Abstract
Continuous disruption of circadian rhythms, as seen in human shift workers, has been associated with the development of a number of adverse mental and physiological conditions. However, scientific evidence linking circadian disruption to overall health, particularly in animal models, is not well documented. In this study, we have demonstrated that exposing C57BL/6J mice to 12-h phase shifts every 5 days for 3 mo had no effect on body weight or intestinal physiology. However, when animals were further challenged with dextran sodium sulfate to induce colitis, chronic shifting of the light-dark cycle led to a dramatic increase in the progression of the colitis as indicated by reduced body weight, abnormal intestinal histopathology, and an exacerbated inflammatory response. These data indicate that circadian disruption is an important predisposing factor that may provoke the onset or worsening of various disease states such as inflammatory disorders. This study provides further evidence for continued investigations using animal models of circadian disruption to examine the consequences of circadian disruption on health when organisms are faced with a “challenging” environment.
Keywords: circadian rhythms, shift work, circadian disorganization
throughout the animal kingdom, a robust 24-h diurnal rhythmicity is exhibited at the cell, tissue, organ, and behavioral levels of the organism (9, 23). Recent studies have found that the core molecular transcriptional-translational circadian regulatory feedback loop(s) is present in most cells/organs of the body (e.g., heart, lung, intestine) and that this 24-h molecular clock regulates the diurnal timing of the expression of hundreds, and possibly thousands, of clock-controlled genes (17). The circadian clock is the mechanism by which organisms achieve synchronization to their external environment and maintain internal synchronization among physiological systems. Despite the ubiquitous nature of the circadian clock and the myriad of biochemical, cellular, physiological, and behavioral processes that are under its organizational control, there are surprisingly few examples in the literature demonstrating clear, robust effects of circadian disruption on the health status or disease state in humans or animal models.
It could be argued that the adverse mental and physical effects associated with human shift work, including several chronic diseases such as diabetes, cardiovascular disease, and gastrointestinal disorders, represent strong evidence that chronic disruption of circadian rhythmicity at the whole organism level imposes a serious challenge to human health (2, 4, 8). However, despite a few sporadic reports in rodents, there is very little evidence in animal models that continuous disruption of normal circadian temporal organization is detrimental to the health of the organism or that circadian disorganization is a risk factor for specific organ diseases (3, 12, 13, 18). An increase in mortality recently was observed in old mice undergoing repeated phase shifts (6-h advances or delays) of the light-dark (LD) cycle (3), and changes in body weight have been recorded in rodents exposed to long-term repeated changes in the LD cycle (25–27). This sparse evidence supporting the importance of maintaining normal and consistent internal circadian organization for the health and well-being of animals raises the possibility that the adverse health consequences of human shift work may not be due to internal circadian disruption per se, but instead could be due to other factors such as chronic sleep loss or the social consequences associated with working and sleeping out of synchrony with the societal norm (2). It also is possible that circadian perturbation has minimal effects in healthy animals but may provoke the onset or exacerbate the severity of conditions to which the organism is predisposed due to either environmental or genetic factors. In the present study we have demonstrated that phase shifting the LD cycle by 12 h every 5 days for 3 mo had no effect on body weight or intestinal histopathology in C57BL/6J (B6) mice compared with nonshifted mice; however, chronic shifting of the LD cycle led to a drastic increase in weight loss and intestinal histopathology in animals “challenged” by chemically induced inflammation (colitis) compared with nonshifted challenged animals. These data indicate that circadian disruption may represent an important predisposing factor that could provoke the onset or worsening of various disease states.
MATERIALS AND METHODS
Phase shifting the LD cycle and DSS treatment.
