Abstract
Vascular resistance and blood pressure (BP) are reduced during late normal pregnancy (Norm-Preg). In contrast, studies in human preeclampsia and in animal models of hypertension in pregnancy (HTN-Preg) have suggested that localized reduction in uterine perfusion pressure (RUPP) in late pregnancy is associated with increased systemic vascular resistance and BP; however, the vascular mechanisms involved are unclear. Because Ca2+ is a major determinant of vascular contraction, we hypothesized that the intracellular free calcium concentration ([Ca2+]i) signaling of vasoconstriction is differentially regulated in systemic microvessels during normal and RUPP in late pregnancy. Pressurized mesenteric microvessels from Norm-Preg and RUPP rats were loaded with fura 2 in preparation for simultaneous measurement of diameter and [Ca2+]i (presented as fura 2 340/380 ratio). Basal [Ca2+]i was lower in RUPP (0.73 ± 0.03) compared with Norm-Preg rats (0.82 ± 0.03). Membrane depolarization by 96 mM KCl, phenylephrine (Phe, 10−5 M), angiotensin II (ANG II, 10−7 M), or endothelin-1 (ET-1, 10−7 M) caused an initial peak followed by maintained vasoconstriction and [Ca2+]i. KCl caused similar peak vasoconstriction and [Ca2+]i in Norm-Preg (45.5 ± 3.3 and 0.89 ± 0.02%) and RUPP rats (46.3 ± 2.1 and 0.87 ± 0.01%). Maximum vasoconstriction to Phe, ANG II, and ET-1 was not significantly different between Norm-Preg (28.6 ± 4.8, 32.5 ± 6.3, and 40 ± 4.6%, respectively) and RUPP rats (27.8 ± 5.9, 34.4 ± 4.3, and 38.8 ± 4.1%, respectively). In contrast, the initial Phe-, ANG II-, and ET-1-induced 340/380 ratio ([Ca2+]i) was reduced in RUPP (0.83 ± 0.02, 0.82 ± 0.02, and 0.83 ± 0.03, respectively) compared with Norm-Preg rats (0.95 ± 0.04, 0.93 ± 0.01, and 0.92 ± 0.02, respectively). Also, the [Ca2+]i-vasoconstriction relationship was similar in KCl-treated but shifted to the left in Phe-, ANG II-, and ET-1-treated microvessels of RUPP compared with Norm-Preg rats. The lower agonist-induced [Ca2+]i signal of vasoconstriction and the leftward shift in the [Ca2+]i-vasoconstriction relationship in microvessels of RUPP compared with Norm-Preg rats suggest activation of [Ca2+]i sensitization pathway(s). The similarity in vasoconstriction in RUPP and Norm-Preg rats suggests that such a [Ca2+]i sensitization pathway(s) may also provide a feedback effect on Ca2+ mobilization/homeostatic mechanisms to protect against excessive vasoconstriction in systemic microvessels during RUPP in late pregnancy.
Keywords: resistance vessels, vascular smooth muscle, intracellular free calcium concentration, preeclampsia
during normal pregnancy(Norm-Preg) increases in plasma volume, heart rate, and renal blood flow, as well as decreases in systemic vascular resistance, blood pressure (BP), and vascular reactivity to circulating vasoconstrictors are often observed (8, 18, 37, 65). In contrast, in 5–7% of pregnancy, women develop a condition called preeclampsia, which is characterized by increased intravascular coagulation, proteinuria, increased systemic vascular resistance, and hypertension in pregnancy (HTN-Preg; Refs. 24, 25, 32, 59, 60, 63, 66). Although HTN-Preg is a major cause of maternal morbidity, fetal mortality, and low-birth-weight (2, 3, 27), its exact mechanism has not been clearly identified.
Because of the difficulty to perform mechanistic studies in women with preeclampsia, animal models of HTN-Preg have been developed (1, 13, 14, 44, 57, 58). Studies in these animal models have led to the concept that reduction in the uteroplacental perfusion pressure and the ensuing placental ischemia/hypoxia during late pregnancy may represent the initiating events that eventually lead to increased systemic vascular resistance and HTN-Preg. In support of this concept, studies (5, 6, 14, 26, 63) have demonstrated that reduction of uterine perfusion pressure (RUPP) in late pregnant rats is associated with significant increases in renal vascular resistance and BP; however, the vascular and cellular mechanisms involved have not been clearly elucidated.
Vascular smooth muscle (VSM) contraction is triggered by an increase in intracellular free Ca2+ concentration ([Ca2+]i) due to initial Ca2+ release from the intracellular stores and maintained Ca2+ entry from the extracellular space (39–41, 51). Ca2+ binds calmodulin to form a complex that activates myosin light chain kinase, causes myosin light chain phosphorylation, initiates actin-myosin interaction, and produces VSM contraction (30, 62). Previous studies (51) in isolated VSM cells suggested that phenylephrine (Phe)-induced contraction and [Ca2+]i are reduced in aortic VSM of female compared with male rats. Also, Phe-induced vascular contraction is reduced in aortic segments of Norm-Preg compared with virgin rats but significantly enhanced in rat models of HTN-Preg produced by administration of the nitric oxide synthase (NOS) inhibitor Nω-nitro-l-arginine (l-NAME) or by inducing RUPP in late pregnancy (14, 15, 36, 45). We have also shown that both contraction and [Ca2+]i are enhanced in renal arterial VSM cells isolated from RUPP rats compared with Norm-Preg rats (50). However, the vascular responses during pregnancy may not be uniform and may vary depending on the vascular bed studied and the vessel size down the arterial tree, i.e., large, intermediate, small, and microvessels. The differences in the responses of various blood vessels can be related to differences in vasoconstrictor receptor distribution, receptor-coupling mechanisms, and postreceptor mechanisms particularly [Ca2+]i control mechanisms and [Ca2+]i sensitization pathways (7, 31, 45). Previous studies (6) have shown that the mesenteric vascular resistance is elevated in rat models of HTN-Preg. Also, the mesenteric vascular resistance is reduced in Norm-Preg compared with nonpregnant normotensive rats (61, 68, 74) and in Norm-Preg compared with nonpregnant spontaneously hypertensive rats (12). However, little is known about the pregnancy-associated changes in the mechanisms of vasoconstriction in the small microvessels of the systemic circulation, which are directly relevant to the changes in BP. Also, in our previous studies (15, 49, 50), we examined the pregnancy-associated changes in vascular contraction and [Ca2+]i in response to only one agonist, making it difficult to appreciate whether the observed alterations are specific to a particular agonist/receptor or represent changes in a common signaling mechanism downstream from receptor activation.
In the present study, we tested the hypothesis that the Ca2+-dependent mechanisms of vasoconstriction in the systemic microvessels are differentially regulated under conditions of normal and RUPP in late pregnancy. We used small mesenteric microvessels isolated from the well-described RUPP rat model of HTN-Preg and control Norm-Preg rats to determine the following: 1) whether the mesenteric microvessel reactivity to four different vasoconstrictor stimuli is altered in RUPP compared with Norm-Preg rats; 2) whether the alterations in microvessel reactivity in RUPP compared with Norm-Preg rats reflect differences in the microvessel [Ca2+]i; and 3) whether the alterations in microvessel reactivity in RUPP compared with Norm-Preg rats reflect differences in the microvessel vasoconstriction sensitivity to [Ca2+]i.
MATERIAL AND METHODS
Animals.
Time-pregnant (day 12) female Sprague-Dawley rats (12 wk of age) were purchased from Charles River Laboratories (Wilmington, MA). The rats were housed in the animal facility and maintained on ad libitum standard rat chow and tap water in 12-h light-dark cycle. All surgical procedures were performed using aseptic technique and proper anesthetics and analgesics in accordance with the National Institutes of Health Guide for the Care of Laboratory Animal Welfare Act, the guidelines of the Animal Care and Use Committee at Harvard Medical School, and the American Physiological Society.
Protocol for RUPP.
