Abstract
Glucagon-like peptide-2 (GLP-2) is a nutrient-dependent, intestinotrophic hormone derived from posttranslational processing of proglucagon in the distal bowel. GLP-2 is thought to act through indirect mediators, such as IGF-I. We investigated whether intestinal expression of GLP-2 and IGF-I system components are increased with the mucosal growth induced by enteral nutrient (EN) and/or a low dose of GLP-2 in parenterally fed rats. Rats were randomized to four treatment groups using a 2×2 design and maintained with parenteral nutrition (PN) for 7 days: PN alone, EN, GLP-2, and EN+GLP-2; n = 7–9. The two main treatment effects are ±GLP-2 (100 μg·kg body wt−1·day−1) and ±EN (43% of energy needs, days 4–6). Combination treatment with EN+GLP-2 induced synergistic intestinal growth in ileum, resulting in greater mucosal cellularity, sucrase segmental activity, and gain of body weight (EN×GLP-2, P < 0.04). In addition, EN+GLP-2 induced a significant 28% increase in plasma concentration of bioactive GLP-2, a significant 102% increase in ileal proglucagon mRNA with no change in ileal dipeptidyl peptidase-IV (DPP-IV) specific activity, and significantly reduced plasma DPP-IV activity compared with GLP-2. This indicates that EN potentiates the intestinotrophic action of GLP-2. Proliferation of enterocytes due to GLP-2 infusion was associated with greater expression of ileal proglucagon, GLP-2 receptor, IGF-I, IGF binding protein-3 mRNAs, and greater IGF-I peptide concentration in ileum (P < 0.032). Ileal IGF-I mRNA was positively correlated with expression of proglucagon, GLP-2R, and IGFBP-5 mRNAs (R2 = 0.43–0.56, P < 0.0001). Our findings support the hypothesis that IGF-I is one of the downstream mediators of GLP-2 action in a physiological model of intestinal growth.
Keywords: parenteral nutrition, proglucagon, insulin-like growth factor binding protein-3 and -5, glucagon-like peptide-2 receptor
glucagon-like peptide-2 (glp-2) is a potent intestinotrophic hormone derived from tissue-specific posttranslational processing of proglucagon in the endocrine L cells of the ileum and colon (13). GLP-2 is secreted in response to nutrient intake and is rapidly inactivated by dipeptidyl peptidase-IV (DPP-IV) with a biological half-life of ∼7 min (10). Previous studies indicate that GLP-2 is a key mediator of intestinal adaptive growth through stimulation of epithelial cell proliferation and inhibition of apoptosis in a variety of models of intestinal dysfunction in mice, rats, and pigs (1, 6, 11). Moreover, exogenous GLP-2 and a GLP-2 analog resistant to degradation by DPP-IV improve intestinal morphology and energy absorption in humans with short bowel syndrome (16, 17). Given the therapeutic potential for use of GLP-2 in humans with compromised intestinal function, there is great interest in understanding the mechanisms by which GLP-2 regulates intestinal epithelial growth. Expression of the GLP-2 receptor (GLP-2R), a G protein-coupled receptor, occurs primarily in the gastrointestinal tract and central nervous system, with limited expression in lung, cervix, and vagal afferents (24, 27). Within the intestine, the GLP-2R has been localized to enteroendocrine cells, enteric neurons, and subepithelial myofibroblasts (10, 27). In contrast, the target cells for intestinal growth, crypt epithelial cells, and enterocytes do not express the GLP-2R. This has led to the hypothesis that GLP-2 action requires downstream mediators to induce intestinal growth and regulate other aspects of intestinal physiology (10).
IGF-I has been identified as a potential downstream mediator of the intestinal growth effects of GLP-2. Recent work in mice showed that exogenous GLP-2 improves gut morphology and increases proliferative indices in wild-type, but not IGF-I knockout mice, suggesting an essential role for IGF-I in the intestinotrophic action of GLP-2 (11). Moreover, administration of either exogenous IGF-I or GLP-2 reverses the mucosal atrophy induced by parenteral nutrition in rodents (4, 7). IGF-I is a growth hormone-dependent anabolic hormone that stimulates whole body and intestinal growth in response to nutritional status. IGF-I has been well characterized as a potent stimulator of intestinal growth via endocrine mechanisms associated with administration of IGF-I, as well as paracrine mechanisms associated with local synthesis of IGF-I in the intestine (34). The intestine synthesizes IGF-I in smooth muscle and subepithelial myofibroblasts, which expresses the GLP-2R (30), and epithelial IGF-I receptors are widespread in the intestine (28). Thus, GLP-2 may increase IGF-I synthesis in subepithelial myofibroblasts with IGF-I acting as the downstream mediator to promote the intestinal growth induced by GLP-2 (12).
Enteral nutrients (EN) provide the primary stimulus for intestinal growth both directly by providing energy and protein to enterocytes and indirectly by stimulating hormonal mediators such as GLP-2 and IGF-I. The parenteral nutrition (PN) rat model is ideal to study the intestintrophic effects of nutrient-regulated hormones such as GLP-2 and IGF-I because it provides precise control of nutrient intake and induces 40–50% mucosal atrophy without the systemic alterations induced by fasting (7). The objective of the current study was twofold. First, we sought to determine whether the synergistic intestinal growth response to combination treatment with EN and a low dose of GLP-2 occurs during the condition of PN-induced mucosal atrophy without the trophic stimulus of intestinal resection (21). Second, to further understanding of the hypothesis that IGF-I is one of the downstream mediators of GLP-2 action, we characterized intestinal expression of GLP-2 and IGF-I system components that occur in conjunction with the intestinal growth induced by EN and GLP-2.
MATERIALS AND METHODS
Animals and Experimental Design
The animal facilities and research protocols were approved by the University of Wisconsin Madison Institutional Animal Care and Use Committee. Male, Sprague-Dawley rats (Harlan, Madison, WI) initially weighing 175–200 g were housed in individual, stainless-steel cages with unlimited access to water in a room maintained at 22°C on a 12:12-h light-dark cycle. All rats were acclimated to the facility for 6 days, while being fed a semipurified diet ad libitum (7).
Rats were randomly assigned to four PN treatment groups (n = 9) using a 2×2 factorial design as follows: PN alone, EN, GLP-2, and EN+GLP-2. Rats in these four groups were maintained with PN for 7 days. The two main treatment effects are ±EN (days 4–6) and ±GLP-2 (100 μg·kg body wt−1·day−1). The final sample size for each group was PN alone, n = 7; EN, n = 9; GLP-2, n = 8; and EN+GLP-2, n = 9. A nonsurgical group of rats fed a semipurified diet ad libitum was included for reference (Oral, n = 9).