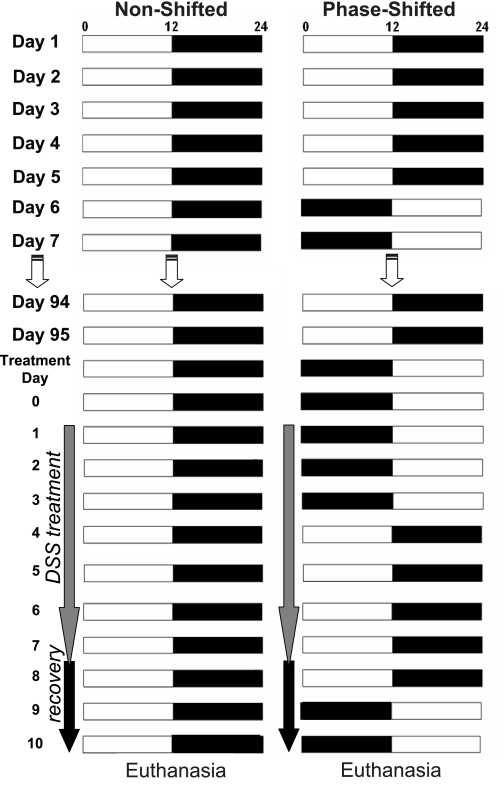
Twenty-eight-week-old male C57BL/6J (B6) mice were singly housed and entrained to a 12:12-h LD cycle for 2 wk. Animals had free access to food (Harlan Teklad, no. 7012) and water throughout the study. After the entrainment period, one group (n = 16) was subjected to multiple phase shifts of the LD cycle by extending the period of light or dark every 5th day by 12 h for 3 mo. A nonshifted group (n = 15) remained on a constant 12:12 LD cycle for the 3-mo period. Between phase shifts 19 and 20, a colitis challenge was introduced to a subset of animals from the phase-shifted and nonshifted groups (11 of each group) by adding 2% dextran sodium sulfate (DSS; MP Biomedicals) to the daily water supply for 7 days (30, 31). The remaining mice in each group served as a DSS control and continued to receive pure water. After the 7 days of DSS treatment, mice were given pure drinking water for a 3-day recovery period. The phase-shifted animals remained on the phase shifting schedule during DSS treatment and thus were shifted on treatment days 3 and 8 of the 10-day DSS challenge/recovery period. An illustrative overview of the experimental protocol is provided in Fig. 1. All procedures in this experiment were conducted with Animal Care and Use Committee-approved protocols.
Fig. 1.
Protocol for phase shifting the light-dark (LD) cycle and colitis induction. The diagram illustrates the applied phase-shift paradigm over the 107 days of the study. Fifteen animals (n = 15) were kept continuously in a 12:12-h LD environment (nonshifted), whereas 16 mice were subjected to 12-h reversals of the LD cycle every 5 days continuously for 3 mo (phase shifted). Two percent dextran sodium sulfate (DSS) was added to the drinking water to a subset of animals (n = 11) from both the nonshifted and phase-shifted groups at the beginning of day 98 (treatment day 1). After 7 days of DSS administration, the water supply was replaced with DSS-free water for a 3-day recovery period before euthanasia and tissue collection. The phase-shifting LD cycle continued during the DSS treatment and recovery period (right).
Body weight loss is the most consistent and reliable continuous marker for the severity of disease state in the DSS model of colitis (30, 31). Therefore, we monitored body weight to assess both the severity and progression of the DSS-induced colitis in the phase-shifted and nonshifted animals throughout the 7 days of treatment and the 3-day recovery period.
Necropsy for tissue collection.
At the end of the 10-day DSS protocol, animals were euthanized using an isoflurane chamber, and necropsies were performed to remove colon tissues from each animal. The total colon was separated into three equal-size parts from the distal to the proximal end, and each part was further separated into two pieces; one was fixed in 4% (wt/vol) paraformaldehyde in PBS and used for tissue histopathology, whereas the other was used for myeloperoxidase (MPO) activity analysis.
Thick (7 μm) sections of all fixed tissue samples were stained with hematoxylin and eosin to examine histological changes, as described previously (22, 29). All samples were scored blindly by a gastroenterological pathologist (see Acknowledgments) to determine intestinal inflammation and tissue damage. Seven different score categories were evaluated measuring destruction, inflammation, and repair. Total scores for the three separate colon sections were averaged for each individual animal. Scoring criteria and validation have been described previously (22, 29).
MPO measurement.
For MPO activity assays, colon tissue samples were homogenized (50 mg/ml) by sonification in ice-cold 50 mM potassium phosphate buffer (pH 6.0) containing 0.5% hexadecyltrimethylammonium bromide (Sigma, St Louis, MO) according to a previously described protocol (1). The samples were then centrifuged at 12,000 rpm for 12 min at 4°C. The supernatant was added to a solution of O-dianisidine (Sigma) and hydrogen peroxide. The absorbance of the colorimetric reaction was measured using a spectrophotometer. MPO was expressed in units of activity per milligram of wet tissue, with 1 unit being the quantity of enzyme able to convert 1 μmol of hydrogen peroxide to water in 1 min at room temperature. MPO activity serves as a marker for neutrophil infiltration, which is a potent predictor for tissue (colon) inflammation (1). High neutrophil infiltration rates indicate severe inflammation in the target area. The relative MPO activity value was established by averaging three colon sections collected from each individual mouse to avoid effects of inflammatory spottiness in the colon area.