On day 13 of pregnancy, pregnant rats destined to be in the RUPP group were anesthetized by inhalation of isoflurane, the abdominal cavity was opened by a midline incision, the lower abdominal aorta was exposed, and a silver clip (0.203 mm ID) was placed around the aorta above the iliac bifurcation as previously described (1, 6, 14, 26, 50). This procedure has been shown to reduce uterine perfusion pressure in the gravid rat by ∼40% (20). Since compensation of blood flow to the placenta occurs through an adaptive increase in ovarian blood flow (53), a silver clip (0.1 mm ID) was also placed on the main uterine branches of both the right and left ovarian arteries. Norm-Preg rats were sham operated. RUPP rats in which the clipping procedure resulted in maternal death or total resorption of the fetuses were excluded from the data analyses. With the use of the same RUPP protocol, the BP was ∼25–35 mmHg greater in RUPP rats compared with Norm-Preg rats as previously reported (1, 5, 6, 14, 22, 26, 50, 56).
Tissue preparation.
On gestational day 19, the rats were euthanized by inhalation of CO2. The abdominal cavity was opened, the pups and placentas were removed, the pups were weighed, and the litter size was recorded. The small intestine, adjacent mesentery, and mesenteric arterial arcade were excised and placed in ice-cold oxygenated Krebs solution. Small mesenteric arteries third or fourth order were dissected free of surrounding adipose tissue under microscopic visualization.
Pressurized microvessels.
A 1- to 2-mm microvessel segment was transferred to a temperature-controlled perfusion chamber (5 ml), mounted between two glass micropipettes (cannulas), and secured with 10–0 ophthalmic suture (Living Systems Instrumentation, Burlington, VT). The microvessel bath was placed on the stage of a Nikon inverted microscope with attached video camera. The lumen of the artery was filled with Krebs solution, one micropipette was clamped off, and the other micropipette was connected to a pressure servo control to maintain the intraluminal pressure at 60 mmHg. Applying the same constant pressure in the microvessels should limit potential fluctuations in endothelial cell production of vasodilators associated with changes in the microvessel pressure, flow, and sheer stress. The Krebs solution within the microvessel was not renewed during pressurization; however, the microvessel was bathed in 5 ml of Krebs and was continuously superfused with fresh Krebs at a rate of 1 ml/min using a peristaltic mini-pump (Master-Flex; Cole-Parmer, Vernon Hills, IL), which should maintain the ionic environment constant throughout the duration of the experiment. The temperature of Krebs solution was kept at 37°C. Drugs were added abluminally to the bath solution. Microvessels were unacceptable if they show leaks or if they fail to produce >30% constriction to 96 mM KCl or 80% dilation with sodium nitroprusside (10−5 M).
Simultaneous measurement of microvessel diameter and [Ca2+]i.
The mesenteric microvessles were continuously monitored using a video camera connected to a television monitor, and the microvessel diameter was measured using automatic edge-detection system (Crescent Electronics, Sandy, UT) and digitized at 1 Hz using a personal computer. Snap-pictures of the microvessel were taken at specific time points using a digital camera (Cool-Snap, Photometrics, Tucson, AZ). For measurement of [Ca2+]i, microvessels were incubated in Krebs solution containing the Ca2+ indicator fura 2/AM (5 μM) and pluronic F-127 (0.01%) for 60 min. The microvessel was excited alternately at 340 and 380 nm, and the emitted light was collected at 510 nm using Felix Fluorescence data acquisition and analysis software (Photon Technology International, Birmingham, NJ). The 340/380 ratio was calculated and represented the changes in [Ca2+]i. The signal-to-noise ratio was improved by averaging 10 consecutive 340/380 fluorescence ratio readings.
The sensitivity of the contractile response to KCl and Phe has previously been published by our group and other laboratories in the aorta, uterine, and mesenteric arteries of Norm-Preg and RUPP rats (5, 6, 14, 23). Our initial experiments demonstrated that the KCl and Phe-induced changes in [Ca2+]i were rather small, and we could not detect with confidence concentration-dependent changes in [Ca2+]i. Also, the angiotensin II (ANG II) response in rat tissue is notoriously tachyphylactic, making it difficult to construct a cumulative concentration-constriction or concentration-[Ca2+]i curve. Additionally, the endothelin-1 (ET-1) response was relatively slow in onset, particularly at low concentrations, and a cumulative-constriction response curve would require prolonged exposure to excitation light, which would cause significant photobleaching of fura 2 and affect the accuracy of [Ca2+]i measurements. Therefore, to accurately compare the [Ca2+]i-dependent constriction induced by various agonists, we used maximal concentrations and a 10-min exposure time. The maximal concentrations of KCl, Phe, ANG II, and ET-1 used were based on previous reports (4–6, 14, 23) from our laboratory and other groups, which examined the concentration-constriction curves for KCl, Phe, ANG II, and ET-1 in the aorta, uterine, and mesenteric vessels of nonpregnant, Norm-Preg, and RUPP rats. In all experiments, the microvessel was first stimulated with 96 mM KCI and the initial and maintained vasoconstriction and 340/380 ratio were measured. Once the constriction reached a plateau, the microvessel was washed with Krebs solution for 15 min. The microvessel was then stimulated with either Phe (10−5 M), ANG II (10−6 M), or ET-1 (10−7 M), and the initial and maintained vasoconstriction and 340/380 ratio were recorded and used to construct the 340/380 ratio ([Ca2+]i)-vasoconstriction relationship for microvessels from Norm-Preg and RUPP rats.
Solutions and drugs.
Normal Krebs solution contained the following (in mM): 120 NaCl, 5.9 KCl, 25 NaHCO3, 1.2 NaH2PO4, 11.5 dextrose, 2.5 CaCl2, 1.2 MgCl2 pH 7.4, and then it was bubbled with 95% O2-5% CO2. Ninety-six millimolars were prepared as Krebs solution with equimolar substitution of NaCl with KCl. Stock solutions of Phe, ANG II, and ET-1 (Sigma, St. Louis, MO) were prepared in distilledd water. All other chemicals were of reagent grade or better.
Statistical analysis.
Data from Norm-Preg (n = 10) and RUPP rats (n = 9) were analyzed and presented as means ± SE. Student's t-test for unpaired data was used for comparison of two means. Differences were considered statistically significant if P < 0.05.
RESULTS
Effect of RUPP on pups.
On the day of the experiment (day 19 of gestation), examination of the pup litter demonstrated that there was a significant reduction in litter size in RUPP (7.7 ± 1.3 pups) compared with Norm-Preg (12.7 ± 0.5 pups; P = 0.002). Also, the average pup weight was significantly reduced in RUPP rats (2.14 ± 0.17 g) compared with that in Norm-Preg rats (2.68 ± 0.12 g; P = 0.017).
Resting diameter and basal [Ca2+]i.
Cumulative data in unstimulated pressurized mesenteric microvessels demonstrated that the resting internal diameters were 240.8 ± 11.2 μm in Norm-Preg rats and were not significantly different from that in RUPP rats (237.9 ± 18.1 μm; P = 0.575). The thickness of the microvessel wall was 40.4 ± 3.3 μm in Norm-Preg rats and was not significantly different in RUPP rats (45.7 ± 5.5 μm). Also, the wall thickness to luminal diameter was not significantly different in Norm-Preg compared with RUPP rats. On the other hand, the basal 340/380 ratio ([Ca2+]i) was significantly reduced in RUPP rats (0.73 ± 0.03) compared with Norm-Preg rats (0.82 ± 0.03; P < 0.05).
Effect of KCl.