Rats were fasted for 18 h before surgical placement of intravenous catheters. Rats were anesthetized by inhalation of isoflurane (IsoFlo; Abbot Laboratories, North Chicago, IL) via an anesthesia machine before surgery. Intravenous catheters were placed in the superior vena cava as previously reported (7, 20). Infusion of PN solution was initiated using a Harvard syringe pump (Harvard Apparatus, Holliston, MA) at 1.0 ml/h immediately following surgery (day 0), advanced to 1.67 ml/h on day 1, and maintained at full-strength infusion of 2.5 ml/h (60 ml/day) for groups not receiving EN for days 2–6. Rats given PN alone and GLP-2 on days 2–6 received the following daily nutrient intake: 64 kcal, 2.6 g protein (16.5% energy), 1.7 g fat (24% energy), and 11 g dextrose (59% energy). The composition and preparation of the nutritionally complete PN solution were previously reported (7). Treatment groups given oral EN received 2.5 ml/h PN solution for days 2–4, and then the infusion was gradually decreased to 1.67 ml/h on day 5 and 1.0 ml/h on day 6. A low-residue, semielemental liquid diet containing intact and hydrolyzed protein, safflower oil, medium-chain triglycerides, sucrose, and maltodextrins (Vital, donated by Ross Products Division, Abbott Laboratories, Columbus, OH) was offered ad libitum in graduated feeding tubes on days 4–6, providing 1 kcal/ml with 16.7% energy from protein, 9.5% energy from fat, and 73.8% energy from carbohydrate. Thus, ∼43% of energy needs was provided by EN, and 57% was provided by PN during the last 3 days of the study for EN and EN+GLP-2 groups.
Rats infused with GLP-2 received 100 μg human GLP-2·kg body wt−1·day−1 concurrent with continuous intravenous infusion of PN solution for days 1–7. Human GLP-2 (preproglucagon 126–158; California Peptide Research, Napa, CA) was diluted in PBS, pH = 7.4 1 day before surgery and added to the PN solution daily. Vehicle was infused in rats not given GLP-2 (21). Assay of bioactive GLP-2 in the residual PN solution after daily infusion indicated greater than 95% recovery.
Body weights, infusion of PN solution, and the amount of EN consumed were recorded daily. Urine was collected into containers with 0.1% boric acid for determination of nitrogen balance (35). After 7 days of PN, rats were anesthetized with isoflurane and killed by exsanguination.
Intestinal Composition, Histology, Proliferation, and Sucrase Activity
Rats received an intravenous bolus of 0.2 mg/g body wt of bromo-2′-deoxyuridine (BrdU; Sigma Chemical, St. Louis, MO) to label enterocytes for measuring proliferation at 1 h before euthanasia. Rats were anesthetized and killed by cardiac exsanguination within 10 min of stopping the PN infusion. The entire small and large bowel and liver were removed for analysis. The bowel was sectioned into duodenum, defined as pylorus to ligament of Treitz; jejunum, defined as ligament of Treitz to ileum; ileum, defined as the final 25 cm of small bowel proximal to cecum; and colon. All sections of bowel were immediately flushed with ice-cold saline and put on a chilled glass plate to be sectioned. The first 3 cm of duodenum and colon and from 6–9 cm of jejunum and ileum were used for determining mucosal dry mass. The next 1 cm was fixed in 10% buffered formalin for histology, and the next 3 cm were collected for determination of protein (bicinchoninic acid protein assay; Pierce Chemicals, Rockford, IL), DNA (19), and sucrase activity in jejunum (5). The first 5 cm and the remaining tissue of jejunum and ileum were snap-frozen intact in liquid nitrogen and stored at −70°C for RNA extraction and determination of IGF-I content by RIA, respectively. Fixed tissue for histology was paraffin embedded, cut into 5-μm sections and processed for immunoperoxidase staining of BrdU-labeled cells and stained with hematoxylin and eosin for histomorphology, as previously described (8). For each rat, 10–12 crypts were counted.
Biochemical Analyses
Immunoreactive IGF-I and bioactive GLP-2.
Blood was collected in chilled tubes containing a final concentration of 1 mg/ml EDTA, 0.1 mM diprotin A (MP Biomedicals, Aurora, OH), and 0.01 mM aprotinin (Calbiochem, La Jolla, CA). Plasma was isolated by centrifugation at 1,800 g for 15 min at 4°C and was stored at −70°C. Plasma bioactive GLP-2 was measured by RIA using an antibody that has an absolute requirement for the intact N terminal amino acid sequence of GLP-2 that confers its bioactivity, i.e., GLP-2, 1–33 (15). Plasma IGF-I was measured by RIA after removing binding proteins by HPLC under acidic conditions; the recovery of IGF-I was 85–90% (29). Intact jejunum and ileum samples were homogenized in ammonium formate, spun at 14,000 g for 15 min, and the supernatant was applied to a C-2 bond elute column (Varian, Harbor City, CA), as previously described (14). Immunoreactive IGF-I was extracted in 45% acetonitrile-3% trifluoroacetic acid, and IGF-I concentration was determined by RIA (14, 29).
DPP-IV activity.
DPP-IV activity was measured in plasma and homogenates of ileal mucosa and intact colon using the discontinuous direct photometric method of Nagatsu et al. (25). The assay mixture included 5 μl of sample and 25 μl of 0.15 M glycine/NaOH pH 8.7 buffer. Substrate (25 μl of 3 mM glycylproline-p-nitroanilide tosylate) was quickly added to the wells, and plates were incubated at 37°C for 30 min. The reaction was stopped by rapidly adding 150 μl of 1 M sodium acetate, pH 4.2. The amount of p-nitroaniline liberated per minute was used to determine enzyme activity based on the standard curve that contained 0–50 nmol p-nitroaniline. One unit of DPP-IV activity is defined as the amount of enzyme that hydrolyzes 1 μmol of substrate/min. Specific activity of DPP-IV in ileum and colon was expressed as unit of DPP-IV activity per gram of protein.
IGF-I, IGF binding protein-3 and -5, GLP-2R, and proglucagon mRNA.
Total RNA was extracted from intact jejunum and ileum using TRIzol reagent (GIBCO-BRL Life Technologies, Grand Island, NY) and quantified spectrophotometrically at 260 nm. Integrity was confirmed by visualization of 18S and 28S rRNA on an agarose-formaldehyde gel with ethidium bromide staining. IGF-I, IGF binding protein-3 (IGFBP-3), and IGFBP-5 mRNAs were measured by RNase protection assay (RPA) using a HybSpeed RPA kit (Ambion, Austin, TX), according to the procedure described previously (14). Quantification of proglucagon mRNA was done using a Northern Max kit (Ambion) (6). All bands were quantified by light densitometry using OptiQuant software package (Packard Instruments, Meriden, CT). GLP-2R, IGF-I, and proglucagon mRNA expression were also measured in a two-step quantitative reverse transcriptase-real-time PCR (RT-qPCR) using SYBR Green detection method, as described previously (26). Sequences for forward and reverse primers (Integrated DNA Technologies, Coralville, IA) are shown in Table 1. Data were analyzed using 7000 system software (Applied Biosystems), and relative quantification was done using ΔΔCt method (22) with 18S as the internal control and the oral group as a reference control.