Statistical analysis.
Analysis of variance (ANOVA) was used to detect group × time effects in body weight measurements (see Fig. 2A) and in MPO scores (see Fig. 3B). The t-test was used to assess differences in body weight (see Fig. 2B) and histology scores (see Fig. 3A). Significance levels were set at P < 0.05 in all analyses. Statistics were performed using Statistica (StatSoft, Tulsa, OK).
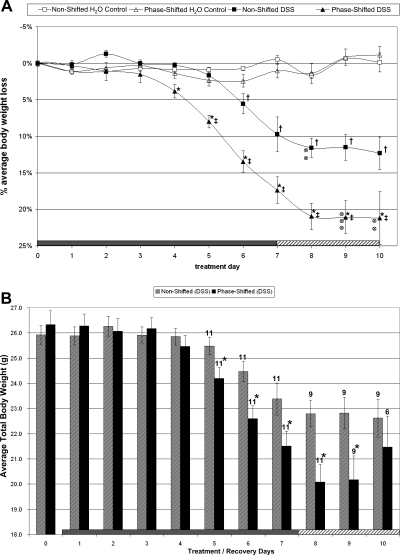
Fig. 2.
Body weight change during DSS challenge and recovery period. A: ANOVA for body weight for all mice alive at the end of study. The progression of weight loss (%change from day 0, means ± SE) during a 7-day inflammatory challenge (DSS was added to water supply from days 1 to 7) and a 3-day recovery period (animals received pure drinking water) is presented. Before this 10-day protocol, mice were maintained on either a stable LD schedule or a 12-h phase-shifting schedule every 5 days for 3 mo, as shown in Fig. 1. Body weight was maintained in the phase-shifted and nonshifted control groups on pure drinking water throughout the 10-day period. The effects of the DSS challenge were more pronounced in the phase-shifted group as demonstrated by an earlier onset and significantly increased loss of body weight compared with the nonshifted group. Two animals in the DSS nonshifted group and 5 animals in the DSS-treated shifted group (indicated by ⊗) died during the recovery period and were not included in the repeated-measures ANOVA analysis of body weight. *P < 0.05, phase shifted (DSS) vs. nonshifted (DSS). ‡P < 0.05, phase shifted (DSS) vs. phase-shifted H2O control. †P < 0.05, nonshifted (DSS) vs. nonshifted H2O control. B: t-test analysis of body weight for all surviving animals on each day of treatment and recovery: This graph depicts the body weight averages (means ± SE) for all DSS-challenged mice in the nonshifted and phase-shifted groups. The 2 nonshifted and 5 shifted animals that died during the recovery period were removed from analysis only on the respective days of their death. Significance was determined by a t-test comparing the body weights of all living mice on each individual day. *P < 0.05, non shifted (DSS) vs. phase shifted (DSS). The number of animals in each group beginning on treatment day 5 is indicated above each bar. Significant differences in body weight between surviving DSS-challenged animals on the nonshifted LD cycle and phase-shifted cycle were observed on days 5–9.
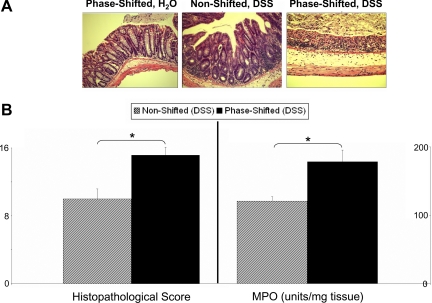
Fig. 3.
