Abstract
Background and Aims
Nitrogen (N) remobilization is the major source of N for grain filling in wheat, the other being N uptake after anthesis (Nup); however, variations in remobilization efficiency are not fully understood. It is hard to tell whether the source or the sink effects predominate, because N in the culm at anthesis (Nant) correlates strongly with both N remobilization (Nrem) and grain number (Gn), respectively the main source and the main sink.
Methods
A pot experiment was thus designed to assess the relative contributions of the source and sink to Nrem regulation. Using two cultivars of winter wheat (Triticum aestivum, ‘Apache’ and ‘Autan’), three pre-anthesis and two post-anthesis N fertilization levels were applied in order to vary the N sources, while ear trimming at anthesis reduced sink size.
Key Results
Unlike results observed at a scale of m2, the equation binding Nant to Nrem exhibited a negative intercept, challenging the concept of nitrogen remobilization efficiency. Before ear trimming, Gn fitted well to Nant, with a slope dependent on genotype. To obtain a sink variable that was less correlated with Nant, the difference δGn was calculated between actual grain number and that which could be predicted from culm N before trimming. A multiple regression then predicted Nrem (r2 = 0·95) from Nant, Nup and δGn, with fitting unbiased by fertilization treatment, trimming or genotype.
Conclusions
In untrimmed culms, δGn had a negligible effect, so that Nrem could be fitted to Nant and Nup only: grain N filling appeared to be determined by sources only (Nant and Nup), not by sink, and the reduction of Nrem by Nup was quantified. In these ‘normal’ cases, the regulation of Nrem should thus be located within the N sources themselves. In contrast, ear-trimming needs to be considered with caution as it introduced a sink limitation on Nrem; moreover one with an important genotype effect.
Key words: Triticum aestivum, winter wheat, source/sink, grain filling, nitrogen uptake, grain number, nitrogen harvest index, nitrogen remobilization efficiency, genotype × environment
INTRODUCTION
Grain protein concentration (GPC) is one of the principal criteria listed in grain specifications for both the processing of wheat flour (Triticum aestivum) and the export of grain (Gooding et al., 1994). In a world seeing an ever-growing demand for grain production, increasing grain nitrogen (N) yield is the only way to improve GPC. Thus many genotype and/or management research programs have been undertaken to improve N yield under a range of climatic conditions (Brancourt-Hulmel et al., 2003, 2005). Environmental concerns also require optimized use of the two sources of grain N filling, i.e. (1) the remobilization of nitrogen (Nrem) already present in vegetative parts at anthesis (Nant), and (2) post-anthesis nitrogen uptake (Nup; see Table 1 for a list of abreviations used in the text).
Table 1.
Abbreviations used in the text
| Nant | N amount in vegetative parts, including roots, at anthesis (after ear trimming, if performed) |
| Nant,i | N amount before ear trimming |
| Gn | Ear grain number measured at the end of grain filling |
| δGn | Difference between actual Gn and its predicted value obtained from the genotype-dependant correlation to Nant,i |
| Nup | Nitrogen uptake by the culm after anthesis |
| Nrem | Nitrogen remobilization from vegetative parts during grain filling |
| GPC | Grain protein concentration at the end of grain filling |
| NRE | Nitrogen remobilization efficiency (ratio of Nrem to Nant) |
However, the factors that determine N fluxes during grain filling remain far from clear. According to Martre et al. (2003), grain N yield is mostly source-limited, while Barbottin et al. (2005) suggested that sink capacity was the main determinant for variations in Nrem. In fact, it is difficult to distinguish between sink and source, as they are so strongly correlated that grain number can be accurately modelled using Nant (Abbate et al., 1995; Oscarson, 2000; Demotes-Mainard and Jeuffroy, 2001). Some modellers have even suggested discarding the use of grain number, leading to sink-less models (Sinclair and Jamieson, 2006, 2008). As early as 1979, Martinez-Carrasco and Thorne used surgical ear trimming at around anthesis to disconnect the sink and source, and numerous subsequent papers have reported similar treatments. Both GPC and amount of N per grain always increased following trimming, reaching levels far higher than those observed in control plants, while N yield per ear generally declined, with marked genotype and treatment interactions. According to Ma et al. (1996), N yield was only maintained in some cultivars following moderate trimming (25 % of grains removed); however, trimming experiments did not provide clear evidence for a sink limitation of grain N filling. Indeed, several reports have suggested that as well as modifications to the sink, trimming could also induce a fall in Nrem or Nup, and thus cause a decrease in the source (Mi et al., 2000). Data on the quantitative N balance at the whole-plant level would therefore be welcome in trimming studies, but have seldom been reported.
The simplest way to modify the source/sink ratio during grain N filling may be to increase Nup by delaying the last application of fertilizer. This type of crop management has been widely employed in France during the past 10 years; nitrogen application rates are split into three parts so that the last fertilization is delayed to around heading. However, fertilization cannot be delayed indefinitely, because the efficiency of N uptake rapidly declines after anthesis, possibly because of leaf senescence. The improved N yield of ‘stay green’ cultivars largely arises from their continued N uptake after anthesis, probably because of delayed leaf senescence (Borrell et al., 2001). Generally speaking, it appears that a higher level of N uptake after anthesis leads to delayed N decline in vegetative tissues (Martre et al., 2006). In an opinion paper, Barneix (2007) even suggested N uptake and remobilization might be mutually incompatible due to reciprocal inhibition: N remobilization would only – and irreversibly – start once N uptake had slowed down. This point of view is difficult to sustain at the whole-plant level, as early leaves have already senesced well before anthesis, at a time when N uptake is very high. Instead, it should be noted that Nrem is actually a balance between N input and output from vegetative tissues, the former probably increasing in line with Nup levels. Regarding the whole period of grain filling, late fertilizer applications have also been shown to reduce the efficiency of N remobilization (Gooding et al., 2007). Conversely, Triboï and Triboï-Blondel (2002) noted that plants with a lower Nant, and hence a lower Nrem capacity, have a greater propensity to take up nitrogen after anthesis.