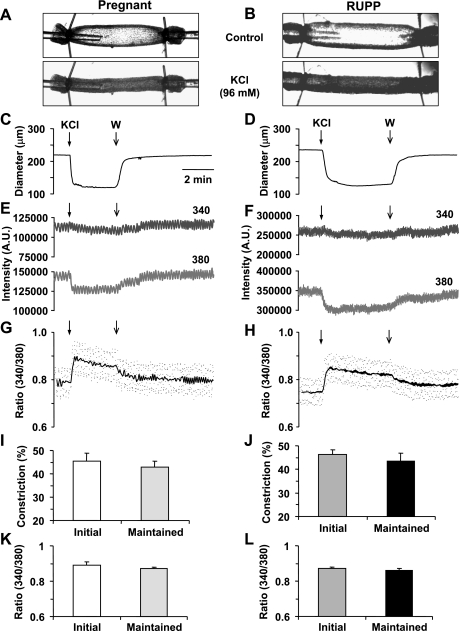
KCl (96 mM) caused a significant decrease in the diameter of microvessels of both Norm-Preg (Fig. 1A) and RUPP rats (Fig. 1B). The KCl-induced response demonstrated an initial maximum followed by maintained vasoconstriction (Fig. 1, C and D). Also, in microvessels of both Norm-Preg and RUPP rats, KCl caused a slight change in the 340-nm fura 2 fluorescence signal, a significant decrease in the 380-nm fluorescence signal (Fig. 1, E and F), and an increase in the 340/380 fluorescence ratio (Fig. 1, G and H), indicating a simultaneous increase in [Ca2+]i during KCl-induced vasoconstriction. In both Norm-Preg and RUPP rats, the KCl-induced [Ca2+]i preceded and reached peak before the maximum vasoconstriction (Table 1). Also, in microvessels of Norm-Preg and RUPP rats stimulated with KCl, the time to steady-state [Ca2+]i coincided with the time to steady-state vasoconstriction (Table 1). Cumulative data indicated that the KCl-induced initial and maintained vasoconstriction (Fig. 1, I and J) and [Ca2+]i (Fig. 1, K and L) was not significantly different between microvessels of Norm-Preg and RUPP rats.
Fig. 1.
Effect of KCl (96 mM) on vasoconstriction and intracellular free calcium concentration ([Ca2+]i) in mesenteric microvessels of late normal pregnancy (Norm-Preg) and reduction in uterine perfusion pressure (RUPP) rats. Images of fura 2 loaded microvessel from Norm-Preg (A) and RUPP rat (B) were taken at rest and after stimulation with KCl. Simultaneous measurements of diameter, 340- and 380-nm fluorescence signal, and 340/380 ratio were recorded in the isolated microvessel of Norm-Preg (C, E, G) and RUPP rat (D, F, H). Bar graphs represent means ± SE of vasoconstriction and [Ca2+]i measurements in 10 microvessels of Norm-Preg (I, K) and 9 microvessels of RUPP rats (J, L).
Table 1.
Time-to-peak and time-to-steady-state vasoconstriction and [Ca2+]i in response to KCl (96 mM), Phe (10−5 M), ANG II (10−7 M), and ET-1 (10−7 M) in Norm-Preg and RUPP rats
|
Norm-Preg |
RUPP
|
|||||||
|---|---|---|---|---|---|---|---|---|
| KCl | Phe | ANG II | ET-1 | KCl | Phe | ANG II | ET-1 | |
| Time-to-maximum constriction, s | 38.4±2.4 | 34.4±6.2 | 33.3±5.8 | 46.5±6.5 | 26.8±4.3 | 29.4±9.2 | 33.8±7.8 | 39.7±11.1 |
| Time-to-peak [Ca2+]i, s | 19.8±3.1 | 10.8±0.9 | 9.3±1.9 | 13.5±1.5 | 22.1±6.6 | 12.6±2.4 | 12.8±1.5 | 16.5±4.4 |
| Time-to-steady-state constriction, s | 106.4±20.1 | 86.6±15.7 | 125±20.7 | 97±9 | 115.8±12.1 | 88.8±19.9 | 135±12.1 | 98±16.8 |
| Time-to-steady-state [Ca2+]i, s | 105.3±8.8 | 85.8±14.2 | 108.7±6.9 | 148.8±11.8 | 114.3±12.9 | 89.5±16.1 | 92.2±2.2 | 147.3±16.8 |
[Ca2+]i, intracellular free calcium concentration; ED-1, endothelin-1; Norm-Preg, late normal pregnancy. RUPP, reduction in uterine perfusion pressure; Phe, phenylalanine.
Effect of Phe.
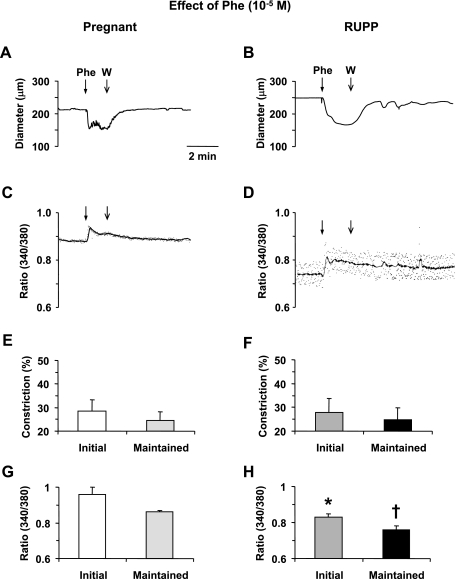
Mesenteric microvessels of both Norm-Preg and RUPP rats showed an initial vasoconstriction followed by maintained decrease in diameter in response to the α-adrenergic agonist Phe (10−5 M; Fig. 2, A and B). The Phe-induced vasoconstriction was preceded by an initial spike followed by a smaller, but maintained, increase in [Ca2+]i (Fig. 2, C and D). In microvessels of both Norm-Preg and RUPP rats, the Phe-induced [Ca2+]i preceded and reached peak before the maximum vasoconstriction (Table 1). Also, in microvessels of Norm-Preg and RUPP rats stimulated with Phe, the time to steady-state [Ca2+]i coincided with the time to steady-state vasoconstriction (Table 1). Cumulative data demonstrated no significant difference in Phe-induced initial or maintained vasoconstriction between Norm-Preg and RUPP rats (Fig. 2, E and F). In contrast, the Phe-induced initial and maintained [Ca2+]i presented as the 340/380 ratio was significantly reduced in RUPP compared with Norm-Preg rats (P < 0.05; Fig. 2, G and H).
Fig. 2.
Effect of phenylephrine (Phe; 10−5 M) on vasoconstriction and [Ca2+]i in mesenteric microvessels of Norm-Preg and RUPP rats. Simultaneous measurements of Phe-induced changes in diameter and 340/380 fluorescence ratio were recorded in isolated microvessels from Norm-Preg (A, C) and RUPP rat (B, D). Bar graphs represent means ± SE of vasoconstriction and [Ca2+]i measurements in 10 microvessels of Norm-Preg (E, G) and 9 microvessels of RUPP rats (F, H). *,†[Ca2+]i (340/380 ratio) measurements in RUPP rats are significantly different (P < 0.05) from corresponding measurements in Norm-Preg rats.
Effect of ANG II.
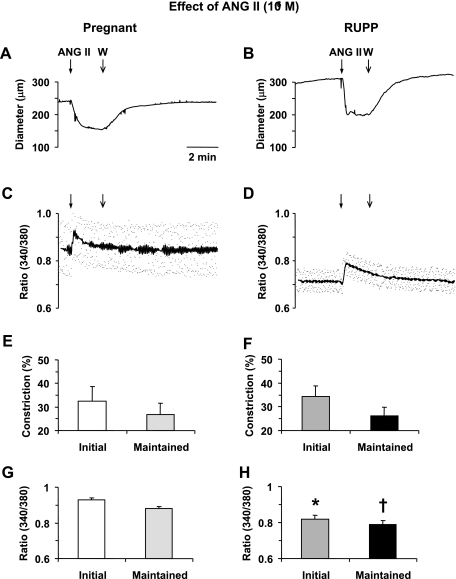
In mesenteric microvessels of both Norm-Preg and RUPP rats ANG II (10−7 M) caused a rapid decrease in diameter that reached a maximum in ∼30 s (Fig. 3, A and B). ANG II also caused an initial peak followed by a maintained increase in [Ca2+]i in microvessels of both Norm-Preg and RUPP rats (Fig. 3, C and D). In both Norm-Preg and RUPP rats the ANG II-induced [Ca2+]i preceded and reached peak and steady-state levels before the maximum and steady-state vasoconstriction, respectively (Table 1). Cumulative data demonstrated no significant difference in the ANG II-induced initial and maintained vasoconstriction (Fig. 3, E and F). In contrast, the ANG II-induced initial and maintained [Ca2+]i presented as the 340/380 ratio was significantly reduced (P < 0.05) in RUPP compared with Norm-Preg rats (Fig. 3, G and H).
Fig. 3.