Intestinal pathophysiology. A: histological damage stains. Representative histological stains are from phase-shifted animals with no DSS challenge (left), nonshifted animals challenged with 2% DSS (middle), and phase-shifted 2% DSS-challenged animals (right). There was no significant histology change in the colon of phase-shifted mice (left) compared with that of nonshifted H2O control mice (stain not shown). The colitis induced by 2% DSS in mice is evidenced by mild mucosal infiltration of inflammatory cells and a reduction of goblet cells (middle). The DSS-induced colitis is exacerbated as evidenced by more extensive destruction of mucosal layer and mucosal ulceration (right). These stains were used for quantitative histological analysis of inflammation (see B). B: histological damage score and myeloperoxidase (MPO) activity. The quantitative measurement for tissue damage was achieved by blindly scoring 3 independent colon sections from each animal (representative stains are shown in A) collected posteuthanasia for visual characterization of destruction, inflammation, and repair using multiple subcategories. The total score (see materials and methods) for each animal was calculated and averaged for the nonshifted and phase-shifted groups. No histological damage could be observed in any of the non-DSS-treated animals, and thus no scores are presented. The DSS challenge led to increased damaged tissue in both groups, but significantly higher levels of tissue damage were observed in the phase-shifted colons (left). MPO activity was determined from 3 colon sections from each animal. Activity levels of both H2O control groups were indistinguishable and at baseline levels of neutrophil activity (data not shown). Two percent DSS-challenged animals in both groups showed a significant 20- to 40-fold increase in MPO activity in all samples compared with the baseline activity, indicating increased neutrophil infiltration into the colon. The neutrophil infiltration process was significantly increased in the phase-shifted mice (right) compared with nonshifted mice, suggesting higher levels of inflammation in these animals. The standard errors shown indicate the variation between animals in each group, not between individual colon sections (*P < 0.01).
RESULTS
Body weight.
Body weights of the phase-shifted (26.01 ± 0.42 g, n = 16) and nonshifted animals (25.95 ± 0.30 g, n = 15) were indistinguishable from one another at the beginning of the DSS challenge (treatment day 0), indicating that persistent phase shifts of the LD cycle throughout the previous 95 days did not have an effect on body weight.
In response to DSS, 2 of 11 nonshifted animals died and 5 of 11 phase-shifted animals died toward the end of the 10-day protocol. Based on χ2 testing, the difference in deaths between groups was not significant. In the repeated-measures ANOVA presented below, the 7 animals that died during days 8–10 after initiation of DSS treatment were not included in the statistical analyses.
As expected, the body weights of the control (non-DSS) nonshifted and shifted mice did not change over the 10-day experimental period (Fig. 2A). However, there was a significant decrease in body weight in the nonshifted group receiving DSS compared with the nonshifted H2O control group [group × time interaction, F(1,99) = 17.7, P < 0.001]. This significant difference in body weight first appeared on treatment day 6 (nonshifted DSS vs. nonshifted H2O control, P < 0.01; Fig. 2A). In the phase-shifted group receiving DSS, there was a dramatic reduction in body weight compared with the phase-shifted H2O control group [group × time interaction, F(1,99) = 34.8, P < 0.001; Fig. 2A] with the first significant decrease in body weight observed on treatment day 5 (phase-shifted DSS vs. phase-shifted H2O control, P < 0.01).
In a final comparison, phase-shifted DSS mice exhibited a significantly lower body weight compared with nonshifted DSS mice [group × time interaction, F(1,117) = 6.8, P < 0.001; Fig. 2A]. This group difference emerged on treatment day 4 (phase-shifted DSS vs. nonshifted DSS, P < 0.01) and persisted throughout the treatment and recovery periods. These data indicate a significant interaction between phase shifting and DSS with a magnitude greater than DSS alone.
To analyze the weight loss progression of all DSS-treated animals, including mice that died near the end of the protocol, which were not included in the repeated measurement statistics above, we compared body weight differences for all living animals on each of the seven DSS treatment days as well as during the three recovery days. The analysis revealed more advanced body weight loss in the phase-shifted mice beginning on treatment day 5 (Fig. 2B, t-test: phase-shifted DSS vs. nonshifted DSS, P < 0.05, days 5–9).
Tissue histology and myeloperoxidase measurements.
Animals in the nonshifted and phase-shifted control (non-DSS) groups did not exhibit any histological colon tissue damage (Fig. 3A, left). As expected, the DSS-treated groups had a clear elevation in the histological damage score (22, 29) (representative stains in Fig. 3A). The colitis induced by 2% DSS in the nonshifted mice is evidenced by mild mucosal infiltration of inflammatory cells and reduction of goblet cells (Fig. 3A, middle). The DSS-induced colitis is worsened in phase-shifted mice as evidenced by more extensive destruction of mucosal layer and mucosal ulceration (Fig. 3A, right). The degree of DSS-induced histological damage was significantly greater in the phase-shifted DSS group compared with the nonshifted DSS group [Fig. 3B, left; t(13) = 3.31, P < 0.01].