In fact, most of the evidence for antagonism between Nrem and Nup is based on variations in N concentrations in vegetative parts at the end of the grain filling period. When available, quantitative N balances at the whole-plant level tend to suggest that the losses in N yield due to antagonism between Nup and Nrem are quite small. This may be due to the fact that under current crop management methods, Nup is much lower than Nrem during grain filling (Van Sanford and MacKown, 1987); therefore any Nup antagonism to Nrem would remain moderate and can thus be ignored. This situation is favourable for farmers; however, it could be reversed, because current trends in both plant breeding and crop management are leading to an increase in post-anthesis N uptake. The antagonism between Nrem and Nup thus needs to be quantified under conditions that lead to higher Nup/Nrem ratios, because gains in N availability through an increase in Nup may be counteracted by a decrease in Nrem; in which case, the apparent efficiency of fertilizers would be reduced by increasing the amount of N wasted in straw.
This paper focuses on the regulation of Nrem using two wheat cultivars that differ in terms of their grain N filling. A broad range in Nant/Nup ratios was obtained by combining three levels of N fertilization before anthesis with two levels afterwards. The specific effect of grain number reduction through ear trimming was also studied. Analyses of variance and multiple correlations were used to explore the regulatory relationships between Nrem, Nup and grain N filling.
MATERIALS AND METHODS
Culture and treatments
Two modern cultivars (‘Apache’ and ‘Autan’) of winter wheat (Triticum aestivum L.) were chosen because of their contrasting patterns of N metabolism. In preliminary field trials, ‘Apache’ always produced more ears per m2, while grain numbers per ear (main culm and tiller mixed) were similar in the two genotypes. N uptake per m2 before anthesis was greater in ‘Apache’, but N uptake after anthesis was higher in ‘Autan’. The mean grain weight, GPC and amount of N per grain were higher in ‘Autan’.
Seeds were sown on 20 October 2004 in an experimental field at the INRA station in Thiverval-Grignon, France (48°50′N; 1°57′E) at a density of 250 seeds m−2, under either high or no N fertilization (no-N). Highly fertilized plots were sown in a silt loam, a typical Eutrochrept soil (according to soil taxonomy), while no-N plots were sown in the poorest areas in a sandy embankment. On 25 March, plants in the plots had reached the beginning of stem elongation (growth stage = 31, according to Zadoks et al., 1974). No-N plots exhibited a nitrogen nutrition index of 0·4 (NNI; Justes et al., 1994), while the NNI of highly fertilized plots was 0·9. About 560 plants were carefully collected from each of the no-N plots and 280 plants from each of the high-N plots. Their roots were washed extensively before plants were transplanted into perlite-filled pots (two plants per 1·7-L pot) that were then placed outside. The average temperature was 11·5 °C during the next 2 months before anthesis and 17·4 °C during the 2 months after that. During pot culture prior to anthesis, one half of the plants transplanted from the no-N plot received 2·7 mg N per plant on a weekly basis; all other plants received 10·8 mg N per plant. Apart from nitrogen, all plants received the same full fertilization each week, and water when required depending on the weather. Three levels of early fertilization (applied before anthesis) were thus attained: some plants had received low fertilization (low in field, then low in pot), while others had received increasing fertilization (low in field, then high in pot), and others had received high fertilization (high in field, then high in pot).
Ear emergence was recorded for every main culm in order to select 84 pots bearing synchronous plants within each cultivar and early fertilization treatment group. After anthesis, one half of the selected plants received low fertilization, while the other half received high fertilization (1·8 and 7·2 mg N weekly per plant, respectively). Obviously, this latter fertilization could only be absorbed after anthesis; however, because the pots were not washed at anthesis, some of the fertilizer applied before anthesis may have been absorbed afterwards. Thus early and late fertilization could not be regarded as strictly equivalent to N uptake before and after anthesis, respectively. Conversely, N supply and N harvest were balanced over the whole season, suggesting that over this time scale (that of the study) no N leaching occurred from pots.
Within 2 d of anthesis, the ears on one half of the plants were trimmed by removing all the odd-numbered spikelets. The experiment as a whole thus consisted 24 treatments with four factors: two genotypes; three early fertilizations; two late fertilizations; and two ear-trimming treatments (Table 2). This provided a fully cross-factor experimental set-up designed to analyse individual effects and interactions. Each treatment was applied to 24 pots, but hereafter only the first and last sampling times are considered, i.e. six pots per treatment, which were harvested as detailed below.
Table 2.
Summary of the different genotypes and treatments (crossing of genotype, fertilization before/after anthesis and ear reduction) used in the study. Treatment identities are used in Fig. 3
| Treatment identity | Genotype | Early fertilization | Late fertilization | Ear reduction |
|---|---|---|---|---|
| A | ‘Apache’ | Low | Low | Control |
| B | ‘Apache’ | Low | Low | 50 % trimming |
| C | ‘Apache’ | Low | High | Control |
| D | ‘Apache’ | Low | High | 50 % trimming |
| E | ‘Apache’ | Increasing | Low | Control |
| F | ‘Apache’ | Increasing | Low | 50 % trimming |
| G | ‘Apache’ | Increasing | High | Control |
| H | ‘Apache’ | Increasing | High | 50 % trimming |
| I | ‘Apache’ | High | Low | Control |
| J | ‘Apache’ | High | Low | 50 % trimming |
| K | ‘Apache’ | High | High | Control |
| L | ‘Apache’ | High | High | 50 % trimming |
| M | ‘Autan’ | Low | Low | Control |
| N | ‘Autan’ | Low | Low | 50 % trimming |
| O | ‘Autan’ | Low | High | Control |
| P | ‘Autan’ | Low | High | 50 % trimming |
| Q | ‘Autan’ | Increasing | Low | Control |
| R | ‘Autan’ | Increasing | Low | 50 % trimming |
| S | ‘Autan’ | Increasing | High | Control |
| T | ‘Autan’ | Increasing | High | 50 % trimming |
| U | ‘Autan’ | High | Low | Control |
| V | ‘Autan’ | High | Low | 50 % trimming |
| W | ‘Autan’ | High | High | Control |
| X | ‘Autan’ | High | High | 50 % trimming |
Sampling procedure
Three pots containing two plants each were harvested from each treatment at anthesis (17–26 May, depending on the treatment) and at physiological maturity (around 15 July). The roots and above-ground parts were collected, and within the above-ground parts the main culms were separated from tillers. Roots were recovered quantitatively, but they were usually fragmented and therefore could not be directly attributed to either the main culm or the tillers. Instead, the N ratio of the main culm to tillers was calculated for all above-ground parts of each sample, and used to split the corresponding root nitrogen between the main culm and tillers. The samples were dried for 48 h at 80 °C except for the ears, which were freeze-dried. The grains were then separated from the chaff. The spikelets removed from the trimmed ears were also reserved for further analysis. Tiller number and growth varied considerably within a treatment group. Moreover, under some treatments tillers appeared after anthesis and headed without elongation of their stem. The data concerning tillers should therefore be regarded as being of little significance; hereafter in this paper, only the main culm is considered.