Effect of ANG II (10−7 M) on vasoconstriction and [Ca2+]i in mesenteric microvessels of Norm-Preg and RUPP rats. Simultaneous measurements of ANG II-induced changes in diameter and 340/380 fluorescence ratio were recorded in isolated microvessels from Norm-Preg (A, B) and RUPP rat (B, D). Bar graphs represent means ± SE of vasoconstriction and [Ca2+]i measurements in 10 microvessels of Norm-Preg (E, G) and 9 microvessels of RUPP rats (F, H). *,†[Ca2+]i (340/380 ratio) measurements in RUPP rat are significantly different (P < 0.05) from corresponding measurements in Norm-Preg rats.
Effect of ET-1.
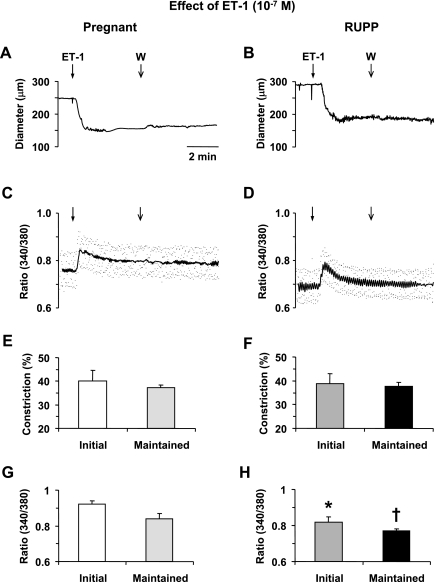
ET-1 (10−7 M) caused a relatively slow developing decrease in diameter of microvessels of Norm-Preg and RUPP rats that reached a steady state in ∼45 s. The ET-1-induced vasoconstriction was prolonged and could not be reversed despite that the microvessels were washed several times with Krebs solution (Fig. 4, A and B). The ET-1-induced vasoconstriction was associated with an initial peak in [Ca2+]i followed by smaller but maintained increase in [Ca2+]i (Fig. 4, C and D). In both Norm-Preg and RUPP rats, the ET-1-induced [Ca2+]i preceded and reached peak before the maximum vasoconstriction (Table 1). In contrast, in microvessels stimulated with ET-1 the time to steady-state [Ca2+]i lagged behind the time to steady-state vasoconstriction in both Norm-Preg (P = 0.003) and RUPP rats (P = 0.054) (Table 1). Cumulative data demonstrated no significant difference in ET-1-induced initial or maintained vasoconstriction between microvessels of Norm-Preg and RUPP rats (Fig. 4, E and F). In contrast, the ET-1-induced initial and maintained [Ca2+]i was lower in RUPP compared with Norm-Preg rats (Fig. 4, G and H).
Fig. 4.
Effect of endothelin-1 (ET-1; 10−7 M) on vasoconstriction and [Ca2+]i in mesenteric microvessels of Norm-Preg and RUPP rats. Simultaneous measurements of ET-1-induced changes in diameter and 340/380 fluorescence ratio were recorded in isolated microvessels from Norm-Preg (A, C) and RUPP rat (B, D). Bar graphs represent means ± SE of vasoconstriction and [Ca2+]i measurements in 10 microvessels of Norm-Preg (E, G) and 9 microvessels of RUPP rats (F, H). *,†[Ca2+]i (340/380 ratio) measurements in RUPP rats are significantly different (P < 0.05) from corresponding measurements in Norm-Preg rats.
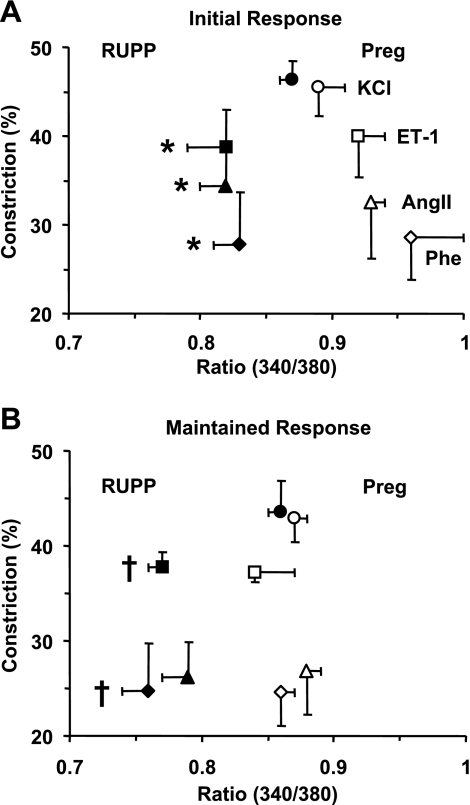
[Ca2+]i-vasoconstriction relationship.
The initial and maintained 340/380 ratio ([Ca2+]i) and vasoconstriction in response to KCl, Phe, ANG II, and ET-1 were used to construct the [Ca2+]i-vasoconstriction relationship in microvessels of Norm-Preg and RUPP rats. Both the initial and maintained KCl [Ca2+]i-vasoconstriction relationship were not different between Norm-Preg and RUPP rats. In contrast, the initial and maintained [Ca2+]i-vasoconstriction relationship for Phe, ANG II, or ET-1 was shifted to the left in RUPP rats compared with Norm-Preg rats (Fig. 5).
Fig. 5.
[Ca2+]i-vasoconstriction relationship in mesenteric microvessels from Norm-Preg and RUPP rats. Mesenteric microvessels from Norm-Preg and RUPP rats were stimulated with either KCl (96 mM), Phe (10−5M), ANG II (10−7M), or ET-1 (10−7M) and the initial (A) and maintained (B) changes in vasoconstriction and [Ca2+]i (340/380 ratio) were used to construct a [Ca2+]i-vasoconstriction relationship. The initial and maintained Phe, ANG II, and ET-1 induced [Ca2+]i-vasoconstriction relationships were shifted to the left (less [Ca2+]I) in RUPP rats compared with Norm-Preg rats. *,†[Ca2+]i (340/380 ratio) measurements in RUPP rats are significantly different (P < 0.05) from corresponding measurements in Norm-Preg rats.
DISCUSSION
The main findings of the present study are as follows: 1) the maximal mesenteric microvessel vasoconstriction to KCl and the more physiological vasoconstrictor stimuli Phe, ANG II, and ET-1 is not different between RUPP and Norm-Preg rats; 2) the KCl-induced [Ca2+]i is not different, but the basal and agonist-induced initial and maintained [Ca2+]i is reduced in RUPP compared with Norm-Preg rats; and 3) the [Ca2+]i-vasoconstriction relationship for KCl is similar, while that for Phe, ANG II, or ET-1 is shifted to the left (enhanced) in microvessels of RUPP compared with Norm-Preg rats.
Previous studies (6) have shown that the mesenteric vascular resistance is elevated in rat models of HTN-Preg. Studies have also shown that the mesenteric vascular resistance is reduced in Norm-Preg compared with nonpregnant normotensive rats (61, 68, 74) and in Norm-Preg compared with nonpregnant spontaneously hypertensive rats (12). Studies (11) have also shown that the vascular reactivity to electrical stimulation or intraarterial norepinephrine, ANG II, and arginine vasopressin are decreased in the in situ blood perfused mesenteries of Norm-Preg rats compared with nonpregnant controls. Other studies (12) in the in situ blood perfused mesenteric resistance vessels of 18- to 20-day pregnant spontaneously hypertensive rats have shown much lower vascular response to electrical stimulation or intraarterial norepinephrine than either pregnant or nonpregnant spontaneously hypertensive rats. Although the in situ blood-perfused mesenteries could provide important physiological vascular reactivity information, they may not allow further investigation of underlying cellular mechanisms, particularly measurement of [Ca2+]i.