There was a trace amount of MPO activity in both phase-shifted and nonshifted H2O control groups attributable to normal basal activity (<10 U/mg tissue) with no significant difference between the H2O control groups (data not shown). MPO activity significantly increased following DSS administration in both the phase-shifted and nonshifted groups compared with the respective H2O control groups [drug main effect, F(1,21) = 179.7, P < 0.001]. The MPO activity was significantly greater in the phase-shifted DSS treated mice compared with the nonshifted DSS-treated animals [Fig. 3B, right; condition × drug interaction, F(1,21) = 7.5, P < 0.01].
DISCUSSION
In the present study, male B6 mice exposed to a 12-h shift of the LD cycle every 5 days for a total of 3 mo exhibited body weights and measures of intestinal physiology that were similar to mice on a constant 12:12-h LD cycle for the same period of time. However, when animals were presented with a physiological challenge by mimicking a model disease state (e.g., a DSS challenge to imitate an intestinal inflammatory disorder, colitis) significant effects of circadian disruption were revealed. In response to DSS treatment, the phase-shifted mice exhibited more severe colitis symptomatology compared with DSS-treated animals that were not phase shifted, as indicated by greater body weight loss, colonic inflammation (MPO levels), and tissue injury (histopathology score). After DSS treatment, there was a trend for increased mortality in the phase-shifted DSS group (5 of 11) compared with the nonshifted DSS group (2 of 11), whereas none of the phase-shifted control (pure water) animals died. These data reveal that in healthy control (non-DSS) mice, repeated phase shifts of the LD cycle had little effect on body weight or intestinal physiology, whereas phase shifting had detrimental effects in animals that manifested coexisting colitis symptomatology. More broadly, we were able to utilize an animal pharmacological model of colitis to support the generalizable hypothesis that chronically phase shifting the 24-h LD cycle in the context of acute and/or chronic illness can aggravate the severity and/or progression of an existing disease state.
A basic function of the circadian clock is to entrain the organism to the external environment via cues such as the LD cycle and food availability (23). In most organisms, the endogenous circadian pacemaker oscillates with a period slightly different from 24 h. Therefore, the circadian clock makes small adjustments (i.e., entrains) each day to remain synchronized to environmental time cues. In situations where the demands of entrainment are severe, such as a time zone change or shift work, the circadian clock may not be able to fully adjust, which results in a state of acute or chronic circadian desynchronization. The most commonly studied example of circadian desynchrony in humans is shift work, where the phase relationship between external time cues and internal rhythms is chronically disrupted, due to working either a steady or a rotating shift schedule. In today's modern industrialized society, a large percentage (15–20%) of the work force is engaged in some form of shift work (2). The occurrence of gastroenterological disorders, obesity, diabetes, cardiovascular disease, infertility, and some forms of cancer are more common in shift workers compared with non-shift workers (2, 4, 8). These epidemiological findings provide support for the hypothesis that the chronic disruption of circadian organization may lead to, or aggravate, abnormal functioning in multiple physiological systems and increase the risk for disease.
Many fundamental questions about the links between circadian rhythms and human disease remain to be elucidated. For example, does circadian disruption lead directly to the development and onset of adverse health outcomes, and/or do alterations in circadian processes trigger or aggravate underlying conditions that already exist or for which the individual is at risk to develop (e.g., genetic predisposition)? Why do particular individuals suffer more consequences from shift work than others, and what determines which organ systems will be affected in different individuals? Furthermore, are the effects of circadian perturbation rooted mainly in behavioral (i.e., whole body) circadian disruption, or do they extend more deeply to the molecular and genetic levels? An importance advance will be to understand what physiological and molecular factors are involved in the links between circadian regulation/dysregulation and the function of multiple physiological systems. One approach to better define the role of circadian rhythms in physiology is to develop animal models of circadian disruption under “challenging” environmental conditions. Surprisingly, there are only a few studies in the animal literature that have used experimental paradigms to alter circadian rhythms to examine the relationship between disrupted circadian organization and physiological outcomes (3, 10, 11, 16).
Davidson et al. (3) found an increase in mortality in old mice (27–31 mo of age) subjected to repeated weekly 6-h phase advances or delays of the LD cycle compared with nonshifted animals. Penev et al. (18) demonstrated that weekly reversal of the LD cycle in cardiomyopathic hamsters reduced median life span by 11% compared with nonshifted cardiomyopathic control animals. These studies provide “rare” examples of the consequences of circadian disruption when combined with risk factors, such as advanced age or an underlying cardiovascular disease. Considering the high odds ratios for cardiometabolic disease in human shift work (8), it will be important to more fully characterize the role of circadian rhythms in energy metabolism and cardiovascular function under challenging environmental conditions.
