by CRFR1 (8, 42), but we did not find any change in corticosterone release during restraint in any of the RRS rats in any of the studies described here. It should be noted that the single measure of corticosterone concentration made during restraint (60 min) would not have identified any change in the temporal pattern of adrenal response to stress. Blood samples were collected on the 2nd day of restraint because corticosterone release during restraint starts to attenuate by the 3rd day of RRS, but it is the same on the 2nd as on the 1st day (20). ANT did suppress corticosterone in ANT/Control rats during the period of restraint in experiment 2, which suggests that corticosterone release during RRS results from an integration of multiple mechanisms. For example, Turnbull et al. (43) demonstrated that basal release of ACTH in CRFR1-deficient mice is controlled by AVP, whereas ACTH release in response to inflammation is stimulated by a mechanism that is independent of both CRF and AVP. Thus, the failure to inhibit corticosterone release of RRS rats treated with CRFR1 antagonists was most likely due to redundancy in mechanisms available to activate the HPA axis in conditions of stress. In experiment 2, basal corticosterone concentrations measured before the start of restraint on the 2nd day of RRS were elevated in ANT-treated rats, representing a sustained effect of the ANT injection from the previous day. Without more extensive measures of ACTH and corticosterone in these rats, it is not possible to determine whether the elevated corticosterone was due to a shift in the normal diurnal cycle of HPA activity, prolonged activation of the pituitary gland, or abnormal activity of the adrenal gland.
The long-term downregulation of body weight is not the only sustained change that we have observed in RRS animals. Rats and mice subjected to RRS show an exaggerated response to novel mild stress in the poststress period measured as activation of the HPA axis (18), stress-related hypophagia (18), or anxiety-type behavior (11). Therefore, an additional objective of the studies described here was to test whether antagonism of CRFR during RRS would block the poststress hyperreactivity of the rats, but we did not find any effect of any of the interventions on the endocrine response of the rats to mild stress in the post-RRS period. This indicates that the mechanisms that change processes that determine body weight are independent of those that modify sensitivity of the HPA axis. The increased reactivity in rats that have been subjected to RRS could be due to an elevation of basal levels of ligand production so that the increase required to activate stress responses is smaller than usual, a change in receptor number or amplification of the postreceptor response. We have not tested any of these aspects of the hyperreactivity in RRS rats but have previously reported that increased stress-responsiveness of high-fat-fed rats is associated with changes in CRF and Ucn I mRNA expression in basal conditions (26).
In conclusion, the experiments described here demonstrate that specific antagonism of CRFR1 during RRS attenuates the sustained reduction in body weight, but not the poststress hyperreactivity of the HPA axis, in RRS rats. This chronic effect of RRS on body weight appears to be independent of the inhibition of food intake or the stimulation of corticosterone release on the days of restraint. Future studies will investigate the role of CRFR1 in individual brain sites in the forebrain and how the effects of CRFR1 and CRFR2 activation interact to produce the acute and the enduring changes in body weight regulation, HPA activity, and anxiety-type behavior in animals exposed to RRS.
Perspectives and Significance
The studies described here tested the importance of specific CRFR subtypes in causing a prolonged reduction in body weight of rats exposed to RRS. This model of disruption of body weight regulation is of interest because weight loss caused by other interventions, such as food restriction (19) or cold exposure (27), is rapidly reversed once the animal is returned to its normal environment. Understanding the mechanisms responsible for the prolonged downregulation of weight in stressed rats may provide information not only on the interactions between systems that are activated by stress and energy balance regulation, but also on the factors that contribute to weight regain following weight loss in conditions other than stress. The studies described here clearly show that CRFR1 located in the forebrain are critical to the initiation of events that lead to the sustained reduction in body weight of RRS rats, and future studies will focus on identifying the location of these receptors.
GRANTS
This work was supported by National Institute of Mental Health Grant MH06828101 and by Georgia Agricultural Experiment Station grant CSREES/GEO00932 awarded to R. B. S. Harris.
