Abstract
Sleep is understood to possess recuperative properties and, conversely, sleep loss is associated with disease and shortened life span. Despite these critical attributes, the mechanisms and functions by which sleep and sleep loss impact health still are speculative. One of the most consistent, if largely overlooked, signs of sleep loss in both humans and laboratory rats is a progressive increase in circulating phagocytic cells, mainly neutrophils. The destination, if any, of the increased circulating populations has been unknown and, therefore, its medical significance has been uncertain. The purpose of the present experiment was to determine the content and location of neutrophils in liver and lung tissue of sleep-deprived rats. These are two principal sites affected by neutrophil migration during systemic inflammatory illness. The content of neutrophils in the intestine also was determined. Sleep deprivation in rats was produced for 5 and 10 days by the Bergmann-Rechtschaffen disk method, which has been validated for its high selectivity under freely moving conditions and which was tolerated and accompanied by a deep negative energy balance. Comparison groups included basal conditions and 48 h of sleep recovery after 10 days of sleep loss. Myeloperoxidase (MPO), an enzyme constituent of neutrophils, was extracted from liver, lung, and intestinal tissues, and its activity was determined by spectrophotometry. Leukocytes were located in vasculature and interstitial spaces in the liver and the lung by immunohistochemistry. Heme oxygenase-1, also known as heat shock protein-32 and a marker of cellular stress, and corticosterone also were measured. The results indicate neutrophil migration into extravascular liver and lung tissue concurrent with cell stress and consistent with tissue injury or infection induced by sleep loss. Plasma corticosterone was unchanged. Recovery sleep was marked by increased lung heme oxygenase-1, increased intestinal MPO activity, and abnormally low corticosterone, suggesting ongoing reactive processes as a result of prior sleep deprivation.
Keywords: sleep deprivation, sleep rebound, granulocytes, myeloperoxidase, heme oxygenase-1, leukocytosis
since the early 1920s, it has been known that sleep deprivation in humans results in a progressive increase in circulating white blood cells, mainly granulocytes (cited in Ref. 39). This outcome has since been repeatedly demonstrated (13, 30, 38, 44), and it is one of the few consistent physiological findings in humans. In the clinical setting, mild leukocytosis usually would be taken as a sign of underlying infectious disease or inflammatory processes. However, in the research field, this outcome has been considered nonspecific, presumably because subjects did not seem ill or have fevers, and because the traffic pattern of the circulating leukocytes is unknown (e.g., Ref. 5, 13). Also, scientific endeavors largely have been focused on cells and molecules associated with the classical immune system, rather than those of the innate immune system. Investigators in recent years have reported increases in various circulating proinflammatory molecules with different time courses in both human (2, 31, 47, 51, 63, 72) and animal (14, 32) sleep-deprived subjects, but there is a dearth of evidence of any physiological causes, consequences, or sequelae.
In a nearly identical manner to humans, rats sleep deprived under the present method show a progressive increase in circulating leukocytes, due mostly to an early increase in the population of immature neutrophilic granulocytes (i.e., “left shift” toward earlier stages of maturation) and days later to an added increase in monocytes (14). The leukocytosis is considered regenerative because the myeloid-to-erythroid ratio in bone marrow is high. Lymphocyte numbers in the circulation tend to decrease, which is consistent with findings in humans sleep deprived in excess of 76 h (Refs. 13 and 44, as cited in 39). The increase in circulating phagocytes is accompanied by increases in circulating proinflammatory molecules, especially interleukin-1β and macrophage chemotactic protein-1, and a polyclonal-like increase in all major immunoglobulin classes (14). Associated signs in sleep-deprived rats include a progressive, negative energy balance (16), and suppression of several anabolic hormones at the level of the hypothalamus (17, 20, 21). With the exception of marked hyperphagia, the profile of sleep deprivation in the laboratory rat resembles chronic systemic inflammatory response syndrome in humans (reviewed in Refs. 4 and 37), including poor microbial control and eventual lethal septicemia (15).
Inflammatory processes generally are considered at the etiological root of several medical evils. Therefore, inflammatory processes that may be induced by sleep deprivation are believed to have clinical and biological relevance, as well as potentially far-reaching implications. The main purpose of the present study was to investigate whether the increases in phagocytes in the circulation indicate migratory traffic into tissues, which is an inflammatory response. This profile can be contrasted with two other categories of mild leukocytosis that might arguably be operative during sleep deprivation. One of these is physiological neutrophilia, which occurs during sympathetic activation, “fight or flight” response, and/or exercise without muscle damage. In physiological neutrophilia, leukocytosis results from the movement of granulocytes from the marginated pool of adherence to endothelium to the circulating pool without a net change in the number of neutrophils that emigrate from bone marrow or migrate into tissue. The other form is corticosterone-induced neutrophilia, which may occur during psychological distress or pain and is characterized by increased emigration of neutrophils from bone marrow into the circulation. In this case, there is not an appreciable change in migration into tissues due to the fact that the neutrophils are rendered less sticky (reviewed in Ref. 35, 45).
In the present study, rats were sleep deprived for 5 or 10 days, which is long enough for metabolic changes and mild neutrophilia to become manifested (14), but short enough to preclude accumulated changes that eventuate in advanced morbidity by an average of 20 days (15, 16, 24). Organs chosen for study were the liver and the lung, because these sites are affected in states of systemic inflammation (reviewed in Refs. 1 and 34). We also measured the neutrophil content of the intestine to determine whether neutrophils appear drawn there. This is because the intestine has been implicated as a site of bacterial translocation in sleep-deprived rats (23) without histological abnormalities found by bright-field microscopy (15). To quantify neutrophils in organs, we first measured the tissue content of myeloperoxidase (MPO), an enzyme virtually exclusive to neutrophils. MPO activity is directly proportional to neutrophil number (3, 43). On the basis of a preliminary outcome of high MPO activity in liver and lung under conditions of sleep deprivation, we next addressed whether the neutrophils were adherent to the blood vessels (marginated pool) or had completed steps of diapadesis and chemotaxis and were present in interstitial spaces. Leukocytes in the liver and the lung were located by means of immunohistochemistry and found to be predominantly interstitial. And lastly, in a step toward understanding the extent to which migration of neutrophils may be physiologically important, we measured the tissue content of heme-oxygenase-1, an inducible heat shock protein that increases in response to inflammation and oxidative stress. Heme oxygenase-1 was significantly increased in both the liver and the lung tissue during sleep loss. Results also showed that recovery sleep was not quiescent but was marked by peak induction of heme oxygenase-1 in the lung and twofold increases in MPO in the intestine, suggesting ongoing recuperative processes. Corticosterone did not increase during sleep loss, consistent with earlier findings (17, 21), but decreased to abnormally low levels during sleep recovery.
MATERIALS AND METHODS
Animals and surgical procedures.