Assessments of N balances
The dry weight of each sample was measured and then finely ground, and N concentrations were subsequently determined using Dumas' combustion method. N uptake (Nup) and net remobilization from vegetative organs (Nrem) were derived from Ruske et al. (2003) except that, unless specifically indicated, these equations also involved root N:
| 1 |
| 2 |
Hereafter in this paper N at anthesis (Nant) and grain number (Gn) both refer to the values obtained after ear trimming, in cases where this was done. In some specified cases, estimates prior to trimming (Ni,ant and Gi,n) are also used. The initial grain number (Gi,n) in trimmed ears could be estimated as being twice the value of Gn recorded after trimming. Trimming also removed some chaff N, which was measured in corresponding samples. Nitrogen before trimming (Ni,ant) was calculated by correcting Nant for the nitrogen discarded in the spikelets that were removed.
Data analysis
Analyses of variance were performed using Statgraphics Plus (Manugistics, Inc., Rockville, MA) to examine the effects of different genotypes and treatments on various N remobilization parameters. The individual effects of genotype, early fertilization, late fertilization and ear trimming were analysed, as well as their first-level interactions. Statistically significant differences were then determined using the Newman–Keuls test with an overall error rate α = 0·01.
The contributions of Nant, Gn and Nup to Nrem were analysed using simple and multiple linear regressions. Slopes and intercepts of the regression lines between genotypes were tested for significance and compared using the specific Statgraphics Plus procedure. Finally, analyses of variance were performed on the residuals of the regressions thus described, in order to detect any bias linked to either genotype, early fertilization, late fertilization or ear trimming.
RESULTS
General features of treatments
The various genotypes and treatments resulted in broad variations in main-culm nitrogen at anthesis (Nant; Table 3). Analysis of variance indicated no significant difference in Nant between genotypes under low, early fertilization (17 ± 3 mg culm−1; mean ± s.e.), while under increasing or high fertilization, Nant was higher in ‘Apache’ than in ‘Autan’ (up to 48 ± 5 vs. 39 ± 2 mg culm−1, respectively). Despite the fact that trimming discarded some chaff N (4 ± 1 mg culm−1, averaged over all treatments), Nant was not significantly lower in trimmed plants (P > 0·05).
Table 3.
(a) Nitrogen variables in main culms for the different genotypes and treatments, as listed in Table 2. Anthesis N (Nant) is the amount of N in vegetative parts (including roots) at anthesis (after ear trimming, in cases where this was done); ear grain number (Gn) was obtained at the end of grain filling; N uptake (Nup) and remobilization (Nrem) were calculated from the differences between data at anthesis and at the end of grain filling. (b) ANOVA was performed by fully crossing the effects of genotype, early fertilization, late fertilization and ear reduction, as well as first-order interactions between the different factors
| (a) N variables | ||||||
|---|---|---|---|---|---|---|
| Nant (mg) | Gn | Grain N (mg) | Nup (mg) | Nrem (mg) | NRE | |
| Genotype (G) | ||||||
| ‘Apache’ | 30a | 38b | 1·4a | 26a | 21b | 0·67b |
| ‘Autan’ | 26a | 29a | 1·5b | 28a | 15a | 0·56a |
| Early fertilization (E) | ||||||
| Low | 17a | 24a | 1·4a | 24a | 10a | 0·57a |
| Increasing | 27b | 33b | 1·5a | 30a | 17b | 0·61ab |
| High | 42c | 42c | 1·5a | 27a | 28c | 0·66b |
| Late fertilization (L) | ||||||
| Low | 28a | 34a | 1·3a | 23a | 19a | 0·64a |
| High | 28a | 33a | 1·5b | 31b | 17a | 0·58a |
| Ear reduction (R) | ||||||
| Control | 30a | 44b | 1·2a | 30a | 21b | 0·68a |
| 50 % trimming | 27a | 22a | 1·7b | 24a | 16a | 0·55a |
| (b) ANOVA | ||||||
|---|---|---|---|---|---|---|
| Nant (mg) | Gn | Grain N (mg) | Nup (mg) | Nrem (mg) | NRE | |
| G | *** | ** | ns | *** | *** | |
| E | *** | *** | ns | ns | *** | * |
| L | nd | ns | *** | * | ns | * |
| R | ns | *** | *** | *** | *** | |
| G × E | *** | ns | ** | ns | ||
| G × L | nd | ns | ns | ns | ns | ns |
| G × R | ns | ns | ** | ** | ||
| E × L | nd | ns | ns | ns | ns | ns |
| E × R | ns | *** | * | ns | ns | |
| L × R | nd | ns | ns | ns | ns | ns |
In (a) different letters indicate a significant difference between means (P < 0·01; n = 72).
In (b) * P < 0·01; ** P < 0·001; *** P < 0·0001; ns denotes non-significant effects (P > 0·05); nd indicates that the effect of late fertilization did not apply to Nant.
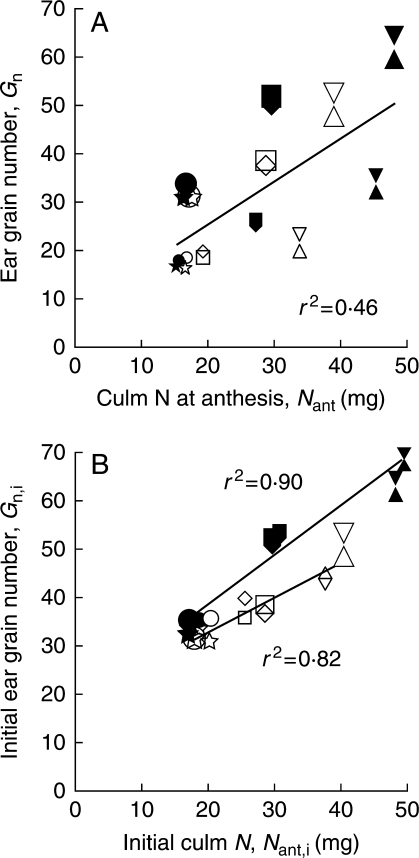
Grain number (Gn) ranged from 16·3 ± 0·9 in trimmed ears of ‘Autan'following low, early fertilization to 63·2 ± 2·5 in control ears of ‘Apache’ following high, early fertilization. The experimental conditions resulted in very large variations in both Nant and Gn, but source and sink always remained correlated, except in the case of the trimming treatment. The overall correlation of Nant to Gn was moderate, with r2 = 0·46 for n = 72 (Fig. 1A), whereas estimates of culm N and grain number before trimming (Nant,i and Gn,i, respectively) were very strongly correlated (Fig. 1B), with the slope of the regression being significantly affected by genotype (P < 0·01):
| 3a |
| 3b |
Fig. 1.