Acknowledgments
The authors thank Ms. Jessica Davenport and Dr Joanna Miragaya for their help with these studies.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Akana SF, Dallman MF. Chronic cold in adrenalectomized, corticosterone (B)-treated rats: facilitated corticotropin responses to acute restraint emerge as B increases. Endocrinology 138: 3249–3258, 1997. [DOI] [PubMed] [Google Scholar]
- 2.American PS Guiding principles for research involving animals and human beings. Am J Physiol Regul Integr Comp Physiol 283: R281–R283, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Bale TL, Anderson KR, Roberts AJ, Lee KF, Nagy TR, Vale WW. Corticotropin-releasing factor receptor-2-deficient mice display abnormal homeostatic responses to challenges of increased dietary fat and cold. Endocrinology 144: 2580–2587, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Bale TL, Contarino A, Smith GW, Chan R, Gold LH, Sawchenko PE, Koob GF, Vale WW, Lee KF. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat Genet 24: 410–414, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol 44: 525–557, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Bamshad M, Aoki VT, Adkison MG, Warren WS, Bartness TJ. Central nervous system origins of the sympathetic nervous system outflow to white adipose tissue. Am J Physiol Regul Integr Comp Physiol 275: R291–R299, 1998. [DOI] [PubMed] [Google Scholar]
- 7.Bhatnagar S, Dallman M. Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience 84: 1025–1039, 1998. [DOI] [PubMed] [Google Scholar]
- 8.Bradbury MJ, McBurnie MI, Denton DA, Lee KF, Vale WW. Modulation of urocortin-induced hypophagia and weight loss by corticotropin-releasing factor receptor 1 deficiency in mice. Endocrinology 141: 2715–2724, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Caggiula AR, Antelman SM, Aul E, Knopf S, Edwards DJ. Prior stress attenuates the analgesic response but sensitizes the corticosterone and cortical dopamine responses to stress 10 days later. Psychopharmacology (Berl) 99: 233–237, 1989. [DOI] [PubMed] [Google Scholar]
- 10.Chance WT, Dayal R, Friend LA, Thomas I, Sheriff S. Mediation of burn-induced hypermetabolism by CRF receptor-2 activity. Life Sci 80: 1064–1072, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Chotiwat C, Harris RB. Increased anxiety-like behavior during the post-stress period in mice exposed to repeated restraint stress. Horm Behav 50: 489–495, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Coste SC, Kesterson RA, Heldwein KA, Stevens SL, Heard AD, Hollis JH, Murray SE, Hill JK, Pantely GA, Hohimer AR, Hatton DC, Phillips TJ, Finn DA, Low MJ, Rittenberg MB, Stenzel P, Stenzel-Poore MP. Abnormal adaptations to stress and impaired cardiovascular function in mice lacking corticotropin-releasing hormone receptor-2. Nat Genet 24: 403–409, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Cullen MJ, Ling N, Foster AC, Pelleymounter MA. Urocortin, corticotropin releasing factor-2 receptors and energy balance. Endocrinology 142: 992–999, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Dautzenberg FM, Kilpatrick GJ, Hauger RL, Moreau J. Molecular biology of the CRH receptors—in the mood. Peptides 22: 753–760, 2001. [DOI] [PubMed] [Google Scholar]
- 15.de Groote L, Penalva RG, Flachskamm C, Reul JM, Linthorst AC. Differential monoaminergic, neuroendocrine and behavioural responses after central administration of corticotropin-releasing factor receptor type 1 and type 2 agonists. J Neurochem 94: 45–56, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Funk CK, Zorrilla EP, Lee MJ, Rice KC, Koob GF. Corticotropin-releasing factor 1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biol Psychol 61: 78–86, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grill HJ Distributed neural control of energy balance: contributions from hindbrain and hypothalamus. Obesity 14 Suppl 5: 216S–221S, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Harris RB, Gu H, Mitchell TD, Endale L, Russo M, Ryan DH. Increased glucocorticoid response to a novel stress in rats that have been restrained. Physiol Behav 81: 557–568, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Harris RB, Kasser TR, Martin RJ. Dynamics of recovery of body composition after overfeeding, food restriction or starvation of mature female rats. J Nutr 116: 2536–2546, 1986. [DOI] [PubMed] [Google Scholar]