Table 1.
Primer sequences used in RT-qPCR
| Target | Primer Sequences | Amplicon Size |
|---|---|---|
| IGF-I | 5′-AAC CTG CAA AAC ATC GGA AC-3′ | 73 bp |
| 5′-GGA AAT GCC CTA CTC TGA AA-3′ | ||
| proglucagon | 5′-GAA TTC ATT GCT TGG CTG GT-3′ | 72 bp |
| 5′-TTC CTC AGC TAT GGC GAC TT-3′ | ||
| GLP-2R | 5′-CGA CGA CCA AGT TCA AGG AT-3′ | 109 bp |
| 5′-TCC ATT GGC AAA GCC ATA CT-3′ | ||
| 18S | QuantumRNA 18S Internal Standards (Ambion, Austin, TX) |
RT-qPCR, 2-step reverse transcriptase-real time PCR; IGF-I, insulin-like growth factor I; GLP-2R, glucagon-like peptide 2 receptor.
Statistical Analyses
Treatment groups were analyzed using general linear models, and individual differences between the treatment groups were identified by one-way ANOVA followed by the protected least significant difference technique (SAS version 8.2; SAS Institute, Cary, NC). Main effects of EN and GLP-2, and their interaction, were assessed using two-way ANOVA. Statistics were performed on log-transformed data for data showing unequal variance among groups. All data are presented as means ± SE. P < 0.05 was considered statistically significant.
RESULTS
Body Weight and Nitrogen Balance
There were no significant differences in body weights among the groups before surgery (Fig. 1). There were no significant differences in energy intake, body weight, and nitrogen retention among the treatment groups before the introduction of EN (day 0–day 4).
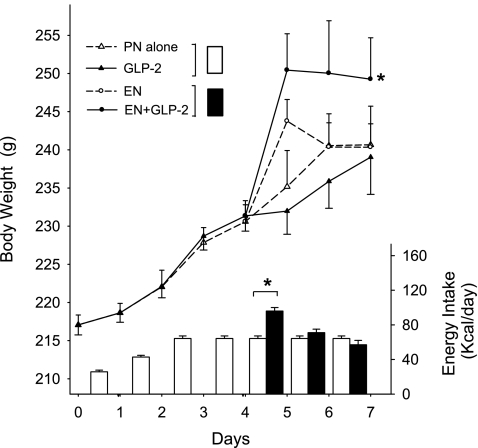
Fig. 1.
Daily changes in body weight (g) and energy intake (kcal/day) in rats maintained with parenteral nutrition (PN) for 7 days as follows: PN alone, enteral nutrition (EN), glucagon-like peptide-2 (GLP-2), and EN+GLP-2. Rats in all groups showed similar gain of body weight during the period after surgery and before EN treatment, day 0 to day 4. During the period when rats received partial EN (day 4 to day 7), EN+GLP-2 induced significantly greater gain of body weight compared with other groups (Time×EN×GLP-2, P = 0.037). *Significantly different final body weight or energy intake compared with other groups, P < 0.05. Values are expressed as means ± SE, n = 7–9.
During the period when rats received both PN and EN (day 4–day 7), neither EN nor GLP-2 had a significant effect on change in body weight. However, in the same period, combination treatment with EN+GLP-2 induced significantly greater gain in body weight (Time×EN×GLP-2, P = 0.037). Total energy intake was significantly greater in the two groups receiving EN on day 4 (96 ± 4; kcal/day) compared with the two groups who received only PN (64 kcal/day), but not significantly different on days 5 and 6. Total energy intake was not significantly different in the two groups given EN for days 4–6. Thus, in spite of similar energy intake, rats given combination treatment with EN+GLP-2 showed significantly greater gain in body weight, although differences in fluid retention cannot be ruled out. Liver mass was significantly greater in rats given EN or GLP-2 treatment compared with PN alone.
Mucosal Adaptive Growth
Rats given PN alone exhibited significant mucosal atrophy in the duodenum, jejunum, and ileum, but not in the colon compared with oral reference (Fig. 2). Data for duodenum and colon are not shown. Mucosal atrophy in the small bowel was not altered with individual treatment with EN, such that mucosal dry mass and concentrations of protein and DNA were not significantly different compared with PN alone. However, individual treatment with GLP-2 reversed the PN-induced mucosal atrophy in all sections of the small bowel, such that mucosal cellularity was similar or greater compared with the oral reference.
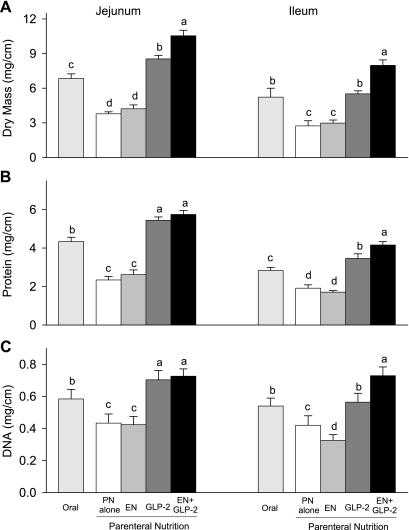
Fig. 2.
Jejunal and ileal mucosal dry mass (A), protein (B), and DNA (C) in rats fed orally or maintained with parenteral nutrition (PN) for 7 days as follows: PN alone, EN, GLP-2, and EN+GLP-2. Values are expressed as means ± SE; n = 7–9. a,b,c,dMeans with different letter superscripts are significantly different (P < 0.05).
The significant mucosa growth induced by GLP-2 was most pronounced in the jejunum and ileum. GLP-2 or EN+GLP-2 induced greater jejunum and ileum mucosal dry mass, and concentrations of protein and DNA by 34–132% or 67–192%, respectively, compared with PN alone (Fig. 2). Indices of mucosal growth in the ileum showed significant interaction (EN×GLP-2, P < 0.03), indicating a synergistic effect of treatment with EN+GLP-2 to further enhance mucosal adaptive growth beyond that expected by additive effects of the two treatments in the ileum. Treatments with GLP-2 resulted in a significantly greater (P < 0.001) ratio of protein to DNA in jejunal and ileal mucosa, suggesting that the greater mass of jejunum and ileum was due to greater cell size and cell number compared with PN alone.