Relationships between culm N at anthesis (including roots) and grain number. (A) Results after selected plants were trimmed at anthesis by removing one longitudinal half of the ear. (B) Genotype-dependant correlations between initial parameters before trimming. Each point is the mean of three pots containing two main culms. Closed symbols represent the genotype ‘Apache’ and open symbols the genotype ‘Autan’; large symbols represent culms with whole ears and small symbols culms with trimmed ears. The various symbols refer to level of N fertilization before/after anthesis: low/low, circles; low/high, stars; medium/low, squares; medium/high, diamonds; high/low, triangles; high/high, inverted triangles. In (A) the regression line and correlation coefficient apply to the whole data set (d.f. = 71); in (B) two correlations are presented for the genotypes ‘Apache’ (closed symbols) and ‘Autan’ (open symbols).
The amount of N per grain was significantly lower in ‘Apache’ than in ‘Autan’ (P < 0·001; Table 3), but a highly significant interaction in genotype × ear reduction (G × R; P < 0·001) was observed because this difference was no longer significant when trimming had been performed. In trimmed ears, the amount of N per grain increased up to 1·7 ± 0·1 mg grain−1 in both genotypes. The amount of N per grain was increased by late fertilization (P < 0·0001), but not by early fertilization (P > 0·05) A significant interaction in early fertilization × ear reduction (E × R; P < 0·01), however, suggested that both increasing and high, early fertilization resulted in higher N amounts per grain, but only in trimmed ears.
N uptake after anthesis (Nup) ranged from 12 ± 2 to as much as 45 ± 3 mg culm−1 under the A and W treatments (see Table 2), respectively (Table 3), while its ratio to final N yield ranged from 0·4 ± 0·1 to 0·9 ± 0·3. No significant effect of early fertilization on Nup was observed (P > 0·05), while late fertilization clearly enhanced Nup (P < 0·01). N uptake declined following ear trimming in ‘Autan’ (33 ± 3 vs. 22 ± 3 mg culm−1), whereas it was not affected in ‘Apache’ (27 ± 2 vs. 26 ± 2 mg culm−1). N uptake did not correlate with either Nant or Gn (r2 < 0·12 for n = 72).
The amount of N remobilized from vegetative parts after anthesis (Nrem) ranged from 7 to 36 mg culm−1, and exhibited highly significant effects of genotype, early fertilization and ear trimming (P < 0·0001); only the effect of late fertilization on Nrem was not significant (P > 0·05). The average decrease in Nrem due to trimming was 3 ± 1 and 7 ± 2 mg culm−1 in ‘Apache’ and ‘Autan’, respectively. In ‘Autan’ therefore, but not in ‘Apache’, the decrease in Nrem due to trimming exceeded the amount of N in discarded spikelets. A highly significant G × E interaction (P < 0·001) was observed because the response of Nrem to early fertilization was stronger in ‘Apache’ than in ‘Autan’ (after low, increasing and high fertilization before anthesis, respectively: 10 ± 1, 19 ± 1 and 34 ± 2 mg culm−1 in ‘Apache’ vs. 9 ± 1; 15 ± 3 and 22 ± 2 mg culm−1 in ‘Autan’).
Lastly, N remobilization efficiency (NRE: ratio of Nrem to Nant; Table 3) ranged from 0·28 ± 0·13 to 0·76 ± 0·02, and varied highly significantly with genotype and ear trimming (P < 0·0001). Indeed, highly significant G × R interactions (P < 0·001) were observed because trimming clearly reduced NRE in ‘Autan’, but hardly at all in ‘Apache’. High, early fertilization increased NRE when compared to low, early fertilization (P < 0·01), whereas high, late fertilization decreased NRE (P < 0·01).
Determining N remobilization from vegetative parts
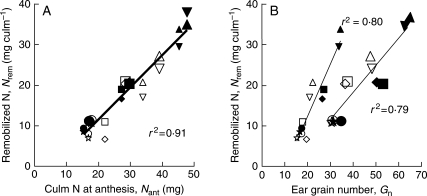
Figure 2A indicates that Nrem was very strongly correlated to Nant (r2 = 0·91; n = 72), with an intercept significantly different from zero. Analysis of variance of the residuals of this regression indicated that it was biased by genotype (P < 0·0001), early (P < 0·01) and late (P < 0·001) fertilization, as well as by ear trimming (P < 0·0001). Quite clearly, Nant is a major determinant of Nrem, but it is not alone. Grain number was also well correlated with Nrem (Fig. 2B), but a comparison of the regression lines indicates they were markedly affected by trimming treatment (P < 0·001); the two regression lines are shown in Fig. 2, indicating r2 at 0·79 and 0·80 (n = 36) for control and trimmed culms, respectively. However, analysis of variance of the residuals indicated that this trimming-dependent correlation was in turn biased by genotype (P < 0·01) and early fertilization (P < 0·001), but not by late fertilization (P > 0·05). Lastly, despite very marked variations in Nup, within the same range as for Nant, no correlation was observed when Nup was plotted against Nrem (r2 = 0·03, n = 72, data not shown).
Fig. 2.
Relationship between the amount of remobilized nitrogen from vegetative parts, including roots (Nrem) and (A) nitrogen in vegetative parts at anthesis, after trimming (Nant), and (B) actual grain number (Gn). Each point is the mean of three pots containing two main culms. Symbols represent the various genotypes and treatments as in Fig. 1. In (A) the regression line and correlation coefficient apply to the whole data set (d.f. = 71), while in (B) two correlations are given for whole ears (large symbols) and trimmed ears (small symbols).
