Abstract
The “developmental origins of health and disease” (DOHAD) hypothesis derives from clinical observations, indicating long-term health consequences for persons of low birth weight. There is growing evidence, primarily from animal studies, that supports the idea that processes put in motion during development that contribute to DOHAD do not necessarily reflect as significantly compromised growth and altered birth weight. Throughout the body of work investigating the DOHAD hypothesis, several themes have emerged; the importance of the placenta, the presence of critical periods of vulnerability, the involvement of the kidney in programmed hypertension, the presence of sex differences in the progression and development of adult diseases. Despite compelling findings in recent studies, much remains unclear regarding the impact of biological sex in the progression of human diseases, in general, and in the mechanisms underlying developmentally programmed responses, in particular. Although the contribution of biological sex to DOHAD is increasingly recognized, it also appears that it may exert distinctly different influences during fetal and adult life. The mechanisms by which biological sex contributes to these processes remains nebulous at present; nevertheless, several intriguing mechanistic candidates have been proposed ranging from differences in the amounts of sex hormones (e.g., estrogens, androgens) to recently described sexual dimorphism in the transcriptome of a variety of mammalian tissues. Recognizing the influences of biological sex or sex hormones on DOHAD uniquely situates research in this area to provide significant insights into the development and progression of many diseases, recent examples of which are the subject of this review.
Keywords: placenta, estrogen, maternal nutrient restriction, hypertension
gestation is a considerable physiological stress, and adaptation to this stress requires synchronized adjustments to the maternal physiological state. Not only are the proper regulation of energy, fluid, and electrolyte balance critical to the maintenance of maternal homeostasis during pregnancy; simultaneously, the needs of a rapidly growing conceptus must be met. When this maternal-fetal balance is not maintained, a suboptimal intrauterine environment is created and long-term consequences for health and well-being may result.
Developmental programming, defined as the response by the developing mammalian organism to a specific challenge during critical periods that alter the normal trajectory of development qualitatively and/or quantitatively, with resulting persistent effects on phenotype, is now recognized as an important determinant of adult health. Acceptance and understanding of this concept derive from human epidemiological studies suggesting that cardiovascular disease (6), chronic kidney disease (68), end stage renal disease (60), and low glomerular filtration rate (52) are associated with low birth weight. Epidemiological studies have examined several forms of maternal-fetal stressors during early development—uteroplacental insufficiency, hypertension, maternal nutrient deprivation, hyperemesis gravidarum, nutrient excess, and glucocorticoid excess chief among them—that provide convincing evidence in support of this belief (7, 29, 116). In addition, a wealth of carefully controlled animal investigations, primarily in rodents and sheep (summarized in Table 1), has provided further support and mechanistic insights (3, 25, 29, 30, 32, 33, 37, 38, 62, 63, 72, 73, 79, 81, 82, 91, 132–134). Because developing organisms pass more biological milestones before parturition than during any other time in their lives, it is not surprising that significant deviations in the timing or nature of these developmental steps have functional consequences in later life. It is becoming accepted that the development of each individual's specific phenotype, although based on a specific genome (of which chromosomal sex is a key factor), is influenced considerably to a varying extent by epigenetic and environmental factors. Hence, it is vitally important to understand early life gene-environment interactions that can increase predisposition to adult disease.
Table 1.
Long-term consequences of developmental programming
| Programming Insult | Adult Outcomes Reported in the Literature |
|---|---|
| Maternal overnutrition | Hypertension (females), reduced vascular compliance, Endothelial dysfunction, aortic hypoplasia, decreased renal Na+, K+ ATPase activity, decreased locomotor activity (female>male) |
| Maternal undernutrition | Hypertension (male>female), growth restriction, altered expression of renin-angiotensin system |
| Placental insufficiency | Hypertension (male>female), growth restriction |
| Maternal renal insufficiency | Hypertension (female>male) |
| ANG II receptor inhibition | Hypertension, decreased nephron number, glomerulosclerosis (male>female), interstitial fibrosis (male>female) |
| Glucocorticoid excess | Hypertension (sex and age dependent), glomerulosclerosis |
Numerous studies have documented sex differences in the incidence and severity of cardiovascular diseases such as coronary artery disease, heart failure, cardiac hypertrophy, and sudden cardiac death (29, 36, 90). These differences in the expression of cardiovascular disease may be related in part to intrinsic sex differences in myocardial function. Many recent studies have provided evidence that indicates a sex dichotomy also exists in the physiological responses to developmental challenges as they relate to the programming of subsequent cardiorenal function. These studies have largely been interpreted in one of two ways: 1) that male and female fetuses adapt differently to developmental stressors; or 2) that male and female sex steroids have a profound influence on the development and progression of developmentally programmed disease states. Moreover, since sex differences are apparent quite early in embryonic development and are independent of sex hormones, developing a third line of reasoning to suggest innate differences between the sexes play a role may yield particularly useful insights. Viewed in concert, several primary remaining questions emerge: Do innate sex differences with their roots in fetal life play a significant role in predisposition to adult diseases, in general, and developmentally programmed outcomes, in particular? Do postnatal sex differences interact with fetal adaptations to in utero stressors to generate differential outcomes? Or is it some combination of these scenarios? The goal of this review is to evaluate and place into perspective the current body of knowledge in the rapidly growing area of sex differences in developmental programming.
Innate Sex Differences
While the existence of sexually dimorphic phenotypes is rather obvious, the mechanisms that underlie this process have remained a matter of interest. Using a theoretical model to examine the evolutionary association between X-linkage and sexually dimorphic phenotypes, Rice concluded that “sex chromosomes facilitate the evolution of sexual dimorphism and that X-linked genes have a predominant role in coding for sexually dimorphic traits” (102). In the ensuing 25 years, support for this thesis has grown to include functional grouping of X chromosome gene content. Genes expressed in the brain (142), for example, are particularly abundant on the X chromosome. In contrast, and perhaps of importance to potential paternal contributions to the interactions between fetus and the maternal environment, placentally expressed genes are relatively rare on the X chromosome (57).
It has been recognized in humans that blood pressure is higher in men than in women (16) and that this difference originates during adolescence and persists into adulthood (141). Numerous studies have documented sex differences in the incidence, severity, and progression of cardiovascular and renal disease (47, 77, 113) and that men are often at higher risk for cardiovascular disease than premenopausal women of similar age (101, 129). The differences in the rates of cardiovascular disease may be related, in part, to intrinsic sex differences in cardiovascular and/or renal function (84, 112). There appears to be a sex dichotomy in cardiac morphology that may contribute to altered function (77) that is not the case for kidney structure (88), for example. In addition, sex-related differences in blood pressure regulation and progression of cardiorenal diseases could also be a consequence of differing endocrine milieu in men and women (23, 101). From a developmental orgins of health and disease (DOHAD) perspective, these factors likely interact to produce sex differences in developmentally programmed outcomes, as illustrated in Fig. 1.
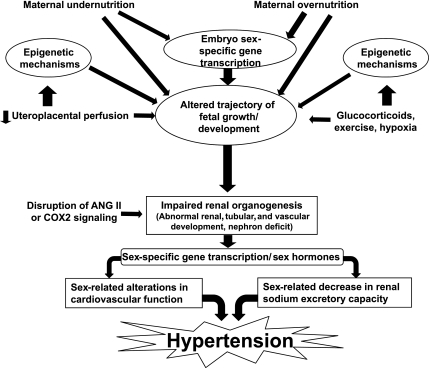
Fig. 1.
Overview of pathways by which various maternal and fetal stressors may interact with sex and sex hormones to result in adult hypertension. Environmental factors, such as undernutrition and overnutrition and impaired placental perfusion, may influence epigenetic regulatory mechanisms in addition to restricting fetal growth and development. Although alterations in the fetal ANG II signaling are common to most models of developmental programming, dysregulation of PG via cyclooxygenase-2 (COX2) has recently been reported. Biological sex of the fetus influences the response to these factors and may determine the extent of suppression or activation of the ANG II or PG pathways. In addition to disruption of renal nephro- and tubulo-genesis during fetal life, programmed alterations in sex hormones contribute to cardiorenal dysfunction and result in adult hypertension.
Differences observed during embryonic development.
Differences between the sexes appear both morphologically and in the transcriptome at a very early time in mammalian development. It has been known for some time that male and female preimplantation embryos differ in their mRNA expression patterns. Several genes located on the X chromosome are more expressed in bovine and human female vs. male embryos (39, 97, 121, 136), while autosomal genes expressed in trophoblast, such as those for interferon-τ (65), human choriogonadotropic hormone (41), and numerous other imprinted genes (24, 58, 95) are also not expressed or methylated the same across the sexes. In addition, morphological differences are frequently reported, such as the observation that early male and female embryos differ in their rates of development as early as the first few days postfertilization. Bovine (5, 139), murine (126), and ovine embryos (11) produced in vitro often fall into fast-cleaving and slow-cleaving groups that are predominantly male and female, respectively. Interestingly, Sood et al. (117) reported a sex dichotomy in the genes expressed in male and female placentas. Employing Significance Analysis of Microarrays to identify genes in villus samples that were expressed differently between the placentas of male and female fetuses, these authors demonstrate genes expressed at higher levels in female placentas, including those with roles in immune regulation like JAK1, IL2RB, Clusterin, LTBP, CXCL1, and IL1RL1. These authors also reported sex differences in placental gene expression that revealed interesting candidates for pathways involved in fetal development, physiology, and birth weight (see online supplemental material associated with Ref. 115).