Interestingly, in young healthy rats, 12-h phase shifts of the LD cycle twice per week resulted in a significant increase in body weight compared with nonshifted rats (26, 27). Using a different phase-shifting paradigm, Salgado-Delgado et al. (25) held the LD cycle constant but phase shifted the sleep-wake cycle in rats by placing them in slowly rotating wheels during either the light or dark phase on alternating weeks, which led to an increase in weight gain compared with control animals. In contrast, phase shifting the LD cycle in CD1F2 female mice had no effect on body weight (16), similar to the results in the present study for the phase-shifted, non-DSS-treated mice. Since few studies have been performed, there is not enough information to determine whether there are species or strain differences in the effects of circadian disruption on health and disease.
Because so few studies have been carried out in animals to examine the effects of disrupting circadian rhythms on specific disease states or organ systems, it is not known whether different environmental (e.g., phase reversal or phase shift in the LD cycle, or environmental-endogenous period mismatch) or genetic perturbations have similar or differing negative consequences. In the present study, we used a complete reversal of the LD cycle every 5 days in an attempt to cause a maximal disruption of circadian organization. It is of interest to determine how different circadian disrupting protocols affect the pathological progressions of various disease states in view of 1) the increase in mortality seen in old mice exposed to chronic phase shifts in the LD cycle (3) and 2) the many different ways humans phase shift their work-rest schedules without any knowledge of the adverse health consequences of any particular type of phase-shifting environment.
In the past few years, tremendous advances have been made in demonstrating the presence of cell autonomous circadian clock gene networks in central, as well as most peripheral, tissues of the body, including the liver, heart, lungs, pancreas, skeletal and smooth muscle, and the gut (11, 19). These discoveries have provided a framework to begin to elucidate how the circadian clock is involved in mediating the function of multiple tissues and how different physiological processes are integrated for optimal systems level function. Circadian clock mutant animals may prove to be particularly valuable in developing new insights into the importance of the circadian clock in health. Mice harboring a mutation in the canonical circadian gene, Clock, have alterations in sleep regulation, energy metabolism, reproduction, and various neurocognitive functions (14, 15, 28). Deletion of the dimeric partner of Clock, Bmal1, results in cardiometabolic phenotypes (24), and Per2 mutant mice are susceptible to tumor formation (6). Although the effects of disrupting the molecular circadian clock appear to be widespread across various tissues and organs, many questions remain unanswered. For example, do perturbations in clock gene rhythms, and/or clock-controlled gene rhythms, alter physiological function in an organ-specific manner? To what extent do changes in the phase relationship of circadian rhythms between various organ systems contribute to disease states? Furthermore, do clock genes have pleiotropic (i.e., circadian independent) roles that serve critical functions in noncircadian molecular pathways? Answers to these questions are expected to lead to a more detailed understanding of how whole body circadian desynchronization, such as occurs during shift work or persistent LD phase reversal, leads to internal physiological malfunction.
The molecular circadian clock is now known to regulate the rhythm of expression of many important regulatory genes that control critical processes involved in various diseases, including those that involve immunoinflammatory cascades (5). A key nuclear transcription gene that has been shown to be under the control of circadian clock genes is NF-κB (5). NF-κB is involved in cell survival and is an important factor controlling intracellular inflammatory processes via production of proinflammatory cytokines. NF-κB has been implicated in many local and systemic inflammatory disorders, including the metabolic syndrome, rheumatological disorders, inflammatory bowel disease, and cancer (10). Circadian desynchronization could potentially result in dysregulation of NF-κB activation, leading to initiation and/or exacerbation of the inflammatory cascade with an associated negative impact in the disease process. This mechanism may represent one explanation of the observed exacerbation of colitis in our phase-shifted mice. Further studies are needed to directly investigate the relationship between NF-κB activation and circadian misalignment in animals and humans.
