Abstract
Background and Aims
Most tropical lianas have specialized organs of attachment such as twining stems, hooks or tendrils but some do not. Many climbers also have an early self-supporting phase of growth and in some species this can produce treelet-sized individuals. This study focuses on how a liana can climb without specialized attachment organs and how biomechanical properties of the stem are modulated between self-supporting treelets and canopy-climbing lianas.
Methods
Biomechanics and stem development were investigated in self-supporting to climbing individuals of Manihot aff. quinquepartita (Euphorbiaceae) from tropical rain forest at Saül, central French Guiana. Bending tests were carried out close to the site of growth. Mechanical properties, including Young's elastic modulus, were observed with reference to habit type and changes in stem anatomy during development.
Key Results
This liana species can show a remarkably long phase of self-supporting growth as treelets with stiff, juvenile wood characterizing the branches and main stem. During the early phase of climbing, stiff but unstable stem segments are loosely held in a vertical position to host plants via petiole bases. The stiffest stems – those having the highest values of Young's modulus measured in bending – belonged to young, leaning and climbing stems. Only when climbing stems are securely anchored into the surrounding vegetation by a system of wide-angled branches, does the plant develop highly flexible stem properties. As in many specialized lianas, the change in stiffness is linked to the development of wood with numerous large vessels and thin-walled fibres.
Conclusions
Some angiosperms can develop highly effective climbing behaviour and specialized flexible stems without highly specialized organs of attachment. This is linked to a high degree of developmental plasticity in early stages of growth. Young individuals in either open or closed marginal forest conditions can grow as substantial treelets or as leaning/climbing plants, depending on the availability of host supports. The species of liana studied differs both in terms of development and biomechanics from many other lianas that climb via twining, tendrils or other specialized attachment organs.
Key words: Biomechanics, bending, developmental plasticity, French Guiana, liana, Manihot aff. quinquepartita (Euphorbiaceae), treelet, branch angle climber, Young's modulus
INTRODUCTION
Lianas are climbing plants that develop a significant amount of wood during their development. After a surge of interest in liana biology over the last two decades, it is increasingly clear that lianas play very important roles in tropical forest ecosystems (Putz and Mooney, 1991). Lianas may represent high percentages of species diversity in some forests and have long been thought to influence tree growth and regeneration because of their climbing growth mode (Putz, 1984; Schnitzer and Bongers, 2002). They can physically link adjacent trees, sometimes carpet substantial areas of forest canopy or ground vegetation and also influence forest regeneration, partly because of the ability of many species to survive and re-sprout after tree-falls. More recently, liana growth has been discussed with reference to changes in carbon sequestering in forest ecosystems, possibly linked to increased atmospheric CO2 and/or increased forest disturbance (Phillips et al., 2002; Wright et al., 2004), although the putative increase of lianas in forest ecosystems and the potential influence of CO2 in explaining it are both controversial (Mohan et al., 2006). Lianas have evolved many times and in many groups of angiosperms (Gentry, 1991) and show a bewildering array of developmental traits linked to aspects of their life history, wood production and highly derived mechanical and hydraulic functioning. Besides the difficulties of studying and measuring lianas in the field, it is perhaps this functional diversity of these often bizarre growth forms that contributes to difficulties in interpreting their ecology and distribution (Schnitzer, 2005; van der Heijden and Phillips, 2008) and their apparent increase in forest ecosystems (Wright et al., 2004; Londré and Schnitzer, 2006).
An aspect of lianas that is possibly linked to their ecological and evolutionary success is their developmental plasticity. Many liana species show a developmental transition from a self-supporting to a climbing phase, but in some species this difference is more marked and potentially linked to environmental cues such as growth in open or closed conditions (e.g. Gartner, 1991; Gallenmüller et al., 2004; Spector and Putz, 2006). Different species of lianas in tropical rain forests can show radically different attachment mechanisms such as twining stems, twining branches (Peñalosa, 1982; Putz and Chai, 1987), angled branches and many forms of sensitive tendrils, adhesive structures and sensitive or non-sensitive hooks (Ewart, 1898). Such widely varying functional traits probably play important roles in their habitat preferences and distribution. An aspect of their life history that has been generally observed concerns the potential mechanical constraints linked to their mode of climbing (Putz, 1995). Different liana species with different modes of climbing can show different ecological preferences (Peñalosa, 1982; Putz and Chai, 1987; Hegarty, 1991; Caballé, 1993; Dewalt et al., 2000).