Differences observed during fetal development.
As gestation progresses and the embryo becomes a fetus, the sex dimorphism observed in early development reappears around midgestation as male fetuses become larger than age-matched females (21, 44, 96). From clinical studies, we know that this size difference persists to term (44, 96). Interestingly, much less focus has been placed on this issue in animal studies, although we have observed differences between male and female ovine (30) and baboon fetuses (28) that are of similar magnitude to those reported in human studies (44, 96).
Sex differences at the molecular level also persist from embryonic into fetal life. Baserga et al. (8) have reported that gestation in the rat cyclooxygenase-2 (COX-2) levels were higher in the female than the male kidney at day of gestation (DG) 8, although not significantly increased at DG 21. In contrast, 11β-hydroxysteroid dehydrogenase 2 (11β-HSD2) levels were higher in the male control kidney at DG 21. Both of these gene products play important roles in renal function, and alterations in either could have developmental and/or functional effects in the kidney (8). We have recently shown that considerable sex differences are observed in the response to maternal nutrient restriction (MNR) between male and female baboon fetuses near term (19). That gene expression of key components of the renin-angiotensin system (RAS) are down-regulated in MNR males compared with females lends support to the idea that compromise of this crucial system plays a key role in renal and hypertensive diseases in the male in adult life. Of further interest is the observation that gene expression in pathways involved in energy regulation, such as insulin signaling, are up-regulated in MNR females and not in males (19). Response patterns such as these may explain why females are at lower risk. In contrast to the many studies that suggest males' susceptibility to cardiorenal disease due to the lack of a protective effect of estrogens present in adult females, these data support the view that male susceptibility to cardiorenal disease is innate rather than a consequence.
In the ovine fetus, we have previously shown that there is a sex difference in the ontogeny of gene expression in the RAS (30). ANG II type-1 receptor (AT1) protein was increased from 78 (mid-gestation) to 135 DG (late gestation; term at 148 DG) in male but not female fetuses. In contrast, ANG II type-2 receptor (AT2) protein decreased in the female but not male fetuses from middle to late gestation. Interestingly, no sex differences were apparent in angiotensin-converting enzyme (ACE) or renin protein expression at either middle or late gestation in the sheep. In contrast, we have found that ACE protein is increased in the female compared with male fetal kidney of the baboon at mid-gestation (DG 90) (28). Although the origin and the purpose of these sex differences in fetal protein expression remain unclear, it should be considered that these observations simply reflect different trajectories of fetal development between the sexes; that is, fetal development between the sexes may just be different at any given gestational age.
Differences observed following delivery.
The transcriptome continues to display sex differences in adulthood, such as in differences in expression of mRNA for osmoregulatory and drug-metabolizing proteins in the murine kidney (103), for example. Similarly, genes encoding drug and steroid metabolism are also reported to be differentially expressed between the sexes in the liver (103). It is, therefore, not unreasonable to hypothesize that sex differences exist within a molecular framework. There are also recognized sex differences in arterial pressure and the progression of renal disease, both of which are thought to involve actions of the RAS (110, 114). Moreover, recent clinical studies show that females are more responsive to the effects of ACE inhibition than men and that this occurs in an estrogen-independent manner (99). It seems plausible that there are many potential avenues, from embryonic life on into adulthood, through which sex differences may interact with developmental programming stimuli or programmed adaptations to result in sex-specific cardiorenal disease susceptibility.
Sex Differences in Developmental Programming
As introduced above, males, both human and animal, show an enhanced propensity to progress toward renal injury and decreased renal function than do females (88, 100, 110). Although the roots of this difference have been linked to the RAS (84), a role for an alteration in the ratio of sex steroids has also been proposed. Androgens have been linked with the progression of renal injury (100, 110), while estrogens have been proposed as being protective of renal function (107, 110). Moreover, it seems that sex may exert distinctly different influences during fetal and adult life. Whereas male fetuses may be more susceptible to in utero nutrient deprivation (30), female fetuses appear to have increased susceptibility to gestational overnutrition (55). The reasons for this are not clear; however, one clue may be held in the long-observed differences in growth rates exhibited by male and female fetuses in utero (96). Figure 1 provides an overview of the possible interactions between various maternal stressors and sex and how they may interact to produce a hypertensive phenotype. Despite contemporary findings that seem to clearly identify sex hormones as a likely culprit, recent efforts have raised many further questions and much remains unclear regarding the role of sex in programmed hypertension.
Human studies.
A small number of clinical studies have investigated sex differences in renal function as it relates to developmentally programmed hypertension. The larger body of work in this area has detailed differences in cardiovascular parameters and stress responses. Nevertheless, several interesting findings have been reported that confirm the idea that women are “reno-protected” during early adulthood. A recent report from the Nord Trøndelag Health Study (1995–1997) in Norway found that intrauterine growth restriction (IUGR), high blood pressure, and low normal renal function were associated in 20- to 30-yr olds (40). Although the degree of impaired renal function was small in these young adults, it was significant and more consistent in men than women (40). Similarly, Kistner et al. (56) reported that women born preterm had increased blood pressure but no signs of adverse renal function as young adults.
Other studies have evaluated cardiovascular responses between male and female subjects that were growth restricted in utero. In one such study, Ward and colleagues (127) reported women that were born small were far more susceptible to stress-induced increases in systolic blood pressure (127). A recent study by Jones et al. (49) has shown that there are marked sex differences in the way that size at birth is associated with alterations in cardiovascular physiology established in childhood. Specifically, they reported that smaller size at birth is associated with higher arterial pressure systemic vascular resistance following stress in boys. In girls, they reported evidence of increased cardiac sympathetic activation at rest and during stress (49).
Further evidence that markers of impaired fetal growth are related to autonomic cardiovascular control involving modulation of both sympathetic and parasympathetic function but in a sex-specific manner has also been provided in an adult Australian cohort by the same group (50). The authors reported that women but not men who were small at birth demonstrated increased low-frequency blood pressure variability at rest and during stress, reduced levels of high-frequency heart period variability, and a reduction in baroreflex sensitivity. These observations suggest that, similar to the findings from animal studies, intrauterine influences in humans can have lasting although different effects on cardiovascular function in males and females and that these effects are evident before the endocrinologic events associated with puberty.
Animal studies.
Studies utilizing animal models have employed a range of stressors in a variety of species to induce fetal growth restriction and test hypotheses regarding the developmental origins of disease. Perhaps the most common model to date has focused on MNR, either as a decrease in total caloric intake or an isocaloric decrease in protein content; studies to understand the consequences of maternal obesity from the DOHAD perspective are gaining (27, 36, 54, 55, 79, 122).
Small animal models of dietary nutrient restriction.
Evidence from MNR studies suggest that female progeny are less affected than their male siblings (81, 82, 94, 135), although these observations may depend on the extent of the nutrient restriction (46). These studies generally show decreased nephron endowment and altered expression of components of the intrarenal RAS (46, 81, 82, 94, 135). Hemmings et al. (43) have reported impairment of the myogenic response in the mesenteric vascular bed of pregnant adult females that had been exposed to MNR during their own development. MNR during the preimplantation period in the rat resulted in elevated BP in male offspring only (59). It should be noted, however, that the blood pressures of the control male rats in the latter experiments were lower than those of the control females, and it is the reversal of the dichotomy that is associated with the observation of relative hypertension in male offspring. Restriction of specific nutrients other than protein has also been evaluated. A maternal low-sodium diet in rats has recently been associated with increased maternal plasma renin activity and correlated with IUGR, increased blood pressure, and reduced creatinine clearance in female offspring but not in males (9). Although it currently appears that MNR is associated with increased risk to the male compared with the female offspring, the mechanisms underlying this observation remain unclear. Moreover, it remains unclear whether the increased risk to the males is a result of gene × environment interactions originating during or after gestation. Further studies are needed to thoroughly investigate these possibilities.
Large animal models of dietary nutrient restriction.
Although not all large animal models show clear effects of MNR on the offspring, several large animal models have been evaluated for sex differences; we have shown that similar to the rodent models, the male ovine and baboon fetuses appear to be more susceptible to the effects of poor maternal nutrition (28, 30, 32). Our work in sheep has shown that maternal global caloric restriction impairs nephrogenesis and alters intrarenal immunoreactive AT1, AT2, and renin expression in gestational age and gender-specific ways (30). Further, we have found that only male offspring of these MNR ewes are hypertensive (32). While the mechanisms by which MNR alters gene expression remains unclear in our model, data from Lillycrop et al. (69–71) and Burdge et al. (15), both employing protein restriction in the rat suggest that deficiency of methyl donors may alter gene methylation patterns and, in turn, affect expression.
Although we have not found a decrease in fetal growth after 30% MNR in the baboon, we have previously reported sex-specific alterations in renal gene expression (28). AT1 expression is increased in the MNR fetal male baboon kidney at mid-gestation compared with the control male at both the mRNA and protein level. Further, when AT1 and AT2 are expressed as an AT1:AT2 ratio, we find that this ratio is relatively constant across all groups except in the nutrient-restricted male, in which it is greatly increased. We have also reported that immunoreactive ACE is increased by MNR in the male but decreased in the female (28). This is another truly unique observation that may reflect disparities between MNR models in primate and nonprimate species.