One goal of the present study was to investigate the mechanisms of vasoconstriction in small systemic microvessels of Norm-Preg and RUPP rats. Arteries of internal diameter <300 μm are by and large considered resistance vessels (47, 48, 75). Specifically, the small mesenteric feed arteries and microcirculatory vessels have been used in several studies (6, 21, 52) as representative of resistance vessels. These small resistance size arteries have a significant myogenic tone that primarily maintains constant blood flow and provide a baseline diameter that is modulated by vasoconstrictors and vasodilators (10, 16, 75). Therefore, we used isolated mesenteric microvessels to determine the [Ca2+]i-signaling mechanism underlying the vascular changes in Norm-Preg and RUPP rats. The average internal diameter of the mesenteric microvessels used in the present study was 241 in Norm-Preg and 238 μm in RUPP rats, well in the range of resistance arteries. Also, all microvessels tested produced ∼45% constriction in response to KCl and significant vasoconstriction to three different physiological agonists namely Phe, ANG II, and ET-1, confirming viability of the microvessel preparation. Furthermore, during stimulation by KCl and other agonists the time to peak [Ca2+]i always preceded the time to maximum vasoconstriction (Table 1), supporting the contention that the increased [Ca2+]i triggers the vasoconstriction.
Previous studies (1, 14, 22, 26) have shown that RUPP in late pregnant rats is associated with significant increases in renal vascular resistance and BP. Also, we (14) have shown that Phe-induced vascular contraction is greater in aortic strips isolated from RUPP compared with Norm-Preg rats. Although the differences in aortic contraction were partially related to reduced endothelium-dependent nitric oxide-mediated vascular relaxation in RUPP rats, differences in contraction were still observed in endothelium-denuded aortic strips of RUPP compared with Norm-Preg rats, suggesting additional differences in the mechanisms of aortic VSM contraction (14). In support of this view, studies (5, 70) have shown enhanced Phe-induced contraction in isolated uterine arteries from RUPP or transgenic preeclampsia rats compared with Norm-Preg rats.
In search for the cellular mechanisms involved in the enhanced vasoconstriction during HTN-Preg, our previous experiments on the aorta have shown that the Phe- and caffeine-induced contraction in Ca2+-free solution are not different in RUPP rats compared with Norm-Preg rats, suggesting that the IP3-sensitive and the Ca2+-induced Ca2+ release mechanisms from the intracellular stores are not different between the RUPP rat model of HTN-Preg and Norm-Preg rats (23). On the other hand, the aortic contractile response to membrane depolarization by KCl, which stimulates Ca2+ entry from the extracellular space, is reduced in Norm-Preg compared with virgin rats but significantly enhanced in RUPP rats (15, 23, 36). Also, our studies (50) in isolated renal arterial VSM cells have shown that the basal and ANG II-stimulated [Ca2+]I is reduced in Norm-Preg compared with virgin rats but significantly elevated in RUPP rats. These data suggest that the Ca2+ entry mechanisms of vascular contraction are enhanced in the aorta and renal artery of RUPP rats compared with Norm-Preg rats.
Based on previous measurements of VSM contraction and Ca2+ in the aorta, renal, and uterine arteries (5, 15, 36, 50), we hypothesized that the Ca2+-dependent mechanisms of vasoconstriction are most likely enhanced in small mesenteric microvessels of RUPP compared with Norm-Preg rats. Contrary to our prediction, the KCl-induced vasoconstriction and [Ca2+]i were not different in mesenteric microvessels of RUPP and Norm-Preg rats. Because KCl largely stimulates Ca2+ influx through voltage-gated Ca2+ channels, the present data suggest that this Ca2+ entry mechanism of vasoconstriction is not different in mesenteric microvessels of Norm-Preg and RUPP rats. We should note that while the KCl-induced response is thought to be mainly due to Ca2+ entry from the extracellular space, the KCl-induced Ca2+ entry can also activate Ca2+ release from internal stores by Ca2+-induced Ca2+ release mechanism and the contribution of this mechanism to the observed KCl response cannot be ruled out.
The present data also demonstrate that the mesenteric microvessel reactivity to Phe was not significantly different between RUPP and Norm-Preg rats. Because different parts of the circulation may have different distribution of α-adrenergic receptors, we hypothesized that the lack of change in the responsiveness to Phe in mesenteric microvessels of RUPP rats compared with the previously reported enhanced sensitivity to Phe in the aorta and uterine artery may be related to decreased amount of α-adrenergic receptors in the mesenteric vessels. If this is the case, then the mesenteric microvessels of RUPP rats should be more responsive to other agonists/receptors. Thus a second goal of the present study was to investigate whether the differences in the mechanisms of vasoconstriction in systemic microvessels of Norm-Preg and RUPP rats are specific to a particular agonist/receptor or represent difference in a common signaling pathway downstream from receptor activation.
ANG II, which stimulates angiotensin type 1 receptor in VSM, caused significant vasoconstriction of mesenteric microvessels that was similar in RUPP and Norm-Preg rats. Also, ET-1, which stimulates ETA and perhaps ETB2 receptor in VSM, induced similar vasoconstriction in RUPP and Norm-Preg rats. Nevertheless, similar to the Phe response, the ANG II- and ET-1-induced [Ca2+]i was lower in RUPP compared with Norm-Preg rats. These data suggest that the decreased [Ca2+]i signaling of vasoconstriction in the RUPP rats in response to the α-adrenergic agonist Phe is shared by other agonists such as ANG II and ET-1, which act on different sets of receptors. Alternatively, the decreased [Ca2+]i signaling of vasoconstriction in response to Phe, ANG II, and ET-1 in RUPP compared with Norm-Preg rats may represent a difference in a common [Ca2+]i regulatory pathway downstream from receptor activation. Agonists such as Phe, ANG II, and ET-1 are known to activate Ca2+ influx through ligand-gated and store-operated Ca2+ channels in VSM (17). The decreased [Ca2+]i signaling of vasoconstriction in response to Phe, ANG II, and ET-1 in RUPP compared with Norm-Preg rats may therefore reflect reduced expression/activity of ligand-gated and/or store-operated Ca2+ channels. However, if [Ca2+]i is the sole regulator of the microvessel vasoconstriction, then the reduced [Ca2+]i in microvessels of RUPP rats should be associated with reduced vasoconstriction. This is not the case, as the microvessel vasoconstriction was similar in magnitude in RUPP rats and Norm-Preg rats, suggesting activation of other control or signaling mechanisms in addition to [Ca2+]i.
An important observation is that the basal [Ca2+]i was lower in mesenteric microvessels of RUPP rats compared with Norm-Preg rats. Ca2+ homeostasis is controlled by Ca2+ extrusion mechanisms in the plasma membrane including the Ca2+-ATPase and Na+-Ca2+ exchanger (40). The decreased basal [Ca2+]i in microvessels of RUPP rats may be related to enhanced Ca2+ extrusion mechanisms. Similarly, the reduced [Ca2+]i response to vasoconstrictor agonists in RUPP rats could be partly explained by increased activity of Ca2+ extrusion mechanisms. Another possibility is that the vasoconstriction sensitivity to [Ca2+]i is altered in microvessels of RUPP rats. We found that the [Ca2+]i-vasoconstriction relationship was similar in KCl-stimulated microvessels of Norm-Preg and RUPP rats. In contrast, the [Ca2+]i-vasoconstriction relationship was enhanced in Phe-, ANG II-, and ET-1-stimulated microvessels of RUPP compared with Norm-Preg rats. These findings suggest activation of a [Ca2+]i regulatory pathway that increases the myofilament sensitivity to [Ca2+]i. Several studies (28–30, 63, 73) have shown that in addition to the role of Ca2+, calmodulin and myosin light chain kinase, Rho kinase, and mitogen-activated protein kinase may contribute to VSM contraction. Also, PKC has been suggested to play an important role in the regulation of VSM contraction, in part by increasing the [Ca2+]i sensitivity of the contractile proteins (38, 41, 54, 35, 62, 72). Additionally, previous studies in rat small mesenteric arteries have shown that norepinephrine-induced Ca2+ sensitization is associated with increased myosin light chain phosphorylation and suggested decreased myosin light chain phosphatase activity that is mediated through PKC (9, 55). Collectively, the present and previous studies suggest that while Ca2+-dependent myosin light chain phosphorylation is a major regulator of vasoconstriction in both Norm-Preg and RUPP rats, other [Ca2+]i sensitization pathways such as PKC or Rho kinase may be involved in the regulation of vasoconstriction in microvessels of RUPP rats.
