Abstract
The primary afferent neurotransmitter triggering the spinal micturition reflex after complete spinal cord injury (SCI) in the rat is unknown. Substance P detected immunohistochemically in the sacral parasympathetic nucleus was significantly higher in 12 SCI rats than in 12 spinally intact rats (P = 0.008), suggesting substance P as a plausible candidate for the primary afferent neurotransmitter. The effects of the tachykinin NK1 receptor antagonist L-733060 on the spinal micturition reflex were then determined by performing conscious cystometry in an additional 14 intact rats and 14 SCI rats with L-733060 (0.1–100 μg) administered intrathecally at L6-S1. L-733060 was without effect in intact rats, but blocked the spinal micturition reflex in 10 of 14 SCI rats and increased the intermicturition interval in 2 of 4 others at doses ranging from 10 to 100 μg. Both phasic and nonphasic voiding contractions, differentiated according to the presence of phasic external urethral sphincter (EUS) activity, were present in most SCI rats. Both types of contractions were blocked by high doses of L-733060. Interestingly, there was a relative decline in phasic voiding contractions at high doses as well as a decline in contraction amplitude in nonphasic voiding contractions. In other respects, cystometric variables were largely unaffected in either spinally intact or SCI rats. L-733060 did not affect tonic EUS activity at any dose except when the spinal micturition reflex was blocked and tonic activity was consequently lost. These experiments show that tachykinin action at spinal NK1 receptors plays a major role in the spinal micturition reflex in SCI rats.
Keywords: spinal cord injury, bladder, tachykinins, external urethral sphincter
micturition in spinally intact rats is controlled by a spinobulbospinal reflex (9), in which afferent signals from the bladder are relayed to the pontine micturition center; an afferent-activated neuronal “switch” is thrown when the bladder reaches its capacity for innocuous stretch, and thereafter descending activation of parasympathetic preganglionic neurons in the sacral parasympathetic nucleus (SPN) induces bladder contraction and micturition. Axons of both ascending and descending portions of the reflex pathway are interrupted by spinal cord transection and the spinobulbospinal reflex is lost. However, a purely spinal micturition reflex develops for volume-evoked micturition within ∼1 wk (47).
The neurotransmitters used by primary afferent neurons in the spinal micturition reflex are unknown. In spinally intact rats, the neurotransmitters most likely to be used by bladder primary afferent neurons are neuropeptides and glutamate. Neuropeptides (e.g., substance P, neurokinin A, calcitonin gene-related peptide) are used most prominently by C-fiber afferent neurons, whereas glutamate is used not only by nonpeptidergic C-fiber and Aδ-fiber afferent neurons but also as cotransmitters by peptidergic C-fiber afferent neurons (2, 8, 17). Glutamate could be the sole primary afferent neurotransmitter responsible for the spinobulbospinal micturition reflex, given that intrathecal glutamate NMDA and AMPA receptor antagonists block the reflex in conscious, spinally intact rats, whereas intrathecal tachykininergic antagonists are without effect in conscious, spinally intact rats. However, glutamate cannot be the sole primary afferent neurotransmitter responsible for the spinal micturition reflex of chronic spinal cord injury (SCI) rats because intrathecal glutamate NMDA and AMPA receptor antagonists do not block the reflex in conscious chronic SCI rats (28, 43, 44).
Among the neuropeptides, the tachykinins substance P and NKA (both arising from preprotachykinin) are interesting candidates to be involved in the spinal micturition reflex. Intrathecal tachykinin antagonists have little effect on the spinobulbospinal micturition reflex induced by innocuous bladder filling but do modulate the reflex with noxious bladder filling or cystitis in conscious, spinally intact rats (reviewed in Refs. 21, 22, 32). Given the strong relationship between neuronal tachykinin expression and capsaicin sensitivity, it is especially likely that tachykinins are involved in the genesis of nonvoiding contractions (NVCs) in chronic SCI rats, which can be eradicated by capsaicin pretreatment even as voiding contractions are unaffected (4, 6). We were surprised that we were unable to find any studies in which the effects of intrathecal application of NK1 antagonists in chronic SCI rats were established, which is the topic of this paper.
MATERIALS AND METHODS
Animals.
A total of 52 female Sprague-Dawley rats (Charles River, Wilmington, DE) with an initial body mass of 175–200 g was used. The experimental protocol was approved by the Animal Care and Use Committee of the Durham Veterans Affairs Medical Center where these experiments were performed.
SCI.
SCI was produced in 26 rats by transection at the T9 vertebral (T10 spinal cord) level; terminal study occurred 2 wk posttransection. Following anesthetization with isoflurane, laminectomy was performed, and the dura mater and spinal cord were cut with microscissors. Transection was considered complete when the two ends of the spinal cord retracted to produce a gap between the cut ends and the ventral surface of the vertebral bone could be visualized. A sterile Gelfoam sponge was placed between the cut ends of the spinal cord, the muscle and skin were then sutured, and the rat was returned to its cage. Rats received prophylactic enrofloxacin (5–10 mg/kg sc) twice daily for 5 days following surgery and buprenorphine (0.1–0.5 mg/kg sc) twice daily for 3 days following surgery for analgesia. Voiding was induced twice daily by transabdominal compression of the bladder (Credé maneuver) following spinal cord transection, and the volume expressed in the morning was measured as an indicator of bladder capacity (29).
Spinal cord substance P labeling and quantitation.
A total of 12 intact and 12 SCI rats were anesthetized with 50 mg/kg of pentobarbital sodium after which they were euthanized by bilateral thoracotomy. While the heart was still beating, 0.05 ml of 1,000 U/ml heparin (Baxter, Deerfield, IL) was injected by intracardiac puncture. A cannula inserted through the ventricular apex was tied into the ascending aorta. After cutting a vent in the right atrium, the rats were perfused first with 400 ml of ice-cold phosphate-buffered saline and then with 500 ml of ice-cold 4% paraformaldehyde in PBS. The lumbosacral spinal cord was then immediately removed into additional fixative, the dura opened, and an additional 2 h of fixation allowed. The L6 segment was processed through an ascending sucrose series (through 30%) over the course of at least 3 days prior to embedding and freezing in Tissue-Tek OCT (Sakura Finetek, Torrance, CA). Serial frozen sections were cut at 40 μm and stored in successive wells of multiwell plates containing cryoprotecting buffer [5% ethylene glycol, 25% glycerol, 0.05 M phosphate buffer; (11)]. Every sixth section from the L6 segment (8–10 stained sections/rat) was stained via indirect immunofluorescence for demonstration of substance P and choline acetyltransferase (ChAT). The primary antibodies were guinea pig anti-substance P (cat. no. GP14103, Neuromics, Minneapolis, MN) used at 1:1,000 and goat anti-ChAT (cat. no. AB144P, Chemicon, Temecula, CA) used at 1:100 to label cholinergic neurons, including the parasympathetic preganglionic neurons of the SPN. Reproducible fluorescent illumination was assured by examination of a piece of nonfading fluorescent plastic and careful adjustment of incident light so that the emitted standard fluorescence was the same each day. For production of the photographs of Fig. 2, fields to be photographed were chosen looking only at the green channel to localize ChAT-positive neurons (i.e., substance P staining was not examined to select fields). Acquisition time and processing for substance P was identical for all micrographs, whereas ChAT processing was varied slightly to best reveal the parasympathetic preganglionic neurons. For quantitative study of substance P in the SPN, the spinal cord was examined looking only at the green channel, and the outline of the SPN was defined as a smooth envelope just including all the ChAT-positive neurons. One micrograph per section was sufficient to reveal the SPN on one side. Micrographs were taken on both the red channel (for substance P) and the green channel (ChAT). Using the program ImageJ (National Institutes of Health, Bethesda, MD, http://rsb.info.nih.gov/ij/, 1997–2006) the area in the red channel and within the outlined SPN that was of suprathreshold intensity on each micrograph was measured. Similar approaches have been used previously for spinal cord neuropeptides (40, 48).
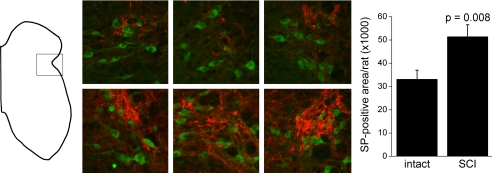
Fig. 2.