Despite the considerable alterations observed at the molecular level in the fetal kidney (19, 20, 28, 30, 32, 37, 80–82), the observation that moderate MNR during the first half of gestation has no effect on fetal size is not unreasonable because of the normally constrained growth potential of the fetus during early gestation. Early to mid-gestation is regarded more as the period of placental proliferation rather than a period of accelerated fetal growth, such as that seen during the latter half of gestation. Interestingly, we have noted that pregnant baboons carrying male fetuses in the control group had a greater average consumption of kilocalories during the first 13 wk of gestation than the pregnant baboons carrying female fetuses (unpublished observations), and similar reports have been made in the clinical literature (120). These observations highlight a primary strength of large animal models, the ability to more closely approximate the gestational environment that is realized by pregnant women. Nevertheless, further studies that are designed to evaluate sex differences that may originate in utero will have to be performed in both large and small animal model systems.
Models of utero-placental insufficiency.
Models of utero-placental insufficiency are quite intriguing, as they are relevant to multiple maternal health issues, as well as to the developmental programming of hypertension. Alexander and colleagues (3, 37) have shown that reduced uterine perfusion pressure during the last trimester of pregnancy in the rat programs hypertension in the offspring and in a sex-specific manner (3, 37). Further, in this model both the RAS and sex steroids have been implicated in the observed sex differences in hypertension (37, 91, 92). In contrast, the two kidney-one wrapped kidney (2K, 1W) model of hypertension resulted in hypertension in 30-wk-old female offspring only (22). Interestingly, plasma renin activity was significantly lower in the female offspring of hypertensive mothers at 10 wk of age (P < 0.05), suggesting that development of the RAS was altered. The differences in the factors elaborated by the ischemic placenta and poorly perfused kidney illustrate the complexity of the interactions between the maternal endocrine milieu and fetal development. Whereas reduced renal perfusion primarily activates the RAS, the ischemic placenta produces a variety of humoral and locally acting factors such as sFlt-1 (soluble fms-like tyrosine kinase-1) and tumor necrosis factor (TNF)-α that have far-reaching effects.
Recent studies in the rat and baboon have shown that chronic reductions of utero-placental blood flow results in increased levels of sFlt-1 in the placenta, amniotic fluid, and maternal plasma (34, 76). In the rat, this has been associated with decreased fetal growth and subsequent hypertension that is sex dependent (3, 91). Recent studies in rodents have shown that elevated sFlt-1 levels alone results in fetal growth restriction (14, 75). Furthermore, Lu et al. (74) have followed the mouse offspring of these pregnancies and reported sex-specific effects regarding the development of hypertension as only male mice have higher blood pressure in this model (74). Viewed together, these studies strongly suggest that in addition to the immediate well-being of the mother, a long-term outlook with regard to the well-being of the fetus must also be considered during complicated and/or high-risk pregnancies.
Maternal obesity.
Maternal obesity is associated with a plethora of conditions, including maternal hypertension, hypertriglyceridemia, hyperglycemia, and insulin resistance (130), that have each been independently correlated with a suboptimal in utero environment and consequently linked to DOHAD. Several human studies have described a positive correlation between maternal weight and/or adiposity and blood pressure of teenage children (17, 64, 66), leading Boney et al. (13) to conclude from their examination of large-for-gestational-age babies and the incidence of childhood metabolic syndrome, that “given the increased obesity prevalence in children exposed to either maternal diabetes or maternal obesity, there are implications for perpetuating the cycle of obesity, insulin resistance, and their consequences in subsequent generations.” Few, if any, of the studies in humans include offspring sex as a covariable.
Important information with regard to maternal nutrient excess and sex-associated difference comes largely from animal models. Langley-Evans (61) described hypertension in male offspring after exposure to a maternal diet high in saturated fat (or low in linoleic acid) in rats that was not true of female offspring. In contrast, Elahi et al. (25) have shown that mice fed high-fat diets long before the onset of gestation are hypercholesterolemic and hypertensive and produce female offspring that are hypertensive and hypercholesterolemic and have reduced locomotor activity. Moreover, treatment of the dams with pravastatin lowered blood pressure and cholesterol levels and increased activity in the female offspring (25). The numerous pleiotropic effects of statins and the mechanisms for these effects remain unclear; nevertheless, these observations provide insights that may lead to further studies.
In a model more resembling high-fat food consumption in humans, Armitage et al. (4) demonstrated that a diet rich in fat fed to pregnant rats results in male offspring gaining more body weight and presenting with decreased renal renin activity compared with females (4). Offspring from this model of maternal high-fat diet have been shown to be hypertensive and to exhibit increased aortic stiffness, decreased aortic smooth muscle cell number, endothelial dysfunction, and decrease renal Na+-K+-ATPase activity. The bulk of these changes were independent of sex, except for increased blood pressure in which female offspring were hypertensive, while the males were not (55, 109). Further, Khan et al. (55) reported that female offspring have reduced locomotor activity at 180 days of age compared with male offspring of pregnant rats fed a high-fat diet during pregnancy. In addition, this research group used cross-fostering techniques after birth to show that the hypertension in females is attained whether exposure to maternal high-fat diet occurs before and during pregnancy or during the suckling period (54). Innate sex differences, such as lower plasma aldosterone concentration, greater renal weight, increased glomerular number, and volume in males were also noted, independent of maternal diet. While the mechanisms responsible for programming due to high-fat diets remain unclear, the report that statin treatment has beneficial effects on the offspring highlights at least one potential mechanism, alterations in lipid metabolism (25). In addition, it has been suggested that high levels of butyric acid that may result from a high-fat diet could lead to changes in chromatin structure and result in epigenetic alterations (51). Taken together, these observations indicate that a maternal high-fat diet alters mesenteric artery, conduit artery, and renal function in offspring, engendering hypertension in which the RAS is implicated.
Models of maternal renal compromise.
Another intriguing area of investigation that is garnering recent attention involves the role of the maternal RAS during pregnancy and/or lactation in pregnancy outcome and offspring health. These approaches may be in the form of administration of RAS inhibitors (108), altered sodium diet, as described above (9), or the previously discussed 2K, 1W Page hypertension (22). RAS inhibition at the level of the AT1 receptor is reported to have several sex-specific effects that manifest postpartum (73, 106, 108). Saez et al. (106) found that AT1 inhibition reduces nephron number similarly in male and female rats, but the subsequent glomerulosclerosis and interstitial fibrosis are greater in males than in females. Further, the male rats are also reported to have a significant papillary atrophy. Functional differences include impaired urinary-concentrating ability during a prolonged dehydration in the male offspring (73) and impaired excretory capacity following acute volume expansion (72). In another study from the same group, the authors reported that during exposure to a high-salt diet at 11–12 mo of age, the putative renoprotective effect of female sex hormones observed earlier in the development of their model, and which was proposed to prevent proteinuria despite elevated systolic blood pressure (106), seemed to be exceeded (108). A high-salt diet during pregnancy in rats has also been evaluated and reported to alter the response to restraint stress in female offspring. Further, female high-salt diet offspring had increased corticotrophin-releasing hormone mRNA levels in the PVN than did normal-salt diet females (98).
Another intriguing area of investigation involves COX-2 inhibition during renal development. It has long been observed that interplay exists between the RAS and prostaglandins, although it has only been recently that clear links have been made to its developmental importance (42, 83). Recent studies indicate that deletion of the COX-2 gene in mice has profound effects on blood pressure, primarily in male mice (140). In addition, the authors reported that genetic background also played an important role as COX-2−/− mice of different backgrounds did not respond the same way to the deletion (140). Although the present data are clear that pharmacological inhibition of the RAS during pregnancy has well-defined and deleterious effects on renal development and function in the offspring, current studies are less clear on the effects of more subtle perturbations of the RAS (e.g., via dietary alterations, etc.) and other pathways, such as prostaglandins (via COX-2) on the long-term health of the offspring. Further work in these areas will help define the importance of these pathways in the developmental programming of health and disease.
Models of maternal stress and glucocorticoid excess.
Other stressors such as corticosteroid administration and exercise during pregnancy have also received attention. With respect to the latter, Gilbert et al. (31) have investigated the effects of exercise during pregnancy in spontaneously hypertensive rats (31). The authors reported that a moderate volume of exercise lowered blood pressure in female offspring and increased body density in both male and female progeny. In contrast, a high volume of exercise resulted in postnatal growth failure followed by catch-up growth in male and female offspring, but only females suffered exacerbated hypertension (31). Using a dexamethazone model, O'Regan et al. (89) showed similar effects on BP in males and females, but the magnitude of hypertension and a greater stress-induced hypertension was observed in males. Interestingly, the response to catecholamine release was similar in both sexes (89). In another study, prenatal dexamethasone (DEX) treatment significantly enhanced the arterial pressure response to acute stress only in female Wistar-Kyoto rats, while DEX augmented the elevation in heart rate during stress only in male rats (10).
Ortiz et al. (93) have shown that antenatal DEX elevates blood pressure in female offspring at 3 wk of age, while only male offspring had increased blood pressure at 6 mo of age. Interestingly, despite the observation that only male DEX-treated rats were hypertensive at 6 mo of age, both male and female offspring showed signs of glomerulosclerosis compared with control rats (93). Similar work has shown that a postnatal diet rich in ω-3 (n-3) fatty acids attenuates the effects of DEX on blood pressure in the offspring (138). Moreover, these authors also reported the same lowered blood pressure in female control animals vs. those fed a control diet (138). With the wide-ranging effects reported in the glucocorticoid models, it is clear that continued studies are required to tease out the mechanisms underpinning the sex-specific responsivity in this programming model.