Biomechanical studies of a wide range of lianas suggest that mode of attachment is probably coupled with a specific type of mechanical organization of the stem (Rowe et al., 2006; Rowe and Speck, 1998). Species deploying relatively ‘loose’ attachment modes such as open hooks possibly have rigid mechanical architectures with well-developed shrub-like or self-supporting growth during early development. Many species that attach themselves quickly and securely by stem twining, possibly show a rapid transition from stiff to flexible stems relatively soon in development (Rowe and Speck, 1996).
Possible reasons for this are that different types of attachment are likely to introduce different mechanical constraints between climber and host. Climbing stems that are securely attached to the host will readily experience forces generated by bending, torsion, tension and shear with potentially damaging effect when the host tree and its branches move in the wind. Climbing stems that are more loosely attached, for example, via open hooks or spines, will probably experience less mechanical constraints because of more slack between climber and support. Loosely attached species possibly need to retain relatively stiff mechanical properties for a relatively long part of their development because, although less firmly attached and thus less likely to break from swaying and movement, they are more likely to become unattached from the host and fall to the ground. So it is an advantage for such stems to retain a degree of rigidity in order to maintain their position in the host vegetation (Rowe et al., 2006; Isnard and Rowe, 2008).
An intriguing aspect of liana diversity and life history is therefore centred on attachment type and the well-documented change in anatomical and mechanical organization from young to old growth. Such trait combinations are possibly linked to many potential life-history processes such as preference for light or shaded habitats (Gallenmüller et al., 2004), understorey density, size and shape of host supports (Putz, 1984; Putz and Holbrook, 1991) as well as the evolutionary and developmental constraints acting on phylogenetically disparate groups (Speck et al., 2003; Rowe and Speck, 2004; Lahaye et al., 2005).
This paper is part of a wider project focusing on growth form diversity in climbing plants, including the genus Manihot (Euphorbiaceae), and studies of growth form evolution during domestication of M. esculenta (manioc or cassava). A detailed study of plants identified as Manihot aff. quinquepartita was carried out in the vicinity of Saül in central French Guiana. In this area the species grows as treelet-like individuals over 3 m in height as well as woody lianas capable of establishing themselves at canopy heights of 20–30 m. First observations suggested two aspects of its growth form that differ from many other lianas: (1) the absence of specialized attachment structures such as twining stems, hooks, or tendrils; (2) the ability to develop as treelets and as lianas with comparable maximum stem diameters. The latter feature differs from most other lianoid species observed, in which the initial self-supporting phase is shorter and its stem diameter smaller than in the mature climbing phase. The variation of growth forms for the species has been recorded recently in a recent flora of the Saül area as ‘… scandent shrubs or lianas …’ that are reported to be ‘… growing into canopy of low disturbed forest.’ (Gillespie, 2002).
In the present study, the following questions concerning the growth form and life-history of Manihot aff. quinquepartita are addressed: (a) How does the plant climb if it does not have specialized climbing organs? (b) Do climbing individuals produce flexible lianoid stems typical of many specialized twiners, hook climbers and tendril climbers? If so, what macro-anatomical traits characterize the transitions from self-supporting to climbing phases of growth? (c) What is the significance of the fact that individuals can grow as robust self-supporting treelets or climbers?