- 20.Harris RB, Mitchell TD, Simpson J, Redmann SM Jr, Youngblood BD, Ryan DH. Weight loss in rats exposed to repeated acute restraint stress is independent of energy or leptin status. Am J Physiol Regul Integr Comp Physiol 282: R77–R88, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Harris RB, Palmondon J, Leshin S, Flatt WP, Richard D. Chronic disruption of body weight but not of stress peptides or receptors in rats exposed to repeated restraint stress. Horm Behav 49: 615–625, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Harris RB, Zhou J, Youngblood BD, Rybkin II, Smagin GN, Ryan DH. Effect of repeated stress on body weight and body composition of rats fed low- and high-fat diets. Am J Physiol Regul Integr Comp Physiol 275: R1928–R1938, 1998. [DOI] [PubMed] [Google Scholar]
- 23.Higelin J, Py-Lang G, Paternoster C, Ellis GJ, Patel A, Dautzenberg FM. 125I-Antisauvagine-30: a novel and specific high-affinity radioligand for the characterization of corticotropin-releasing factor type 2 receptors. Neuropharmacology 40: 114–122, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Inoue K, Valdez GR, Reyes TM, Reinhardt LE, Tabarin A, Rivier J, Vale WW, Sawchenko PE, Koob GF, Zorrilla EP. Human urocortin II, a selective agonist for the type 2 corticotropin-releasing factor receptor, decreases feeding and drinking in the rat. J Pharmacol Exp Therap 305: 385–393, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Larauche M, Bradesi S, Million M, McLean P, Tache Y, Mayer EA, McRoberts JA. Corticotropin-releasing factor type 1 receptors mediate the visceral hyperalgesia induced by repeated psychological stress in rats. Am J Physiol Gastrointest Liver Physiol 294: G1033–G1040, 2008. [DOI] [PubMed] [Google Scholar]
- 26.Legendre A, Papakonstantinou E, Roy MC, Richard D, Harris RB. Differences in response to corticotropin-releasing factor after short- and long-term consumption of a high-fat diet. Am J Physiol Regul Integr Comp Physiol 293: R1076–R1085, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Leung PM, Horwitz BA. Free-feeding patterns of rats in response to changes in environmental temperature. Am J Physiol 231: 1220–1224, 1976. [DOI] [PubMed] [Google Scholar]
- 28.Lewis K, Li C, Perrin MH, Blount A, Kunitake K, Donaldson C, Vaughan J, Reyes TM, Gulyas J, Fischer W, Bilezikjian L, Rivier J, Sawchenko PE, Vale WW. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc Natl Acad Sci USA 98: 7570–7575, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miragaya JR, Harris RB. Antagonism of corticotrophin-releasing factor receptors in the fourth ventricle modifies responses to mild but not restraint stress. Am J Physiol Regul Integr Comp Physiol 295: R404–R416, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press, 1998.
- 31.Pelleymounter MA, Joppa M, Ling N, Foster AC. Behavioral and neuroendocrine effects of the selective CRF2 receptor agonists urocortin II and urocortin III. Peptides 25: 659–666, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Pelleymounter MA, Joppa M, Ling N, Foster AC. Pharmacological evidence supporting a role for central corticotropin-releasing factor(2) receptors in behavioral, but not endocrine, response to environmental stress. J Pharmacol Exp Therap 302: 145–152, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Perrin MH, Sutton SW, Cervini LA, Rivier JE, Vale WW. Comparison of an agonist, urocortin, and an antagonist, astressin, as radioligands for characterization of corticotropin-releasing factor receptors. J Pharmacol Exp Therap 288: 729–734, 1999. [PubMed] [Google Scholar]