Protocols for animal care and use were approved by institutional animal care and use committees at The Medical College of Wisconsin and the Zablocki Veterans Administration Medical Center. Subjects were 85 male Sprague-Dawley rats obtained from Harlan (Madison, WI) that weighed 470 (SD 39) g and were 23 (SD 2) wk old at the time of study. The rats were kept in constant light to diminish the amplitude of circadian rhythm for all groups because sleep deprivation is known to affect both the amplitude and the phase of the circadian rhythm (50, 70). Ambient temperature was maintained by means of thermostatically controlled heat lamps at 28°C during baseline and experimental periods, which is within the thermoneutral zone for rats (68).
Surgery was performed to implant macroelectrodes for recording cortical electroencephalographic and cortical theta signals (collectively, the EEG) and electromyographic (EMG) activity for the purpose of identifying wakefulness and specific sleep stages, as previously described (7). Surgical anesthesia and analgesia were produced by ketamine hydrochloride (100 mg/kg ip), xylazine hydrochloride (2.4 mg/kg im), and atropine sulfate (0.1 mg/kg im) after brief inhalant anesthesia with halothane. Supplementary doses of ketamine hydrochloride (10 mg/kg ip) were administered as needed to maintain the surgical plane of anesthesia. Animals were allowed to recover from surgery for ≥7 days. Each of 44 live-animal experiments was composed of a set of procedures administered to two rats that shared an experimental enclosure, described below.
Procedure for producing sleep-deprived and yoked animals.
Sleep deprivation in rats was produced by the Bergmann-Rechtschaffen method (7, 24) for 5 or 10 days, which was tolerated and produced hyperphagia and a negative energy balance, as already reported (19). Two freely moving rats were studied at a time. Each of the two rats was housed on one half of a large platform (45-cm diameter) divided into two sides within an open-air enclosure. The plug assembly of EEG and EMG connections on the head of each rat was fastened to a long recording cable that was connected to a 360° commutator and counterbalanced boom assembly to provide freedom of movement. At a short distance beneath the housing platform was a shallow pan of water that extended to the periphery of the enclosure. While the rats were not restricted from entering and walking around in the water, which was only 2 to 3 cm deep, they almost always avoided the water and stayed on the platform where they would engage in normal behaviors, such as eating and lying to sleep. EEG and EMG signals were filtered, displayed online as analog and digital signals, and verified by observation of the rats. During a baseline period of at least 7 days, the platform was automatically rotated once per hour to acclimate the rats to platform movement and to remove debris as the platform rotated beneath sponges located between the two cages.
During the sleep deprivation phase, sleep onset in the rat to be sleep deprived was detected by a computer program (8) that compared the amplitudes of digitized EEG and EMG signals to criteria set to match sleep, as determined by analog chart recordings and by behavioral observation. Sleep onset triggered a brief ambulatory requirement by means of rotation of the housing platform for 6 s, which prompted both rats to walk to remain fully and comfortably on the platform. When the sleep-deprived rat was awake, the platform was stationary, and there was no interruption of other behaviors. The method has been validated and reliably produces a consistent amount of sleep reduction in our studies to <10% of total time, composed mostly of transitional sleep and highly fragmented non-rapid eye movement (NREM) sleep (comparable to human stages I-IV or N1-N3) (15, 16, 22, 24). The ambulatory requirement does not increase as the duration of sleep deprivation increases, but rather it remains stable at 18–22% of time (16, 24). The paired rat, known as the yoked rat or partially sleep-deprived rat, has the same ambulatory requirement because it is housed on the same platform as the totally sleep-deprived rat. The difference is that the ambulatory requirement is not contingent on sleep onset. In these yoked rats, sleep is both fragmented and reduced in amount because of ambulatory requirements that occur during their own sleep and sleepiness bouts. Total sleep in yoked rats averages 38% of time in NREM sleep and 3% in paradoxical sleep [PS; also known as rapid eye movement (REM) sleep] (15, 16, 22, 24). Yoked rats typically exhibit signs in the same direction as do totally sleep-deprived rats, but to a lesser extent (e.g., Ref. 16). Under baseline conditions, during which sleep is permitted nearly ad libitum, sleep occurs an average of 54% of the time and is composed of 48% NREM sleep and 6% PS.
Rats that composed the sleep recovery groups were permitted 48 h of recovery sleep after 10 days of sleep deprivation or partial sleep-deprivation (i.e., yoked) conditions. The 48-h period of measurement was based on previous findings of a return to near basal levels of energy expenditure (18) and normalization of the liver content of glutathione and catalase activity, which are principal free radical scavengers (19). Rats in the sleep recovery groups were injected 6 h before tissue harvest with 25 to 50 mg of bromodeoxyuridine (BrdU)/kg body wt for further studies of cell renewal. Because of this added procedure, the effect of BrdU treatment on intestinal MPO activity was completed in control rats, and the results were negative. Rats that composed the baseline control group were operated on for EEG and EMG implants, and housed in the apparatuses during a 7-day baseline period. Tissue harvests and necropsy procedures were conducted after completion of the planned durations of study and at the same time of day. Rats were exsanguinated by cardiac puncture under deep anesthesia or by decapitation, depending on the tissues collected and variables of interest. Not all variables were measured in all subjects. In experiments in which MPO activity and leukocyte destination were measured in lung tissue, bronchial lavage was performed for further study of fluid contents. The lavage consisted of repeated infusion and withdrawal of 7 ml isotonic saline via the isolated trachea. The liver and the lung tissues were among those quickly dissected, rinsed in saline if needed, blotted, parceled, and fast frozen in liquid nitrogen or embedded and frozen in tissue-freezing medium. The small intestine was sectioned into parts, rinsed three times through with sterile saline to remove contents, and subsections were either fast frozen or preserved in buffered formalin for follow up. Aliquots of some of these tissues were studied for antioxidant parameters, as previously reported (19).
MPO activity in liver, lung, and intestine.
Determinations of MPO activity were based on procedures by Bradley et al. (10) and optimization by Graff et al. (25), in which N-ethylmaleimide (NEM) is used to overcome interference by glutathione. Furthermore, we adapted the procedure via a series of control experiments to take measurements with a temperature-controlled plate reader (Bio-tek FL600). Tissue aliquots of 50 to 100 mg were homogenized on ice in ice-cold NEM buffer at pH 7.4 and twice centrifuged at 12,000 g and 4°C and decanted. MPO then was extracted from cells by flushing the pellets with 0.5% hexadecyltrimethylammonium bromide (HTAB) buffer at pH 6.0, followed by three sonication and freeze-thaw cycles. These were followed by centrifugation of the specimens at 12,000 g, decantation, and suspension of the pellets in HTAB buffer for the collection of three post-NEM supernatants, in which MPO was measured. Reactions were initiated by the addition of 50 mM potassium phosphate buffer containing 0.167 mg/ml o-dianisidine dihydrochloride and 0.0005% H2O2 at pH 6.0 and measured by kinetic specrophotometry at 465 nm. One unit of MPO activity degrades 1 μmol H2O2/min at 25°C. Data are expressed as MPO activity per gram wet weight. Determinations were made in quadruplicate. The interassay coefficient of variance was <4%.