Substance P and choline acetyltransferase (ChAT) in the L6 sacral parasympathetic nucleus. The spinal gray matter schematic (left; dorsal surface up) shows the approximate site from which the color pictures were taken. The color panels show 3 examples from intact rats (top) and 3 from spinal cord injury (SCI) rats (bottom) stained for substance P (red) and ChAT (green). ChAT-positive neurons are parasympathetic preganglionic neurons. All pictures were identically processed for substance P; ChAT was slightly altered from picture to picture for best definition of the parasympathetic preganglionic neurons. Right: total area positive for substance P (SP) per rat (mean ± SE). Difference is significant at P = 0.008.
Conscious cystometry.
Conscious cystometry was required for these experiments because anesthetized and conscious rats have different responses to NK1 receptor antagonists (21, 32) and because bladder-sphincter dyssynergia is a uniform feature of chronic spinal cord-transected rats when under urethane anesthesia but not when conscious. Cystometry was performed as we have previously described in detail (10, 12) with necessary modifications for conscious cystometry. Beginning 1 wk before cystometry, rats spent 1–2 h/day accommodating to the Ballman restraint cage. On the day of cystometry, rats were prepared as follows: anesthetize with isoflurane, expose the bladder via a midline abdominal incision, pass the distal end of a PE-50 polyethylene catheter (heated to create a flared end) through a small incision at the apex of the bladder dome, tighten a purse-string suture around the catheter, pass the proximal end of the catheter through the abdominal incision, suture the abdomen in layers around the catheter, place bipolar electrodes periurethrally for EUS-EMG recording, place the rat while still anesthetized in the Ballman cage (loosely restrained), and connect the catheter and electrode leads to the recording system. The cage was fitted with a water bottle and feeding trough for the rat's comfort. One hour was allowed for recovery from anesthesia. The bladder was manually emptied and infusion of saline was begun at 0.10 ml/min for intact rats and 0.21 ml/min for SCI rats, adjusting as necessary so that filling time was between 5–10 min. Two modes of cystometry were employed: filling cystometry wherein the bladder was emptied via the catheter before the cystometrogram was begun and infusion was stopped when voiding began, and free-running (continuous) cystometry, wherein the infusion of saline is continuous and the bladder is not emptied between micturitions. Filling cystometry provides information on more variables than free-running cystometry; free-running cystometry permits collection of more data per unit time for SCI rats because the bladder is partially filled (due to the residual volume) at all times. Bladder capacity was taken as the volume infused before voiding occurred, micturition volume as the volume voided, and residual volume as the volume remaining in the bladder (and removed by syringe) after voiding.
Intrathecal catheterization.
The site of spinal cord transection was exposed, and a laminectomy performed at T10. The dura was cut, and PE-10 tubing 11-cm long was inserted 2 cm to the lumbar swelling. The catheter was secured by tissue adhesive-soaked cotton balls, and the wound was closed in layers around the exited catheter. The peripheral end of the catheter was attached to the needle of a Hamilton syringe for drug administration. The location of the tip of the intrathecal catheter was confirmed after death in every experiment.
EUS-EMG analysis.
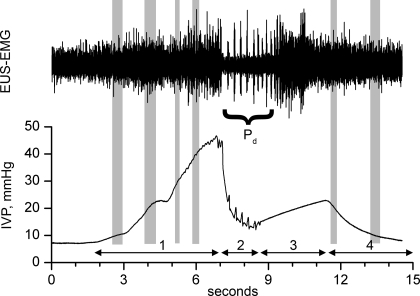
Both tonic and phasic EUS-EMG activity were analyzed. As shown in Fig. 1, the micturition contraction was divided into four phases (7, 38), with phase 1 corresponding to the rise from threshold pressure to the first peak, phase 2 corresponding to the period of phasic EUS-EMG activity and high-frequency oscillations of intravesical pressure (IVP), phase 3 corresponding to a rapid rebound in IVP to a second peak following the cessation of phasic EUS-EMG activity, and phase 4 corresponding to a rapid decline in IVP. Within phase 2, we counted the number of EUS-EMG bursts and pauses (phasic number) and measured the duration of phasic EUS-EMG activity (phasic duration), and from these determined phasic frequency as phasic number divided by phasic duration. Within phases 1 and 4, we measured the mean rectified EUS-EMG activity for each 2-mm wide span around every multiple of 5 mmHg of IVP (i.e., 4–6 mmHg, 9–11 mmHg, 14–16 mmHg, etc.). From these measurements, plots of IVP against mean rectified EUS-EMG activity were made. For NVCs and nonphasic voiding contractions (npVCs), the rising and falling phases of IVP are considered to be phases 1 and 4 (despite their having no phase 2 or 3).
Fig. 1.
Analysis of EUS-EMG waveforms. EUS-EMG and intravesical pressure (IVP) are shown for the 4 phases of a phasic voiding contraction, i.e., 1 showing phasic EUS-EMG activity (bursts and pauses) and high-frequency oscillations of IVP. Phasic duration (Pd) is the time during which periodic burst and pauses of EUS-EMG activity occur (phase 2). For IVP-EMG plots, mean rectified EUS-EMG activity was measured for each 2-mm-wide span around every multiple of 5 mmHg of IVP during phases 1 and 4. Approximations of such spans for multiples of 10 mmHg of IVP are indicated by gray shading. For nonvoiding contractions (NVCs) and nonphasic voiding contractions (npVCs), the rising and falling phases of IVP are considered to be phases 1 and 4 even though there is no phase 2 or 3.
Drugs.
L-733060 hydrochloride [(2S,3S)-3-{[3,5-bis(trifluoromethyl)phenyl]methoxy}-2-phenylpiperidine hydrochloride] (cat. no. 1145; Tocris, Ellisville, MO) was used as an antagonist at NK1 receptors (Ki = 93.13 nM at rat NK1 receptors). L-733060 was dissolved in saline to make a stock solution of 10 mg/ml, from which further dilutions in artificial cerebrospinal fluid were made. The drug was intrathecally delivered at 0.1, 0.3, 1, 3, 10, 30, and 100 μg in 10-μl volume.
Statistics.
Data in the text are given as means ± SE. Differences between intact phasic voiding contractions (intact phVCs), SCI phasic voiding contractions (SCI phVCs), and SCI nonphasic voiding contractions (SCI npVCs), as well as the effects of the NK1 antagonist L-733060 were analyzed using a linear mixed model using maximum likelihood estimation and rat as a random factor. The two fixed factors were contraction type (3 levels: intact phVC, SCI phVC, and SCI npVC) and dose of L-733060 (8 levels: vehicle as 0 μg and 0.1, 0.3, 1, 3, 10, and 100 μg). Logarithmic or square-root transformation of variables or appropriate variance structures were used in the fitting to satisfy statistical assumptions; in all cases diagnostic plots were used to assess the appropriateness and quality of the fitted models. Where the F-test for interaction between contraction type and dose of L-733060 resulted in P ≤ 0.05, indicating that the effect of L-733060 depended on contraction type, we compared doses against vehicle within each contraction type and compared contraction types at each dose (simple effects). Where the interaction was not significant and the F-test for effect of L-733060 yielded P ≤ 0.05, indicating that L-733060 had an effect that was independent of contraction type, we compared the main effects of dose of L-733060 against vehicle. Where the interaction was not significant and the F-test for effect of contraction type yielded P ≤ 0.05, indicating that contraction type had an effect that was independent of L-733060, we compared contraction-type main effects. Comparisons were carried out by using appropriate contrasts in the models fitted. The R programming language and software environment (33) was used for statistical analysis, and lme from package NLME (30) was used for fitting the linear mixed models.
RESULTS
Substance P expression in the L6 SPN.
In the region of the SPN, as defined architectonically and by positive staining for ChAT, substance P-positive terminals were frequent and intensely stained (Fig. 2). Often, much of the substance P staining in this region fell outside the contour line surrounding the parasympathetic preganglionic neurons, particularly dorsally. The number of parasympathetic preganglionic neurons varied from section to section, and the number of substance P-positive varicosities varied roughly in parallel. It is for this reason that the area in which substance P-positive varicosities are counted was limited to the contour defined by the parasympathetic preganglionic neurons as the only easily and sensibly defined region for making measurements. Substance P-positive varicosities were conspicuously more profuse in the SPN of SCI rats, as confirmed by the areas covered by pixels of suprathreshold intensity, which were 33,105 ± 3,900 μm2/rat for intact rats and 51,385 ± 5,127 μm2/rat for SCI rats (Fig. 2; significantly different at P = 0.008).
Types of contractions.