The histology data for jejunum and ileum were consistent with changes in mucosal cellularity. Individual treatment with EN did not alter jejunum and ileum villus height, and crypt depth compared with PN alone (Table 2). Individual treatment with GLP-2 resulted in significantly greater jejunum and ileum villus height and crypt depth compared with PN alone (P < 0.0001). The synergistic effect of combination treatment with EN+GLP-2 was noted for ileum villus height (P = 0.0132) but not for crypt depth.
Table 2.
Histology, number of crypt cells, and BrdU-labeled cells of jejunum and ileum in rats maintained with PN for 7 days, including PN alone, EN, GLP-2, and EN+GLP-2
| PN Alone | EN | GLP-2 | EN+GLP-2 | |
|---|---|---|---|---|
| Jejunum | ||||
| Villus height, mm | 0.46±0.02b | 0.49±0.02b | 0.78±0.02a | 0.81±0.03a |
| Crypt depth, mm | 0.11±0.003b | 0.11±0.005b | 0.16±0.005a | 0.15±0.007a |
| No. of crypt cells | 24±1b | 24±1b | 35±1a | 35±1a |
| No. of BrdU-labeled cells | 5±1 | 6±2 | 5±1 | 6±0 |
| Ileum | ||||
| Villus height, mm | 0.29±0.01c | 0.27±0.01c | 0.39±0.01b | 0.45±0.02a |
| Crypt depth, mm | 0.10±0.003b | 0.09±0.004b | 0.12±0.003a | 0.13±0.006a |
| No. of crypt cells | 24±0c | 23±1c | 31±1a | 28±1b |
| No. of BrdU-labeled cells | 6±1b | 5±0b | 10±1a | 8±1a |
Values are expressed as means ± SE; n = 7–9 for histology and n = 3–5 for bromo-2′-deoxyuridine (BrdU)-labeled cells.
Values in the same row with different letter superscripts are significantly different (P < 0.05). PN, parenteral nutrition; EN, enteral nutrition; GLP-2, glucagon-like peptide-2.
Enterocyte proliferation was measured with incorporation of BrdU by crypt cells (Table 2). The number of cells in a crypt column was significantly greater in both jejunum and ileum in animals treated with GLP-2 compared with the two groups not given GLP-2 (main effect, P < 0.0001). The number of BrdU-labeled cells was significantly greater in animals treated with GLP-2 in ileum (main effect, P = 0.0008), but not in jejunum. In summary, GLP-2 treatment increased crypt cell proliferation resulting in a greater total crypt cell population.
Sucrase Activity
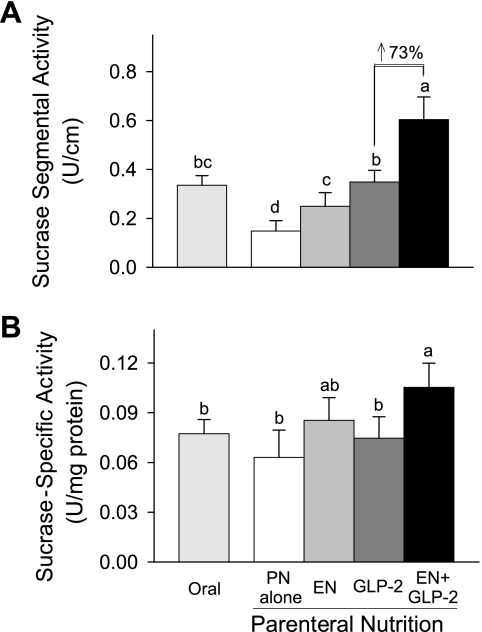
Mucosal sucrase activity reflects the digestive capacity of jejunum. Individual treatment with EN or GLP-2 resulted in significantly greater jejunal sucrase segmental activity compared with PN alone (Fig. 3). Combination treatment with EN+GLP-2 induced significantly greater sucrase segmental activity compared with either treatment alone (EN×GLP-2 interaction, P = 0.0397). When sucrase activity was expressed as specific activity, individual treatment with EN or combination treatment with EN+GLP-2 induced significantly greater sucrase activity (EN, P = 0.002). However, individual treatment with GLP-2 did not alter sucrase-specific activity compared with PN alone, consistent with a population of immature enterocytes in the rats given GLP-2 without EN.
Fig. 3.
Jejunal mucosal sucrase segmental activity (A) and specific activity (B) in rats fed orally or maintained with PN for 7 days as follows: PN alone, EN, GLP-2, and EN+GLP-2. Values are expressed as means ± SE; n = 7 or 8. a,b,cMeans with different letter superscripts are significantly different (P < 0.05).
Plasma Bioactive GLP-2 and DPP-IV Activity
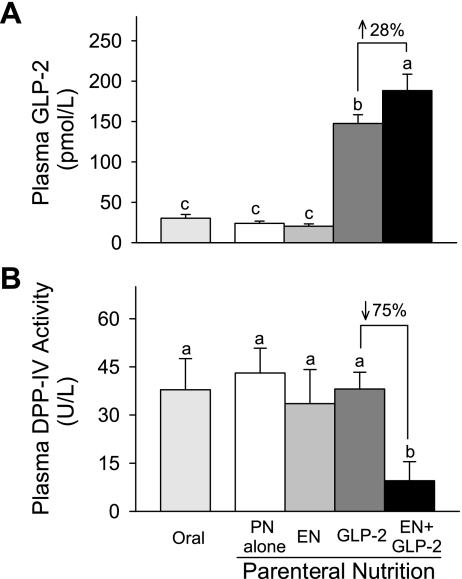
Individual treatment with GLP-2 induced a significantly greater concentration of bioactive GLP-2 in plasma than did treatment with EN or PN alone (Fig. 4). The plasma concentration of bioactive GLP-2 due to combination treatment with EN and GLP-2 was significantly greater by 28% compared with GLP-2 alone. There were no significant differences in DPP-IV activity in plasma among the individual treatment groups with the exception of the EN+GLP-2 group. Combination treatment with EN and GLP-2 significantly reduced plasma DPP-IV activity by 75% compared with GLP-2 alone (EN×GLP-2 interaction, P = 0.031). No significant main effect of EN, GLP-2, and their interaction was found in DPP-IV specific activity in ileum and colon, data not shown.
Fig. 4.
Plasma concentration of bioactive GLP-2 (A) and DPP-IV activity (B) in rats fed orally or maintained with PN for 7 days as follows: PN alone, EN, GLP-2, and EN+GLP-2. Values are expressed as means ± SE. Sample size was n = 7–9 for plasma concentration of bioactive GLP-2. Sample size was n = 5 for plasma DPP-IV activity except for the EN group where n = 3. a,b,cMeans with different letter superscripts are significantly different (P < 0.05).