Localization of granulocytes.
Frozen embedded liver and lung specimens were sectioned 4 μm thick on a cryostat and mounted. Each of three sections was stained with either hematoxylin-and-eosin or one of two antibodies for immunohistochemistry, counterstained with Mayer's hematoxylin. The HIS48 antibody reacts with an antigen found on all granulocytes (BD Pharmingen, San Diego, CA). Anti-rat CD45 antibody reacts with all molecular forms of CD45 (leukocyte common antigen) on all hematopoietic cells except erythrocytes. The respective isotype controls were mouse IgM and mouse BALB/c IgG. Sectioning and staining of tissues were completed at the Children's Research Institute Histology and Imaging Core Facility (Milwaukee, WI), an affiliate of the Medical College of Wisconsin. Testing and optimization of antibody stains were carried out on positive control tissue collected from rats that had been injected with LPS (2 mg/kg ip). Cells that stained positively for HIS48 or CD45 in separate slides were counted in 10 fields at ×400 magnification, and the locations were tallied under the categories of 1) within the lumen of a blood vessel, 2) adherent to or within a vessel wall, or 3) within the tissue extravascular spaces.
Detection of inducible heme oxygenase-1.
Inducible heme oxygenase-1 was measured in homogenized tissues by immunoblot. Lysates were prepared from tissue samples by homogenization in RIPA lysis buffer (Santa Cruz Biotechnology, Santa Cruz, CA) and centrifugation. Positive control tissue was harvested from animals injected with LPS (2 mg/kg ip), phorone (250 mg/kg), and buthionine (900 mg/kg ip) to cause sickness and systemic oxidative stress, while negative control tissue was harvested from animals not subjected to procedures except those for inducing deep anesthesia and analgesia prior to cardiac puncture. Protein contents of supernatants were quantified by Bio-Rad RC DC protein assay. Proteins were separated by SDS-PAGE on a 12% nitrocellulose membrane (Bio-Rad, Hercules, CA) with recombinant rat heme oxygenase-1 protein as a standard (liver, 30 ng/μl; lung, 6 ng/μl; and intestine, 0.5 ng/μl). Proper loading of lanes was evaluated visually by Ponceau S staining. The membrane was blocked with 5% nonfat milk and incubated at 4°C overnight with rabbit anti-rat heme oxygenase-1 (Hsp32) polyclonal antibody (Stressgen/Assay Design, Ann Arbor, MI) and then at room temperature with goat anti-rabbit IgG antibody (Stressgen/Assay Design). After standard wash steps, chemiluminescence was detected using ECL Western blot analysis reagents (Super Signal West Femto Maximum Sensitivity Substrate; Pierce, Rockford, IL), and intensity was measured on a Kodak Imaging Station (2000 MMT). Data are expressed as the relative intensity, which is the ratio of the mean intensity of the specimen to that of the recombinant rat heme oxygenase-1 protein per gel. For comparisons of experimental groups for lung heme oxygenase-1, baseline (n = 5) and colony control (n = 2) values were considered together, weighted with respect to number, to increase the degrees of freedom for descriptive statistics.
Plasma corticosterone.
Blood from cardiac puncture was transferred to sterile glass tubes containing K2 EDTA, rotated, and centrifuged at 4°C and 8 000 g for 10 min. Plasma was separated into aliquots, and these were frozen at −80°C for batch assay. Corticosterone was measured in duplicate by using a commercial RIA kit (Diagnostic Systems Laboratories, Webster, TX). The low and high detection limits were 20 and 2,000 ng/ml, and the intra- and interassay CVs were both <5%.
Data analysis.
Data were analyzed by means of conditional planned comparisons (29). The computations for a one-way ANOVA were first carried out to provide the mean square error and the sum of squares between groups to complete the comparison tests. Statistical significance for treatment effects determined by ANOVA was set at P < 0.05. The family-wise error for planned and post hoc comparisons was held constant at P < 0.016, based on three a priori comparisons (baseline vs. sleep deprivation at 5 days or 10 days, and sleep deprivation at 10 days vs. recovery) and 95% confidence. For cases in which there was not a significant difference between time points at 5 or 10 days for the same condition, these data were combined to compose a “sleep-deprived” treatment or a “yoked” treatment for comparison with baseline and recovery groups. Not all possible weighted comparisons were made or reported, but rather just those relating to specific questions. Nonstatistical significant differences are designated as NS. Data from six to nine animals usually compose each group at each time point for different variables, but occasionally data were obtained from only 5 animals, due to technical matters. Values are expressed as the means (SD), unless designated otherwise as SE.
RESULTS
MPO activity in liver, lung, heart, and intestinal tissues.
The amount of MPO activity in the lung showed strong effects of experimental treatment (F6,35 = 6.04, P < 0.0002). As can be seen in Fig. 1, MPO activity in the lungs of sleep-deprived rats during day 10 was nearly twofold that of baseline controls (P < 0.001) and more than 1.5-fold that of rats sleep deprived for 5 days (P < 0.01). Recovery sleep was associated with MPO activity in sleep-deprived rats that was substantially lower than that on day 10 (P < 0.016) and not significantly different from baseline. The amount of MPO activity in the liver of sleep-deprived rats had a similar profile. MPO activity in the liver was increased to 1.85-fold compared with baseline (P < 0.016) during day 10 and was not significantly different from baseline 48 h after sleep recovery was permitted. Upward trends in MPO activity in yoked rats during days 5 and 10 were not statistically significant. An opposite profile emerged for MPO in intestinal tissue. MPO activity was significantly higher during the recovery phase in sleep-deprived rats compared with baseline and with 5 and 10 days of sleep loss (recovery after sleep deprivation compared with baseline, P < 0.001; 5 days or 10 days, P < 0.01; Fig. 1); otherwise significant differences were not found. The average increase in MPO activity in the intestines of yoked rats during the recovery phase was not significant in post hoc analyses.
Fig. 1.
Myeloperoxidase (MPO) activity during sleep loss and sleep recovery. MPO activities in the lung (left), liver (middle), and intestine (right) from baseline controls (open bars) and from yoked (shaded bars) and sleep-deprived rats (solid bars) during 5 and 10 days of sleep deprivation and during 2 days of recovery sleep subsequent to 10 days of sleep deprivation are shown. Values are means (SE); n = 6 per group at each time point. P < .016 or better for the comparison with *baseline controls (Bsln), or †day 5 or ¶day 10 of the same group. P < .001 for the comparison with **baseline control or ‡day 5 of the same group.
Localization of phagocytes by immunohistochemistry in liver and lung tissue.