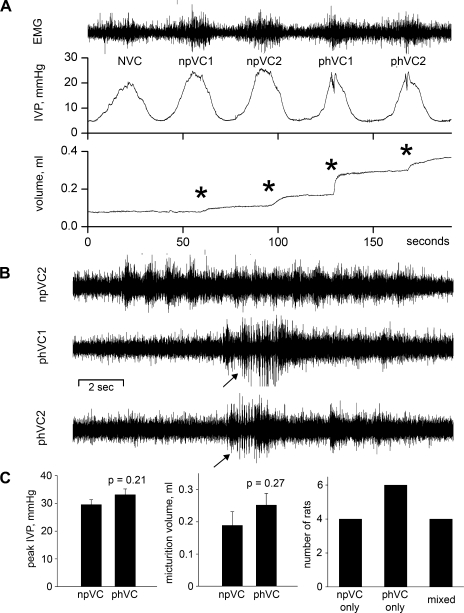
In conscious, spinally intact rats, all substantial bladder contractions are associated with voiding, phasic EUS-EMG activity, and high-frequency oscillations of IVP. The same is not true of conscious 2-wk SCI rats, which showed three different types of contractions as pictured in the continuous cystometrogram of Fig. 3. The division of contractions into NVC (Fig. 3) and voiding contractions is conventional. The division of voiding contractions into npVC and phVC (Fig. 3) reflects the fact that even after sufficient time is allowed for the development of both powerful micturition contractions and phasic EUS-EMG activity, many strong voiding contractions in conscious rats lack phasic EUS-EMG activity. This confirms recent data from Leung et al. (23).
Fig. 3.
NVCs, npVCs, and phasic voiding contractions (phVC) in an SCI rat. A: EUS-EMG activity, IVP, and micturition volume for the 3 types of contractions. *Onsets of detection of voiding. B: comparison of EUS-EMG activities for the 17 s centered around the peaks of the micturition contractions for the second npVC (npVC2) and the 2 phVCs (phVC1, phVC2). Arrows mark beginning of phasic EUS-EMG activity. C: two left-hand plots show that neither peak IVP nor micturition volume differ significantly between npVCs and phVCs. Right: plot shows the distribution of npVCs and phVCs among the 14 SCI rats; for the 4 rats with a mixture of npVCs and phVCs, a mean of 64.5% of all voiding contractions were phVCs (range 43.5–85.7%).
Bladders were hypertrophied in SCI rats, with bladder mass being 0.14 ± 0.01 g in spinally intact rats and 0.54 ± 0.04 g in SCI rats (P < 0.001). It is conceivable that bladder hypertrophy contributes to the occurrence of npVCs. However, Spearman's correlation coefficient for bladder mass vs. the percentage of npVCs among all voiding contractions was only −0.18 (P = 0.62), indicating no significant correlation between these variables.
Phasic EUS-EMG activity in intact and SCI phVCs.
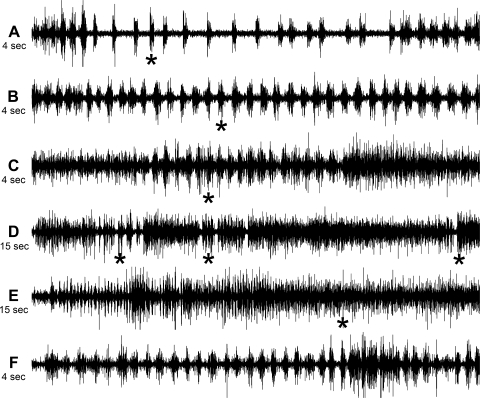
Figure 4 shows EUS-EMG activity associated with voiding in one spinally intact rat and several SCI rats. The EUS-EMG of spinally intact rats was associated with long pauses between brief bursts, except at the beginning and end of bursting where the pauses were quite brief; onset of voiding was detected quickly after phasic activity began (Fig. 4A). In EUS-EMG recordings from SCI rats, the rate of bursting was typically much higher and the pauses shorter (Fig. 4B), to the point where they could be somewhat difficult to detect (Fig. 4C); the onset of voiding continued to occur quickly after phasic activity began. SCI rats, whose contraction duration is known to be long compared with spinally intact rats (4, 6, 23) sometimes showed multiple clusters of phasic EUS-EMG activity, usually with renewed onset of voiding at each cluster (Fig. 4D; note the different time base). One SCI rat consistently showed an unusual anomaly, that phasic EUS-EMG activity was not associated with voiding. Fig. 4E from this rat, with an expanded time base, shows that the onset of voiding came long after phasic activity began; Fig. 4F, from the beginning of Fig. 4E, and at the same time base as Fig. 4, A–C, shows that the phasic activity was quite normal for an SCI rat.
Fig. 4.
Variations in the EUS-EMG activity in SCI phasic voiding contractions. *Onset of detection of voiding. A: entire phasic portion of spinally intact rat EUS-EMG. Frequency of bursts is higher at the beginning and end of phasic activity; pauses are often quite long in between. B: part of a long stretch of EUS-EMG activity in an SCI rat; frequency of bursts is much higher than for the spinally intact rat in A. C: short stretch of EUS-EMG activity in an SCI rat. Pauses are almost obliterated, which is not uncommon in an SCI rat. D: long stretch of EUS-EMG activity in an SCI rat (note change in time base) in which multiple periods of bursting occurred associated with repeated onset of voiding. E and F: long stretch in EUS-EMG activity in an SCI rat, where phasic EUS-EMG activity was not associated with voiding. For comparison, the segment in F from the beginning of the recording in E is on the same time base as A–C.
Effects of L-733060 on the micturition reflex.
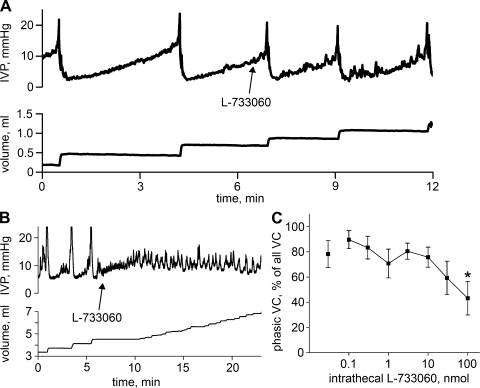
The effects of intrathecal L-733060 on the spinal micturition reflex were ultimately quite different for spinally intact rats than for SCI rats. Figure 5A shows a cystometrogram from a spinally intact rat during the course of a dose-response study of L-733060 before and after the intrathecal administration of 100 μg of L-733060. Essentially no change is observed, which was uniformly true for spinally intact rats. On the other hand, in 10 of 14 SCI rats, the administration of 30 or 100 μg intrathecal L-733060 was sufficient to block the spinal micturition reflex for 21.6 ± 4.7 min, and in two of 14 SCI rats the intermicturition interval was increased by 4.6 ± 0.4 min. As shown in Fig. 5B, intrathecal application of 100 μg L-733060 resulted in the loss of micturition contractions, followed by the gradual increase of pressure to the point where small and rapid pressure oscillations developed and resulted in overflow incontinence. Due to the limited solubility of L-733060, higher doses could not be used without increasing injection volume, which we avoided. In two additional rats, only a single 100-μg dose of L-733060 was administered and found to block the spinal micturition reflex, indicating that diffusion of L-733060 from the injection site during the course of repeated doses of L-733060 is not a likely explanation for its effects. Both phVCs and npVCs were blocked. In five of the 10 SCI rats with phasic voiding contractions, L-733060 blocked phasic contractions before npVCs were blocked; in the other five, both were first blocked at the same dose.
Fig. 5.
Effects of L-733060 on reflex voiding in spinally intact and SCI rats. A: spinally intact rat; administration of 100 μg L-733060 is without effect. B: SCI rat; administration of the same dose of L-733060 blocks the spinal micturition reflex. C: L-733060 causes a decrease in the %phVCs among all voiding contractions in SCI rats, which becomes significant at 100 μg.
Interestingly, the percentage of phVCs among all voiding contractions in the 10 rats that showed phVCs fell as the intrathecal dose of L-733060 rose (Fig. 5C) with the decline reaching statistical significance at 100 μg (P = 0.007). This suggests that phVCs were inhibited or were converted to npVCs.
Differences between intact phVCs, SCI phVCs, and SCI npVCs.