In summary, the literature on the subject of sex differences in the developmental of hypertension and cardiovascular disease is divided. On the one hand, differences between the sexes have been argued to have their roots in the hormonal milieu (primarily androgen/estrogen). On the other, more fundamental differences at the level of gene expression have been argued to be of primary importance to these observations. Adding to the complexity of the debate is the fact that differences in response due to sex may differ between species. In nonhuman primates, sheep and rats decreased fetal nutrient availability increases expression of the renal AT1 receptor in the kidneys of males. However, in mice, it is the norm for females to exhibit increased AT1A expression and for protein restriction to normalize the differences in expression between the sexes (46). The investigation of developmental programming is uniquely positioned to address some of this ongoing debate since differences between developmental responses to nutrient deprivation or glucocorticoid excess are less likely to be under the influence of reproductive endocrinology than are the changes occurring immediately before and after puberty.
Potential Mechanisms Underlying Sex Differences in Developmental Programming
A variety of mechanisms have been postulated with regard to DOHAD (summarized in Fig. 2). While the contribution of sex to the developmental origins of disease is widely recognized, it seems sex may exert distinctly different influences during fetal and adult life. For example, while male fetuses may be more susceptible to in utero nutrient deprivation (30), female fetuses may have increased susceptibility to gestational overnutrition (55). The reasons for this remain nebulous; however, one clue may be held in the long-observed differences in growth rates exhibited by male and female fetuses in utero (96). Hence, a faster growing male fetus may experience greater or lesser degrees of these nutritional insults compared with a female counterpart. Differences in the rate at which the male develops compared with the female likely contribute to gender differences in the response to MNR reported in the literature (94). One unresolved question is whether male fetuses have increased metabolism compared to female fetuses. Hence, the chromosomal complement of the fetus may affect maternal metabolism, and as the mother carrying a male fetus endures MNR, the male fetus will face greater hardship than a female fetus in an equivalent pregnancy. In contrast, the female fetus in a pregnancy with an overnourished mother could face similar hardship, albeit via different pathways.
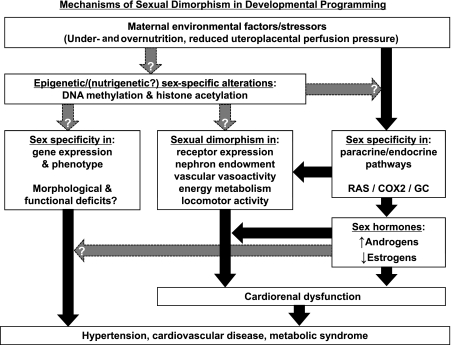
Fig. 2.
Mechanisms underlying sexual dimorphism in developmental programming of hypertension. The mechanisms underlying the processes of developmental programming can be broadly grouped into epigenetic (and possibly nutrigenetic) and endocrine/nervous system categories. Several pathways, including the RAS, prostaglandins via cyclooxygenase-2 (COX2), glucocorticoids (GC), and sex steroids (androgens and estrogens) have been identified as playing a mechanistic role in the developmentally programmed hypertension. Although a large body of work clearly supports the importance of endocrine pathways in developmental programming of cardiorenal disease, the epigenetic mechanisms are poorly defined. A working hypothesis is that epigenetic alterations directly induce changes in gene expression and ultimately phenotype; whereas endocrine/nervous system changes directly influence gene expression and/or physiological function. Alterations in gene expression and organogenesis contribute to functional deficits and ultimately disease states such as hypertension, cardiorenal disease and the metabolic syndrome. Although the manner in which epigenetic phenomena contribute to the programming of cardiorenal dysfunction remains unclear, the role of sex steroids has been studied extensively. While sex steroids appear to interact with the programmed phenotype of the offspring and contribute to cardiorenal dysfunction and hypertension, the altered levels of those hormones may be a result of epigenetic alterations. Black arrows indicate relationships supported by experimental evidence; gray arrows indicate relationships that are currently in question or are suggested but as yet unproven.
Epigenetic mechanisms.
Epigenetic phenomena appear to be central to the induction of persistent and heritable changes in gene expression that occur without alteration of DNA sequence (2, 12, 45, 137). While most cells in an organism contain the same DNA, gene expression varies widely across various tissues. Epigenetic mechanisms underlie this tissue- and cell-type-specific gene expression (128) and include CpG methylation, histone modification (acetylation), and the activity of autoregulatory DNA-binding proteins (53). Moreover, since DNA methylation and histone acetylation are implicated in the silencing of gene expression, X-inactivation and X-linked dosage differences (18), one might argue that sex bias in differential gene expression linked to DOHAD also has its roots in methylation. Indeed, these processes appear to have many sex-specific features (for a thorough review, see Ref. 52).
Because moderate folate depletion can induce genome-wide DNA methylation (48), genomic methylation may be useful as an integrative biomarker of methyl donor nutritional status (78). While considerable work has been initiated in this area with regard to developmental programming, little work has focused specifically on sex differences. Interestingly, sheep exposed to a methyl-deficient diet during pregnancy produce hypertensive male offspring compared with females of similar rearing, as well as to male and female controls (115). The authors then evaluated 1,400 CpG sites (primarily gene promoter associated) in fetal liver at 90 days of gestation (term=150) and reported that more than half of the affected loci were specific to males. These observations suggest male-specific demethylation that could provide a mechanistic basis for the phenotypic sex differences observed in that study (115). In addition, the emerging fields of nutrigenetics and metabolomics (35, 87) seem poised to shed further light on the operational characteristics of these mechanisms.
Alternatively, it has also been hypothesized that when genes are expressed in multiple tissues or serve several functions, they should show less sex bias than genes that are more specialized (26). The genes, such as those involved in the RAS, are certainly expressed in multiple tissues, yet these genes are also closely associated with sex differences in the developmental origins of cardiorenal diseases. Clearly, there is a tremendous gap in our understanding of these complex topics, and further studies are needed to clarify these matters, particularly, in the light of the differences reported regarding fetal gender and the developmental response to maternal overnutrition and undernutrition.
Sex steroids.
In contrast to the sex-related dichotomy observed in response to nutritional stressors, when faced with a robust stressor such as AT1 antagonism (72, 73, 106, 108), severe protein restriction (133), or chronic reductions in uterine perfusion pressure (3, 91), both male and female fetuses are affected similarly in utero. Nonetheless, a dichotomy emerges later in life with females being less impacted by their suboptimal in utero experience (72, 73, 106, 108). The apparent benefit of being female in scenarios such as these is supported by recent work that suggested estrogens confer a protective effect on intrauterine growth-restricted females that prevents the development of programmed hypertension (91). Moreover, the observation that ovariectomy leads to a significant increase in blood pressure in growth-restricted females with no significant effect in controls makes a strong case for the postdevelopmental involvement of estrogens. Estrogen replacement reversed the effect of ovariectomy on blood pressure in growth-restricted offspring as did RAS blockade (91).
Another possible mechanism may be through interactions between the RAS and female sex steroids (119). Rogers et al. (105), studying the role of sex hormones in expression of components of renal renin angiotensin in healthy Sprague-Dawley rats, have suggested that an estrogen-mediated attenuation of renal AT1 binding is a potential mechanism by which estrogen exerts protection from vascular and renal disease in females (105). When this inhibition is lifted following ovariectomy in their model, or in diabetes or menopause, the resulting increased ANG II signaling increases both the degree of susceptibility to vascular and renal disease and the rate of existing disease progression (105).
Testosterone has also been implicated in the progression of hypertension in male growth-restricted offspring (92). The potential underlying mechanisms have been studied by Sullivan et al. (118) who described a relationship between androgens and the development of albuminuria, and the renal protection afforded by estrogen, in spontaneously hypertensive rats. There is some evidence to suggest that both overactivity of the RAS and oxidative stress likely contributing to sex differences in the progression to renal injury. Treatment with either an AT1 blocker and/or an ACE inhibitor blunts the occurrence of renal injury in males (67). Antioxidant treatment improves renal function and decreases markers of renal injury in males supporting the contribution of oxidative stress to renal disease (111). Male spontaenously hypertensive rats, exhibit androgen-dependent increases in blood pressure and albuminuria that are independent of renal cortical ANG II levels and oxidative stress (118).
Interestingly, the cardiorenal protective effects of estrogens has not been a universal finding (108). Considering the differences between the models employed by different laboratories, one possibility could be the magnitude of the insult to the kidney during development has an influence on the extent of protection that may be afforded by female sex hormones in later life. It is widely recognized that differences in sex hormones contribute to considerable sexual dimorphism in the transcriptome of a variety of mammalian tissues and organs (104); however, it has only recently been recognized that androgen/estrogen-independent mechanisms may operate at the transcriptional level to regulate sex differences (124). This possibility represents an alternate pathway that may be at work contributing to the observations that the relationship between sex hormones and blood pressure is far more complex than simply the balance of estrogen vs. testosterone (91). Taken together, it appears that the influence of sex on the developmental origins of disease may reach far beyond the widely recognized role of sex hormones.
Alternatively, recent work implicates growth hormone (GH) in sex-dependent differences in renal expression of glomerular AT1 during hypertrophy following uninephrectomy; male rat kidneys show increased glomerular AT1 expression, whereas females do not (86). Because there is sexual dimorphism in GH release these observations may hold implications for both normal and pathological growth and development of the kidney. Further evidence comes from the regulation of hepatic genes and has revealed the existence of numerous examples of gender-dependent transcriptional regulation by growth hormone (1). Moreover, a host of transcription factors have been identified as possible contributors to these regulatory mechanisms (123, 125, 131).