If activation of a Ca2+ regulatory pathway such as PKC causes an increase in the vasoconstriction sensitivity to [Ca2+]i in RUPP rats, then the question is why the initial and maintained vasoconstriction is similar in microvessels of Norm-Preg and RUPP rats? Studies (35, 62) have suggested that PKC may activate feedback mechanisms involving uncoupling of the surface receptors from the GTP-binding protein, inhibition of phospholipase C, inhibition of Ca2+ mobilization via the Ca2+ release or Ca2+ entry channels, activation of Ca2+ extrusion mechanisms, and phosphorylation and inhibition of myosin light chain kinase. Interestingly, during microvessel stimulation with most of the agonists tested in the present study the time to steady-state [Ca2+]i preceded or coincided with the time to steady-state vasoconstriction, suggesting that [Ca2+]i remains a major determinant of vasoconstriction during steady state. A clear exception was the ET-1 response in which the time to steady-state [Ca2+]i lagged behind the time to steady-state vasoconstriction. We (46) have previously shown that ET-1 promotes VSM contraction via activation of PKC, raising the possibility that an ET-1-induced activation of PKC during maximal contraction would cause feedback control of Ca2+ entry and therefore a delay in the [Ca2+]i steady state. PKC is a family of Ca2+-dependent and Ca2+-independent isoforms that exhibit different enzyme properties, substrates, functions, and subcellular distributions in various blood vessels (35, 55, 62), and therefore, activation of PKC may have different effects in various vascular beds particularly during pregnancy. We (15) have previously shown that the vascular sensitivity to Ca2+ entry was enhanced in the aorta of l-NAME treated compared with control Norm-Preg rats. We (33, 34) have also shown that the activity of the Ca2+-dependent α-PKC and the Ca2+-independent δ-PKC is enhanced in l-NAME-treated compared with control Norm-Preg rats. Although our previous data point to PKC as a possible regulator of Ca2+ signaling in the l-NAME treated rat model of HTN, the specific role of PKC or other Ca2+ sensitization pathways such as Rho kinase in the RUPP model of HTN-Preg is unclear at the present time and should be further examined in future studies.
We should note that previous wire myography studies in first order mesenteric arteries have shown enhanced vasoconstrictor responses to a range of KCl, Phe, and ANG II concentrations in RUPP rats compared with Norm-Preg rats (6). The differences in the results could be related to differences in recording techniques (wire myography vs. pressurized microvessels) or in the size of the vessels studied (1st order arteries compared with 3rd to 4th branch mesenteric microvessels). Our (69, 71) results in the RUPP rats are consistent with studies in human vessels thatdemonstrated no significant differences in basal myogenic tone or constrictor responses to KCl, Phe, or ANG II in subcutaneous resistance arteries from women with preeclampsia compared with those from Norm-Preg women. It has been suggested that the vasoconstrictor sensitivity of arteries may be different in isobaric vs. isometric conditions for reasons related to both the mounting technique and mechanical loading (9, 67). It has also been suggested that conditions associated with medial hypertrophy might result in a higher maximal tension development to vasoconstrictors in isometric arteries, while isobaric arteries may show similar maximal constrictions as long as the pressure load is not too high (69).
Thus the Ca2+ regulatory mechanisms in mesenteric microvessels appear to be different from those previously demonstrated in the aorta, uterine arteries, or main mesenteric arteries of RUPP rats compared with Norm-Preg rats (5, 6, 14, 23). Whether the difference represents different adaptation mechanisms in the mesenteric microvessels compared with other vascular beds or large conduit vessels needs to be further examined. The microvessel vasoconstriction to three different physiological agonists acting through different receptors is associated with smaller increases in [Ca2+]i in RUPP compared with Norm-Preg rats. The smaller [Ca2+]i signaling of vasoconstriction in microvessels of RUPP compared with Norm-Preg rats suggests activation of additional Ca2+ regulatory pathway(s) that increase the vasoconstriction sensitivity to [Ca2+]i. The similarity in the initial and maintained vasoconstriction in RUPP and Norm-Preg rats suggests that such Ca2+ regulatory pathway(s) may have a feedback effect on Ca2+ mobilization/homeostatic mechanisms to protect against excessive vasoconstriction in systemic microvessels during RUPP in late pregnancy.
Perspectives
Preeclampsia is associated with increased total vascular resistance, which is thought to cause generalized organ hypoperfusion and multisystem disorder. Vascular resistance is determined by vascular tone, which depends on vascular smooth muscle [Ca2+]i and the Ca2+ sensitivity of the contractile proteins. Studying the vascular reactivity and [Ca2+]i in animal models of HTN-Preg should help to elucidate the mechanisms of preeclampsia in women. Studies in the aorta, uterine, and main mesenteric arteries suggest an increase in vascular reactivity in rat models of HTN-Preg (5, 6, 14, 23). In contrast, the present study suggests no difference in reactivity of mesenteric microvessels of RUPP rats compared with Norm-Preg rats, a finding that is consistent with previously reported lack of difference in vascular reactivity in subcutaneous resistance arteries from preeclamptic and Norm-Preg women (69, 71). The present results suggest that the vascular responses during HTN-Preg are not uniform and highlight the importance of studying other vascular beds including the renal, coronary, and cerebral resistance arteries in future studies. The study also highlights the importance of measuring [Ca2+]i and Ca2+-sensitization pathways in blood vessels to demonstrate potential abnormalities in the underlying cellular mechanisms, despite apparent lack of changes in vascular reactivity. Many factors could affect vascular [Ca2+]i in preeclampsia, including depolarization of the membrane potential, maladjustment of Ca2+ influx across the plasma membrane and Ca2+ release from intracellular stores in addition to abnormal transport of sodium and magnesium, and alteration of Ca2+ metabolism and plasma Ca2+ (19, 42, 71), and these factors should be thoroughly examined in future investigations.