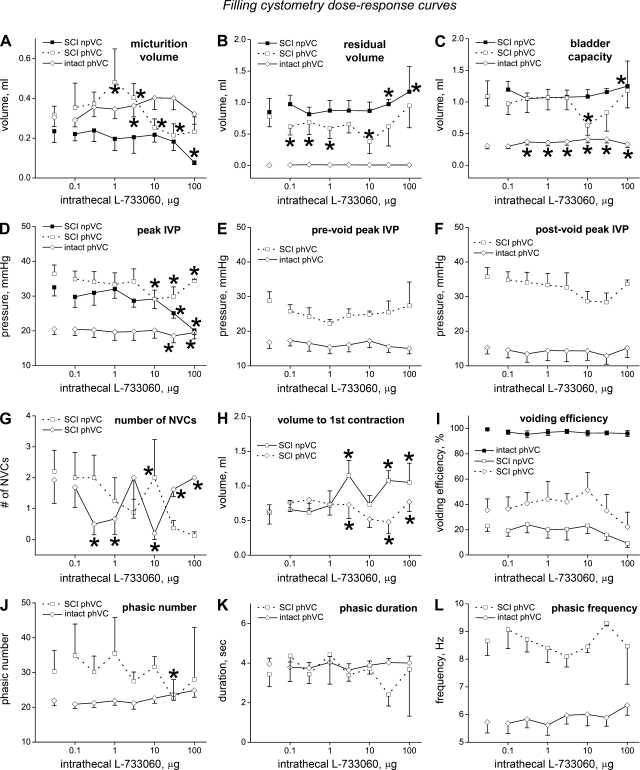
Dose-response curves for 12 cystometric variables against L-733060 obtained from filling cystometry are shown in Fig. 6 for spinally intact phVCs and SCI phVCs and npVCs. The first point for each curve (separated from the following points) is for the value obtained with vehicle administration, and thus provides information to assess differences between the three types of voiding contraction. Somewhat surprisingly, micturition volume (Fig. 6A) was not significantly different between any of the voiding contraction types, whereas residual volume (Fig. 6B) and bladder capacity (Fig. 6C) were both significantly higher for both types of SCI voiding contraction than for intact phVCs (P < 0.001 for all comparisons), whereas values from SCI phVCs and SCI npVCs were not significantly different from each other (P = 0.31 for residual volume, P = 0.57 for capacity). As expected from the literature, peak IVP (Fig. 6D) was significantly higher for both types of SCI contraction (36.4 ± 2.6 mmHg for SCI phVCs and 32.5 ± 2.5 mmHg for SCI npVCs) than for intact phVCs (20.5 ± 1.6 mmHg; P < 0.001 vs. both SCI phVCs and SCI npVCs), with SCI phVCs and SCI npVCs not being significantly different (P = 0.38). Considering only phasic voiding contractions, the peak prevoiding IVP (opening pressure, Fig. 6E) and peak postvoiding IVP (at the end of phase 3, Fig. 6F) were significantly greater for SCI than intact phVCs (28.8 ± 2.6 mmHg for SCI prevoiding peak IVP vs. 16.8 ± 1.8 mmHg for intact, P = 0.0011; 35.7 ± 2.6 mmHg for SCI postvoiding peak IVP vs. 15.3 ± 1.9 mmHg for intact, P < 0.001). During filling cystometry, prevoiding peak IVP exceeded postvoiding peak IVP in 67% of contractions in intact rats vs. 17% of contractions in SCI rats (P < 0.001); the results were much the same during continuous cystometry (70% vs. 22%; P < 0.001). In spinally intact rats, the prevoiding peak has been shown to be strongly influenced by ATP release, whereas the postvoiding peak is more strongly influenced by acetylcholine release (38). Whether the same is true for SCI rats is unknown and is likely to be affected by the much greater contraction duration (4, 6, 23). NVCs (Fig. 6G) were similarly infrequent for both SCI phVCs and SCI npVCs (2.20 ± 0.69 for npVCs, 1.93 ± 0.76 for phVCs; P = 0.15). The volume to the first contraction exceeding 15 mmHg, whether that contraction was an NVC or a voiding contraction, was not different between SCI phVCs (0.64 ± 0.19 ml) and npVCs (0.61 ± 0.12 ml; P = 0.96). Voiding efficiency (Fig. 6I) was essentially 100% for intact phVCs and markedly less (P < 0.001 in both cases) for SCI phVCs and npVCs (32.1% ± 7.2% for SCI phVCs and 18.5% ± 4.2% for SCI npVCs); voiding efficiency was not significantly different for SCI phVCs vs. npVCs (P = 0.14). Phasic number per micturition (Fig. 6J) was not significantly different (P = 0.53) in SCI phVCs (30.3 ± 6.0) than intact phVCs (21.9 ± 1.4), and phasic duration per micturition (Fig. 6K) was essentially the same (3.44 ± 0.63 sec for SCI vs. 3.96 ± 0.30 s, respectively, P = 0.26). Phasic frequency (Fig. 6L) was strikingly different between SCI phVCs and intact phVCs (8.66 ± 0.53 for SCI vs. 5.73 ± 0.39 for intact, P < 0.001), as suggested by comparison of Fig. 4, A and B and reflected in the decline in pause length recently reported by Leung et al. (23).
Fig. 6.
Filling cystometry dose-response curves for intrathecal L-733060 in intact and SCI rats. Separate values at left, which are not connected to other points, are vehicle values; bars indicate SEs. *Statistically significant differences for a given contraction type between the marked response and vehicle; other statistical information is presented in the text.
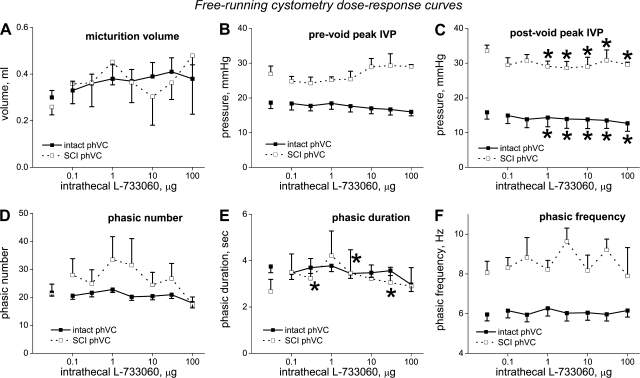
Dose-response curves for L-733060 obtained from free-running cystometry are shown in Fig. 7 for intact and SCI phVCs; again, the first point for each curve is for values obtained with vehicle administration. As for filling cystometry, there was no difference in micturition volume between intact and SCI phVCs (Fig. 7A). The peak prevoiding and postvoiding IVPs were significantly greater for SCI than intact phVCs (Fig. 7, B and C) as was also the case for filling cystometry. Finally, neither phasic number (Fig. 7D) nor phasic duration (Fig. 7E) were significantly different for SCI phVCs vs. intact phVCs, but phasic frequency (Fig. 7F) was again strikingly elevated (8.97 ± 1.67 for SCI vs. 5.96 ± 1.20 for intact, P < 0.001).
Fig. 7.
Free-running cystometry dose-response curves for effects of intrathecal L-733060 on phVCs in intact and SCI rats. Separate values at left, which are not connected to other points, are vehicle values; bars indicate SEs. *Statistically significant differences for a given contraction type between the marked response and vehicle; other statistical information is presented in the text.
Effects of L-733060 on cystometric variables.
Several cystometric variables showed a dose-dependent change, sometimes dependent on contraction type. The dose-response curves for filling cystometry are shown in Fig. 6, with asterisks on the plots as appropriate to indicate statistically significant differences between individual doses and vehicle for a given contraction type. For micturition volume (Fig. 6A), the effects of L-733060 were dependent on contraction type. Intact and SCI phVCs showed scattered significant changes from vehicle without any clear pattern of response; SCI npVCs showed a slow decline at higher doses, which became significant at 100 μg (P = 0.049). Significant differences between contraction types were few and scattered except for consistently lower values for SCI npVCs vs. intact phVCs at doses from 1–100 μg. For residual volume (Fig. 6B), the effects of L-733060 were dependent on contraction type. Significant differences in residual volume for L-733060 doses compared with vehicle showed no consistent pattern, whereas residual volume rose significantly for SCI npVCs at 30 (P = 0.011) and 100 μg (P < 0.001). Not surprisingly, residual volumes for both types of SCI contraction were different from intact phVCs at every dose; additionally, residual volume was significantly higher for SCI npVCs than phVCs at every dose of L-733060 except 30 μg. For bladder capacity (Fig. 6C), the effects of L-733060 were dependent on contraction type. Bladder capacity was significantly different from vehicle at all drug doses for intact phVCs, but the tiny size of the dose effects strongly suggests there is no biologically significant difference. SCI npVCs showed a small increase at the highest dose, which was significant (P = 0.01), whereas SCI phVCs showed one significant (and aberrant) difference at 10 μg. SCI phVCs and SCI npVCs were only significantly different from one another at one dose, whereas both were significantly different from intact phVCs at every dose. For peak IVP (Fig. 6D), the effects of L-733060 were dependent on contraction type. Peak IVP changed significantly at higher doses (10–100 μg) for all types of contractions, but only for SCI npVCs was there a sizeable and consistent (downward) trend (P = 0.015 at 30 μg and P < 0.001 at 100 μg). Neither prevoiding peak IVP (Fig. 6E) nor postvoiding peak IVP (Fig. 6F) showed dose-dependent changes for either contraction type (Figs. 6E and 6F). The number of NVCs (Fig. 6G) showed scattered and unsystematic statistically significant differences, which must be of dubious biological significance (Fig. 6G); the same was true for volume to first contraction for SCI phVCs, although the increase in SCI npVCs was more consistent and likely meaningful (Fig. 6H). For voiding efficiency (Fig. 6I), there was no dose-dependent change for any contraction type, with both SCI phVCs and npVCs showing dose-dependent declines at higher doses that were not statistically significant. For phasic number per micturition (Fig. 6J), the effects of L-733060 were found to be dependent on contraction type; however, only one contraction type (SCI phVC) at one dose (30 μg) showed a statistically significant difference from vehicle, rendering this of unlikely biological significance. For phasic duration per micturition (Fig. 6K) and phasic frequency (Fig. 6L), L-733060 was without significant effect.