Concluding Remarks
From the clinical perspective, it is hoped that a better understanding of developmental programming will lead to better diagnostic, preventive, and therapeutic measures. The persistence of programmed effects is likely due to covalent modifications of the genome, resulting from changes in promoter methylation and histone acetylation. The emerging fields of metabolomics and nutrigenetics suggest many of these alterations are likely a result of changes in the metabolic flux within an organism. While epigenetic phenomena are central to the induction of persistent and heritable changes in gene expression that occur without alteration of DNA sequence, their contribution to the intensively studied sex differences in developmental programming remains uncertain. Reversal of these molecular changes may be possible and may improve loss of function in existing structures, but if developmental plasticity is no longer present, it is very unlikely that the structural changes can be reversed. For example, it is difficult to see how any deficit in nephron endowment can be made good. Nevertheless, continued investigation using hypothesis-driven mechanistic studies that incorporate sexual dimorphism into the models rather than attempt to control for sex differences are needed to identify target pathways for possible intervention.
Acknowledgments
M. J. Nijland is supported by National Institutes of Health Grant HD 21350.
REFERENCES
- 1.Ahluwalia A, Clodfelter KH, Waxman DJ. Sexual dimorphism of rat liver gene expression: regulatory role of growth hormone revealed by deoxyribonucleic acid microarray analysis. Mol Endocrinol 18: 747–760, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Akintola AD, Crislip ZL, Catania JM, Chen G, Zimmer WE, Burghardt RC, Parrish AR. Promoter methylation is associated with the age-dependent loss of N-cadherin in the rat kidney. Am J Physiol Renal Physiol 294: F170–F176, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Alexander BT Placental insufficiency leads to development of hypertension in growth-restricted offspring. Hypertension 41: 457–462, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Armitage JA, Lakasing L, Taylor PD, Balachandran AA, Jensen RI, Dekou V, Ashton N, Nyengaard JR, Poston L. Developmental programming of aortic and renal structure in offspring of rats fed fat-rich diets in pregnancy. J Physiol 565: 171–184, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avery B, Jorgensen CB, Madison V, Greve T. Morphological development and sex of bovine in vitro-fertilized embryos. Mol Reprod Dev 32: 265–270, 1992. [DOI] [PubMed] [Google Scholar]
- 6.Barker DJ Fetal origins of coronary heart disease. Br Heart J 69: 195–196, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barker DJ, Clark PM. Fetal undernutrition and disease in later life. Rev Reprod 2: 105–112, 1997. [DOI] [PubMed] [Google Scholar]
- 8.Baserga M, Hale MA, Wang ZM, Yu X, Callaway CW, McKnight RA, Lane RH. Uteroplacental insufficiency alters nephrogenesis and downregulates cyclooxygenase-2 expression in a model of IUGR with adult-onset hypertension. Am J Physiol Regul Integr Comp Physiol 292: R1943–R1955, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Battista MC, Oligny LL, St Louis J, Brochu M. Intrauterine growth restriction in rats is associated with hypertension and renal dysfunction in adulthood. Am J Physiol Endocrinol Metab 283: E124–E131, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Bechtold AG, Vernon K, Hines T, Scheuer DA. Genetic predisposition to hypertension sensitizes borderline hypertensive rats to the hypertensive effects of prenatal glucocorticoid exposure. J Physiol 586: 673–684, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernardi ML, Delouis C. Sex-related differences in the developmental rate of in-vitro matured/in-vitro fertilized ovine embryos. Hum Reprod 11: 621–626, 1996. [DOI] [PubMed] [Google Scholar]
- 12.Bird AP CpG-rich islands and the function of DNA methylation. Nature 321: 209–213, 1986. [DOI] [PubMed] [Google Scholar]
- 13.Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: Association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 115: e290–e296, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Bridges JP, Gilbert JS, Colson D, Dukes M, Babcock SA, Ryan MJ, Granger JP. Soluble Flt-1 induces hypertension and vascular dysfunction in pregnant rats. FASEB J 22: 969, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burdge GC, Slater-Jefferies J, Torrens C, Phillips ES, Hanson MA, Lillycrop KA. Dietary protein restriction of pregnant rats in the F0 generation induces altered methylation of hepatic gene promoters in the adult male offspring in the F1 and F2 generations. Br J Nutr 97: 435–439, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burt VL, Whelton P, Roccella EJ, Brown C, Cutler JA, Higgins M, Horan MJ, Labarthe D. Prevalence of hypertension in the US adult population : results from the third national health and nutrition examination survey, 1988–1991. Hypertension 25: 305–313, 1995. [DOI] [PubMed] [Google Scholar]
- 17.Cho NH, Silverman BL, Rizzo TA, Metzger BE. Correlations between the intrauterine metabolic environment and blood pressure in adolescent offspring of diabetic mothers. J Pediatr 136: 587–592, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Chow JC, Yen Z, Ziesche SM, Brown CJ. Silencing of the mammalian X chromosome. Annu Rev Gen Hum Genet 6: 69–92, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Cox LA, Glenn J, Schiabritz-Loutsevitch NE, Nathanielsz PW, Nijland MJ. Sex effects of maternal nutrient restriction (MNR) on renal transcriptome expression in the 0.9 gestation (G) fetal baboon. Reprod Sci 15: 120A, 2008. [Google Scholar]
- 20.Cox LA, Nijland MJ, Gilbert JS, Schlabritz-Loutsevitch N, Hubbard GB, McDonald TJ, Shade RE, Nathanielsz PW. Effect of thirty percent maternal nutrient restriction from 0.16 to 0.5 gestation on fetal baboon kidney gene expression. J Physiol 572: 67–85, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crawford MA, Doyle W, Meadows N. Gender differences at birth and differences in fetal growth. Hum Reprod 2: 517–520, 1987. [DOI] [PubMed] [Google Scholar]
- 22.Denton KM, Flower RL, Stevenson KM, Anderson WP. Adult rabbit offspring of mothers with secondary hypertension have increased blood pressure. Hypertension 41: 634–639, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Dubey RK, Oparil S, Imthurn B, Jackson EK. Sex hormones and hypertension. Cardiovasc Res 53: 688–708, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Durcova-Hills G, Burgoyne P, McLaren A. Analysis of sex differences in EGC imprinting. Dev Biol 268: 105–110, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Elahi MM, Cagampang FR, Anthony FW, Curzen N, Ohri SK, Hanson MA. Statin treatment in hypercholesterolemic pregnant mice reduces cardiovascular risk factors in their offspring. Hypertension 51: 939–944, 2008. [DOI] [PubMed] [Google Scholar]
- 26.Ellegren H, Parsch J. The evolution of sex-biased genes and sex-biased gene expression. Nat Rev Genet 8: 689–698, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Gallou-Kabani C, Vige A, Gross MS, Boileau C, Rabes JP, Fruchart-Najib J, Jais JP, Junien C. Resistance to high-fat diet in the female progeny of obese mice fed a control diet during the periconceptual, gestation, and lactation periods. Am J Physiol Endocrinol Metab 292: E1095–E1100, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Gilbert JS, Cox L, Babcock SA, Shade R, Nathanielsz PW, Nijland MJ. Gender-specific effects of moderate maternal nutrient restriction (NR) in the first half of pregnancy on fetal renal renin-angiotensin system (RAS). J Soc Gynecol Investig 13: 207A, 2006. [Google Scholar]
- 29.Gilbert JS, Cox LA, Mitchell G, Nijland MJ. Nutrient-restricted fetus and the cardio-renal connection in hypertensive offspring. Expert Rev Cardiovasc Ther 4: 227–237, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Gilbert JS, Ford SP, Lang AL, Pahl LR, Drumhiller MC, Babcock SA, Nathanielsz PW, Nijland MJ. Nutrient restriction impairs nephrogenesis in a gender-specific manner in the ovine fetus. Pediatr Res 61: 42–47, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Gilbert JS, Knoblich PR, Steck S. Effects of regular, voluntary gestational exercise on the development of hypertension in offspring. FASEB J 16: A1141, 2002. [Google Scholar]