Acknowledgments
This work was supported by grants from National Heart, Lung, and Blood Institute (HL-65998 and HL-70659).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Alexander BT, Kassab SE, Miller MT, Abram SR, Reckelhoff JF, Bennett WA, Granger JP. Reduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxide. Hypertension 37: 1191–1195, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Alexander BT Divergent origins of slow fetal growth: relevance to adult cardiovascular disease. Hypertension 50: 465–466, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Alexander BT Placental insufficiency leads to development of hypertension in growth-restricted offspring. Hypertension 41: 457–462, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Allahdadi KJ, Walker BR, Kanagy NL. ROK contribution to endothelin-mediated contraction in aorta and mesenteric arteries following intermittent hypoxia/hypercapnia in rats. Am J Physiol Heart Circ Physiol 293: H2911–H2918, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Anderson CM, Lopez F, Zhang HY, Pavlish K, Benoit JN. Reduced uteroplacental perfusion alters uterine arcuate artery function in the pregnant Sprague-Dawley rat. Biol Reprod 72: 762–766, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Anderson CM, Lopez F, Zhang HY, Shirasawa Y, Pavlish K, Benoit JN. Mesenteric vascular responsiveness in a rat model of pregnancy-induced hypertension. Exp Biol Med (Maywood) 231: 1398–1402, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Belfort MA, Saade GR, Yared M, Grunewald C, Herd JA, Varner MA, Nisell H. Change in estimated cerebral perfusion pressure after treatment with nimodipine or magnesium sulfate in patients with preeclampsia. Am J Obstet Gynecol 181: 402–407, 1999. [DOI] [PubMed] [Google Scholar]
- 8.Brodszki J, Länne T, Laurini R, Strevens H, Wide-Swensson D, Marsál K. Vascular mechanical properties and endothelial function in pre-eclampsia with special reference to bilateral uterine artery notch. Acta Obstet Gynecol Scand 87: 154–162, 2008. [DOI] [PubMed] [Google Scholar]
- 9.Buus CL, Aalkjaer C, Nilsson H, Juul B, Møller JV, Mulvany MJ. Mechanisms of Ca2+ sensitization of force production by noradrenaline in rat mesenteric small arteries. J Physiol 510: 577–590, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chlopicki S, Nilsson H, Mulvany MJ. Initial and sustained phases of myogenic response of rat mesenteric small arteries. Am J Physiol Heart Circ Physiol 281: H2176–H2183, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Chu ZM, Beilin LJ. Mechanisms of vasodilatation in pregnancy: studies of the role of prostaglandins and nitric-oxide in changes of vascular reactivity in the in situ blood perfused mesentery of pregnant rats. Br J Pharmacol 109: 322–329, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chu ZM, Beilin LJ. Nitric oxide-mediated changes in vascular reactivity in pregnancy in spontaneously hypertensive rats. Br J Pharmacol 110: 1184–1188, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conrad KP Animal models of pre-eclampsia: do they exist? Fetal Med Rev 2: 67–88, 1990. [Google Scholar]
- 14.Crews JK, Herrington JN, Granger JP, Khalil RA. Decreased endothelium-dependent vascular relaxation during reduction of uterine perfusion pressure in pregnant rat. Hypertension 35: 367–372, 2000. [DOI] [PubMed] [Google Scholar]
- 15.Crews JK, Novak J, Granger JP, Khalil RA. Stimulated mechanisms of Ca2+ entry into vascular smooth muscle during NO synthesis inhibition in pregnant rats. Am J Physiol Regul Integr Comp Physiol 276: R530–R538, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiol Rev 79: 387–423, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Davis MJ, Wu X, Nurkiewicz TR, Kawasaki J, Gui P, Hill MA, Wilson E. Regulation of ion channels by protein tyrosine phosphorylation. Am J Physiol Heart Circ Physiol 281: H1835–H1862, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Duvekot JJ, Peeters LL. Renal hemodynamics and volume homeostasis in pregnancy. Obstet Gynecol Surv 49: 830–839, 1994. [DOI] [PubMed] [Google Scholar]
- 19.Ebose EJ, Campbell PI, Okorodudu AO. Electrolytes and pH changes in pre-eclamptic rats. Clin Chim Acta 384: 135–140, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Eder DJ, McDonald MT. A role for brain angiotensin II in experimental pregnancy-induced hypertension in laboratory rats. Clin Exp Hyper Hyper Preg B6: 431–451, 1987. [Google Scholar]
- 21.Fenger-Gron J, Mulvany MJ, Christensen KL. Intestinal blood flow is controlled by both feed arteries and microcirculatory resistance vessels in freely moving rats. J Physiol 498: 215–224, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gadonski G, LaMarca BB, Sullivan E, Bennett W, Chandler D, Granger JP. Hypertension produced by reductions in uterine perfusion in the pregnant rat: role of interleukin 6. Hypertension 48: 711–716, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Giardina JB, Cockrell KL, Granger JP, Khalil RA. Low-salt diet enhances vascular reactivity and Ca2+ entry in pregnant rats with normal and reduced uterine perfusion pressure. Hypertension 39: 368–374, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Gilbert JS, Ryan MJ, LaMarca BB, Sedeek M, Murphy SR, Granger JP. Pathophysiology of hypertension during preeclampsia: linking placental ischemia with endothelial dysfunction. Am J Physiol Heart Circ Physiol 294: H541–H550, 2008. [DOI] [PubMed] [Google Scholar]
- 25.Granger JP, Alexander BT, Bennett WA, Khalil RA. Pathophysiology of pregnancy-induced hypertension. Am J Hypertens: 178S–185S, 2001. [DOI] [PubMed]
- 26.Granger JP, LaMarca BB, Cockrell K, Sedeek M, Balzi C, Chandler D, Bennett W. Reduced uterine perfusion pressure (RUPP) model for studying cardiovascular-renal dysfunction in response to placental ischemia. Methods Mol Med 122: 383–392, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Herse F, Staff AC, Hering L, Müller DN, Luft FC, Dechend R. AT1-receptor autoantibodies and uteroplacental RAS in pregnancy and pre-eclampsia. J Mol Med 86: 697–703, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Hilgers RH, Todd J Jr, Webb RC. Increased PDZ-RhoGEF/RhoA/Rho kinase signaling in small mesenteric arteries of angiotensin II-induced hypertensive rats. J Hypertens 25: 1687–1697, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Hilgers RH, Webb RC. Molecular aspects of arterial smooth muscle contraction: focus on Rho. Exp Biol Med (Maywood) 230: 829–835, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Horowitz A, Menice CB, Laporte R, Morgan KG. Mechanisms of smooth muscle contraction. Physiol Rev 76: 967–1003, 1996. [DOI] [PubMed] [Google Scholar]
- 31.Hynes PG, Friel AM, Smith TJ, Morrison JJ. Beta-adrenoceptor subtype expression in human placenta and umbilical arteries in normal and preeclamptic pregnancies. Hypertens Pregnancy 27: 169–181, 2008. [DOI] [PubMed] [Google Scholar]
- 32.Ilekis JV, Reddy UM, Roberts JM. Preeclampsia–a pressing problem: an executive summary of a National Institute of Child Health and Human Development workshop. Reprod Sci 14: 508–523, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Kanashiro CA, Alexander BT, Granger JP, Khalil RA. Ca2+-insensitive vascular protein kinase C during pregnancy and NOS inhibition. Hypertension 34: 924–930, 1999. [DOI] [PubMed] [Google Scholar]
- 34.Kanashiro CA, Cockrell KL, Alexander BT, Granger JP, Khalil RA. Pregnancy-associated reduction in vascular protein kinase C activity rebounds during inhibition of NO synthesis. Am J Physiol Regul Integr Comp Physiol 278: R295–R303, 2000. [DOI] [PubMed] [Google Scholar]
- 35.Kanashiro CA, Khalil RA. Signal transduction by protein kinase C in mammalian cells. Clin Exp Pharmacol Physiol 25: 974–985, 1998. [DOI] [PubMed] [Google Scholar]
- 36.Khalil RA, Crews JK, Novak J, Kassab S, Granger JP. Enhanced vascular reactivity during inhibition of nitric oxide synthesis in pregnant rats. Hypertension 31: 1065–1069, 1998. [DOI] [PubMed] [Google Scholar]
- 37.Khalil RA, Granger JP. Vascular mechanisms of increased arterial pressure in preeclampsia: lessons from animal models. Am J Physiol Regul Integr Comp Physiol 283: R29–R45, 2002. [DOI] [PubMed] [Google Scholar]
- 38.Khalil RA, Lajoie C, Resnick MS, Morgan KG. Ca2+-independent isoforms of protein kinase C differentially translocate in smooth muscle. Am J Physiol Cell Physiol 263: C714–C719, 1992. [DOI] [PubMed] [Google Scholar]
- 39.Khalil RA, van Breemen C. Intracellular free calcium concentration/force relationship in rabbit inferior vena cava activated by norepinephrine and high K+. Pflügers Arch 416: 727–734, 1990. [DOI] [PubMed] [Google Scholar]
- 40.Khalil RA, van Breemen C. Mechanisms of calcium mobilization and homeostasis in vascular smooth muscle and their relevance to hypertension. In: Hypertension: Pathophysiology, Diagnosis, and Management, edited by JH Laragh, BM Brenner. New York: Raven, p. 523–540, 1995.