The results from free-running cystometry for phVCs were similar to those for filling cystometry (Fig. 7). Micturition volume (Fig. 7A) was not affected by state (P = 0.24) nor by L-733060 (P = 0.27). Prevoiding peak IVP (Fig. 7B) depended on state at all doses of L-733060 (P < 0.001) being higher in SCI phVCs; L-733060 had no significant effect on response (P = 0.0873). Postvoiding peak IVP (Fig. 7C) depended on both state (P < 0.0001) and L-733060 (P = 0.0024) being higher in SCI phVCs at all doses of L-733060 (P < 0.001); for both SCI and intact phVCs the slow decline in IVP with dose compared with vehicle was significant for 1 μg and above. Phasic number per micturition (Fig. 7D) was not significantly influenced by state (P = 0.65) or L-733060 (P = 0.72). Phasic duration per micturition (Fig. 7E) was similarly independent of L-733060 (P = 0.60) but depended on state (P = 0.016). For phasic frequency (Fig. 7F), the effect of L-733060 was dependent on state. However, phasic frequency for intact phVCs was independent of dose and only sporadic significant differences from vehicle were found for SCI phVCs, rendering the dose-dependence of dubious biological significance.
Taken together, these results suggest that the effects of L-733060 were less on the cystometric variables than on blocking the spinal micturition reflex. The sole exception that we found persuasive was the conjoint decline in peak IVP and voiding efficiency and increase in residual volume and capacity seen for SCI npVCs at the highest doses.
Effects of L-733060 on tonic EUS-EMG activity.
npVCs may simply be NVCs that are sufficiently powerful to overcome bladder-sphincter dyssynergia. If so, then nonphasic and phVCs may be activated by different pathways involving different afferent neurons as has been reported for NVCs (dependent primarily on capsaicin-sensitive afferent neurons) and voiding contractions (dependent primarily on capsaicin-insensitive afferent neurons) (4, 6). Because different pathways could differentially engage tonic EUS-EMG activity, we next investigated the relationship between IVP and tonic EUS-EMG activity as described in materials and methods.
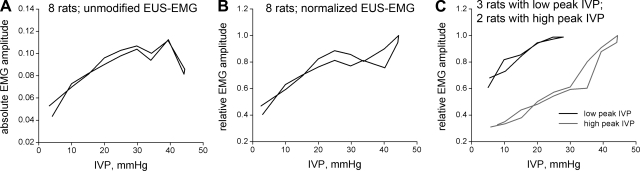
In Fig. 8A, the IVP-EMG plot for vehicle-treated npVCs averaged across 8 rats is shown rising rapidly, then leveling off and perhaps even dropping at high pressures. However, EUS-EMG amplitude varies from rat to rat, at least in part due to variations in the separation between the electrodes and the EUS. We thus replotted the same data in Fig. 8B after normalizing each rat's EMG values to the maximum value it obtained during the micturition cycle. This resulted in a more linear plot, except that it still seemed to level off except at the highest pressures. Because all eight rats participated in the generation of the mean up to 25 mmHg but only two participated at the higher pressures, we separately plotted the mean values from the rats with the three lowest and two highest peak IVPs (peak IVPs were 26.0 ± 1.0 and 41.5 ± 2.9, respectively). The result, shown in Fig. 8C, is two fairly linear plots with similar slopes but different ranges and intercepts.
Fig. 8.
IVP-EMG relationships. A: IVP is plotted against mean rectified EUS-EMG activity across the 8 SCI rats that exhibited npVCs following vehicle. The plot rises to a peak around 40 mmHg, then falls. Knowing that EUS-EMG amplitude varies from rat to rat, at least in part due to variations in the separation between the electrodes and the EUS, we replotted the data in B. Here, the peak value of rectified EUS-EMG within each rat is used to normalize the rectified EUS-EMG values. This results in a plot that is fairly linear but which, except at pressures over 40 mmHg, seems to level off after 25 mmHg or so. Because all 8 rats participate in the generation of the mean up to 25 mmHg but only 2 participated at the higher pressures, we separately plotted the mean values from the rats with the 3 lowest and 2 highest peak IVPs. The result, shown C, is 2 fairly linear plots with similar slopes but different intercepts and ranges.
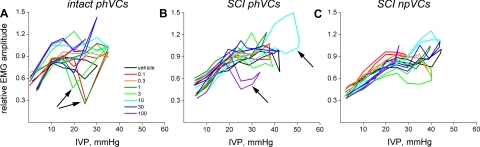
We then used normalization to compare intact phVCs with SCI phVCs and npVCs (Fig. 9). We did not investigate high- and low-IVP contractions separately. The red plots are for vehicle-treated rats, and are quite similar (except for range of IVP) for all three panels. Results obtained with increasing doses of L-733060 are shown in approximately rainbow order. A number of anomalies are present in these plots, which in every case arise where there are very few measurements so that values are not coerced toward a stable mean. In Fig. 9A for intact phVCs, the dips in several curves (arrows) at ∼20–25 mmHg are all from phase 1 of the micturition cycle, and occur because the only rat having opening pressures this high also exhibited very little phase 1 EUS-EMG activity despite exhibiting pronounced phase 4 EUS-EMG activity. The anomalous ballooning of the 10 μg curve in Fig. 9B for SCI phVCs arises for a similar reason: whereas 10 rats contributed data to the curves at 15 mmHg, only one rat (and only one contraction) contributed data to this high-pressure part of the curve. For the anomalous portion of the 100 μg curve in Fig. 9B, again only one rat contributed to data at 25 mmHg and above.
Fig. 9.
Effect of L-733060 on relationship between IVP and tonic mean rectified EUS-EMG activity in intact phVCs (A), SCI phVCs (B), and SCI npVCs (C). The dips in several curves in A (arrows), most pronounced at ∼20–25 mmHg, are all from phase 1 of the micturition cycle, and occur because the only rat having opening pressures this high also exhibited very little EUS-EMG activity during phase 1 but had pronounced activity during phase 4. The anomalous portion of the 10 μg curve in B arises because, whereas 10 rats contribute data to the curves at 15 mmHg, only 1 rat (and only 1 contraction) contributed data to this high-pressure part of the curve. For the anomalous portion of the 100 μg curve in B, again only 1 rat contributed to data at 25 mmHg and above. With such anomalies accounted for, there appears to be little difference between the curves for intact phVCs, SCI phVCs, and SCI npVCs (other than the lower peak pressure for intact phVCs as per Fig. 6D), and there is no indication that L-733060 affects tonic EUS-EMG activity.
Looking at all three panels of Fig. 9 without considering these anomalies, two facts are apparent. First, there is little difference between contraction types in the enlistment of tonic EUS-EMG activity as IVP rises; thus, there is no evidence of differential engagement of the tonic EUS-EMG reflex between these three types of contractions. Second, it is apparent that L-733060 has little, if any, effect on the IVP-EMG relationship.
Contraction type and Credé volume in SCI rats.