Another possible mechanism for why the DSS-induced colitis may have been more severe in the phase-shifted animals is that there may be a decrease in the amount of sleep or in sleep quantity in the phase-shifted animals. In addition to disturbed circadian organization, a hallmark of shift work in humans is chronically reduced sleep time (U.S. Congress, Office of Technology Assessment, Biological Rhythms: Implications for the Worker, OTA-BA-463, Washington, DC: U.S. Government Printing Office, September 1991). Indeed, we have recently demonstrated that sleep deprivation without phase shifting the LD cycle can also exacerbate the effects of DSS-induced colitis in mice (Tang Y and Preuss F, unpublished results). We are unaware of any data in the literature on the effects of chronic phase shifts in the LD cycle on total sleep time. It may well be that some of the adverse effects associated with circadian disruption may be due to the impact of this disruption on sleep amount and/or sleep continuity. Given the long-term adverse health effects associated with shift work in humans, it is clearly of great importance to determine the roles of circadian disorganization and sleep loss in mitigating these disease conditions.
Our data suggest that circadian disruption has deleterious effects when it is combined with other pathological events and thus can negatively impact the pathological course of the disease. The present results in mice provide a scientific foundation for examining the impact of circadian desynchronization and misalignment, as occurs in shift workers, on inflammatory disorders. These findings may have important implications not only for specific disorders associated with the gastrointestinal tract but also for the potential importance of circadian synchronization for overall health and management of patients with systemic diseases.
Perspectives and Significance
Colitis is an inflammatory bowel disease of the large intestine characterized by acute or chronic episodes of abdominal discomfort and pain, frequent diarrhea, loss of body weight, and fatigue, among other symptoms. If left untreated, patients have an increased risk of colon cancer. The underlying cause of colitis is unknown, and similarly, it is unclear what provokes the onset of acute flare-ups. Interestingly, a few clinical studies have shown perturbations to the sleep-wake cycle to be associated with inflammatory episodes (7, 20, 21); however, no controlled clinical studies have been performed to determine the causative relationship. In combination with clinical reports, our finding that chronic phase shifting of the LD cycle exacerbates the symptoms of colitis in mice strongly supports the hypothesis that circadian disturbance may contribute to the occurrence and severity of acute and/or chronic colitis episodes and raises the possibility that chronic circadian disruption may impact the progression of other pathological conditions.
GRANTS
This work was supported by National Institutes of Health Grants P01 AG11412 (to F. W. Turek) and AA013745 (to A. Keshavarzian).
Acknowledgments
We thank Matthew Augustine for technical assistance and Dr. Shriram Jakate for performing the histopathological analysis.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol 78: 206–209, 1982. [DOI] [PubMed] [Google Scholar]
- 2.Costa G Shift work and occupational medicine: an overview. Occup Med (Lond) 53: 83–88, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Davidson AJ, Sellix MT, Daniel J, Yamazaki S, Menaker M, Block GD. Chronic jet-lag increases mortality in aged mice. Curr Biol 16: R914–R916, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haus E, Smolensky M. Biological clocks and shift work: circadian dysregulation and potential long-term effects. Cancer Causes Control 17: 489–500, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Hayashi M, Shimba S, Tezuka M. Characterization of the molecular clock in mouse peritoneal macrophages. Biol Pharm Bull 30: 621–626, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Hua H, Wang Y, Wan C, Liu Y, Zhu B, Yang C, Wang X, Wang Z, Cornelissen-Guillaume G, Halberg F. Circadian gene mPer2 overexpression induces cancer cell apoptosis. Cancer Sci 97: 589–596, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keefer L, Stepanski EJ, Ranjbaran Z, Benson LM, Keshavarzian A. An initial report of sleep disturbance in inactive inflammatory bowel disease. J Clin Sleep Med 2: 409–416, 2006. [PubMed] [Google Scholar]