Multiple regression analysis of N remobilization
Nrem was linearly correlated with the main determinants identified above, leading to the following multiple regression (r2 = 0·94 for n = 72):
| 4 |
The coefficients for Nant and Gn were positive and highly significant (P < 0·0001), but the negative coefficient for Nup was much less significant (P > 0·01). Lastly, the negative intercept of the relationship was clearly different from zero (P < 0·0001). Such a correlation should be regarded with caution, however, as Nant and Gn were far from being independent from each other. Moreover, the correlation exhibited bias for genotype and late fertilization (P < 0·01), as well as a G × R interaction (P < 0·0001): eqn (4) under-estimated Nrem in trimmed ‘Apache’ by −1·7 ± 0·2 mg culm−1 and over-estimated Nrem in trimmed ‘Autan’ by 1·8 ± 0·1 mg culm−1. In addition, the regression did not discriminate between genotypes despite the fact that their reaction to trimming was clearly different.
To obtain a sink variable that was less correlated to Nant than Gn, the difference δGn was then calculated between Gn and grain number, which could be predicted from Nant,i using eqn (3). Using δGn instead of Gn, a new multiple regression analysis of Nrem was obtained (eqns 5a, b), which had a high level of significance (r2 = 0·95 for n = 72) and a constant that did not differ significantly from zero (P > 0·05), provided the slope for δGn was kept genotype-dependent:
| 5a |
| 5b |
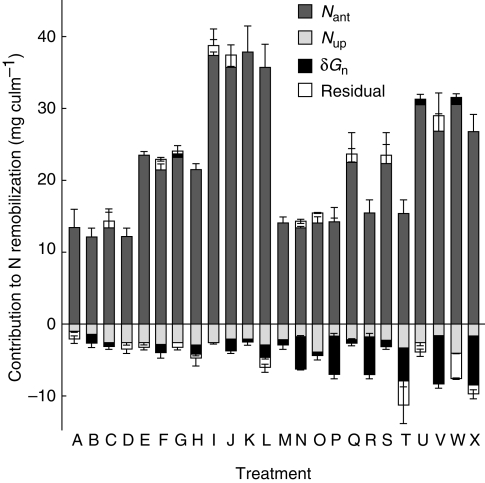
The coefficients for Nant and Nup, as well as that for δGn in ‘Autan’, were highly significant (P < 0·0001), but this was not the case for δGn in ‘Apache’ (P > 0·01). The only significant bias (P < 0·01) was observed for trimmed culms: in these plants, Nrem was under-estimated by −1·0 ± 0·5 mg culm−1 under low, late fertilization and over-estimated by 1·2 ± 0·5 mg culm−1 under high, late fertilization. The genotype effect on the coefficient for δGn suggested that there was almost no sink limitation in ‘Apache’, in contrast to ‘Autan’; this is indicated in Fig. 3, which shows the respective contributions of the terms in eqn (5) to Nrem. As the root-mean-square error (RMSE) of the fit was low (2·1 mg culm−1), the residuals of Nrem in its estimation by eqns (5a) and (5b) were within 8 % of the actual Nrem in all but one case (exhibiting a very low Nrem). The major role of Nant in determining Nrem was clear, while Nup led to a decrease in Nrem from −1 to −5 mg culm−1, generally accounting for between −10 % and −30 % of Nrem. Lastly, the influence of δGn was markedly genotype-dependent in trimmed culms: the corresponding loss was −8 ± 1 % Nrem in ‘Apache’ vs. −60 ± 9 % Nrem in ‘Autan’. In contrast, the influence of δGn was very small in untrimmed plants (± 0·5 mg culm−1, less than 3 % Nrem), and in this case the multiple regression became genotype-independent, as the coefficient of δGn did not significantly differ from zero for both genotypes (P > 0·05), leading to the following, simpler equation (r2 = 0·99 for n = 36; RMSE = 1·6 mg culm−1):
| 6 |
Fig. 3.
Contribution to N remobilization in main culm (Nrem) for the various treatments as detailed in Table 2. The various terms used in eqn (5) are shown, as well as the residuals of the multiple correlation to Nrem of Nant, Nup and δGn. Nant is the amount of N in vegetative parts at anthesis (after trimming), Nup is the N uptake after anthesis, and δGn refers to the difference between Gn and grain number as predicted from Nant,i (before trimming) by eqn (3). Bars indicate the s.e.m. for three replicates.
The coefficients for Nant and Nup were then highly significant (P < 0·0001 and P < 0·001, respectively), without significant bias for either genotype or fertilization treatment, as suggested by analysis of variance for the correlation residuals (data not shown).
Predictions of remobilization without root measurements
All the data presented so far in this paper have involved the quantification of root N using the experimental design described. However, root sampling is impossible in field assays, and in such cases Nant, Nup and Nrem are commonly estimated using Nant,a, Nup,a and Nrem,a, obtained by applying eqns (1) and (2) to above-ground parts rather than the whole culm. The values of Nant,a, Nup,a and Nrem,a were affected by genotype, fertilization and ear trimming, in a way similar to Nant, Nup and Nrem, respectively (Table 4). Both Nant,a/Nant and Nrem,a/Nrem ratios averaged 0·85, with variations that were not significantly linked to genotype, fertilization or ear trimming. NRE and its estimation using the Nrem,a/Nant,a ratio did not differ significantly according to a paired sample comparison (P > 0·05). The value of Nup,a (Table 4) considered remobilization from the roots as an N uptake, and therefore over-estimated Nup by between 1 % and 27 %, depending on genotype (P < 0·01) and early fertilization (P < 0·0001), but not on late fertilization (P > 0·01) or ear trimming (P > 0·05). Therefore, Nup could not be precisely evaluated from Nup,a measurements in above-ground parts only. Nevertheless, a direct estimate of Nrem in untrimmed culms was obtained from Nant,a and Nup,a (r2 = 0·97 for n = 36; RMSE = 1·8 mg culm−1):
| 7 |
Table 4.