Proglucagon mRNA and GLP-2 Receptor mRNA Expression
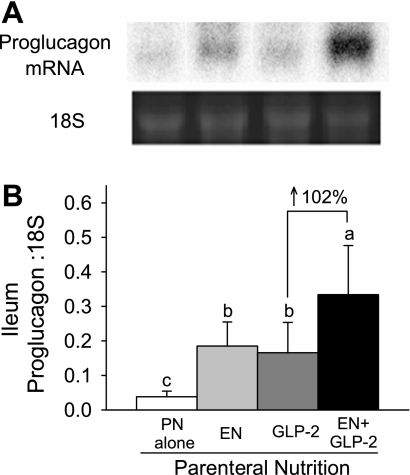
Ileum and colon are the primary sites for proglucagon expression, the precursor for GLP-2. Individual treatment with EN or GLP-2 significantly increased proglucagon mRNA expression in the ileum (Fig. 5). Ileal proglucagon mRNA expression due to combination treatment with EN and GLP-2 was 102% greater compared with GLP-2 alone (EN×GLP-2 interaction, P < 0.045). In the colon, EN and GLP-2 showed significant main effects to increase proglucagon mRNA expression without significant interaction (for EN, P = 0.0015, for GLP-2 P = 0.0303; data not shown).
Fig. 5.
Representative bands from Northern blot analysis of proglucagon mRNA and 18S rRNA (A) and ileal proglucagon mRNA expression (B) in rats maintained with PN for 7 days as follows: PN alone, EN, GLP-2, and EN+GLP-2. Values are expressed as means ± SE; n = 6–8. a,b,cMeans with different letter superscripts are significantly different (P < 0.05).
Individual treatment with EN did not significantly alter the expression of GLP-2R mRNA in jejunum and ileum. Interestingly, individual treatment with GLP-2 and combination treatment with EN+GLP-2 induced a significantly greater expression of GLP-2R mRNA in the ileum compared with PN alone (PN alone, 0.86 ± 0.19; EN, 1.25 ± 0.17; GLP-2, 1.89 ± 0.37; EN+GLP-2, 1.60 ± 0.25; fold change compared to oral reference; GLP-2 main effect, P = 0.0171). GLP-2 treatment did not significantly alter GLP-2R mRNA expression in jejunum.
IGF-I Responses
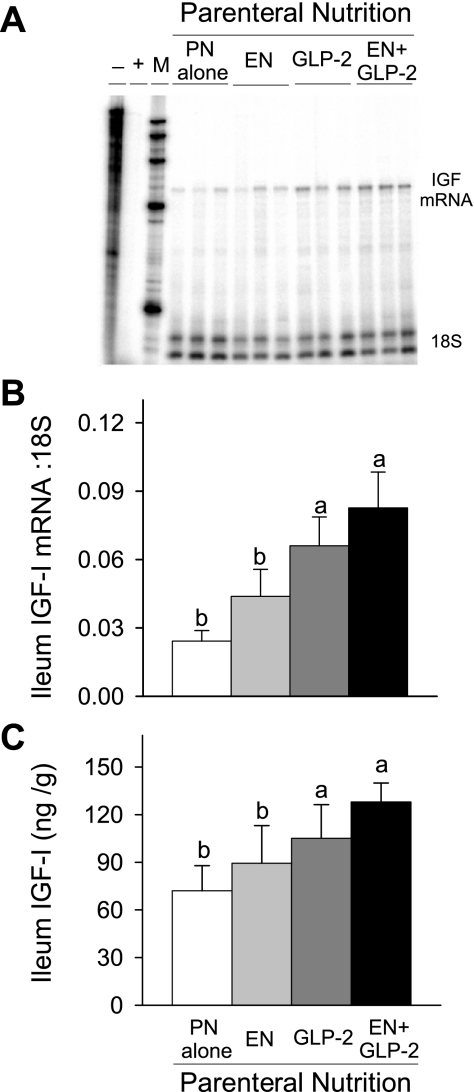
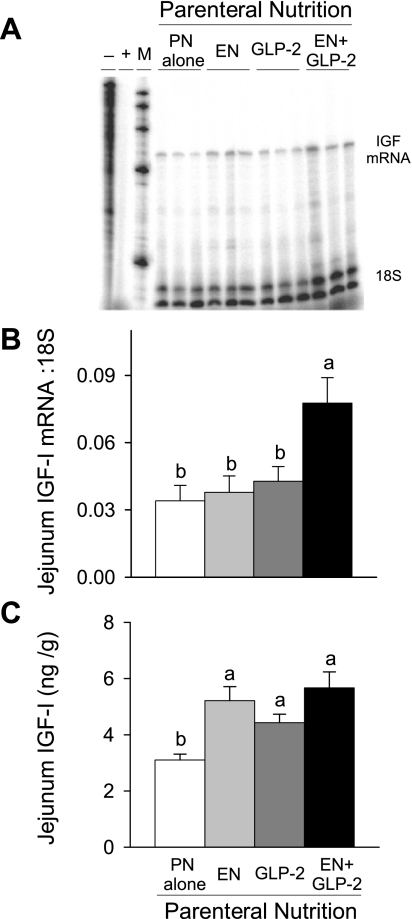
The concentration of IGF-I in plasma was not significantly different between treatment groups (PN alone, 34 ± 8; EN, 41 ± 6; GLP-2, 52 ± 3; EN+GLP-2, 46 ± 9 nmol/l). In conjunction with greater mucosa growth, treatment with GLP-2 and EN+GLP-2, but not individual treatment with EN, induced significantly greater IGF-I mRNA expression and IGF-I peptide concentration in ileum (Fig. 6). In jejunum, significant main effects for both EN (P < 0.025) and GLP-2 (P < 0.045) to increase IGF-I mRNA expression and IGF-I peptide concentration were observed, Fig. 7.
Fig. 6.
Representative bands from RNase protection assay of IGF-I mRNA (A), ileal IGF-I mRNA expression (B), and concentration of IGF-I peptide (C) in rats fed orally or maintained with PN for 7 days as follows: PN alone, EN, GLP-2, and EN+GLP-2. Ileal IGF-I mRNAs were determined by RNase protection assay. Thirty micrograms of ileal RNA was hybridized with 32P-labeled probes followed by RNase digestion and gel electrophoresis. Data were analyzed using OptiQuant software and expressed relative to 18S rRNA levels. Ileal IGF-I peptide concentration was determined by RIA. Values are means ± SE; n = 7–9 for ileal IGF-I mRNAs and n = 3–5 for ileal IGF-I peptide concentration. a,bMeans with different letter superscripts are significantly different (P < 0.05).
Fig. 7.