- 32.Gilbert JS, Lang AL, Grant AR, Nijland MJ. Maternal nutrient restriction in sheep: hypertension and decreased nephron number in offspring at 9 months of age. J Physiol 565: 137–147, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gilbert JS, Lang AL, Nijland MJ. Maternal nutrient restriction and the fetal left ventricle: Decreased angiotensin receptor expression. Reprod Biol Endocrinol 3: 27, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gilbert JS, Babcock SA, Granger JP. Hypertension produced by reduced uterine perfusion in pregnant rats is associated with increased soluble Fms-like tyrosine kinase-1 expression. Hypertension 50: 1142–1147, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Goodacre R Metabolomics of a superorganism. J Nutr 137: 259S–S266, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Grigore D, Ojeda NB, Alexander BT. Sex differences in the fetal programming of hypertension. Gend Med 5 Suppl A: S121–S132, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grigore D, Ojeda NB, Robertson EB, Dawson AS, Huffman CA, Bourassa EA, Speth RC, Brosnihan KB, Alexander BT. Placental insufficiency results in temporal alterations in the renin angiotensin system in male hypertensive growth restricted offspring. Am J Physiol Regul Integr Comp Physiol 293: R804–R811, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gubhaju L, Nathanielsz PW, Nyengaard JR, Nijland MJ, Black MJ. Effects of a thirty percent global reduction in maternal nutrient intake on development of the fetal baboon kidney at term. Early Hum Dev 83: S166–S167, 2007. [Google Scholar]
- 39.Gutierrez-Adan A, Oter M, Martinez-Madrid B, Pintado B, De La FJ. Differential expression of two genes located on the X chromosome between male and female in vitro-produced bovine embryos at the blastocyst stage. Mol Reprod Dev 55: 146–151, 2000. [DOI] [PubMed] [Google Scholar]
- 40.Hallan S, Euser AM, Irgens LM, Finken MJ, Holmen J, Dekker FW. Effect of intrauterine growth restriction on kidney function at young adult age: the Nord Trondelag Health (HUNT 2) Study. Am J Kidney Dis 51: 10–20, 2008. [DOI] [PubMed] [Google Scholar]
- 41.Haning RV, Curet LB, Poole WK, Boehnlein LM, Kuzma DL, Meier SM. Effects of fetal sex and dexamethasone on preterm maternal serum concentrations of human chorionic gonadotropin, progesterone, estrone, estradiol, and estriol. Am J Obstet Gynecol 161: 1549–1553, 1989. [DOI] [PubMed] [Google Scholar]
- 42.Haris RC Cyclooxygenase-2 in the Kidney. J Am Soc Nephrol 11: 2387–2394, 2000. [DOI] [PubMed] [Google Scholar]
- 43.Hemmings DG, Veerareddy S, Baker PN, Davidge ST. Increased myogenic responses in uterine but not mesenteric arteries from pregnant offspring of diet-restricted rat dams. Biol Reprod 72: 997–1003, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Hindmarsh P, Geary M, Rodeck C, Kingdom JC, Cole T. Intrauterine growth and its relationship to size and shape at birth. Pediatr Res 52: 263–268, 2002. [DOI] [PubMed] [Google Scholar]
- 45.Holliday R, Ho T. DNA methylation and epigenetic inheritance. Methods 27: 179–183, 2002. [DOI] [PubMed] [Google Scholar]
- 46.Hoppe CC, Evans RG, Bertram JF, Moritz KM. Effects of dietary protein restriction on nephron number in the mouse. Am J Physiol Regul Integr Comp Physiol 292: R1768–R1774, 2007. [DOI] [PubMed] [Google Scholar]
- 47.Huxley VH Sex and the cardiovascular system: the intriguing tale of how women and men regulate cardiovascular function differently. Adv Physiol Educ 31: 17–22, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jacob RA, Gretz DM, Taylor PC, James SJ, Pogribny IP, Miller BJ, Henning SM, Swendseid ME. Moderate folate depletion increases plasma homocysteine and decreases lymphocyte DNA methylation in postmenopausal women. J Nutr 128: 1204–1212, 1998. [DOI] [PubMed] [Google Scholar]
- 49.Jones A, Beda A, Osmond C, Godfrey KM, Simpson DM, Phillips DIW. Sex-specific programming of cardiovascular physiology in children. Eur Heart J 29: 2164–2170, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jones A, Beda A, Ward AMV, Osmond C, Phillips DIW, Moore VM, Simpson DM. Size at birth and autonomic function during psychological stress. Hypertension 49: 548–555, 2007. [DOI] [PubMed] [Google Scholar]
- 51.Junien C Impact of diets and nutrients/drugs on early epigenetic programming. J Inherit Metab Dis 29: 359–365, 2006. [DOI] [PubMed] [Google Scholar]
- 52.Keijzer-Veen MG, Kleinveld HA, Lequin MH, Dekker FW, Nauta J, de Rijke YB, van der Heijden BJ. Renal function and size at young adult age after intrauterine growth restriction and very premature birth. Am J Kidney Dis 50: 542–551, 2007. [DOI] [PubMed] [Google Scholar]
- 53.Kelly TL, Trasler JM. Reproductive epigenetics. Clin Genet 65: 247–260, 2004. [DOI] [PubMed] [Google Scholar]
- 54.Khan IY, Dekou V, Douglas G, Jensen R, Hanson MA, Poston L, Taylor PD. A high-fat diet during rat pregnancy or suckling induces cardiovascular dysfunction in adult offspring. Am J Physiol Regul Integr Comp Physiol 288: R127–R133, 2005. [DOI] [PubMed] [Google Scholar]
- 55.Khan IY, Taylor PD, Dekou V, Seed PT, Lakasing L, Graham D, Dominiczak AF, Hanson MA, Poston L. Gender-linked hypertension in offspring of lard-fed pregnant rats. Hypertension 41: 168–175, 2003. [DOI] [PubMed] [Google Scholar]
- 56.Kistner A, Celsi G, Vanpee M, Jacobson SH. Increased blood pressure but normal renal function in adult women born preterm. Pediatr Nephrol 15: 215–220, 2000. [DOI] [PubMed] [Google Scholar]
- 57.Ko MS, Threat TA, Wang X, Horton JH, Cui Y, Wang X, Pryor E, Paris J, Wells-Smith J, Kitchen JR, Rowe LB, Eppig J, Satoh T, Brant L, Fujiwara H, Yotsumoto S, Nakashima H. Genome-wide mapping of unselected transcripts from extraembryonic tissue of 7.5-day mouse embryos reveals enrichment in the t-complex and under-representation on the X chromosome. Hum Mol Genet 7: 1967–1978, 1998. [DOI] [PubMed] [Google Scholar]
- 58.Kovtun IV, Therneau TM, McMurray CT. Gender of the embryo contributes to CAG instability in transgenic mice containing a Huntington's disease gene. Hum Mol Genet 9: 2767–2775, 2000. [DOI] [PubMed] [Google Scholar]
- 59.Kwong WY, Wild AE, Roberts P, Willis AC, Fleming TP. Maternal undernutrition during the preimplantation period of rat development causes blastocyst abnormalities and programming of postnatal hypertension. Development 127: 4195–4202, 2000. [DOI] [PubMed] [Google Scholar]
- 60.Lackland DT, Bendall HE, Osmond C, Egan BM, Barker DJP. Low birth weights contribute to the high rates of early-onset chronic renal failure in the southeastern United States. Arch Intern Med 160: 1472–1476, 2000. [DOI] [PubMed] [Google Scholar]
- 61.Langley-Evans SC Intrauterine programming of hypertension in the rat: nutrient interactions. Comp Biochem Physiol A 114: 327–333, 1996. [DOI] [PubMed] [Google Scholar]
- 62.Langley-Evans SC Maternal carbenoxolone treatment lowers birthweight and induces hypertension in the offspring of rats fed a protein-replete diet. Clin Sci (Lond) 93: 423–429, 1997. [DOI] [PubMed] [Google Scholar]
- 63.Langley-Evans SC Fetal programming of cardiovascular function through exposure to maternal undernutrition. Proc Nutr Soc 60: 505–513, 2001. [DOI] [PubMed] [Google Scholar]
- 64.Laor A, Stevenson DK, Shemer J, Gale R, Seidman DS. Size at birth, maternal nutritional status in pregnancy, and blood pressure at age 17: population-based analysis. Br Med J 315: 449–453, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Larson MA, Kimura K, Kubisch HM, Roberts RM. Sexual dimorphism among bovine embryos in their ability to make the transition to expanded blastocyst and in the expression of the signaling molecule IFN-τ. Proc Natl Acad Sci USA 98: 9677–9682, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lawlor DA, Najman JM, Sterne J, Williams GM, Ebrahim S, Smith GD. Associations of parental, birth, and early life characteristics with systolic blood pressure at 5 years of age: findings from the Mater University Study of Pregnancy and Its Outcomes. Circulation 110: 2417–2423, 2004. [DOI] [PubMed] [Google Scholar]
- 67.Lazaro A, Gallego-Delgado J, Justo P, Esteban V, Osende J, Mezzano S, Ortiz A, Egido J. Long-term blood pressure control prevents oxidative renal injury. Antioxid Redox Signal 7: 1285–1293, 2005. [DOI] [PubMed] [Google Scholar]
- 68.Li S, Chen SC, Shlipak M, Bakris G, McCullough PA, Sowers J, Stevens L, Jurkovitz C, McFarlane S, Norris K, Vassalotti J, Klag MJ, Brown WW, Narva A, Calhoun D, Johnson B, Obialo C, Whaley-Connell A, Becker B, Collins AJ. Low birth weight is associated with chronic kidney disease only in men. Kidney Int 73: 637–642, 2007. [DOI] [PubMed] [Google Scholar]