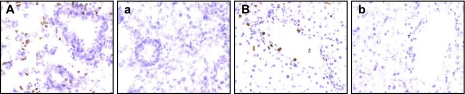
In the lung, counts of HIS48 positive labeling indicated that sleep deprivation of 10 days' duration resulted in a doubling of the number of granulocytes located in the whole tissue, compared with baseline controls. The populations of HIS48-positive cells by location are provided in Table 1. A representative section of lung stained with HIS48 antibody is shown in Fig. 2 along with HIS48 negative control staining. During 10 days of sleep deprivation, 97% of granulocytes were located in the extravascular tissues and the populations of marginated and luminal cells remained at low levels; i.e., <1% and <2%, respectively. As can be determined by the number of HIS48-positive cells counted (Table 1), there was good agreement between the populations of granulocytes identified by HIS48-positive counts and those determined by MPO activity. Differences in magnitude of the changes were likely due to sampling, since only thin tissue sections were used to localize labeled cells by microscopy compared with larger portions of the whole tissue for MPO measurement by biochemistry (43). HIS48-positive cell counts, expressed as a proportion of CD45-positive cell counts, remained stable or increased, indicating a chief contribution by granulocytes to leukocyte cell totals. In yoked rats, HIS48-positive cell numbers averaged between −9 to +22% of baseline and were localized to interstitial tissues 97 to 99% of the time. There was a nonsignificant trend in yoked rats for increased CD45-positive cells in the extravascular spaces at 10 days, which, proportionally, was due to HIS48-negative cells, hinting at recruitment of mononuclear cells.
Table 1.
Localization of granulocytes in the lung and the liver by immunohistochemistry
| Experimental Groups |
Lung |
Liver
|
||||||
|---|---|---|---|---|---|---|---|---|
| n | Interstitial % | Vascular %* | Proportion of CD45+ Cells | n | % Interstitial | % Vascular1 | Proportion of CD45+ Cells | |
| Baseline controls | 380±84 | 98±0.7 | 2±0.7 | 41±7 | 54±17 | 91±4 | 9±4 | 63±13 |
| Partial sleep deprivation | ||||||||
| 5 days | 372±44 | 97±0.8 | 3±0.8 | 45±6 | 70±10 | 95±2 | 5±2 | 44±8 |
| 10 days | 348±68 | 99±0.3 | 1±0.3 | 37±8 | 65±13 | 94±2 | 6±2 | 61±12 |
| Recovery for 48 h | 463±52 | 99±0.3 | 1±0.3 | 54±5 | 74±12 | 87±7 | 13±7 | 59±11 |
| Total sleep deprivation | ||||||||
| 5 days | 481±54 | 98±0.6 | 2±0.6 | 52±5 | 86±12 | 96±2 | 4±2 | 68±14 |
| 10 days | 762±250 | 97±0.8 | 3±0.8 | 54±16 | 97±14 | 94±1 | 6±1 | 81±16 |
| Recovery for 48 h | 588±62 | 99±0.4 | 1±0.4 | 64±11 | 102±21 | 93±3 | 7±3 | 74±9 |
Values are expressed as means ± SE; n = 6–9/group/time.
Cells located in vasculature include those in the lumen and in the blood vessel wall.
Fig. 2.
Representative sections of lung and liver tissue showing immunohistochemical staining of granulocytes and the effect of sleep deprivation Left: representative section of frozen lung stained with HIS48 antibody (A) illustrative of the distribution of granulocytes (stained brown) among bronchiole, alveoli, and vascular interstitium, compared with negative control staining (a). Right: representative section of liver stained with HIS48 antibody showing positive labeling in vasculature and diffusely throughout the extravascular space (B), compared with negative control staining (b). Magnification ×200.
In the liver, total HIS48-positive populations were 59 and 80% greater in sleep-deprived rats during days 5 and 10 compared with those of the baseline animals (Table 1). Most HIS48-positive cells, i.e., 96 and 94%, respectively, were located in the extravascular spaces, compared with 91% during baseline. HIS48-positive cells located on blood vessel walls, not including those in the lumen, remained low in number at <2% of the total population. Representative sections of liver tissue showing positive and negative HIS48 labeling are shown in Fig. 2. During the recovery period, HIS48-positive cells remained elevated 89% above baseline in totally sleep-deprived rats with 93% located in the extravascular tissue. The proportions of HIS48-positive cells relative to CD45-positive cells indicate that the increases in leukocytes were due to the increases in granulocytes. Counts of total HIS48-positive cells in the livers of yoked animals during days 5 and 10, and sleep recovery were 130, 120, and 137% of baseline values, respectively. Of the total counts in livers from yoked rats, 95, 94, and 87% of the total HIS48-positive populations were extravascular. The somewhat lowered percentage during the recovery phase appears to be due to one outlier with many HIS48-positive cells in the blood vessel lumen.
Marker of cellular stress in liver, lung, heart, and intestinal tissues.
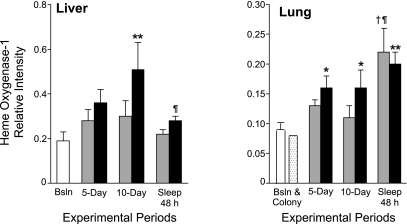
Heme oxygenase-1 in liver showed a strong effect of experimental treatment (F6,43 = P < 0.01), owing to greater induction in sleep-deprived rats than in baseline or yoked rats, shown in Fig. 3. The induction during day 10 was more than 2.5-fold the basal level in sleep-deprived rats (P < 0.001). During the 48-h period of recovery sleep, the value for sleep-deprived rats was significantly diminished from the 10-day time point to a level that was not different from baseline. Liver heme oxygenase-1 levels were not significantly different in yoked animals at any time point in post hoc analyses. Lung heme oxygenase-1 also showed a strong effect of experimental treatment (F7,40 = P < 0.016). The induction was highest during the recovery period in both yoked and totally sleep-deprived rats compared with baseline and colony control values (P < 0.001) (Fig. 3). For intestine, a treatment effect was not found and there was no clear trend and, therefore, these data are not shown.
Fig. 3.
Liver (left) and lung (right) heme oxygenase-1 content during sleep loss and sleep recovery. Data are expressed as the chemiluminescence intensity of the specimen relative to a standard. Data from baseline controls (open bar) included n = 8 for the liver and n = 5 for the lung, the latter of which was combined with data from colony controls (n = 2; stippled bar) to increase the degrees of freedom for statistical tests These data are shown relative to those from yoked (shaded bars) and sleep-deprived animals (solid bars) during 5 and 10 days of sleep deprivation and during 2 days of recovery sleep subsequent to 10 days of sleep deprivation. Values are expressed as means ± SE; n = 5 or 6 per yoked or sleep-deprived group at each time point. P < .016 for the comparison with *baseline+colony, or †day 5 or ¶day 10 of the same group. P < .001 for the comparison with **baseline or baseline+colony.