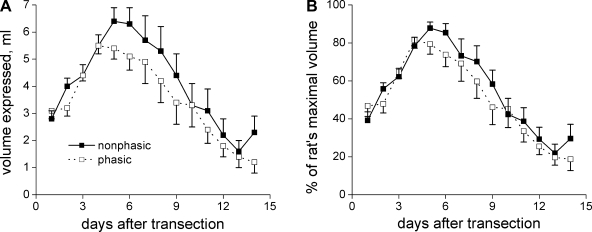
The proportion of voiding contractions, which are phasic during vehicle treatment of a given SCI rat, is quite variable, with some rats having mostly npVCs, some having mostly phVCs, and some having a mixture (Fig. 3). In this study, rats with more phVCs than npVCs outnumbered rats with mostly npVCs (9 mostly phasic, 5 mostly nonphasic). In an as yet unpublished study of the effect of MK-801 on voiding in conscious SCI rats, most of the rats exhibited mostly npVCs, despite the models being prepared in the same way at the same time. We lumped 22 of the SCI rats together, separating them into those that exhibited mostly phasic activity vs. those that exhibited completely nonphasic activity in the presence of vehicle (11 in each group), to answer another question: whether we could identify phasic vs. nonphasic rats by the rate and/or extent of decrease of the volume of fluid expelled from the rats' bladder each morning after spinal cord transection (Credé volume). We hypothesized that rats with mostly phVCs would show earlier recovery to smaller morning Credé volumes. The results are shown in Fig. 10. There was almost no difference between the curves for phasic-dominant vs. nonphasic rats whether considering the raw values or values normalized to each rat's maximal volume.
Fig. 10.
Volume of manually expressed urine on each morning following spinal cord transection for SCI rats that did or did not exhibit phasic EUS-EMG activity during cystometry before L-733060 was administered. A total of 22 SCI rats are included (11 nonphasic, 11 phasic), some of which are from another study but which were identically treated. The difference is surprisingly minor.
DISCUSSION
Substance P and the SPN.
Substance P-positive terminals in both spinally intact and 2-wk SCI rats are numerous not only within that part of the SPN defined by the presence of ChAT-positive parasympathetic preganglionic neurons but also dorsal and particularly dorsolateral to those neurons. Properly speaking, these dorsal and dorsolateral terminals may also innervate the SPN when the SPN is taken to include interneurons and projection neurons, which are fos-positive following innocuous and especially nociceptive infusion of the bladder (41).
That the abundance of ChAT-positive neurons varied from section to section presumably reflects the periodic distribution of those neurons or their transverse dendritic bundles (1) corresponding to the periodic distribution of the afferent neuronal fibers contacting them (27, 47, 48). That the abundance of substance P-positive terminals appeared to be tied to the abundance of ChAT-positive neurons in any given section argues for a role of those terminals in the control of parasympathetic preganglionic neurons. However, they are not necessarily involved in the control of the bladder. Thus, after SCI, some L6-S1 afferent neurons show marked changes that are not shared by bladder afferent neurons (31).
Sprouting of central afferent terminals after SCI has been reported for the SPN (46–48, 50). Central neurotransmitters and neuromodulators that are increased following SCI include CGRP (3, 19, 47, 48), galanin (50), pituitary adenylate cylase-activating peptide (49), and vasoactive intestinal peptide (39). Post-SCI sprouting of central afferent terminals containing substance P has previously been reported for the thoracolumbar intermediolateral nucleus (3, 18), where sympathetic preganglionic neurons are located, and in this paper for the SPN, where parasympathetic preganglionic neurons are located. Sprouting of SPN central afferent terminals containing substance P and CGRP has also been reported for chronic cystitis (40).
What causes the central sprouting of substance P-positive terminals? One strong possibility is the overexpression of nerve growth factor (NGF), which increases central proliferation of C-fiber terminals (3, 34). Whether NGF levels are elevated in the lumbosacral spinal cord following SCI is not completely clear, as one group has reported no change (50), whereas another reported NGF elevation and showed that chronic intrathecal treatment with antibodies to NGF reduces bladder hyperreflexia (36) and detrusor-sphincter dyssynergia (37) in SCI.
Despite the encouraging findings with anti-NGF treatment, prevention of sprouting of substance P-positive terminals in the SPN is not necessarily a good idea. Zinck and Downie (47) noted that sprouting of some types of afferent terminals may promote function even as sprouting of other types impairs function. Our cystometric data argue that tachykininergic neurons are crucial to the spinal micturition reflex emerging after SCI. Ablation of tachykininergic afferent neurons or their targets would help to determine whether this is so, but there are problems in carrying out the relevant experiments. Thus, lumbosacral intrathecal treatment with SSP-saporin ablates superficial NK1-expressing spinal cord neurons but not deeper ones (35). Similarly, systemic treatment with capsaicin eliminates neurons expressing transient receptor potential vanilloid-1 receptors, which includes many tachykininergic afferent neurons; however, there is no one-to-one correspondence between capsaicin-sensitive and tachykininergic neurons. If NK1-expressing neurons are indeed required for the spinal micturition reflex, selective targeting of sprouting would be required as suggested by Zinck and Downie (47). Targeting other growth factors such as brain-derived neurotrophic factor or neurotrophin-3 is one useful approach (26).
Spinally intact rats.
The lack of effect of L-733060 on any cystometric variable in spinally intact rats is not surprising. Whereas NK1 antagonists have been shown to inhibit the volume-evoked spinobulbospinal micturition reflex in anesthetized rats (16, 20, 25), they have been shown to be without effect in conscious unanesthetized rats (15). This is the case only for nonnoxious filling, given that NK1 antagonists administered to spinally intact rats with noxious infusates or bladder inflammation do affect micturition.
That intrathecal NK1 antagonists affect anesthetized but not unanesthetized spinally intact rats is interesting. It has been noted (21) that “anesthesia not only influences the supraspinal excitatory input to the spinal cord but could also modify the activity of descending inhibitory pathways.” Because the lumbosacral spinal cord lacks supraspinal inputs in the spinal cord transected rat, it may on that account somewhat resemble the anesthetized rat. That is, the effectiveness of NK1 antagonists on micturition in SCI but not spinally intact rats may be due wholly or in part to the loss of supraspinal inputs rather than to differences in afferent input or in the anatomic plasticity required for establishment of the spinal micturition reflex. We know of no way to test this hypothesis in the SCI rat, at least until it is understood which descending pathways are important and we are able to pharmacologically replace them.
2-wk SCI rats.
Bladder contractions are usually divided only into nonvoiding and voiding contractions. However, it was our constant experience that most rats were able to void reasonable volumes (comparable to those of spinally intact rats) whether or not phasic EUS-EMG activity was present. This, coupled with the observation that morning Credé volumes of SCI rats were reduced (indicative of reduced residual volume) whether or not the rats showed phVCs while undergoing cystometry, demanded that npVCs be considered separately from NVCs.
That being said, there was little difference between npVCs and phVCs in their response to L-733060; both were eliminated in most rats at higher doses, with the phVCs being slightly more sensitive to L-733060 than npVCs. Additionally, the data shown in Figs. 6 and 7, showing that contractions were relatively normal until the dose at which they were eliminated, strongly suggest that it was the generation of the spinal micturition reflex that was targeted by L-733060 rather than the output of that reflex. If one assumes that tachykininergic primary afferent neurons targeting second-order neurons with NK1 receptors were capsaicin-sensitive neurons, then one would expect that blocking tachykininergic input would, like ablating tachykininergic capsaicin-sensitive afferent neurons, block NVCs and likely npVCs, while leaving phVCs unaffected (6). That phVCs were, if anything, slightly more sensitive to L-733060 suggests that a substantial number of tachykininergic neurons are not capsaicin-sensitive.
This finding is not entirely unexpected, given that while capsaicin sensitivity was once believed to be part of the phenotype of small peptidergic fibers, it has since been shown that the TRPV1 receptor to which it binds is frequently found not only on small peptidergic neurons but also on small nonpeptidergic neurons (13) and on Aδ-fibers as well (24). At some levels of the spinal cord, TRPV1-expressing (and therefore capsaicin-sensitive) afferent neurons rarely express substance P (13). Though this may be less true for bladder afferent neurons (14), we have observed many TRPV1-negative yet substance P-positive terminals near parasympathetic preganglionic neurons of the SPN (Dolber PC, unpublished observations). Our working hypothesis is thus that the spinal micturition reflex depends on capsaicin-insensitive (TRPV1-negative) tachykininergic neurons. If this hypothesis is correct, then intrathecal application of NK1 receptor antagonists in capsaicin-pretreated chronic SCI rats should block the voiding contractions that have been shown to survive under those conditions (5). In anesthetized spinally intact rats, the blockade of micturition contractions by NK1 receptor antagonists is prevented by capsaicin pretreatment (20). However, the situation in chronic SCI rats may be quite different, given that marked changes in bladder afferent neuronal phenotype including capsaicin sensitivity occur (42).