- 69.Lillycrop KA, Phillips ES, Torrens C, Hanson MA, Jackson AA, Burdge GC. Feeding pregnant rats a protein-restricted diet persistently alters the methylation of specific cytosines in the hepatic PPAR alpha promoter of the offspring. Br J Nutr 100: 278–282, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lillycrop KA, Slater-Jefferies JL, Hanson MA, Godfrey KM, Jackson AA, Burdge GC. Induction of altered epigenetic regulation of the hepatic glucocorticoid receptor in the offspring of rats fed a protein-restricted diet during pregnancy suggests that reduced DNA methyltransferase-1 expression is involved in impaired DNA methylation and changes in histone modifications. Br J Nutr 97: 1064–1073, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lillycrop KA, Phillips ES, Jackson AA, Hanson MA, Burdge GC. Dietary protein restriction of pregnant rats induces and folic acid supplementation prevents epigenetic modification of hepatic gene expression in the offspring. J Nutr 135: 1382–1386, 2005. [DOI] [PubMed] [Google Scholar]
- 72.Loria A, Reverte V, Salazar F, Saez F, Llinas MT, Salazar FJ. Changes in renal hemodynamics and excretory function induced by a reduction of ANG II effects during renal development. Am J Physiol Regul Integr Comp Physiol 293: R695–R700, 2007. [DOI] [PubMed] [Google Scholar]
- 73.Loria A, Reverte V, Salazar F, Saez F, Llinas MT, Salazar FJ. Sex and age differences of renal function in rats with reduced ANG II activity during the nephrogenic period. Am J Physiol Renal Physiol 293: F506–F510, 2007. [DOI] [PubMed] [Google Scholar]
- 74.Lu F, Bytautiene E, Tamayo E, Gamble P, Anderson GD, Hankins GD, Longo M, Saade GR. Gender-specific effect of overexpression of sFlt-1 in pregnant mice on fetal programming of blood pressure in the offspring later in life. Am J Obstet Gynecol 197: 418–5, 2007. [DOI] [PubMed] [Google Scholar]
- 75.Lu F, Longo M, Tamayo E, Maner W, Al-Hendy A, Anderson GD, Hankins GDV, Saade GR. The effect of over-expression of sFlt-1 on blood pressure and the occurrence of other manifestations of preeclampsia in unrestrained conscious pregnant mice [Online]. Am J Obstet Gynecol 196: 396, 2007. [DOI] [PubMed] [Google Scholar]
- 76.Makris A, Thornton C, Thompson J, Thomson S, Martin R, Ogle R, Waugh R, McKenzie P, Kirwan P, Hennessy A. Uteroplacental ischemia results in proteinuric hypertension and elevated sFLT-1. Kidney Int 71: 977–984, 2007. [DOI] [PubMed] [Google Scholar]
- 77.Marcus R, Krause L, Weder AB, Dominguez-Meja A, Schork NJ, Julius S. Sex-specific determinants of increased left ventricular mass in the Tecumseh Blood Pressure Study. Circulation 90: 928–936, 1994. [DOI] [PubMed] [Google Scholar]
- 78.Mason JB Biomarkers of nutrient exposure and status in one-carbon (methyl) metabolism. J Nutr 133: 941S–9947S, 2003. [DOI] [PubMed] [Google Scholar]
- 79.McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev 85: 571–633, 2005. [DOI] [PubMed] [Google Scholar]
- 80.McMullen S, Gardner DS, Langley-Evans SC. Prenatal programming of angiotensin II type 2 receptor expression in the rat. Br J Nutr 91: 133–140, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McMullen S, Langley-Evans SC. Maternal low-protein diet in rat pregnancy programs blood pressure through sex-specific mechanisms. Am J Physiol Regul Integr Comp Physiol 288: R85–R90, 2005. [DOI] [PubMed] [Google Scholar]
- 82.McMullen S, and Langley-Evans SC. Sex-specific effects of prenatal low-protein and carbenoxolone exposure on renal angiotensin receptor expression in rats. Hypertension 46: 1374–1380, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mertz HL, Liu J, Valego NK, Stallings SP, Figueroa JP, Rose JC. Inhibition of cyclooxygenase-2: effects on renin secretion and expression in fetal lambs. Am J Physiol Regul Integr Comp Physiol 284: R1012–R1018, 2003. [DOI] [PubMed] [Google Scholar]
- 84.Miller JA, Anacta LA, Cattran DC. Impact of gender on the renal response to angiotensin II. Kidney Int 55: 278–285, 1999. [DOI] [PubMed] [Google Scholar]
- 86.Mok KY, Sandberg K, Sweeny JM, Zheng W, Lee S, Mulroney SE. Growth hormone regulation of glomerular AT1 angiotensin receptors in adult uninephrectomized male rats. Am J Physiol Renal Physiol 285: F1085–F1091, 2003. [DOI] [PubMed] [Google Scholar]
- 87.Mutch DM, Wahli W, Williamson G. Nutrigenomics and nutrigenetics: the emerging faces of nutrition. FASEB J 19: 1602–1616, 2005. [DOI] [PubMed] [Google Scholar]
- 88.Neugarten J, Kasiske B, Silbiger SR, Nyengaard JR. Effects of sex on renal structure. Nephron 90: 139–144, 2002. [DOI] [PubMed] [Google Scholar]
- 89.O'Regan D, Kenyon CJ, Seckl JR, Holmes MC. Prenatal dexamethasone 'programmes' hypotension, but stress-induced hypertension in adult offspring. J Endocrinol 196: 343–352, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ojeda NB, Grigore D, Alexander BT. Intrauterine growth restriction: fetal programming of hypertension and kidney disease. Adv Chronic Kidney Dis 15: 101–106, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ojeda NB, Grigore D, Robertson EB, Alexander BT. Estrogen protects against increased blood pressure in postpubertal female growth restricted offspring. Hypertension 50: 679–685, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ojeda NB, Grigore D, Yanes LL, Iliescu R, Robertson EB, Zhang H, Alexander BT. Testosterone contributes to marked elevations in mean arterial pressure in adult male intrauterine growth restricted offspring. Am J Physiol Regul Integr Comp Physiol 292: R758–R763, 2007. [DOI] [PubMed] [Google Scholar]
- 93.Ortiz LA, Quan A, Zarzar F, Weinberg A, Baum M. Prenatal dexamethasone programs hypertension and renal injury in the rat. Hypertension 41: 328–334, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ozaki T, Nishina H, Hanson MA, Poston L. Dietary restriction in pregnant rats causes gender-related hypertension and vascular dysfunction in offspring. J Physiol 530: 141–152, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Paldi A, Gyapay G, Jami J. Imprinted chromosomal regions of the human genome display sex-specific meiotic recombination frequencies. Curr Biol 5: 1030–1035, 1995. [DOI] [PubMed] [Google Scholar]
- 96.Parker AJ, Davies P, Mayho AM, Newton JR. The ultrasound estimation of sex-related variations of intrauterine growth. Am J Obstet Gynecol 149: 665–669, 1984. [DOI] [PubMed] [Google Scholar]
- 97.Peippo J, Farazmand A, Kurkilahti M, Markkula M, Basrur PK, King WA. Sex-chromosome linked gene expression in in-vitro produced bovine embryos. Mol Human Reprod 8: 923–929, 2002. [DOI] [PubMed] [Google Scholar]
- 98.Porter JP, King SH, Honeycutt AD. Prenatal high-salt diet in the Sprague-Dawley rat programs blood pressure and heart rate hyperresponsiveness to stress in adult female offspring. Am J Physiol Regul Integr Comp Physiol 293: R334–R342, 2007. [DOI] [PubMed] [Google Scholar]
- 99.Pretorius M, Luther JM, Murphey LJ, Vaughan DE, Brown NJ. Angiotensin-converting enzyme inhibition increases basal vascular tissue plasminogen activator release in women but not in men. Arterioscler Thromb Vasc Biol 25: 2435–2440, 2005. [DOI] [PubMed] [Google Scholar]
- 100.Reckelhoff JF, Zhang H, Granger JP. Testosterone exacerbates hypertension and reduces pressure-natriuresis in male spontaneously hypertensive rats. Hypertension 31: 435–439, 1998. [DOI] [PubMed] [Google Scholar]
- 101.Reckelhoff JF Gender differences in the regulation of blood pressure. Hypertension 37: 1199–1208, 2001. [DOI] [PubMed] [Google Scholar]
- 102.Rice WR Sex chromosomes and the evolution of sexual dimorphism. Evolution 38: 735–742, 1984. [DOI] [PubMed] [Google Scholar]
- 103.Rinn JL, Rozowsky JS, Laurenzi IJ, Petersen PH, Zou K, Zhong W, Gerstein M, Snyder M. Major molecular differences between mammalian sexes are involved in drug metabolism and renal function. Dev Cell 6: 791–800, 2004. [DOI] [PubMed] [Google Scholar]
- 104.Rinn JL, Snyder M. Sexual dimorphism in mammalian gene expression. Trends Genet 21: 298–305, 2005. [DOI] [PubMed] [Google Scholar]
- 105.Rogers JL, Mitchell AR, Maric C, Sandberg K, Myers A, Mulroney SE. Effect of sex hormones on renal estrogen and angiotensin type 1 receptors in female and male rats. Am J Physiol Regul Integr Comp Physiol 292: R794–R799, 2007. [DOI] [PubMed] [Google Scholar]
- 106.Saez F, Castells MT, Zuasti A, Salazar F, Reverte V, Loria A, Salazar FJ. Sex differences in the renal changes elicited by angiotensin II blockade during the nephrogenic period. Hypertension 49: 1429–1435, 2007. [DOI] [PubMed] [Google Scholar]