Representative bands from RNase protection assay of IGF-I mRNA (A), jejunal IGF-I mRNA expression (B) and concentration of IGF-I peptide (C) in rats maintained with PN for 7 days as follows: PN alone, EN, GLP-2, and EN+GLP-2. Jejunal IGF-I mRNAs were determined by RNase protection assay. Thirty micrograms of jejunal RNA was hybridized with 32P-labeled probes followed by RNase digestion and gel electrophoresis. Data were analyzed using OptiQuant software and expressed relative to 18S rRNA levels. Jejunal IGF-I peptide concentration was determined by RIA. Values are expressed as means ± SE; n = 7–9 for jejunal IGF-I mRNAs and n = 3–5 for jejunal IGF-I peptide concentration. a,bMeans with different letter superscripts are significantly different (P < 0.05).
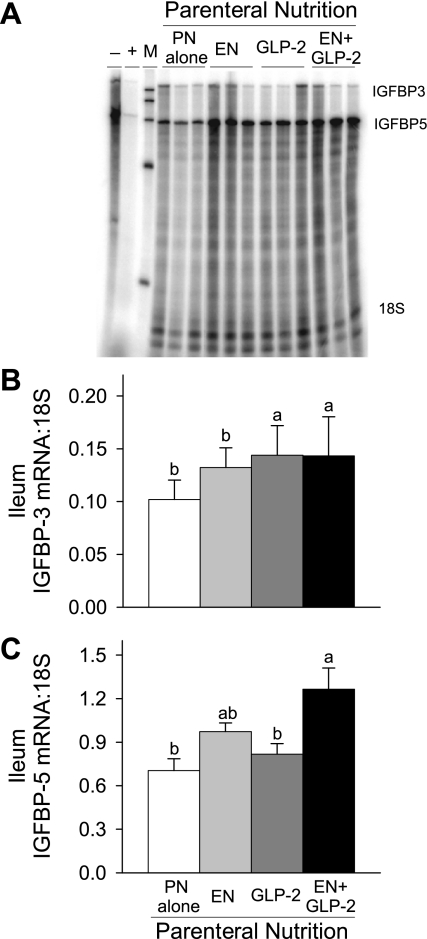
Expression of IGFBP-3 and IGFBP-5 was examined in jejunum and ileum in the same gel. Significant main effects for both EN (P = 0.0007) and GLP-2 (P = 0.0345) to induce greater IGFBP-5 mRNA expression in ileum were observed (Fig. 8). Treatment with GLP-2 and EN+GLP-2 induced significantly greater IGFBP-3 mRNA expression in ileum compared with the PN alone and EN groups. No significant differences between treatment groups were observed in jejunum for IGFBP-3 and IGFBP-5 mRNA expression (data not shown).
Fig. 8.
Representative bands from RNase protection assay of IGFBP-3 and IGFBP-5 mRNA (A), Ileal IGFBP-3 mRNA (B), and IGFBP-5 mRNA (C), expression in rats maintained with PN for 7 days as follows: PN alone, EN, GLP-2, and EN+GLP-2. Ileal IGFBP-3 and IGFBP-5 mRNAs were determined by RNase protection assay in the same gel. Thirty micrograms of ileal RNA was hybridized with 32P-labeled probes followed by RNase digestion and gel electrophoresis. Data were analyzed using OptiQuant software and expressed relative to 18S rRNA levels. Values are expressed as means ± SE; n = 5–8. a,bMeans with different letter superscripts are significantly different (P < 0.05).
Correlations Between GLP-2 and IGF-I System Components
We examined the correlations between IGF-I mRNA and GLP-2 system components in ileum, the primary site for GLP-2 secretion, using qRT-PCR. Significant positive correlations were observed between IGF-I mRNA and proglucagon mRNA expression (R2 = 0.43, P < 0.0001), IGF-I mRNA and GLP-2R mRNA expression (R2 = 0.47, P < 0.0001), and proglucagon mRNA and GLP-2R mRNA expression (R2 = 0.42, P = 0.0003). In addition, we observed a positive correlation between IGF-I mRNA expression and IGFBP-5 mRNA expression using RPA (R2 = 0.56, P < 0.0001).
DISCUSSION
IGF-I and GLP-2 are nutrient-regulated hormones that mediate, in part, the intestinotrophic effects of EN (10, 26, 28). Moreover, the actions of IGF-I and GLP-2 may be interrelated, as evidence from IGF-I knockout mice suggest that IGF-I is an essential mediator of the intestinotrophic actions of GLP-2 (11). The reversal of PN induced mucosal atrophy with GLP-2 administration in the current study was associated with greater expression of proglucagon and the GLP-2R, as well as IGF-I and IGFBP-3 mRNAs, and IGF-I peptide in the ileum. This suggests that the mucosal growth induced by GLP-2 is associated with upregulation of the IGF-I system in the intestine.
Treatment with the combination of EN+GLP-2 induced a synergistic increase in intestinal growth and sucrase segmental activity that was associated with significantly greater gain of body weight compared with EN, GLP-2, or PN alone (21). The GLP-2 receptor has only been identified in tissues found in the brain and the gastrointestinal tract (33). Moreover, several in vivo studies have shown that GLP-2 treatment does not induce changes in body weight or organ weight other than the intestine, which suggests that GLP-2 is an intestine-specific growth factor (1, 4, 32, 33). Using a larger dose of GLP-2 than that used in the present study (240 μg·kg body wt−1·day−1), Martin et al. (23) also noted that GLP-2 infusion reduced loss of body weight in PN rats with proximal bowel resection. Greater circulating IGF-I would improve whole body anabolism; however, plasma concentration of IGF-I was not different among the treatment groups. Thus, enhanced absorption of nutrients due to an enlarged mucosa mass, consistent with greater sucrase activity and the sucrose content of the diet, may have contributed to the greater gain of body weight in rats treated with EN+GLP-2.
Treatment with EN or GLP-2 alone resulted in significantly greater sucrase segmental activity, although only EN stimulated an increase in sucrase-specific activity. These differential changes in segmental and specific-sucrase activity suggest varying effects on enterocyte functional activity due to treatment with either EN or GLP-2, as previously noted in our rat model of short bowel syndrome (21). Thus, the synergistic effect of EN+GLP-2 to improve digestive capacity and gain in body weight reflects the complementary dual actions of EN to stimulate enterocyte differentiation and GLP-2 to stimulate enterocyte proliferation.
GLP-2 is secreted primarily by the ileum in response to EN and is rapidly inactivated by DPP-IV cleavage and cleared by the kidney (10). Rats given EN+GLP-2 showed a significant 28% increase in concentration of plasma GLP-2, a significant 102% increase in ileum proglucagon mRNA expression, no change in ileal DPP-IV specific activity, and a significant 75% decrease in plasma DPP-IV activity compared with GLP-2 alone. This suggests that EN potentiates the intestinotrophic effects of exogenous GLP-2 by increasing GLP-2 synthesis and reducing its cleavage by DPP-IV. Although GLP-2 induces intestinal growth by both endocrine and paracrine mechanisms, the ileum as a primary site for proglucagon synthesis may have a higher local concentration of GLP-2 and display more paracrine effects of GLP-2. This may explain, in part, why ileum showed a greater increase in proglucagon expression and subsequent mucosal growth in response to EN+GLP-2 compared with jejunum.