(a) Importance of taking roots into account for the assessment of anthesis N, post-anthesis N uptake and N remobilization. Data are expressed as either mg per above-ground parts of the culm or relative to the results obtained using the whole culm. Data in this table can be compared with those in Table 3, which were obtained taking roots into account. (b) ANOVA was performed by fully crossing the effects of genotype, early fertilization, late fertilization and ear reduction, as well as first-order interactions between the different factors
| (a) N variables | ||||||
|---|---|---|---|---|---|---|
| Above-ground anthesis N |
Above-ground N increase |
Above-ground N remobilization |
||||
| Total (mg) | Ratio to whole culm | Total (mg) | Ratio to whole culm | Total (mg) | Ratio to whole culm | |
| Genotype (G) | ||||||
| ‘Apache’ | 26a | 0·84a | 30a | 1·13b | 18b | 0·84a |
| ‘Autan’ | 23a | 0·86a | 30a | 1·10a | 13a | 0·85a |
| Early fertilization (E) | ||||||
| Low | 14a | 0·83a | 25a | 1·07a | 8a | 0·83a |
| Increasing | 23b | 0·85a | 32a | 1·08a | 14b | 0·86a |
| High | 35c | 0·85a | 32a | 1·18b | 24c | 0·84a |
| Late fertilization (L) | ||||||
| Low | 24a | 0·85a | 26a | 1·13a | 16a | 0·85a |
| High | 24a | 0·85a | 33b | 1·01a | 15a | 0·85a |
| Ear reduction (R) | ||||||
| Control | 26a | 0·85a | 33a | 1·10a | 18b | 0·86a |
| 50 % trimming | 23a | 0·84a | 26a | 1·12a | 13a | 0·83a |
| (b) ANOVA | ||||||
|---|---|---|---|---|---|---|
| Above-ground anthesis N |
Above-ground N increase |
Above-ground N remobilization |
||||
| Total (mg) | Ratio to whole culm | Total (mg) | Ratio to whole culm | Total (mg) | Ratio to whole culm | |
| G | – | – | ns | * | *** | ns |
| E | *** | ns | – | *** | *** | ns |
| L | nd | nd | * | – | ns | ns |
| R | – | ns | * | ns | *** | – |
| G × E | – | ns | ns | ns | ** | ns |
| G × L | nd | nd | ns | ns | ns | ns |
| G × R | ns | ns | – | ns | – | ns |
| E × L | nd | nd | ns | ns | ns | ns |
| E × R | ns | ns | ns | ns | ns | – |
| L × R | nd | nd | ns | ns | ns | ns |
In (a) different letters indicate a significant difference between means (P < 0·01; n = 72).
In (b) * P < 0·01; ** P < 0·001; *** P < 0·0001; ns denotes non-significant effects (P > 0·05); nd indicates that the effect of late fertilization did not apply to Nant.
Despite it being less relevant from a physiological point of view, the following regression (eqn 8), also restricted to untrimmed culms, could be more easily tested in a field experiment and even compared with previous studies (r2 = 0·97 for n = 36; RMSE = 1·3 mg culm−1):
| 8 |
In both eqns (7) and (8), the coefficients for Nant,a and Nup,a were both highly significant (P < 0·0001 and P < 0·001, respectively), without any significant bias regarding genotype, fertilization treatment or interactions, as suggested by analysis of variance of the regression residuals. It appeared that eqn (8) was indistinguishable from eqn (6), except that it referred to above-ground values.
DISCUSSION
Representativeness of NRE
This experiment was designed to provide for very large Nup by inducing N deficiencies before anthesis (Triboï and Triboï-Blondel, 2002), so that the ratio of Nup to grain N ranged from 0·4 to 0·9 while the values attained in crops are commonly below 0·5 (Van Sanford and MacKown, 1987). However, despite exploring unusual ranges for the Nup/Nrem ratio, the usual values for Nant and Nrem were also represented in the study. Previous field experiments have reported higher values for NRE (0.4 to 0.9) than those found during the present study (0·3 to 0·8). Kichey et al. (2007) suggested a genotype effect on NRE, but Barbottin et al. (2005) noted that the genotype actually interacted with the year and level of fertilization. For this reason, the low NRE levels obtained in the present study could hardly be explained by the choice of cultivars. Unlike field experiments, this study also took roots into account, which are a major N sink according to Andersson et al. (2004). However, despite the fact that root N varied considerably from 7 % to 27 % of N in vegetative parts (depending on the genotype and treatment), the trends observed in whole culms were essentially maintained when only above-ground parts were examined. The sampling with roots therefore may not provide an explanation for the discrepancy between the results of this study and those in the literature.
The absence of high NRE values may have originated from the use of measurements on the scale of the culm, rather than the m2 scale as reported in the literature. The relationships between Nant and Nrem appeared to be affected by the scale considered. At the m2 scale, the simple linear regression of Nant to Nrem shows a positive intercept (e.g. in Barbottin et al., 2005). Consequently a higher Nant thus mathematically led to a higher NRE, which may have occurred when different levels of fertilization prior to anthesis were compared. The higher the fertilization, the higher was Nant and the lower was the NRE, as previously noted by Cox et al. (1986). In contrast, the data in this paper suggest that the simple linear regression of Nant to Nrem displayed a negative intercept at the culm scale. Therefore a higher Nant thus mathematically led to a lower NRE, leading to an effect of early fertilization in contradiction to findings in the literature (Table 3). In fact, Fig. 2A indicates that Nant and Nrem are aligned in the overall correlation; thus the variation in NRE was only based on the negative intercept of the relationship between Nant and Nrem. It is possible to imagine that a certain level of N will be immobilized in the dead tissues of a single culm by the time that anthesis occurs. This would result in the negative intercept observed at the culm level. At a later stage, grain N filling would lead to N remobilization from senescing (but still alive) plant organs. The present data suggest that the corresponding slope, which could be termed the ‘physiological NRE’, was unaffected by fertilization. The ratio of Nrem to Nant, which could be termed the ‘culm NRE’, was, however, biased by the intercept of the correlation, and increased with fertilization. At the m2 level, the relationships between Nant and Nrem become still more complicated because of tillering, resulting in a positive intercept and a ‘crop NRE’ that decreases with fertilization. Therefore, the use of the NRE could lead to confusion.