MATERIALS AND METHODS
Plants attributed to Manihot aff. quinquepartita Rogers & Appan (Gillespie, 2002) were collected in the vicinity of the village of Saül (3°37′N, 53°12′W) in central French Guiana. Climbing individuals were collected from disturbed secondary forest and leaning to self-supporting individuals were located along forest trails and clearings between the village of Saül and in the direction of Mont Boeuf-Mort (see Mori et al., 1997). The plants from Saül closely resemble Manihot quinquepartita from the eastern Amazon basin but differ from that species in having smaller, narrower inflorescence bracts (Gillespie, 2002). The species was readily identified and distinguished from Manihot esculenta (domesticated cassava, plants of which were sometimes found in old fallows and secondary forest) in the area by (a) its deeply palmately divided leaves with five lobes, highly constricted at the base so that the leaf appears palmately compound; and (b) its smooth woody stems that lack the conspicuous ridges around the leaf scars present on woody stems of M. esculenta. The study included all growth form variants of the species including 20 self-supporting individuals, six individuals that were leaning against neighbouring vegetation and eight individuals that were climbing with apical branches effectively fixed within neighbouring vegetation.
Selected plants were photographed in their position of growth and the overall dimensions and growth form recorded; specimens were then harvested for biomechanical study. Plants were transported to a field camp nearby and kept in humid conditions before testing. Three-point bending experiments were carried out on all growth-form types and on segments from the base to the apex of each plant. The study included a total of 239 tested segments from the 34 individuals. Over 194 segments of the complete data set were analysed and the major areas of tissues measured for correlation with mechanical properties.
The bending test protocols as undertaken in the field are described elsewhere (e.g. Isnard et al., 2003a; Lahaye et al., 2005; Rowe et al., 2006). In the present study, segments of plants were cut to appropriate lengths for three-point bending measurements carried out on a portable bending apparatus. All segments tested were inspected for external or internal damage that might influence measured values. The span length (the distance between the two supports in a three-point bending test) was established for the different stem diameters (developmental stages) and different growth forms (self-supporting, leaning and climbing) prior to the bulk of the measurements carried out (Table 1). This is necessary for three-point bending tests in order to avoid the influence of shear on the bending measurement (Vincent, 1990a, b). In M. aff. quinquepartita, the span to depth ratio below which shear forces would be included in the bending test was found to be 35 for all stages of growth varying from young to old for both climbing, leaning and self-supporting stems; hence bending test spans were varied so that the length of the tested stem was no less than 35 times the diameter of the tested segment. After the stem segment was placed on the supports, successive weights (up to five) were suspended from the mid-point of the tested specimen and the deflection observed at 30 s after the application of each weight via a dissecting microscope mounted on the apparatus and fitted with an eyepiece graticule. Depending on the thickness of the tested stem and its required length, weight increments were chosen to provide a total deflection (after five weights) of 0·8–3·5 mm. Generally speaking the larger deflections occurred for the larger specimens, but in general a linear part of the force deflection curve aimed for the beginning of the force deflection curve before the applied force was sufficient for the stem to deform beyond its elastic properties. The latter could be readily detected via direct observation with the microscope as well as by inspection of the plotted force deflection curve. Weights varied between 10 g and 200 g increments with total weight applied varying from 10 g to 1600 g (Table 1). The resultant plot of force (in Newtons, N) applied and deflection (mm) was then used to calculate the flexural rigidity (EI; Nmm).
| 1 |
where l represents the span (mm) of the axis on the apparatus, and b is the slope of the force plotted against deflection (N/mm). [Note: flexural rigidity is expressed as EI, where E is Young's modulus of elasticity and I is the second moment of area relative to the neutral plane of bending; i.e. the plane that neither shortens nor lengthens during deformation.]
Table 1.
Stem diameters, span lengths, and generalized weights applied and maximum deflections used in three-point bending tests on Manihot aff. quinquepartita
| Stem diameter (mm) | Three-point test span (mm) | Weight increment (g) | Maximum weight applied (g) | Maximum deflection (mm) |
|---|---|---|---|---|
| 4·0–6·5 | 180–200 | 10 or 50 | 50–250 | 0·9–3·5 |
| 6·6–9·5 | 250–320 | 50 or 100 | 250–500 | 1·0–4·5 |
| 9·6–13·0 | 320–480 | 50 or 100 | 250–500 | 1·0–3·5 |
| 13–42·0 | 480–1200 | 50, 100 or 200 | 500–1600 | 1·0–3·5 |
The following formula was then used to derive the axial second moment of area, I (mm4), of the tested stem segment using that used for an ellipse:
| 2 |
where A and B represent the radial widths in the direction of the force applied and perpendicular to it, respectively.