Corticosterone.
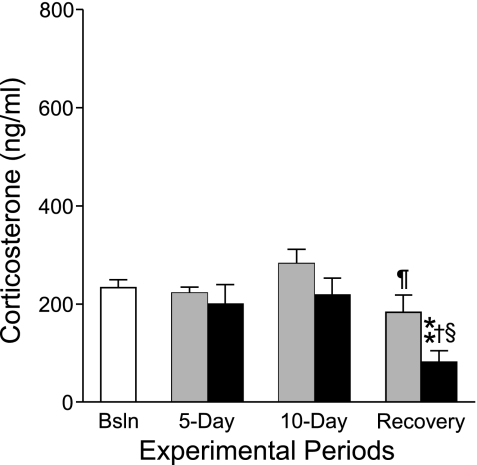
Circulating corticosterone was unchanged by sleep deprivation in totally sleep-deprived rats or by sleep restriction in yoked rats. In contrast, recovery sleep was marked by abnormally low corticosterone concentrations in totally sleep-deprived rats, below basal levels (Fig. 4).
Fig. 4.
Mean basal plasma corticosteroid concentrations during sleep deprivation and sleep recovery. Results from yoked (shaded bars) or sleep-deprived groups (solid bars) were not statistically different from baseline controls (n = 6, open bar) until the recovery period of 48 h of sleep ad libitum after 10 days of partial or total sleep deprivation. Values are expressed as means ± SE, n = 6 per group except sleep-deprived rats studied on day 10, for which there were 5 specimens. P < .016 for the comparison with †day 5 and ¶day 10 of the same group. P < .001 for the comparison with **baseline control and §day 10 of the same group.
DISCUSSION
Sleep deprivation resulted in near doubling of MPO activity in the liver and lung tissue of sleep-deprived rats. The magnitude of the change in MPO activity in the lung or liver was as great as that reported for burn injury (11, 27, 46), hepatic ischemia-reperfusion injury (36), and septic shock (40, 57), although much higher levels have been reported in other models and conditions, such as severe acute pancreatitis with infected necrosis (65). It should be mentioned that most 10-day sleep-deprived rats appear quite healthy looking with a robust appetite that belies evidence of internal abnormalities, such as neutrophil migration, that culminate in severe pathology (16). Localizaton of leukocytes by immunohistochemistry revealed migration into the extravascular spaces of the liver and the lung with a distribution that was diffuse. The majority of leukocytes in sleep-deprived rats were granulocytes, identified by HIS48 labeling, which indicated that the measurements of MPO activity captured the change in leukocyte population in extravascular tissue. This is consistent with previous outcomes from differential cell counts that characterized the increase in circulating white blood cells as granulocytic due mostly to neutrophils, rather than basophils or eosinophils (14). Migration of granulocytes and other leukocytes into extravascular spaces of organs is considered an inflammatory response, thereby attributing functional implications to the leukocytosis induced during sleep loss as distinct from physiological or corticosterone-induced neutrophilia (for a review, see Ref. 45).
Neutrophil migration into interstitial spaces of organs signifies important biochemical changes have taken place in sleep-deprived subjects. The basic steps in neutrophil migration have been well characterized (for reviews, see Refs. 58 and 59). When tissues are injured, mediators diffuse from the site of injury and activate the endothelium and/or tissue. Circulating phagocytes become activated, bind to endothelium, and pass out of the blood vessel by inserting their pseudopodia between the endothelial cells and dissolving the basement membrane. More chemical messengers change the neutrophil phenotype from one that circulates to one able to interact with proteins of the extracellular matrix. Neutrophils migrate further into the tissues based on the strength of the chemotactic gradient formed by alterations in the biochemistry of the tissues. In the present study, an indicator of this altered biochemistry was the up-regulation of heme oxygenase-1, which is widely distributed in systemic tissues, and it is induced in response to both heme and nonheme compounds. Previously, we found biochemical changes characterized by abnormally low liver glutathione and catalase activity, indicating uncompensated oxidative stress (19). The extent to which neutrophils are contributing to, or responding to, the cellular stress reflected by heme oxygenase-1 induction is presently under study. In one way, neutrophils contain abundant cytotoxic factors that can kill parenchymal cells through diffusion of reactive oxygen species and creation of intracellular oxidant stress (28). In another way, neutrophils may be attracted by oxidative stress, tissue injury, and cellular debris, which then modulate their activation (53). Neutophils have been shown, for example, to help facilitate resolution of inflammation by clearing cell debris if the macrophage system is insufficient (60).
Early during the course of sleep deprivation in rats, bacteria overgrow in the intestine and translocate to extraintestinal tissues, where live bacteria can be detected long before pathology culminates in bloodstream infection (15, 23). At first impression then, migration of neutrophils into tissues might be a logical expectation, given their well-known function of ingesting and killing bacterial pathogens as a first line of host defense. However, in the case of sleep-deprived rats, increases in circulating phagocytes, cytokines, chemokines, and immunoglobulins all have been demonstrated, and yet they are insufficient in controlling microbial invasion and eliminating foreign antigens (14). This indicates that abnormal microbial control is not the principal etiological basis for disease progression. In other experimental models and clinical conditions, bacterial overgrowth and translocation have been shown to result from tissue injury and immune suppression, after which there is poor microbial control (6, 12). Systemic inflammatory response syndrome in humans is believed to increase susceptibility to bacterial infections by means of production and release of anti-inflammatory factors that limit damage due to inflammation (4, 37). Furthermore, during trauma and tissue injury, neutrophils migrate to the intestine and break down microvascular endothelial barriers in the gut, as they are able to do in the lung, and thereby augment bacterial translocation (74). These considerations, along with the diffuse pattern of neutrophil infiltration and lack of necrotic foci, favor the view that the aggravating circumstance during sleep deprivation may be tissue injury to which decreased resistance to infectious disease is a consequence. The nature of tissue injury that may result from sleep loss has not yet been elucidated, but injury of cells is indicated by findings of increased plasma aminotransferases in both sleep-deprived humans (33) and rats (19).
Recovery sleep was marked by normalization of MPO activity in the liver and the lung, but increased MPO activity in the intestine in the absence of increased heme-oxygenase-1. We could not discern by our measurements whether the increased MPO activity reflected a change in neutrophil disposal (3, 67) or the possibility that reparative processes involve inflammation and neutrophil recruitment not reflected by increased heme oxygenase-1. For example, in experimental ischemia and reperfusion, reperfusion is vital, yet results in several-fold increases in neutrophil infiltration of the intestines and diminished glutathione and superoxide dismutase in association with additional tissue injury (26, 54). Heme oxygenase-1 was increased in the lung of both partially (yoked) and totally sleep-deprived rats during the recovery period. The same phenomenon previously was observed in measurements of liver glucose-6-phosphate dehydrogenase, 6-phosphogluconate dehydrogenase, and glutathione peroxidase that surged during sleep recovery (19). The increases suggest that either new oxidative burdens are generated during reparation or there is more protection from cell injury than was possible during sleep loss. Others have shown that heme oxygenase-1 provides protection against cellular stress and contributes to the resolution of inflammation (61, 69). The changes in neutrophil migration and markers of cellular stress during recovery sleep indicate ongoing reactions to prior sleep loss that point to properties of sleep recovery.