- 8.Knutsson A Health disorders of shift workers. Occup Med (Lond) 53: 103–108, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Kohsaka A, Bass J. A sense of time: how molecular clocks organize metabolism. Trends Endocrinol Metab 18: 4–11, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Kovalovsky D, Refojo D, Holsboer F, Arzt E. Molecular mechanisms and Th1/Th2 pathways in corticosteroid regulation of cytokine production. J Neuroimmunol 109: 23–29, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Laposky AD, Bass J, Kohsaka A, Turek FW. Sleep and circadian rhythms: key components in the regulation of energy metabolism. FEBS Lett 582: 142–151, 2008. [DOI] [PubMed] [Google Scholar]
- 12.Martino TA, Oudit GY, Herzenberg AM, Tata N, Koletar MM, Kabir GM, Belsham DD, Backx PH, Ralph MR, Sole MJ. Circadian rhythm disorganization produces profound cardiovascular and renal disease in hamsters. Am J Physiol Regul Integr Comp Physiol 294: R1675–R1683, 2008. [DOI] [PubMed] [Google Scholar]
- 13.Martino TA, Tata N, Belsham DD, Chalmers J, Straume M, Lee P, Pribiag H, Khaper N, Liu PP, Dawood F, Backx PH, Ralph MR, Sole MJ. Disturbed diurnal rhythm alters gene expression and exacerbates cardiovascular disease with rescue by resynchronization. Hypertension 49: 1104–1113, 2007. [DOI] [PubMed] [Google Scholar]
- 14.McClung CA, Sidiropoulou K, Vitaterna M, Takahashi JS, White FJ, Cooper DC, Nestler EJ. Regulation of dopaminergic transmission and cocaine reward by the Clock gene. Proc Natl Acad Sci USA 102: 9377–9381, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naylor E, Bergmann BM, Krauski K, Zee PC, Takahashi JS, Vitaterna MH, Turek FW. The circadian clock mutation alters sleep homeostasis in the mouse. J Neurosci 20: 8138–8143, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nelson W, Halberg F. Schedule-shifts, circadian rhythms and lifespan of freely-feeding and meal-fed mice. Physiol Behav 38: 781–788, 1986. [DOI] [PubMed] [Google Scholar]
- 17.Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS, Hogenesch JB. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell 109: 307–320, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Penev PD, Kolker DE, Zee PC, Turek FW. Chronic circadian desynchronization decreases the survival of animals with cardiomyopathic heart disease. Am J Physiol Heart Circ Physiol 275: H2334–H2337, 1998. [DOI] [PubMed] [Google Scholar]
- 19.Ramsey KM, Marcheva B, Kohsaka A, Bass J. The clockwork of metabolism. Annu Rev Nutr 27: 219–240, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Ranjbaran Z, Keefer L, Farhadi A, Stepanski E, Sedghi S, Keshavarzian A. Impact of sleep disturbances in inflammatory bowel disease. J Gastroenterol Hepatol 22: 1748–1753, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Ranjbaran Z, Keefer L, Stepanski E, Farhadi A, Keshavarzian A. The relevance of sleep abnormalities to chronic inflammatory conditions. Inflamm Res 56: 51–57, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Rath HC, Herfarth HH, Ikeda JS, Grenther WB, Hamm TE Jr, Balish E, Taurog JD, Hammer RE, Wilson KH, Sartor RB. Normal luminal bacteria, especially Bacteroides species, mediate chronic colitis, gastritis, and arthritis in HLA-B27/human beta2 microglobulin transgenic rats. J Clin Invest 98: 945–953, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenwasser AM, Turek FW. Physiology of the mammalian circadian system. In: Principles and Practice of Sleep Medicine (4th ed.), edited by Kryger MH, Roth T, and Dement WC. Philadelphia, PA: Elsevier, 2005, p. 351–362.
- 24.Rudic RD, McNamara P, Curtis AM, Boston RC, Panda S, Hogenesch JB, Fitzgerald GA. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol 2: e377, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salgado-Delgado R, Angeles-Castellanos M, Buijs MR, Escobar C. Internal desynchronization in a model of night-work by forced activity in rats. Neuroscience 154: 922–931, 2008. [DOI] [PubMed] [Google Scholar]
- 26.Tsai LL, Tsai YC. The effect of scheduled forced wheel activity on body weight in male F344 rats undergoing chronic circadian desynchronization. Int J Obes 31: 1368–1377, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Tsai LL, Tsai YC, Hwang K, Huang YW, Tzeng JE. Repeated light-dark shifts speed up body weight gain in male F344 rats. Am J Physiol Endocrinol Metab 289: E212–E217, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J. Obesity and metabolic syndrome in circadian Clock mutant mice. Science 308: 1043–1045, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van der Sluis M, De Koning BA, De Bruijn AC, Velcich A, Meijerink JP, Van Goudoever JB, Buller HA, Dekker J, Van Seuningen I, Renes IB, Einerhand AW. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 131: 117–129, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc 2: 541–546, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Zijlstra FJ, Garrelds IM, van Dijk AP, Wilson JH. Experimental colitis in mice: effects of olsalazine on eicosanoid production in colonic tissue. Agents Actions Spec No: C76–C78, 1992. [PubMed]