The relationship between IVP and tonic EUS-EMG activity.
In view of the studies summarized in Fig. 9, it would appear that L-733060 affects tonic EUS-EMG activity in the conscious SCI rat only via affecting either the peak pressure generated or the success of the spinal micturition reflex. Our hypothesis that npVCs and phVCs might be activated by different pathways involving different afferent neurons is weakened by the finding that npVCs and phVCs do not differentially engage tonic EUS-EMG activity.
Morning Credé volumes of rats showing phasic vs. npVCs.
It is well known that Credé volumes rise for several days, peak at ∼1 wk, and then decline thereafter (23, 48). It seemed likely that the decline in volume was associated with both the establishment of the spinal micturition reflex and consequent strong bladder contractions together with the development of phasic voiding contractions. However, upon dividing rats into those that did and did not show phVCs during conscious cystometry, the time course of the growth and decline in morning Credé volume was essentially identical in the two groups. Similar findings were recently reported by Leung et al. (23). The simplest explanation for this is that the force of bladder contraction following attainment of the spinal micturition reflex is sufficiently high to ensure reasonably effective voiding even in the face of bladder-sphincter dyssynergia. It remains possible, however, that some rats that showed no phasic contractions during daytime voiding in the Ballman restraint cage had phasic bladder contractions during the night in their cages while unrestrained. That is, either the time of day or being restrained may have influenced whether phVCs or npVCs occurred.
Possible models explaining differences between phasic and npVCs.
How are phVCs and npVCs in the SCI rat related? One possibility is that npVCs, like NVCs, are triggered by different afferent neurons than are phasic voiding contractions. In this view, npVCs are nothing more than NVCs with sufficient amplitude to overcome detrusor-sphincter dyssynergia. If this is true, then capsaicin treatment of SCI rats should obliterate npVCs just as it obliterates NVCs (4, 6). In this regard, it may be significant that capsaicin did not prevent voiding contractions in any capsaicin-pretreated SCI rats (4, 6). This could be explained if the rats in those experiments, which were studied at 6–8 wk posttransection, did not exhibit any npVCs. However, in ongoing and as yet unreported experiments in our lab, we have found npVCs to be frequent in SCI rats at up 7–10 wk posttransection (5 rats with only npVCs, 2 with an even mixture of npVCs and phVCs, and 1 with phVCs only). Whereas it could be argued that capsaicin treatment eliminated both NVCs and npVCs, volume threshold should have increased with a mixture of nonphasic and phasic voiding contractions, which it did not (4, 6). The question can be answered with study of capsaicin-treated rats whose distribution of npVCs vs. phVCs is known prior to capsaicin treatment, although such a study would not be immune to criticism that nonphasic contractions were lost postcapsaicin due to the passage of time (4 days is the norm for these studies) rather than capsaicin treatment. As regards the present study, the relative decline in phVCs among all voiding contractions would be viewed not as the consequence of a conversion of phVCs to npVCs but as the consequence of an inhibition of phVCs without effect on npVCs.
A second possible relationship between phVCs and npVCs in SCI rats is that npVCs and phVCs are activated by the same afferent input but differ in the ability to engage the lumbar phasic EUS pattern generator in consequence of separate central connections. In this view, the decline in phVCs with increasing NK1 receptor antagonism would be due to compromise of the ability of spinal neurons to activate the phasic EUS pattern generator; that is, there would be a genuine conversion of phVCs to npVCs. In separate work from our laboratory thus far reported only in abstract form, we have shown that apparent conversion can proceed in the opposite direction. Thus, moderate intrathecal doses of the NMDA antagonist MK-801 cause an increase in the fraction of phVCs among all voiding contractions (45). On the other hand, it can be argued that the effect of moderate doses of MK-801 depends on stimulation or inhibition of one type of afferent input over another.
npVCs and phVCs in the present study had essentially identical ability to engage the tonic EUS reflex, which was unaffected by NK1 antagonist (Fig. 9). If npVCs and phVCs have different afferent inputs, then it seems unlikely that the afferent input directly triggers the tonic EUS reflex because it is equally dependent on IVP in either case. Indeed, even with the tonic EUS reflex dependent on the same set of spinal neurons in the two types of contractions it is difficult to understand how reflexes using different afferent input could yield the same output.
Conclusions.
Substance P is increased in the SPN of SCI rats after spinal cord transection, which raises the possibility that substance P may be important in the afferent limb of the spinal micturition reflex that develops about a week after spinal cord transection. Intrathecal administration of the NK1 antagonist L-733060 has no effect on the spinobulbospinal micturition reflex of spinally intact rats but usually blocked the spinal micturition reflex in rats 2 wk after spinal cord transection. Thus, tachykinin action at spinal NK1 receptors plays a major role in the spinal micturition reflex in SCI rats.
GRANTS
This work was supported by a Department of Veterans Affairs Merit Review award (to P. C. Dolber) and National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-61391 (to M. O. Fraser).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Barber RP, Phelps PE, Houser CR, Crawford GD, Salvaterra PM, Vaughn JE. The morphology and distribution of neurons containing choline acetyltransferase in the adult rat spinal cord: an immunocytochemical study. J Comp Neurol 229: 329–346, 1984. [DOI] [PubMed] [Google Scholar]
- 2.Battaglia G, Rustioni A. Coexistence of glutamate and substance P in dorsal root ganglion neurons of the rat and monkey. J Comp Neurol 277: 302–312, 1988. [DOI] [PubMed] [Google Scholar]
- 3.Cameron AA, Smith GM, Randall DC, Brown DR, Rabchevsky AG. Genetic manipulation of intraspinal plasticity after spinal cord injury alters the severity of autonomic dysreflexia. J Neurosci 26: 2923–2932, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng CL, de Groat WC. The role of capsaicin-sensitive afferent fibers in the lower urinary tract dysfunction induced by chronic spinal cord injury in rats. Exp Neurol 187: 445–454, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Cheng CL, Liu JC, Chang SY, Ma CP, de Groat WC. Effect of capsaicin on the micturition reflex in normal and chronic spinal cord-injured cats. Am J Physiol Regul Integr Comp Physiol 277: R786–R794, 1999. [DOI] [PubMed] [Google Scholar]
- 6.Cheng CL, Ma CP, de Groat WC. Effect of capsaicin on micturition and associated reflexes in chronic spinal rats. Brain Res 678: 40–48, 1995. [DOI] [PubMed] [Google Scholar]
- 7.Chien CT, Yu HJ, Lin TB, Chen CF. Neural mechanisms of impaired micturition reflex in rats with acute partial bladder outlet obstruction. Neuroscience 96: 221–230, 2000. [DOI] [PubMed] [Google Scholar]
- 8.De Biasi S, Rustioni A. Glutamate and substance P coexist in primary afferent terminals in the superficial laminae of spinal cord. Proc Natl Acad Sci USA 85: 7820–7824, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Groat WC Central neural control of the lower urinary tract. Ciba Found Symp 151: 27–44, 1990. [DOI] [PubMed] [Google Scholar]
- 10.Dolber PC, Gu B, Zhang X, Fraser MO, Thor KB, Reiter JP. Activation of the external urethral sphincter central pattern generator by a 5-HT(1A) receptor agonist in rats with chronic spinal cord injury. Am J Physiol Regul Integr Comp Physiol 292: R1699–R1706, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med 4: 1313–1317, 1998. [DOI] [PubMed] [Google Scholar]
- 12.Gu B, Fraser MO, Thor KB, Dolber PC. Induction of bladder-sphincter dyssynergia by kappa-2 opioid receptor agonists in the female rat. J Urol 171: 472–477, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Guo A, Vulchanova L, Wang J, Li X, Elde R. Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur J Neurosci 11: 946–958, 1999. [DOI] [PubMed] [Google Scholar]