GLP-2 action is thought to depend on downstream mediators, such as the IGF-I paracrine system because the GLP-2R is not expressed on epithelial cells (10). The presence of the GLP-2R on subepithelial myofibroblasts (30), a primary site for IGF-I synthesis, provides a mechanism by which local GLP-2 may stimulate IGF-I synthesis, interaction with IGF-I receptors that are widespread in intestine, and promote epithelial cell proliferation (28). Consistent with IGF-I acting as a local downstream mediator of GLP-2, we found GLP-2 treatment significantly upregulated expression of GLP-2R mRNA, as well as IGF-I and IGFBP-3 mRNAs, and IGF-I peptide concentration in ileum. Administration of IGF-I or increased local expression of IGF-I is known to increase expression of its carrier protein, IGFBP-3 (31, 34). This study confirms our report in resected rats that a low dose of GLP-2 increases expression of the GLP-2R (18).
Treatment with EN+GLP-2 significantly increased both IGF-I and IGFBP-5 mRNA expression in ileum in association with synergistic ileal mucosal growth. IGFBP-5 is known to potentiate the intestinotrophic effects of IGF-I in PN and resected rats (14, 31), transgenic mice that overexpress IGF-I in mesenchyme (34), and cultured smooth muscle cells derived from the intestine (3, 9). Williams et al. (34) found that IGFBP-5 is specifically increased in the villus and pericryptal regions of the ileal lamina propria of mice that overexpress IGF-I in intestinal mesenchymal cells, and these mice show preferential paracrine growth effects on the ileal mucosal epithelium. Taken together, the synergistic growth in ileum due to treatment with EN+GLP-2 and its association with increased local expression of IGF-I and IGFBP-5, as well as significant positive correlations between IGF-I, proglucagon, and GLP-2R mRNA expression, support the hypothesis that IGF-I acts as a paracrine downstream mediator of GLP-2 in the current study.
EN alone did not show a significant effect to increase plasma concentration of GLP-2 and stimulate mucosal growth (2, 21, 26), in spite of the dramatic mucosal growth induced by treatment with EN+GLP-2. This may reflect the small amount of EN provided, only 43% of energy needs during the last 3 days of the study, as well as the semielemental nature of the liquid diet. Studies in the neonatal pigs indicate that 60% of energy requirements from EN are required to sustain normal plasma concentration of GLP-2 and mucosal growth (2).
In conclusion, we demonstrate for the first time in rats with PN-induced mucosal atrophy that proliferation of enterocytes due to GLP-2 is associated with greater expression of ileal proglucagon, GLP-2R, IGF-I, and IGFBP-3 mRNAs, as well as increased IGF-I peptide in ileum. Thus, our findings support the hypothesis that IGF-I is one of the downstream mediators of GLP-2 action in a physiological model of intestinal growth.
Perspectives and Significance
The primary physiological stimulus for maintaining enterocyte turnover is the presence of nutrients in the gastrointestinal tract. Our data in the model of PN-induced mucosal atrophy show no effect of a small amount of EN alone to induce mucosal growth but a dramatic synergy between the presence of EN and administration of a low-dose of GLP-2 to promote epithelial cell proliferation and digestive capacity. These data emphasize the importance of encouraging EN when GLP-2 is administered to humans with intestinal failure. The hypothesis that IGF-I is a downstream mediator of the intestinotrophic effects of GLP-2 is primarily based on a report in which IGF-I knockout mice did not show growth in response to administration of GLP-2 (11). The current study provides strong evidence in a physiological model of intestinal atrophy and regrowth for this hypothesis, that is, the intestinal paracrine IGF-I system may mediate the intestinotrophic effects of GLP-2. Further studies are needed to understand the cellular mechanisms by which GLP-2 and IGF-I may interact to stimulate mucosal growth.
GRANTS
This work was supported by National Institutes of Health Grants R01-DK-42835 and by funds from the U.S. Department of Agriculture Cooperative State Research, Education and Extension Service, project WISO 3433, University of Wisconsin-Madison.
Acknowledgments
We thank Michael J. Grahn for his expert technical assistance. We acknowledge the generous contribution of Vital formula and the input of Dr. Jeff Baxter at Abbott Nutrition.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Burrin DG, Stoll B, Guan X, Cui L, Chang X, Holst JJ. Glucagon-like peptide 2 dose-dependently activates intestinal cell survival and proliferation in neonatal piglets. Endocrinology 146: 22–32, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Burrin DG, Stoll B, Jiang R, Chang X, Hartmann B, Holst JJ, Greeley GH Jr and Reeds PJ. Minimal enteral nutrient requirements for intestinal growth in neonatal piglets: how much is enough? Am J Clin Nutr 71: 1603–1610, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Bushman TL, Kuemmerle JF. IGFBP-3 and IGFBP-5 production by human intestinal muscle: reciprocal regulation by endogenous TGF-beta1. Am J Physiol Gastrointest Liver Physiol 275: G1282–G1290, 1998. [DOI] [PubMed] [Google Scholar]
- 4.Chance WT, Foley-Nelson T, Thomas I, Balasubramaniam A. Prevention of parenteral nutrition-induced gut hypoplasia by coinfusion of glucagon-like peptide-2. Am J Physiol Gastrointest Liver Physiol 273: G559–G563, 1997. [DOI] [PubMed] [Google Scholar]
- 5.Dahlqvist A Method for assay of intestinal disaccharidases. Anal Biochem 57: 18–25, 1964. [DOI] [PubMed] [Google Scholar]
- 6.Dahly EM, Gillingham MB, Guo Z, Murali SG, Nelson DW, Holst JJ, Ney DM. Role of luminal nutrients and endogenous GLP-2 in intestinal adaptation to mid-small bowel resection. Am J Physiol Gastrointest Liver Physiol 284: G670–G682, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Dahly EM, Guo Z, Ney DM. Alterations in enterocyte proliferation and apoptosis accompany TPN-induced mucosal hypoplasia and IGF-I-induced hyperplasia in rats. J Nutr 132: 2010–2014, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Dahly EM, Guo Z, Ney DM. IGF-I augments resection-induced mucosal hyperplasia by altering enterocyte kinetics. Am J Physiol Regul Integr Comp Physiol 285: R800–R808, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Duan C, Clemmons DR. Differential expression and biological effects of insulin-like growth factor-binding protein-4 and -5 in vascular smooth muscle cells. J Biol Chem 273: 16836–16842, 1998. [DOI] [PubMed] [Google Scholar]