- 107.Sakemi T, Toyoshima H, Shouno Y, Morito F. Estrogen attenuates progressive glomerular injury in hypercholesterolemic male Imai rats. Nephron 69: 159–165, 1995. [DOI] [PubMed] [Google Scholar]
- 108.Salazar F, Reverte V, Saez F, Loria A, Llinas MT, Salazar FJ. Age-sodium sensistive hypertension and sex-dependent renal changes in rats with a reduced nephron number. Hypertension 51: 1184–1189, 2008. [DOI] [PubMed] [Google Scholar]
- 109.Samuelsson AM, Matthews PA, Argenton M, Christie MR, McConnell JM, Jansen EHJ, Piersma AH, Ozanne SE, Twinn DF, Remacle C, Rowlerson A, Poston L, Taylor PD. Diet-iduced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: a novel murine model of developmental programming. Hypertension 51: 383–392, 2008. [DOI] [PubMed] [Google Scholar]
- 110.Sandberg K, Ji H. Sex and the renin angiotensin system: implications for gender differences in the progression of kidney disease. Adv Ren Replace Ther 10: 15–23, 2003. [DOI] [PubMed] [Google Scholar]
- 111.Schnackenberg CG, Welch WJ, Wilcox CS. Normalization of blood pressure and renal vascular resistance in SHR with a membrane-permeable superoxide dismutase mimetic: role of nitric oxide. Hypertension 32: 59–64, 1998. [DOI] [PubMed] [Google Scholar]
- 112.Schwertz DW, Vizgirda V, Solaro RJ, Piano MR, Ryjewski C. Sexual dimorphism in rat left atrial function and response to adrenergic stimulation. Mol Cell Biochem 200: 143–153, 1999. [DOI] [PubMed] [Google Scholar]
- 113.Silbiger S, Neugarten J. Gender and human chronic renal disease. Gend Med 5 Suppl A: S3–S10, 2008. [DOI] [PubMed] [Google Scholar]
- 114.Silva-Antonialli MM, Tostes RCA, Fernandes L, Fior-Chadi DR, Akamine EH, Carvalho MH, Fortes ZB, Nigro D. A lower ratio of AT1/AT2 receptors of angiotensin II is found in female than in male spontaneously hypertensive rats. Cardiovasc Res 62: 587–593, 2004. [DOI] [PubMed] [Google Scholar]
- 115.Sinclair KD, Allegrucci C, Singh R, Gardner DS, Sebastian S, Bispham J, Thurston A, Huntley JF, Rees WD, Maloney CA, Lea RG, Craigon J, McEvoy TG, Young LE. DNA methylation, insulin resistance, and blood pressure in offspring determined by maternal periconceptional B vitamin and methionine status. Proc Natl Acad Sci USA 104: 19351–19356, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Snell LH, Haughey BP, Buck G, Marecki MA. Metabolic crisis: hyperemesis gravidarum. J Perinat Neonatal Nurs 12: 26–37, 1998. [PubMed] [Google Scholar]
- 117.Sood R, Zehnder JL, Druzin ML, Brown PO. Gene expression patterns in human placenta. Proc Natl Acad Sci USA 103: 5478–5483, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sullivan JC, Semprun-Prieto L, Boesen EI, Pollock DM, Pollock JS. Sex and sex hormones influence the development of albuminuria and renal macrophage infiltration in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 293: R1573–R1579, 2007. [DOI] [PubMed] [Google Scholar]
- 119.Sullivan JC Sex and the renin-angiotensin system: inequality between the sexes in response to RAS stimulation and inhibition. Am J Physiol Regul Integr Comp Physiol 294: R1220–R1226, 2008. [DOI] [PubMed] [Google Scholar]
- 120.Tamimi RM, Lagiou P, Mucci LA, Hsieh CC, Adami HO, Trichopoulos D. Average energy intake among pregnant women carrying a boy compared with a girl. Br Med J 326: 1245–1246, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Taylor DM, Handyside AH, Ray PF, Dibb NJ, Winston RML, Ao A. Quantitative measurement of transcript levels throughout human preimplantation development: analysis of hypoxanthine phosphoribosyl transferase. Mol Human Reprod 7: 147–154, 2001. [DOI] [PubMed] [Google Scholar]
- 122.Taylor PD, Khan IY, Hanson MA, Poston L. Impaired EDHF-mediated vasodilatation in adult offspring of rats exposed to a fat-rich diet in pregnancy. J Physiol 558: 943–951, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Teglund S, McKay C, Schuetz E, van Deursen JM, Stravopodis D, Wang D, Brown M, Bodner S, Grosveld G, Ihle JN. Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell 93: 841–850, 1998. [DOI] [PubMed] [Google Scholar]
- 124.Tullis KM, Krebs CJ, Leung JYM, Robins DM. The regulator of sex-limitation gene, Rsl, enforces male-specific liver gene expression by negative regulation. Endocrinology 144: 1854–1860, 2003. [DOI] [PubMed] [Google Scholar]
- 125.Udy GB, Towers RP, Snell RG, Wilkins RJ, Park SH, Ram PA, Waxman DJ, Davey HW. Requirement of STAT5b for sexual dimorphism of body growth rates and liver gene expression. Proc Natl Acad Sci USA 94: 7239–7244, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Valdivia RP, Kunieda T, Azuma S, Toyoda Y. PCR sexing and developmental rate differences in preimplantation mouse embryos fertilized and cultured in vitro. Mol Reprod Dev 35: 121–126, 1993. [DOI] [PubMed] [Google Scholar]
- 127.Ward AM, Moore VM, Steptoe A, Cockington RA, Robinson JS, Phillips DI. Size at birth and cardiovascular responses to psychological stressors: evidence for prenatal programming in women. J Hypertens 22: 2295–2301, 2004. [DOI] [PubMed] [Google Scholar]
- 128.Waterland RA, Michels KB. Epigenetic epidemiology of the developmental origins hypothesis. Ann Rev Nutr 27: 363–388, 2007. [DOI] [PubMed] [Google Scholar]
- 129.Wiinberg N, Hoegholm A, Christensen HR, Bang LE, Mikkelsen KL, Nielsen PE, Svendsen TL, Kampmann JP, Madsen NH, Bentzon MW. 24-h ambulatory blood pressure in 352 normal Danish subjects, related to age and gender. Am J Hypertens 8: 978–986, 1995. [DOI] [PubMed] [Google Scholar]
- 130.Wilson PWF, Grundy SM. The metabolic syndrome: practical guide to origins and treatment: part I. Circulation 108: 1422–1424, 2003. [DOI] [PubMed] [Google Scholar]
- 131.Wiwi CA, Gupte M, Waxman DJ. Sexually dimorphic P450 gene expression in liver-specific hepatocyte nuclear factor 4alpha-deficient mice. Mol Endocrinol 18: 1975–1987, 2004. [DOI] [PubMed] [Google Scholar]
- 132.Woods LL Neonatal uninephrectomy causes hypertension in adult rats. Am J Physiol Regul Integr Comp Physiol 276: R974–R978, 1999. [DOI] [PubMed] [Google Scholar]
- 133.Woods LL, Ingelfinger JR, Nyengaard JR, Rasch R. Maternal protein restriction suppresses the newborn renin-angiotensin system and programs adult hypertension in rats. Pediatr Res 49: 460–467, 2001. [DOI] [PubMed] [Google Scholar]
- 134.Woods LL, Rasch R. Perinatal ANG II programs adult blood pressure, glomerular number, and renal function in rats. Am J Physiol Regul Integr Comp Physiol 275: R1593–R1599, 1998. [DOI] [PubMed] [Google Scholar]
- 135.Woods LL, Ingelfinger JR, Rasch R. Modest maternal protein restriction fails to program adult hypertension in female rats. Am J Physiol Regul Integr Comp Physiol 289: R1131–R1136, 2005. [DOI] [PubMed] [Google Scholar]
- 136.Wrenzycki C, Lucas-Hahn A, Herrmann D, Lemme E, Korsawe K, Niemann H. In vitro production and nuclear transfer affect dosage compensation of the X-linked gene transcripts G6PD, PGK, and Xist in preimplantation bovine embryos. Biol Reprod 66: 127–134, 2002. [DOI] [PubMed] [Google Scholar]
- 137.Wyrwoll CS, Mark PJ, Waddell BJ. Developmental programming of renal glucocorticoid sensitivity and the renin-angiotensin system. Hypertension 50: 579–584, 2007. [DOI] [PubMed] [Google Scholar]
- 138.Wyrwoll CS, Mark PJ, Mori TA, Puddey IB, Waddell BJ. Prevention of programmed hyperleptinemia and hypertension by postnatal dietary ω-3 fatty acids. Endocrinology 147: 599–606, 2006. [DOI] [PubMed] [Google Scholar]
- 139.Yadav BR, King WA, Betteridge KJ. Relationships between the completion of first cleavage and the chromosomal complement, sex, and developmental rates of bovine embryos generated in vitro. Mol Reprod Dev 36: 434–439, 1993. [DOI] [PubMed] [Google Scholar]
- 140.Yang T, Huang YG, Ye W, Hansen P, Schnermann JB, Briggs JP. Influence of genetic background and gender on hypertension and renal failure in COX-2-deficient mice. Am J Physiol Renal Physiol 288: F1125–F1132, 2005. [DOI] [PubMed] [Google Scholar]
- 141.Yong LC, Kuller LH, Rutan G, Bunker C. Longitudinal study of blood pressure: changes and determinants from adolescence to middle age. The Dormont High School Follow-up Study, 1957–1963 to 1989–1990. Am J Epidemiol 138: 973–983, 1993. [DOI] [PubMed] [Google Scholar]
- 142.Zechner U, Wilda M, Kehrer-Sawatzki H, Vogel W, Fundele R, Hameister H. A high density of X-linked genes for general cognitive ability: a run-away process shaping human evolution? Trends Genet 17: 697–701, 2001. [DOI] [PubMed] [Google Scholar]