Corticosterone was decreased 50% below normal in totally sleep-deprived rats during the sleep recovery period. A similar reduction has been previously observed (18). The abnormally low level appears compensatory and points to increased negative feedback to the brain and pituitary, perhaps serving a permissive action for effector functions that tend to be reciprocally related to glucocorticoid action (49). Corticosterone otherwise remained unchanged by sleep loss, consistent with earlier findings in laboratory rats studied under this paradigm (17, 18, 21), and with a lack of a cortisol stress response in human sleep deprivation or extended sleep restriction studies (2, 9, 31, 41, 42, 51, 52, 55, 62, 64, 71, 72). Findings opposite to these, that is, decreased circulating leukocytes and marked increases in corticosterone, have been reported in laboratory rodents sleep deprived by the pedestal technique (73), also known as the platform or inverted flower-pot technique, which is considered nonspecific for sleep deprivation (48, 66). The present data indicate that overactivation of the hypothalamic-pituitary-adrenal axis is neither a necessary nor a sufficient condition by which immune-related alterations during sleep deprivation may be explained, which is in contrast to views expressed by others (56).
Perspectives and Significance
Neutrophil infiltration into liver and lung tissue of sleep-deprived rats is consistent with an inflammatory response and associated with induction of heme oxygenase-1, a marker of cellular stress. Inflammation is considered an etiological source factor for a number of disease states, and this may be one way in which sleep loss interacts with other causal factors to render an individual prone to disease. Recovery sleep was associated with increased lung heme oxygenase-1 induction and increased intestinal MPO, indicating ongoing processes of recuperation in response to prior sleep deprivation.
GRANTS
Research support was provided by the National Heart, Lung and Blood Institute through Grants 59271 and 080744, and the National Institute of Neurological Diseases and Stroke through Grant 38733. Additional support and facilities were provided by the Medical College of Wisconsin, Department of Neurology, and the Department of Veterans Affairs.
Acknowledgments
We express our appreciation to Rebecca Kaleta and Nichole Nellessen for technical assistance during the sleep loss experiments, and to Lynn Gruman at the Children's Research Institute for histology and immunohistochemistry services.
Portions of this work on myeloperoxidase and heme oxygenase-1 were presented respectively at the 19th and 20th annual meetings of the Associated Professional Sleep Societies.
Present address for C. A. Everson: Neurology Research 151, VA Medical Center, Milwaukee, WI 53295 (e-mail: ceverson@mcw.edu).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abraham E Neutrophils and acute lung injury. Crit Care Med 31: S195–S199, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Altemus M, Rao B, Dhabhar FS, Ding W, Granstein RD. Stress-induced changes in skin barrier function in healthy women. J Invest Dermatol 117: 309–317, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Arndt H, Kubes P, Grisham MB, Gonzalez E, Granger DN. Granulocyte turnover in the feline intestine. Inflammation 16: 549–559, 1992. [DOI] [PubMed] [Google Scholar]
- 4.Barie PS, Eachempati SR. Infectious complications following surgery and trauma. In: Infectious Diseases (2nd ed.), edited by Cohen J and Powderly WG. New York: Mosby, 2004, p. 916–917.
- 5.Benca RM, Quintas J. Sleep and host defenses: a review. Sleep 2: 1027–1037, 1997. [PubMed] [Google Scholar]
- 6.Berg RD Bacterial translocation from the gastrointestinal tract. Adv Exp Med Biol 473: 11–30, 1999. [DOI] [PubMed] [Google Scholar]
- 7.Bergmann BM, Kushida CA, Everson CA, Gilliland MA, Obermeyer W, Rechtschaffen A. Sleep deprivation in the rat. II. Methodology. Sleep 12: 5–12, 1989. [DOI] [PubMed] [Google Scholar]
- 8.Bergmann BM, Winter JB, Rosenberg RS, Rechtschaffen A. NREM sleep with low-voltage EEG in the rat. Sleep 10: 1–11, 1987. [DOI] [PubMed] [Google Scholar]
- 9.Born J, Lange T, Hansen K, Molle M, Fehm HL. Effects of sleep and circadian rhythm on human circulating immune cells. J Immunol 158: 4454–4464, 1997. [PubMed] [Google Scholar]
- 10.Bradley P, Priebat D, Christensen R, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol 78: 206–209, 1982. [DOI] [PubMed] [Google Scholar]
- 11.Chen LW, Wang JS, Chen HL, Chen JS, Hsu CM. Peroxynitrite is an important mediator in thermal injury-induced lung damage. Crit Care Med 31: 2170–2177, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Ding LA, Li JS. Gut in diseases: physiological elements and their clinical significance. World J Gastroenterol 9: 2385–2389, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dinges DF, Douglas SD, Zaugg L, Campbell DE, McMann JM, Whitehouse WG, Orne EC, Kapoor SC, Icaza E, Orne MT. Leukocytosis and natural killer cell function parallel neurobehavioral fatigue induced by 64 hours of sleep deprivation. J Clin Invest 93: 1930–1939, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Everson CA Clinical assessment of blood leukocytes, serum cytokines, and serum immunoglobulins as responses to sleep deprivation in laboratory rats. Am J Physiol Regul Integr Comp Physiol 289: R1054–R1063, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Everson CA Sustained sleep deprivation impairs host defense. Am J Physiol Regul Integr Comp Physiol 265: R1148–R1154, 1993. [DOI] [PubMed] [Google Scholar]
- 16.Everson CA, Bergmann BM, Rechtschaffen A. Sleep deprivation in the rat. III Total sleep deprivation. Sleep 12: 13–21, 1989. [DOI] [PubMed] [Google Scholar]
- 17.Everson CA, Crowley WR. Reductions in circulating anabolic hormones induced by sustained sleep deprivation in rats. Am J Physiol Endocrinol Metab 286: E1060–E1070, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Everson CA, Gilliland MA, Kushida CA, Pilcher JJ, Fang VS, Refetoff S, Bergmann BM, Rechtschaffen A. Sleep deprivation in the rat: IX. Recovery. Sleep 12: 60–67, 1989. [PubMed] [Google Scholar]