- 14.Hwang SJ, Valtschanoff JG. Vanilloid receptor VR1-positive afferents are distributed differently at different levels of the rat lumbar spinal cord. Neurosci Lett 349: 41–44, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Ishizuka O, Igawa Y, Lecci A, Maggi CA, Mattiasson A, Andersson KE. Role of intrathecal tachykinins for micturition in unanaesthetized rats with and without bladder outlet obstruction. Br J Pharmacol 113: 111–116, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawatani M, Matsumoto G, Birder LA, Fukuoka H, Yoshiyama M, Misaki A, Suzuki S. Intrathecal administration of NK1 receptor antagonist, CP96345, inhibits the micturition reflex in the rat. Regul Pept 46: 392–395, 1993. [DOI] [PubMed] [Google Scholar]
- 17.Keast JR, Stephensen TM. Glutamate and aspartate immunoreactivity in dorsal root ganglion cells supplying visceral and somatic targets and evidence for peripheral axonal transport. J Comp Neurol 424: 577–587, 2000. [PubMed] [Google Scholar]
- 18.Klimaschewski L Increased innervation of rat preganglionic sympathetic neurons by substance P containing nerve fibers in response to spinal cord injury. Neurosci Lett 307: 73–76, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Krenz NR, Weaver LC. Sprouting of primary afferent fibers after spinal cord transection in the rat. Neuroscience 85: 443–458, 1998. [DOI] [PubMed] [Google Scholar]
- 20.Lecci A, Giuliani S, Garret C, Maggi CA. Evidence for a role of tachykinins as sensory transmitters in the activation of micturition reflex. Neuroscience 54: 827–837, 1993. [DOI] [PubMed] [Google Scholar]
- 21.Lecci A, Maggi CA. Spinal cord tachykinins in the micturition reflex. Prog Brain Res 104: 145–159, 1995. [DOI] [PubMed] [Google Scholar]
- 22.Lecci A, Maggi CA. Tachykinins as modulators of the micturition reflex in the central and peripheral nervous system. Regul Pept 101: 1–18, 2001. [DOI] [PubMed] [Google Scholar]
- 23.Leung PY, Johnson CS, Wrathall JR. Comparison of the effects of complete and incomplete spinal cord injury on lower urinary tract function as evaluated in unanesthetized rats. Exp Neurol 208: 80–91, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma QP Expression of capsaicin receptor (VR1) by myelinated primary afferent neurons in rats. Neurosci Lett 319: 87–90, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Maggi CA, Lecci A, Garret C, Giuliani S. Spinal effects of selective NK-1 and NK-2 receptor antagonists on bladder motility in anesthetized rats. Regul Pept 46: 389–391, 1993. [DOI] [PubMed] [Google Scholar]
- 26.Mitsui T, Fischer I, Shumsky JS, Murray M. Transplants of fibroblasts expressing BDNF and NT-3 promote recovery of bladder and hindlimb function following spinal contusion injury in rats. Exp Neurol 194: 410–431, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Nadelhaft I, Booth AM. The location and morphology of preganglionic neurons and the distribution of visceral afferents from the rat pelvic nerve: a horseradish peroxidase study. J Comp Neurol 226: 238–245, 1984. [DOI] [PubMed] [Google Scholar]
- 28.Nishizawa O, Igawa Y, Satoh T, Yamashiro S, Sugaya K. Effects of glutamate receptor antagonists on lower urinary tract function in conscious unanesthetized rats. Adv Exp Med Biol 462: 275–281, 1999. [DOI] [PubMed] [Google Scholar]
- 29.Pikov V, Wrathall JR. Coordination of the bladder detrusor and the external urethral sphincter in a rat model of spinal cord injury: effect of injury severity. J Neurosci 21: 559–569, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pinheiro J, Bates D, DebRoy S, Sarkar D.NLME: Linear and Nonlinear Mixed Effects Models. R package version 31–87, [http://www.R-project.org]. Vienna, Austria: R Foundation for Statistical Computing, 2008.
- 31.Qiao LY, Vizzard MA. Spinal cord injury-induced expression of TrkA, TrkB, phosphorylated CREB, and c-Jun in rat lumbosacral dorsal root ganglia. J Comp Neurol 482: 142–154, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Quartara L, Maggi CA. The tachykinin NK1 receptor. Part II: Distribution and pathophysiological roles. Neuropeptides 32: 1–49, 1998. [DOI] [PubMed] [Google Scholar]
- 33.R Development Core Team. R: a Language and Environment for Statistical Computing. http://www.R-project.org. Vienna, Austria: R Foundation for Statistical Computing, 2005.
- 34.Rabchevsky AG Segmental organization of spinal reflexes mediating autonomic dysreflexia after spinal cord injury. Prog Brain Res 152: 265–274, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seki S, Erickson KA, Seki M, Nishizawa O, Igawa Y, Ogawa T, de Groat WC, Chancellor MB, Yoshimura N. Elimination of rat spinal neurons expressing neurokinin 1 receptors reduces bladder overactivity and spinal c-fos expression induced by bladder irritation. Am J Physiol Renal Physiol 288: F466–F473, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Seki S, Sasaki K, Fraser MO, Igawa Y, Nishizawa O, Chancellor MB, de Groat WC, Yoshimura N. Immunoneutralization of nerve growth factor in lumbosacral spinal cord reduces bladder hyperreflexia in spinal cord injured rats. J Urol 168: 2269–2274, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Seki S, Sasaki K, Igawa Y, Nishizawa O, Chancellor MB, De Groat WC, Yoshimura N. Suppression of detrusor-sphincter dyssynergia by immunoneutralization of nerve growth factor in lumbosacral spinal cord in spinal cord injured rats. J Urol 171: 478–482, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Streng T, Talo A, Andersson KE. Transmitters contributing to the voiding contraction in female rats. BJU Int 94: 910–914, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Thor KB, Kawatani M, de Groat WC. Plasticity in the reflex pathways to the lower urinary tract of the cat during postnatal development and following spinal cord injury. In: Development and Plasticity of the Mammalian Spinal Cord, edited by Goldberger ME, Gorio A. and Murray M. Padova, Italy: Liviana, 1986, p. 65–80.
- 40.Vizzard MA Alterations in neuropeptide expression in lumbosacral bladder pathways following chronic cystitis. J Chem Neuroanat 21: 125–138, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Vizzard MA Increased expression of spinal cord Fos protein induced by bladder stimulation after spinal cord injury. Am J Physiol Regul Integr Comp Physiol 279: R295–R305, 2000. [DOI] [PubMed] [Google Scholar]
- 42.Yoshimura N, Erdman SL, Snider MW, de Groat WC. Effects of spinal cord injury on neurofilament immunoreactivity and capsaicin sensitivity in rat dorsal root ganglion neurons innervating the urinary bladder. Neuroscience 83: 633–643, 1998. [DOI] [PubMed] [Google Scholar]
- 43.Yoshiyama M, Nezu FM, Yokoyama O, Chancellor MB, de Groat WC. Influence of glutamate receptor antagonists on micturition in rats with spinal cord injury. Exp Neurol 159: 250–257, 1999. [DOI] [PubMed] [Google Scholar]
- 44.Yoshiyama M, Roppolo JR, de Groat WC. Effects of LY215490, a competitive alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptor antagonist, on the micturition reflex in the rat. J Pharmacol Exp Ther 280: 894–904, 1997. [PubMed] [Google Scholar]
- 45.Zhang X, Douglas KL, Jin H, Dolber PC. Ionotropic glutamate NMDA channels are unexpectedly required for the spinal micturition reflex while at lower doses their antagonism surprisingly alleviates detrusor-sphincter dyssynergia in spinal cord injured (SCI) rats (Abstract). J Urol 179: 350–351, 2008. [Google Scholar]
- 46.Zinck NDT, Downie JW. IB4 afferent sprouting contributes to bladder dysfunction in spinal rats. Exp Neurol 213: 293–302, 2008. [DOI] [PubMed] [Google Scholar]
- 47.Zinck NDT, Downie JW. Plasticity in the injured spinal cord: can we use it to advantage to reestablish effective bladder voiding and continence? In: Autonomic Dysfunction After Spinal Cord Injury. Progress in Brain Research, edited by Weaver LC and Polosa C. New York: Elsevier, 2006, vol. 152, p. 147–162. [DOI] [PubMed]
- 48.Zinck ND, Rafuse VF, Downie JW. Sprouting of CGRP primary afferents in lumbosacral spinal cord precedes emergence of bladder activity after spinal injury. Exp Neurol 204: 777–790, 2007. [DOI] [PubMed] [Google Scholar]
- 49.Zvarova K, Dunleavy JD, Vizzard MA. Changes in pituitary adenylate cyclase activating polypeptide expression in urinary bladder pathways after spinal cord injury. Exp Neurol 192: 46–59, 2005. [DOI] [PubMed] [Google Scholar]
- 50.Zvarova K, Murray E, Vizzard MA. Changes in galanin immunoreactivity in rat lumbosacral spinal cord and dorsal root ganglia after spinal cord injury. J Comp Neurol 475: 590–603, 2004. [DOI] [PubMed] [Google Scholar]