After measuring the flexural rigidity EI and calculating the axial second moment of area of each axis segment, Young's modulus E (MPa) was calculated via the following formula:
| 3 |
These parameters have now been widely used for comparing how rigidity and stiffness of the plant stem and branch system may change during development from young to old stages of growth (from the apex to the base) of a plant individual and between different growth forms such as shrubs and climbers.
Following each bending test, the central part of each stem tested was preserved in alcohol for anatomical study. To observe how mechanical properties changed with development of different stem tissues, cross-sections were prepared of each segment tested and the contours of pith, wood and cortex were drawn via a camera lucida and digitized. In this account, the term ‘cortex’ refers to all of the tissues lying outside of the cylinder of wood, including the secondary phloem, the remains of primary cortex tissue and varying amounts of periderm tissues. Overall, the aim was to measure how changes in the two main stem components, wood and cortex, influenced stem bending properties in self-supporting, leaning and climbing phenotypes. Representative samples of stems having juvenile and/or mature lianoid wood were sectioned and stained with safranin to observe cell wall thickness of fibre elements and vessel dimensions of the wood.
RESULTS
Growth form variation
Young genets or ramets are self-supporting (Fig. 1A) and develop as shrubs or treelets in the absence of supports. In dense vegetation, the species readily leans against neighbouring supports (Fig. 1B) and then continues vertical growth as a climber (Fig. 1C). In both illuminated and shaded understorey conditions, self-supporting plants can produce treelets up to 3·2 m in height and 35 mm in basal diameter (Fig. 1D).
Fig. 1.
Growth forms of Manihot aff. quinquepartita: (A) young self-supporting individual over 1 m high in gap conditions of disturbed secondary forest; (B) young individuals leaning against branches of neighbouring vegetation; at this stage of development stems are relatively loosely ‘attached’ to the supports via petiole bases; (C) young climbing individuals over 6 m long, well into the climbing phase of development and anchored in the branches of the host trees; (D) self-supporting treelet, 3 m in height, in open gap conditions of disturbed secondary forest; (E) evidence of thigmo-response of leaf petioles; note the angle of the petiole to the upper left of the stem compared with the reflexed orientation of the two petioles interacting with the narrow branch; (F) mature climbing liana producing pendulous axes bearing immature fruits (April 2006) in openings between adjacent plant hosts – fruiting specimens were only observed on mature climbing individuals.
When self-supporting development is followed by a leaning type of habit, young plants become unstable but retain a more-or-less vertical position in the surrounding vegetation. If supporting plants are moved away, unstable leaning plants undergo Euler buckling and can show permanent lateral deflections from the vertical growth position. Leaning plants use the angles formed between the stem and leaf petioles as hooks (Fig. 1E). Young climbing stems of 10–20 mm diameter are capable of reaching 5–10 m height in the understorey prior to branching (Fig. 1C). Climbing stems continue to lengthen and produce branches that anchor them into the surrounding vegetation. In over 80 individuals observed, mature and aged phenotypes were mostly climbers; green indehiscent fruits about 20 mm in diameter, were only observed on mature climbing individuals (Fig. 1F) but not on leaning or self-supporting plants. Both self-supporting and climbing stems can reach basal diameters over 35 mm in diameter. The broadest diameters and longest stems were observed among aged climbing individuals (Fig. 2A). Mature and aged climbers are more common in the area than aged shrubs or treelets and develop robust branches that part at wide angles from the main stem (Fig. 2B). Well-established climbers could not be pulled out of the surrounding canopy – such was the efficiency of the many wide-angle branches forming an inseparable mesh with neighbouring trees. Overall, the most noticeable aspect of growth form development is the wide developmental plasticity between early growth as self-supporting, leaning or climbing phenotypes, which appears to depend on the availability of supports. The species does not exhibit procumbent or trailing growth forms in the absence of supports even in well-lit conditions, as do many other lianas. Self-supporting shrubs and treelets can attain quite large sizes.