Nrem was essentially source-determined
The principal determinant for Nrem was by far Nant, with slopes in different equations of around 0·75–0·80. This result agreed well with that of Barbottin et al. (2005), who reported that, over a very broad range of Nant, the slope of Nrem vs. Nant was 0·76 under simple regressions regardless of genotype, provided that neither important fertilization was applied at anthesis nor that stresses occurred thereafter. The data also indicated a weaker correlation between Nrem and Gn, which could be associated with the link between Nant and Gn. Studies in the literature have reported a correlation between Nant and Gn at the m2 level, although this was mostly linked to the degree of tillering. This correlation was also observed at the main-culm level, where it was genotype-dependent: ‘Autan’ produced less grain than ‘Apache’, even for the same Nant. This suggests that the plants themselves somehow regulate their sink capacity (Gn) to their source level (Nant) around anthesis (Sinclair and Jamieson, 2006). The use of the δGn difference rather than Gn to characterize the sink led to a reduction in the sensitivity of Nrem determinism to both sink and genotype; in untrimmed culms their effects became negligible. The absence of a constant in eqns (6)–(8), which could therefore probably be extended to the m2 level, indicated that Nrem was fully predicted using source data only (Nant and Nup). However, it did not mean that sink and genotype had no influence on grain N filling, but rather that the sink influence was taken into account by the genotype-dependent relationship through Gn and Nant. Any event disturbing this relationship, either before or after anthesis, could lead to reintroduction of both the genotype and sink effects, for instance when disease or water stress occurr during grain filling, as was observed by Barbottin et al. (2005). Sink and genotype effects also appeared in the response to ear halving, but multiple regressions suggested that the source remained the principal determinant of Nrem, although modulated by sink and genotype. On the other hand, in many cases explicitly accounting for the sink may be redundant, as Jamieson and Semenov (2000) showed that it is simpler to fit grain N filling to sources than to sinks. If regulation somehow occurs, it should be located in sources themselves; according to Hörtensteiner and Feller (2002) and Gregersen and Holm (2007) vegetative parts seem to regulate Nrem themselves by means of a generic senescence program. Nevertheless, despite numerous models in the literature that have described grain N filling regulation by source/sink interactions, to my knowledge no groups have yet described how this regulation could be attained otherwise.
Sink effects in trimmed ears
Ear trimming always resulted in an increase in the amount of N per grain, even though it reduced N yield per ear in most cases. As early as 1991, Jenner et al. suggested that the relationship between source availability and sink activity shifts gradually as a function of the range of the source/sink ratio. If this ratio is low, grain filling would be proportional to source activity, approaching a constant-rate, saturated pattern with higher source availability. These authors also suggested that the dry-matter filling of grain is similar to a saturated pattern, while the grain N filling of whole ears tends to be within the range of a proportional pattern. Ear trimming would thus shift grain N filling to the range of the source/sink ratio leading to a saturated pattern. Bancal and Soltani (2002) modelled this type of mixed source and sink determinism, but only in the case of partitioning between several sinks. In fact, grain N filling is too frequently considered to be a single-sink task, whereas 15N labelling studies have indicated that N absorbed after anthesis may actually be incorporated, and not just temporarily stored, in vegetative parts (Oscarson, 1996; Kichey et al., 2007), thus suggesting that vegetative parts also behave as a sink for newly absorbed N. The model described by Bancal and Soltani (2002) used at least two parameters per sink that mostly affected sink activity at either low or high source/sink ratios, respectively. These parameters might vary according to genotype, which could also explain the genotype effect on trimming response suggested by the genotype-dependent coefficient for δGn. According to Martre et al. (2003), genotypes exhibiting a larger amount of N per grain in untrimmed ears reached their saturation level earlier following trimming, which was also observed during this study in ‘Autan’ vs. ‘Apache’. These hypotheses would not preclude alternative models explaining a trimming effect due to carbon (Dingkuhn et al., 2007) or hormones (Yang, et al., 2003). These puzzling results obtained for trimmed ears should be extended to control plants only with caution.
Negative effect of Nup on Nrem
The negative effect of late fertilization on NRE has long been recognised (Gooding et al., 2007); however, labelling studies have suggested that remobilization is not actually inhibited but counterbalanced by the incorporation of newly absorbed N in vegetative tissues. The harvest index of N absorbed after anthesis is lower than 100 %. Using 15N fertilizers, Kichey et al. (2007) reported that 89·7 % to 93·4 % of this N was translocated to grains, while under eqn (6) of this study the difference from 1 for the slope of Nrem to Nup suggested that 92 ± 2 % of N absorbed after anthesis was translocated to grains in untrimmed ears. Therefore, the unusually high ratios of Nup to grain N did not diminish translocation efficiency. Current trends towards late fertilization or plant breeding to ensure continued N uptake would therefore not result in high levels of N waste in straw.
Conclusions
In untrimmed ears, N yield could be predicted accurately without data for grain number. Sink and genotype effects were only modulations of the major regulation by both sources Nant and Nup, and moreover they only appeared in response to severe stresses (such as ear halving). The results obtained on trimmed ears thus differed qualitatively from the others and should therefore be extended to control plants with caution. Nrem was positively correlated with Nant and negatively with Nup, but even with a very high Nup the negative impact of Nup on Nrem remained small. It will therefore continue to be useful to investigate how the uptake of late nitrogen fertilization can be increased.
ACKNOWLEDGEMENTS
This study was financed by Génoplante [B04 project]. My thanks go to M. Chapon for the management of plant cultures, sampling and measurements.