- 19.Everson CA, Laatsch CD, Hogg N. Antioxidant defense responses to sleep loss and sleep recovery. Am J Physiol Regul Integr Comp Physiol 288: R274–R383, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Everson CA, Nowak TS Jr. Hypothalamic thyrotropin-releasing hormone mRNA responses to hypothyroxinemia induced by sleep deprivation. Am J Physiol Endocrinol Metab 283: E85–E93, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Everson CA, Reed HL. Pituitary and peripheral thyroid hormone responses to thyrotropin-releasing hormone during sustained sleep deprivation in freely moving rats. Endocrinology 136: 1426–1434, 1995. [DOI] [PubMed] [Google Scholar]
- 22.Everson CA, Smith CB, Sokoloff L. Effects of prolonged sleep deprivation on local rates of cerebral energy metabolism in freely moving rats. J Neurosci 14: 6769–6778, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Everson CA, Toth LA. Systemic bacterial invasion induced by sleep deprivation. Am J Physiol Regul Integr Comp Physiol 278: R905–R916, 2000. [DOI] [PubMed] [Google Scholar]
- 24.Everson CA, Wehr TA. Nutritional and metabolic adaptations to prolonged sleep deprivation in the rat. Am J Physiol Regul Integr Comp Physiol 264: R376–R387, 1993. [DOI] [PubMed] [Google Scholar]
- 25.Graff G, Gamache D, Brady M, Spellman J, Yanni J. Improved myeloperoxidase assay for quantitation of neutrophil influx in a rat model of endotoxin-induced uveitis. J Pharmacol Toxicol Methods 39: 169–178, 1998. [DOI] [PubMed] [Google Scholar]
- 26.Grisham MB, Hernandez LA, Granger DN. Xanthine oxidase and neutrophil infiltration in intestinal ischemia. Am J Physiol Gastrointest Liver Physiol 251: G567–G574, 1986. [DOI] [PubMed] [Google Scholar]
- 27.Hansbrough J, Wikstrom T, Braide M, Tenenhaus M, Rennekampff O, Kiessig V, Zapata-Sirvent R, Bjursten L. Effects of E-selectin and P-selectin blockade on neutrophil sequestration in tissues and neutrophil oxidative burst in burned rats. Crit Care Med 24: 1366–1372, 1996. [DOI] [PubMed] [Google Scholar]
- 28.Hasegawa T, Malle E, Farhood A, Jaeschke H. Generation of hypochlorite-modified proteins by neutrophils during ischemia-reperfusion injury in rat liver: attenuation by ischemic preconditioning. Am J Physiol Gastrointest Liver Physiol 289: G760–G767, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Hays WL Statistics. Chicago: Holt, Rinehart and Winston, 1981.
- 30.Heiser P, Dickhaus B, Opper C, Hemmeter U, Remschmidt H, Wesemann W, Krieg JC, Schreiber W. Alterations of host defence system after sleep deprivation are followed by impaired mood and psychosocial functioning. World J Biol Psych 2: 89–94, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Heiser P, Dickhaus B, Schreiber W, Clement HW, Hasse C, Hennig J, Remschmidt H, Krieg JC, Wesemann W, Opper C. White blood cells and cortisol after sleep deprivation and recovery sleep in humans. Eur Arch Psychiatry Clin Neurosci 250: 16–23, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Hu J, Chen Z, Gorczynski CP, Gorczynski LY, Kai Y, Lee L, Manuel J, Gorczynski RM. Sleep-deprived mice show altered cytokine production manifest by perturbations in serum IL-1ra, TNFα, and IL-6 levels. Brain Behav Immun 17: 498–504, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Ilan Y, Martinowitz G, Abramsky O, Glazer G, Lavie P. Prolonged sleep-deprivation induced disturbed liver functions serum lipid levels, and hyperphosphatemia. Eur J Clin Invest 22: 740–743, 1992. [DOI] [PubMed] [Google Scholar]
- 34.Jaeschke H, Hasegawa T. Role of neutrophils in acute inflammatory liver injury. Liver International 26: 912–919, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Jain NC The neutrophils. In: Schalm's Veterinary Hematology (4th ed.), Philadelphia: Lea & Febiger, 1986, p. 676–730.
- 36.Karaman A, Fadillioglu E, Turkmen E, Tas E, Yilmaz Z. Protective effects of leflunomide against ischemia-reperfusion injury of the rat liver. Pediatr Surg Int 22: 428–434, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Keel M, Trentz O. Pathophysiology of polytrauma. Injury 36: 691–709, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Kerkhofs M, Boudjeltia KZ, Stenuit P, Brohee D, Cauchie P, Vanhaeverbeek M. Sleep restriction increases blood neutrophils, total cholesterol and low-density lipoprotein cholesterol in postmenopausal women: A preliminary study. Maturitas 56: 212–215, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Kleitman N Sleep and Wakefulness. Chicago: University of Chicago Press, 1963.
- 40.Koch A, Boehm O, Zacharowski PA, Loer SA, Weimann J, Rensing H, Foster SJ, Schmidt R, Berkels R, Reingruber S, Zacharowski K. Inducible nitric oxide synthase and heme oxygenase-1 in the lung during lipopolysaccharide tolerance and cross tolerance. Crit Care Med 35: 2775–2784, 2007. [DOI] [PubMed] [Google Scholar]
- 41.Kollar EJ, Pasnau RO, Rubin RT, Naitoh P, Slater GG, Kales A. Psychological, psychophysiological, and biochemical correlates of prolonged sleep deprivation. Am J Psychiatry 126: 488–497, 1969. [DOI] [PubMed] [Google Scholar]
- 42.Kollar EJ, Slater GR, Palmer JO, Doctor RF, Mandell AJ. Stress in subjects undergoing sleep deprivation. Psychosom Med 28: 101–113, 1966. [DOI] [PubMed] [Google Scholar]
- 43.Krawisz JE, Sharon P, Stenson WF. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology 87: 1344–1350, 1984. [PubMed] [Google Scholar]
- 44.Kuhn E, Brodan V, Brodanova M, Rysanek K. Metabolic reflection of sleep deprivation. Act Nerv Super (Praha) 11: 165–174, 1969. [PubMed] [Google Scholar]
- 45.Latimer KS, Prasse Leukocytes KW. In: Duncan & Prasse's Veterinary Laboratory Medicine: Clinical Pathology (4th ed.), edited by Latimer KS, Mahaffey EA, and Prasse KW. Ames, Iowa: Iowa State Press, 1994, p. 46–79.
- 46.Magnotti LJ, Xu DZ, Lu Q, Deitch EA. Gut-derived mesenteric lymph: a link between burn and lung injury. Arch Surg 134: 1333–1340; discussion 1340–1331, 1999. [PubMed]
- 47.Meier-Ewert HK, Ridker PM, Rifai N, Regan MM, Price NJ, Dinges DF, Mullington JM. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol 43: 678–683, 2004. [DOI] [PubMed] [Google Scholar]
- 48.Mendelson WB, Guthrie RD, Frederick G, Wyatt RJ. The flower pot technique of rapid eye movement (REM) sleep deprivation. Pharmacol Biochem Behav 2: 553–556, 1974. [DOI] [PubMed] [Google Scholar]
- 49.Miller WL, Tyrrell JB. The adrenal cortex. In: Endocrinology and Metabolism (3rd ed.), edited by Felig P, Baxter JD, and Frohman LA. New York: McGraw-Hill, 1995, p. 555–711.