- 34.Ruhmann A, Bonk I, Lin CR, Rosenfeld MG, Spiess J. Structural requirements for peptidic antagonists of the corticotropin-releasing factor receptor (CRFR): development of CRFR2beta-selective antisauvagine-30. Proc Natl Acad Sci USA 95: 15264–15269, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sawchenko PE Toward a new neurobiology of energy balance, appetite, and obesity: the anatomists weigh in. J Comp Neurol 402: 435–441, 1998. [PubMed] [Google Scholar]
- 36.Sekino A, Ohata H, Mano-Otagiri A, Arai K, Shibasaki T. Both corticotropin-releasing factor receptor type 1 and type 2 are involved in stress-induced inhibition of food intake in rats. Psychopharmacology (Berl) 176: 30–38, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Servatius RJ, Ottenweller JE, Bergen MT, Soldan S, Natelson BH. Persistent stress-induced sensitization of adrenocortical and startle responses. Physiol Behav 56: 945–954, 1994. [DOI] [PubMed] [Google Scholar]
- 38.Seymour PL, Dettloff SL, Jones JE, Wade GN. Corticotropin-releasing factor receptor subtypes mediating nutritional suppression of estrous behavior in Syrian hamsters. Am J Physiol Regul Integr Comp Physiol 289: R418–R423, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Smagin G, Ryan D, De Souza E, Harris R. The role of CRF1 receptors in restraint-, CRF- and urocortin-induced anxiety-like behaviors. Soc Neurosci Abstracts 24: Abstract 1198, 1998. [Google Scholar]
- 40.Smagin GN, Howell LA, Redmann S Jr, Ryan DH, Harris RB. Prevention of stress-induced weight loss by third ventricle CRF receptor antagonist. Am J Physiol Regul Integr Comp Physiol 276: R1461–R1468, 1999. [DOI] [PubMed] [Google Scholar]
- 41.Spina MG, Basso AM, Zorrilla EP, Heyser CJ, Rivier J, Vale W, Merlo-Pich E, Koob GF. Behavioral effects of central administration of the novel CRF antagonist astressin in rats. Neuropsychopharmacology 22: 230–239, 2000. [DOI] [PubMed] [Google Scholar]
- 42.Timpl P, Spanagel R, Sillaber I, Kresse A, Reul JM, Stalla GK, Blanquet V, Steckler T, Holsboer F, Wurst W. Impaired stress response and reduced anxiety in mice lacking a functional corticotropin-releasing hormone receptor 1. Nat Genet 19: 162–166, 1998. [DOI] [PubMed] [Google Scholar]
- 43.Turnbull AV, Smith GW, Lee S, Vale WW, Lee KF, Rivier C. CRF type I receptor-deficient mice exhibit a pronounced pituitary-adrenal response to local inflammation. Endocrinology 140: 1013–1017, 1999. [DOI] [PubMed] [Google Scholar]
- 44.Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science 213: 1394–1397, 1981. [DOI] [PubMed] [Google Scholar]
- 45.Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol 428: 191–212, 2000. [DOI] [PubMed] [Google Scholar]
- 46.Vaughan J, Donaldson C, Bittencourt J, Perrin MH, Lewis K, Sutton S, Chan R, Turnbull AV, Lovejoy D, Rivier C, Rivier J, Sawchenko PE, Vale W. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature 378: 287–292, 1995. [DOI] [PubMed] [Google Scholar]
- 47.Zhao Y, Valdez GR, Fekete EM, Rivier JE, Vale WW, Rice KC, Weiss F, Zorrilla EP. Subtype-selective corticotropin-releasing factor receptor agonists exert contrasting, but not opposite, effects on anxiety-related behavior in rats. J Pharmacol Exp Therap 323: 846–854, 2007. [DOI] [PubMed] [Google Scholar]
- 48.Zorrilla EP, Schulteis G, Ormsby A, Klaassen A, Ling N, McCarthy JR, Koob GF, De Souza EB. Urocortin shares the memory modulating effects of corticotropin-releasing factor (CRF): mediation by CRF1 receptors. Brain Res 952: 200–210, 2002. [DOI] [PubMed] [Google Scholar]
- 49.Zorrilla EP, Tache Y, Koob GF. Nibbling at CRF receptor control of feeding and gastrocolonic motility. Trends Pharmacol Sci 24: 421–427, 2003. [DOI] [PubMed] [Google Scholar]
- 50.Zorrilla EP, Valdez GR, Nozulak J, Koob GF, Markou A. Effects of antalarmin, a CRF type 1 receptor antagonist, on anxiety-like behavior and motor activation in the rat. Brain Res 952: 188–199, 2002. [DOI] [PubMed] [Google Scholar]