LITERATURE CITED
- Abbate PE, Andrade FH, Culot JP. The effects of radiation and nitrogen on number of grains in wheat. Journal of Agricultural Science. 1995;124:351–360. [Google Scholar]
- Andersson A, Johanson E, Oscarson P. Nitrogen redistribution from the roots in post-anthesis plants of spring wheat. Plant and Soil. 2004;264:321–332. [Google Scholar]
- Bancal P, Soltani F. Source–sink partitioning. Do we need Münch? Journal of Experimental Botany. 2002;53:1919–1928. doi: 10.1093/jxb/erf037. [DOI] [PubMed] [Google Scholar]
- Barbottin A, Lecompte C, Bouchard C, Jeuffroy MH. Nitrogen remobilization during grain filling in wheat: genotypic and environmental effects. Crop Science. 2005;45:1141–1150. [Google Scholar]
- Barneix AJ. Physiology and biochemistry of source-regulated protein accumulation in the wheat grain. Journal of Plant Physiology. 2007;164:581–590. doi: 10.1016/j.jplph.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Brancourt-Hulmel M, Doussinault G, Lecomte C, Bérard P, LeBuanec B, Trottet M. Genetic improvement of agronomic traits of winter wheat cultivars released in France from 1946 to 1992. Crop Science. 2003;43:37–45. [Google Scholar]
- Brancourt-Hulmel M, Heumez E, Pluchard P, et al. Indirect versus direct selection of winter wheat for low input or high input levels. Crop Science. 2005;45:1427–1431. [Google Scholar]
- Borrell AK, Hammer GL, van Oosterom EJ. Stay-green: a consequence of the balance between supply and demand for nitrogen during grain filling ? Annals of Applied Biology. 2001;138:81–95. [Google Scholar]
- Cox MC, Qualset CO, Rains DW. Genetic variation for nitrogen assimilation and translocation in wheat. III. Nitrogen translocation in relation to grain-yield and protein. Crop Science. 1986;26:737–740. [Google Scholar]
- Demotes-Mainard S, Jeuffroy MH. Incorporating radiation and nitrogen nutrition into a model of kernel number in wheat. Crop Science. 2001;41:415–423. [Google Scholar]
- Dingkuhn M, Luquet D, Clément-Vidal A, Tambour L, Kim HK, Song YH. Is plant growth driven by sink regulation? Implications for crop models, phenotyping approaches and ideotypes. Wageningen U.R. Frontis. 2007;21:155–168. [Google Scholar]
- Gooding MJ, Smith SP, Davies WP, Kettlewell PS. Effects of late-season applications of propiconazole and tridemorph on disease, senescence, grain development and the breadmaking quality of winter wheat. Crop Protection. 1994;13:362–370. [Google Scholar]
- Gooding MJ, Gregory PJ, Ford KE, Ruske RE. Recovery of nitrogen from different sources following applications to winter wheat at and after anthesis. Field Crops Research. 2007;100:143–154. [Google Scholar]
- Gregersen PL, Holm PB. Transcriptome analysis of senescence in the flag leaf of wheat (Triticum aestivum L.) Plant Biotechnology Journal. 2007;5:192–206. doi: 10.1111/j.1467-7652.2006.00232.x. [DOI] [PubMed] [Google Scholar]
- Hörtensteiner S, Feller U. Nitrogen metabolism and remobilization during senescence. Journal of Experimental Botany. 2002;53:927–937. doi: 10.1093/jexbot/53.370.927. [DOI] [PubMed] [Google Scholar]
- Jamieson PD, Semenov MA. Modelling nitrogen uptake and redistribution in wheat. Field Crops Research. 2000;68:21–29. [Google Scholar]
- Jenner CF, Ugalde TD, Aspinall D. The physiology of starch and protein deposition in the endosperm of wheat. Australian Journal of Plant Physiology. 1991;18:221–226. [Google Scholar]
- Justes E, Mary B, Meynard JM, Machet JM, Thelier-Huche L. Determination of a critical nitrogen dilution curve for winter wheat crops. Annals of Botany. 1994;74:397–407. [Google Scholar]
- Kichey T, Hirel B, Heumez E, Dubois F, Le Gouis J. In winter wheat (Triticum aestivum L.), post-anthesis nitrogen uptake and remobilization to the grain correlates with agronomic and nitrogen physiological markers. Field Crops Research. 2007;102:22–32. [Google Scholar]
- Ma YZ, MacKown CT, Van Sanford DA. Differential effects of partial spikelet removal and defoliation on kernel growth and assimilate partitioning among wheat cultivars. Field Crops Research. 1996;47:201–209. [Google Scholar]
- Martinez-Carrasco R, Thorne GN. Physiological factors limiting grain size in wheat. Journal of Experimental Botany. 1979;30:669–679. [Google Scholar]
- Martre P, Porter JR, Jamieson PD, Triboï E. Modeling grain nitrogen accumulation and protein composition to understand sink/source regulations of nitrogen remobilization for wheat. Plant Physiology. 2003;133:1959–1967. doi: 10.1104/pp.103.030585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martre P, Jamieson PD, Semenov MA, Zyskowski RF, Porter JR, Triboï E. Modelling protein content and composition in relation to crop nitrogen dynamics for wheat. European Journal of Agronomy. 2006;25:138–154. [Google Scholar]
- Mi GH, Tang L, Zhang FS, Zhang JH. Is nitrogen uptake after anthesis in wheat regulated by sink size? Field Crops Research. 2000;68:183–190. [Google Scholar]
- Oscarson P. Transport of recently assimilated 15N nitrogen to individual spikelets in spring wheat grown in culture solution. Annals of Botany. 1996;78:479–488. [Google Scholar]
- Oscarson P. The strategy of the wheat plant in acclimating growth and grain production to nitrogen availability. Journal of Experimental Botany. 2000;51:1921–1929. doi: 10.1093/jexbot/51.352.1921. [DOI] [PubMed] [Google Scholar]
- Ruske RE, Gooding MJ, Jones SA. The effects of triazole and strobilurin fungicide on nitrogen uptake, partitioning, remobilization and grain N accumulation in winter wheat cultivars. Journal of Agricultural Science. 2003;140:395–407. [Google Scholar]
- Sinclair TR, Jamieson PD. Grain number, wheat yield, and bottling beer: an analysis. Field Crops Research. 2006;98:60–67. [Google Scholar]
- Sinclair TR, Jamieson PD. Yield and grain number of wheat: a correlation or causal relationship? Authors' response to ‘The importance of grain or kernel number in wheat: a reply to Sinclair and Jamieson’ by R.A. Fischer. Field Crops Research. 2008;105:22–26. [Google Scholar]
- Triboï E, Triboï-Blondel A-M. Productivity and grain or seed composition: a new approach to an old problem. European Journal of Agronomy. 2002;16:163–186. [Google Scholar]
- Van Sanford DA, MacKown CT. Cultivar differences in nitrogen remobilization during grain fill in soft red winter wheat. Crop Science. 1987;27:295–300. [Google Scholar]
- Yang JC, Zhang JH, Wang ZQ, Zhu QS, Liu LL. Involvement of abscisic acid and cytokinins in the senescence of carbon reserves in wheat subjected to water stress during grain filling. Plant, Cell and Environment. 2003;26:1621–1631. [Google Scholar]
- Zadoks JC, Chang TT, Konzak CF. A decimal code for the growth stages of cereals. Weed Research. 1974;44:415–421. [Google Scholar]