- 50.Mistlberger RE, Belcourt J, Antle MC. Circadian clock resetting by sleep deprivation without exercise in Syrian hamsters: dark pulses revisited. J Biol Rhythms 17: 227–237, 2002. [DOI] [PubMed] [Google Scholar]
- 51.Moldofsky H, Lue FA, Davidson JR, Gorczynski R. Effects of sleep deprivation on human immune functions. FASEB J 3: 1972–1977, 1989. [DOI] [PubMed] [Google Scholar]
- 52.Naitoh P, Kelly TL, Englund C. Health effects of sleep deprivation. Occup Med 5: 209–237, 1990. [PubMed] [Google Scholar]
- 53.Nowak D, Piasecka G, Hrabec E. Chemotactic activity of histones for human polymorphonuclear leukocytes. Exp Pathol (Jena) 40: 111–116, 1990. [DOI] [PubMed] [Google Scholar]
- 54.Otamiri T Oxygen radicals, lipid peroxidation, and neutrophil infiltration after small-intestinal ischemia and reperfusion. Surgery 105: 593–597, 1989. [PubMed] [Google Scholar]
- 55.Ozturk L, Pelin Z, Karadeniz D, Kaynak H, Cakar L, Gozukirmizi E. Effects of 48 hours sleep deprivation on human immune profile. Sleep Res Online 2: 107–111, 1999. [PubMed] [Google Scholar]
- 56.Palma BD, Tiba PA, Machado RB, Tufik S, Suchecki D. Immune outcomes of sleep disorders: the hypothalamic-pituitary-adrenal axis as a modulatory factor. Revista Brasileira de Psiquiatria 29: S33–S38, 2007. [DOI] [PubMed] [Google Scholar]
- 57.Pedoto A, Nandi J, Yang ZJ, Wang J, Bosco G, Oler A, Hakim TS, Camporesi EM. Beneficial effect of hyperbaric oxygen pretreatment on lipopolysaccharide-induced shock in rats. Clin Exp Pharmacol Physiol 30: 482–488, 2003. [DOI] [PubMed] [Google Scholar]
- 58.Roitt I, Brostoff J, Male D. Immunology (6th ed.), New York: Mosby, 2001, p. 480.
- 59.Rosenberg HF, Galin JI. Inflammation. In: Fundamental Immunology (5th ed.), edited by Paul WE. Philadelphia: Lippincott Williams & Wilkins, 2003, p. 1152–1163.
- 60.Rydell-Törmänen K, Uller L, Erjefält JS. Neutrophil cannibalism—a back up when the macrophage clearance system is insufficient. Respir Res 7: 1–7, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ryter SW, Kim HP, Nakahira K, Zuckerbraun BS, Morse D, and Choi AM. Protective functions of heme oxygenase-1 and carbon monoxide in the respiratory system. Antioxidants Redox Signal 9: 2157–2173, 2007. [DOI] [PubMed] [Google Scholar]
- 62.Salin-Pascual RJ, Ortega-Soto H, Huerto-Delgadillo L, Camacho-Arroyo I, Roldan-Roldan G, and Tamarkin L. The effect of total sleep deprivation on plasma melatonin and cortisol in healthy human volunteers. Sleep 11: 362–369, 1988. [DOI] [PubMed] [Google Scholar]
- 63.Shearer WT, Reuben JM, Mullington JM, Price NJ, Lee BN, Smith EO, Szuba MP, Van Dongen HP, Dinges DF. Soluble TNF-α receptor 1 and IL-6 plasma levels in humans subjected to the sleep deprivation model of spaceflight. J Allergy Clin Immunol 107: 165–170, 2001. [DOI] [PubMed] [Google Scholar]
- 64.Spiegel K, Leproult R, L'Hermite-Baleriaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab 89: 5762–5771, 2004. [DOI] [PubMed] [Google Scholar]
- 65.Strobel O, Wachter D, Werner J, Uhl W, Muller CA, Khalik M, Geiss HK, Fiehn W, Buchler MW, Gutt CN. Effect of a pneumoperitoneum on systemic cytokine levels, bacterial translocation, and organ complications in a rat model of severe acute pancreatitis with infected necrosis. Surg Endoscopy 20: 1897–1903, 2006. [DOI] [PubMed] [Google Scholar]
- 66.Suchecki D, Tufik S. Social stability attenuates the stress in the modified multiple platform method for paradoxical sleep deprivation in the rat. Physiol Behav 68: 309–316, 2000. [DOI] [PubMed] [Google Scholar]
- 67.Suratt BT, Young SK, Lieber J, Nick JA, Henson PM, Worthen GS. Neutrophil maturation and activation determine anatomic site of clearance from circulation. Am J Physiol Lung Cell Mol Physiol 281: L913–L921, 2001. [DOI] [PubMed] [Google Scholar]
- 68.Szymusiak R, Satinoff E. Maximal REM sleep time defines a narrower thermoneutral zone than does minimal metabolic rate. Physiol Behav 26: 687–690, 1981. [DOI] [PubMed] [Google Scholar]
- 69.Takahashi T, Shimizu H, Morimatsu H, Inoue K, Akagi R, Morita K, Sassa S. Heme oxygenase-1: a fundamental guardian against oxidative tissue injuries in acute inflammation. Mini-Rev Med Chem 7: 745–753, 2007. [DOI] [PubMed] [Google Scholar]
- 70.Tsai LL, Bergmann BM, Rechtschaffen A. Sleep deprivation in the rat. XVI. Effects in a light-dark cycle. Sleep 15: 537–544, 1992. [DOI] [PubMed] [Google Scholar]
- 71.Tyler DB, Marx W, Goodman J. Effect of prolonged wakefulness on the urinary excretion of 17-ketosteroids. Proc Soc Exp Biol Med 62: 38–40, 1946. [DOI] [PubMed] [Google Scholar]
- 72.Vgontzas AN, Zoumakis E, Bixler EO, Lin HM, Follett H, Kales A, Chrousos GP. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab 89: 2119–2126, 2004. [DOI] [PubMed] [Google Scholar]
- 73.Zager A, Andersen ML, Ruiz FS, Antunes IB, Tufik S. Effects of acute and chronic sleep loss on immune modulation of rats. Am J Physiol Regul Integr Comp Physiol 293: R504–R509, 2007. [DOI] [PubMed] [Google Scholar]
- 74.Zimmerman BJ, Granger DN. Reperfusion-induced leukocyte infiltration: role of elastase. Am J Physiol Heart Circ Physiol 259: H390–H394, 1990. [DOI] [PubMed] [Google Scholar]