- 10.Dube PE, Brubaker PL. Frontiers in glucagon-like peptide-2: multiple actions, multiple mediators. Am J Physiol Endocrinol Metab 293: E460–E465, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Dube PE, Forse CL, Bahrami J, Brubaker PL. The essential role of insulin-like growth factor-1 in the intestinal tropic effects of glucagon-like peptide-2 in mice. Gastroenterology 131: 589–605, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Dube PE, Rowland KJ, Brubaker PL. Glucagon-like peptide-2 activates beta-catenin signaling in the mouse intestinal crypt: role of insulin-like growth factor-I. Endocrinology 149: 291–301, 2008. [DOI] [PubMed] [Google Scholar]
- 13.Estall JL, Drucker DJ. Glucagon-like peptide-2. Annu Rev Nutr 26: 391–411, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Gillingham MB, Kritsch KR, Murali SG, Lund PK, Ney DM. Resection upregulates the IGF-I system of parenterally fed rats with jejunocolic anastomosis. Am J Physiol Gastrointest Liver Physiol 281: G1158–G1168, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Hartmann B, Johnsen AH, Orskov C, Adelhorst K, Thim L, Holst JJ. Structure, measurement, and secretion of human glucagon-like peptide-2. Peptides 21: 73–80, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Jeppesen PB, Hartmann B, Thulesen J, Graff J, Lohmann J, Hansen BS, Tofteng F, Poulsen SS, Madsen JL, Holst JJ, Mortensen PB. Glucagon-like peptide 2 improves nutrient absorption and nutritional status in short-bowel patients with no colon. Gastroenterology 120: 806–815, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Jeppesen PB, Sanguinetti EL, Buchman A, Howard L, Scolapio JS, Ziegler TR, Gregory J, Tappenden KA, Holst J, Mortensen PB. Teduglutide (ALX-0600), a dipeptidyl peptidase IV resistant glucagon-like peptide 2 analogue, improves intestinal function in short bowel syndrome patients. Gut 54: 1224–1231, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koopmann MC, Nelson DW, Murali SG, Liu X, Brownfield MS, Holst JJ, Ney DM. Exogenous glucagon-like peptide-2 (GLP-2) augments GLP-2 receptor mRNA and maintains proglucagon mRNA levels in resected rats. JPEN J Parenter Enteral Nutr 32: 254–265, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Labarca C, Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem 102: 344–352, 1980. [DOI] [PubMed] [Google Scholar]
- 20.Lasekan JB, Rivera J, Hirvonen MD, Keesey RE, Ney DM. Energy expenditure in rats maintained with intravenous or intragastric infusion of total parenteral nutrition solutions containing medium- or long-chain triglyceride emulsions. J Nutr 122: 1483–1492, 1992. [DOI] [PubMed] [Google Scholar]
- 21.Liu X, Nelson DW, Holst JJ, Ney DM. Synergistic effect of supplemental enteral nutrients and exogenous glucagon-like peptide 2 on intestinal adaptation in a rat model of short bowel syndrome. Am J Clin Nutr 84: 1142–1150, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25: 402–408, 2001. [DOI] [PubMed] [Google Scholar]
- 23.Martin GR, Wallace LE, Sigalet DL. Glucagon-like peptide-2 induces intestinal adaptation in parenterally fed rats with short bowel syndrome. Am J Physiol Gastrointest Liver Physiol 286: G964–G972, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Munroe DG, Gupta AK, Kooshesh F, Vyas TB, Rizkalla G, Wang H, Demchyshyn L, Yang ZJ, Kamboj RK, Chen H, McCallum K, Sumner-Smith M, Drucker DJ, Crivici A. Prototypic G protein-coupled receptor for the intestinotrophic factor glucagon-like peptide 2. Proc Natl Acad Sci USA 96: 1569–1573, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagatsu T, Hino M, Fuyamada H, Hayakawa T, Sakakibara S. New chromogenic substrates for X-prolyl dipeptidyl-aminopeptidase. Anal Biochem 74: 466–476, 1976. [DOI] [PubMed] [Google Scholar]
- 26.Nelson DW, Murali SG, Liu X, Koopmann MC, Holst JJ, Ney DM. Insulin-like growth factor I and glucagon-like peptide-2 responses to fasting followed by controlled or ad libitum refeeding in rats. Am J Physiol Regul Integr Comp Physiol 294: R1175–R1184, 2008. [DOI] [PubMed] [Google Scholar]
- 27.Nelson DW, Sharp JW, Brownfield MS, Raybould HE, Ney DM. Localization and activation of glucagon-like peptide-2 receptors on vagal afferents in the rat. Endocrinology 148: 1954–1962, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Ney DM Insulin-like growth factor in relation to gastrointestinal diseases and parenteral nutrition. Totowa, NY: Humana Press, 2005, p. 271–289.
- 29.Ney DM, Yang H, Smith SM, Unterman TG. High-calorie total parenteral nutrition reduces hepatic insulin-like growth factor-I mRNA and alters serum levels of insulin-like growth factor-binding protein-1, -3, -5, and -6 in the rat. Metabolism 44: 152–160, 1995. [DOI] [PubMed] [Google Scholar]
- 30.Orskov C, Hartmann B, Poulsen SS, Thulesen J, Hare KJ, Holst JJ. GLP-2 stimulates colonic growth via KGF, released by subepithelial myofibroblasts with GLP-2 receptors. Regul Pept 124: 105–112, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Peterson CA, Gillingham MB, Mohapatra NK, Dahly EM, Adamo ML, Carey HV, Lund PK, Ney DM. Enterotrophic effect of insulin-like growth factor-I but not growth hormone and localized expression of insulin-like growth factor-I, insulin-like growth factor binding protein-3 and -5 mRNAs in jejunum of parenterally fed rats. JPEN J Parenter Enteral Nutr 24: 288–295, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Scott RB, Kirk D, MacNaughton WK, Meddings JB. GLP-2 augments the adaptive response to massive intestinal resection in rat. Am J Physiol Gastrointest Liver Physiol 275: G911–G921, 1998. [DOI] [PubMed] [Google Scholar]
- 33.Sinclair EM, Drucker DJ. Proglucagon-derived peptides: mechanisms of action and therapeutic potential. Physiology 20: 357–365, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Williams KL, Fuller CR, Fagin J, Lund PK. Mesenchymal IGF-I overexpression: paracrine effects in the intestine, distinct from endocrine actions. Am J Physiol Gastrointest Liver Physiol 283: G875–G885, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Yang H, Grahn M, Schalch DS, Ney DM. Anabolic effect of IGF-I coinfused with total parenteral nutrition in dexamethasone-treated rats. Am J Physiol Endocrinol Metab 266: E690–E698, 1994. [DOI] [PubMed] [Google Scholar]