- 41.Khalil RA, van Breemen C. Sustained contraction of vascular smooth muscle: calcium influx or C-kinase activation? J Pharmacol Exp Ther 244: 537–542, 1988. [PubMed] [Google Scholar]
- 42.Kisters K, Körner J, Louwen F, Witteler R, Jackisch C, Zidek W, Ott S, Westermann G, Barenbrock M, Rahn KH. Plasma and membrane Ca2+ and Mg2+ concentrations in normal pregnancy and in preeclampsia. Gynecol Obstet Invest 46: 158–163, 1998. [DOI] [PubMed] [Google Scholar]
- 43.Kosch M, Hausberg M, Louwen F, Barenbrock M, Rahn KH, Kisters K. Alterations of plasma calcium and intracellular and membrane calcium in erythrocytes of patients with pre-eclampsia. J Hum Hypertens 14: 333–336, 2000. [DOI] [PubMed] [Google Scholar]
- 44.Losonczy G, Brown G, Venuto RC. Increased peripheral resistance during reduced uterine perfusion pressure hypertension in pregnant rabbits. Am J Med Sci 303: 233–340, 1992. [DOI] [PubMed] [Google Scholar]
- 45.Lu Y, Zhang H, Gokina N, Mandala M, Sato O, Ikebe M, Osol G, Fisher SA. Uterine artery myosin phosphatase isoform switching and increased sensitivity to SNP in a rat l-NAME model of hypertension of pregnancy. Am J Physiol Cell Physiol 294: C564–C571, 2008. [DOI] [PubMed] [Google Scholar]
- 46.McNair LL, Salamanca DA, Khalil RA. Endothelin-1 promotes Ca2+ antagonist-insensitive coronary smooth muscle contraction via activation of epsilon-protein kinase C. Hypertension 43: 897–904, 2004. [DOI] [PubMed] [Google Scholar]
- 47.Mulvany MJ, Aalkjaer C, Petersen TT. Intracellular sodium, membrane potential, and contractility of rat mesenteric small arteries. Circ Res 54: 740–749, 1984. [DOI] [PubMed] [Google Scholar]
- 48.Mulvany MJ, Nyborg N. An increased calcium sensitivity of mesenteric resistance vessels in young and adult spontaneously hypertensive rats. Br J Pharmacol 71: 585–596, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murphy JG, Fleming JB, Cockrell KL, Granger JP, Khalil RA. [Ca2+]i signaling in renal arterial smooth muscle cells of pregnant rat is enhanced during inhibition of NOS. Am J Physiol Regul Integr Comp Physiol 280: R87–R99, 2001. [DOI] [PubMed] [Google Scholar]
- 50.Murphy JG, Herrington JN, Granger JP, Khalil RA. Enhanced [Ca2+]i in renal arterial smooth muscle cells of pregnant rats with reduced uterine perfusion pressure. Am J Physiol Heart Circ Physiol 284: H393–H403, 2003. [DOI] [PubMed] [Google Scholar]
- 51.Murphy JG, Khalil RA. Gender-specific reduction in contractility and [Ca2+]i in vascular smooth muscle cells of female rat. Am J Physiol Cell Physiol 278: C834–C844, 2000. [DOI] [PubMed] [Google Scholar]
- 52.Neves LA, Williams AF, Averill DB, Ferrario CM, Walkup MP, Brosniham KB. Pregnancy enhances the angiotensin (Ang)-(1-7) vasodilator response in mesenteric arteries and increases the renal concentration and urinary excretion of Ang-(1–7). Endocrinology 144: 3338–3343, 2003. [DOI] [PubMed] [Google Scholar]
- 53.Nienartowicz A, Link S, Moll W. Adaptation of the uterine arcade in rats to pregnancy. J Dev Physiol 12: 101–108, 1989. [PubMed] [Google Scholar]
- 54.Nishizuka Y Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science 258: 607–614, 1992. [DOI] [PubMed] [Google Scholar]
- 55.Ohanian V, Ohanian J, Shaw L, Scarth S, Parker PJ, Heagerty AM. Identification of protein kinase C isoforms in rat mesenteric small arteries and their possible role in agonist-induced contraction. Circ Res 78: 806–812, 1996. [DOI] [PubMed] [Google Scholar]
- 56.Payne JA, Alexander BT, Khalil RA. Reduced endothelial vascular relaxation in growth-restricted offspring of pregnant rats with reduced uterine perfusion. Hypertension 42: 768–774, 2003. [DOI] [PubMed] [Google Scholar]
- 57.Podjarny E, Baylis C, Losonczy G. Animal models of preeclampsia. Semin Perinatol 23: 2–13, 1999. [DOI] [PubMed] [Google Scholar]
- 58.Podjarny E, Losonczy G, Baylis C. Animal models of preeclampsia. Semin Nephrol 24: 596–606, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roberts JM, Gammill HS. Preeclampsia: recent insights. Hypertension 46: 1243–1249, 2005. [DOI] [PubMed] [Google Scholar]
- 60.Roberts JM, Lain KY. Recent insights into the pathogenesis of pre-eclampsia. Placenta 23: 359–372, 2002. [DOI] [PubMed] [Google Scholar]
- 61.Ross GR, Yallampalli C. Vascular hyperresponsiveness to adrenomedullin during pregnancy is associated with increased generation of cyclic nucleotides in rat mesenteric artery. Biol Reprod 76: 118–123, 2007. [DOI] [PubMed] [Google Scholar]
- 62.Salamanca DA, Khalil RA. Protein kinase C isoforms as specific targets for modulation of vascular smooth muscle function in hypertension. Biochem Pharmacol 70: 1537–1547, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sholook MM, Gilbert JS, Sedeek MH, Huang M, Hester RL, Granger JP. Systemic hemodynamic and regional blood flow changes in response to chronic reductions in uterine perfusion pressure in pregnant rats. Am J Physiol Heart Circ Physiol 293: H2080–H2084, 2007. [DOI] [PubMed] [Google Scholar]
- 64.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev 83: 1325–1358, 2003. [DOI] [PubMed] [Google Scholar]
- 65.Sowers JR, Zemel MB, Walsh MF, Standley PR, Zemel PC, Bronsteen RA, Kraniak J, Sokol RJ. Effects of normal pregnancy on cellular cation metabolism and peripheral vascular resistance. Am J Hypertens 3: 16–22, 1990. [DOI] [PubMed] [Google Scholar]
- 66.Stennett AK, Khalil RA. Neurovascular mechanisms of hypertension in pregnancy. Curr Neurovasc Res 3: 131–148, 2006. [DOI] [PubMed] [Google Scholar]
- 67.van Bavel E, Mulvany MJ. Role of wall tension in the vasoconstrictor response of cannulated rat mesenteric small arteries. J Physiol 477: 103–115, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van Eijndhoven HW, Janssen GM, Aardenburg R, Spaanderman ME, Peeters LL, De Mey JG. Mechanisms leading to increased vasodilator responses to calcitonin-gene-related peptide in mesenteric resistance arteries of early pregnant rats. J Vasc Res 45: 350–356, 2008. [DOI] [PubMed] [Google Scholar]
- 69.van Wijk MJ, Boer K, van der Meulen ET, Bleker OP, Spaan JA, VanBavel E. Resistance artery smooth muscle function in pregnancy and preeclampsia. Am J Obstet Gynecol 186: 148–154, 2002. [DOI] [PubMed] [Google Scholar]
- 70.Verlohren S, Niehoff M, Hering L, Geusens N, Herse F, Tintu AN, Plagemann A, LeNoble F, Pijnenborg R, Muller DN, Luft FC, Dudenhausen JW, Gollasch M, Dechend R. Uterine vascular function in a transgenic preeclampsia rat model. Hypertension 51: 547–553, 2008. [DOI] [PubMed] [Google Scholar]
- 71.Wimalasundera RC, Thom SA, Regan L, Hughes AD. Effects of vasoactive agents on intracellular calcium and force in myometrial and subcutaneous resistance arteries isolated from preeclamptic, pregnant, and nonpregnant woman. Am J Obstet Gynecol 192: 625–632, 2005. [DOI] [PubMed] [Google Scholar]
- 72.Xiao D, Huang X, Longo LD, Pearce WJ, Zhang L. Regulation of baseline Ca2+ sensitivity in permeabilized uterine arteries: effect of pregnancy. Am J Physiol Heart Circ Physiol 291: H413–H420, 2006. [DOI] [PubMed] [Google Scholar]
- 73.Xiao D, Zhang L. Adaptation of uterine artery thick- and thin-filament regulatory pathway to pregnancy. Am J Physiol Heart Circ Physiol 288: H142–H148, 2005. [DOI] [PubMed] [Google Scholar]
- 74.Yallampalli C, Kondapaka SB, Lanlua P, Wimalawansa SJ, Gangula PR. Female sex steroid hormones and pregnancy regulate receptors for calcitonin gene-related peptide in rat mesenteric arteries, but not in aorta. Biol Reprod 70: 1055–1062, 2004. [DOI] [PubMed] [Google Scholar]
- 75.Zhang RZ, Gashev AA, Zawieja DC, Davis MJ. Length-tension relationships of small arteries, veins, and lymphatics from the rat mesenteric microcirculation. Am J Physiol Heart Circ Physiol 292: H1943–H1952, 2007. [DOI] [PubMed] [Google Scholar]