Fig. 2.
Growth forms of climbing Manihot aff. quinquepartita. (A) Large-bodied mature liana. The base of the main stem (lower left) extends to the right, producing numerous branches, which are irreversibly anchored into the vegetation above. This organization is quite unlike that of aged self-supporting individuals. (B) Wide-angled branches of mature climbing individual. Such branches act as highly effective grappling hooks in host vegetation. Plants that have developed this degree of branch attachment cannot be removed from the canopy.
Bending properties (E modulus) of the stem and their changes during development
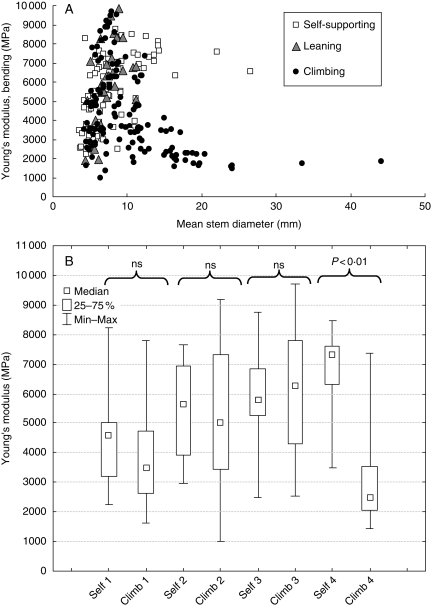
Values of E for all the stem segments tested varied from approx. 1800 to just under 10 000 MPa (Fig. 3A). Up to a diameter of approx. 10 mm all three growth forms showed a wide range of values. These were highly variable for stem diameters between 4 mm and 10 mm diameter and showed three broad clusters of points, each of which increases in modulus with increasing diameter up to a diameter of about 10 mm. This variation was mostly related to position on the plant and growth form: among stems <10 mm diameter the stiffest stems (Fig. 3A) between 6000 MPa and 10 000 MPa were mostly middle portions of leaning stems and relatively young self-supporting and climbing stems. The middle cloud of points ranging from 4000 MPa to 6000 MPa (0–10 mm diameter) includes (a) bases of self-supporting stems, (b) bases and apices of leaning stems and (c) medium-sized self-supporting and climbing forms. The stems with the lower values of E, 2000–4000 MPa comprise the relatively flexible apical portions of all growth forms. The stems showing the very highest values of E (over 8500 MPa) included mostly those of climbing (12 segments) or leaning (three segments) stems, with only one self-supporting segment (Fig. 3A).
Fig. 3.
(A) Young's elastic modulus measured in three-point bending, plotted against stem diameter for the three main types of growth form – self-supporting, leaning and climbing. All growth form categories increase in stiffness with increasing stem diameter up to about 10 mm diameter. After this point, larger self-supporting stems retain high values of E, whereas equivalent diameter climbing plants are more flexible. (B) Pair-wise comparisons of elastic moduli for self-supporting and climbing growth forms. Self-supporting and climbing stem classes are grouped in terms of stem diameter: 1, <5·4 mm; 2, 5·5–7·4 mm; 3, 7·5–10·5 mm; 4, > 10·5 mm. Only the largest and oldest category (>10·5 mm) shows statistical significance between median values of E for self-supporting and climbing stem stiffness (P < 0·01, Mann–Whitney test).
In stem diameters >10 mm, self-supporting individuals retain high values of E between 6000 and 8000 MPa, whereas climbing individuals show a marked drop and then continuous decline in modulus to values of around 1800 MPa in stems between 20 mm and 35 mm diameter. A comparison of values of E between self-supporting and climbing stems grouped according to size indicated a significant difference (Mann–Whitney P < 0·01) between self-supporting and climbing stems with a diameter >10·5 mm. There was a significant difference only in values of E between large-diameter self-supporting stems and climbing stems (Fig. 3B).
Tissue development and changes in bending properties
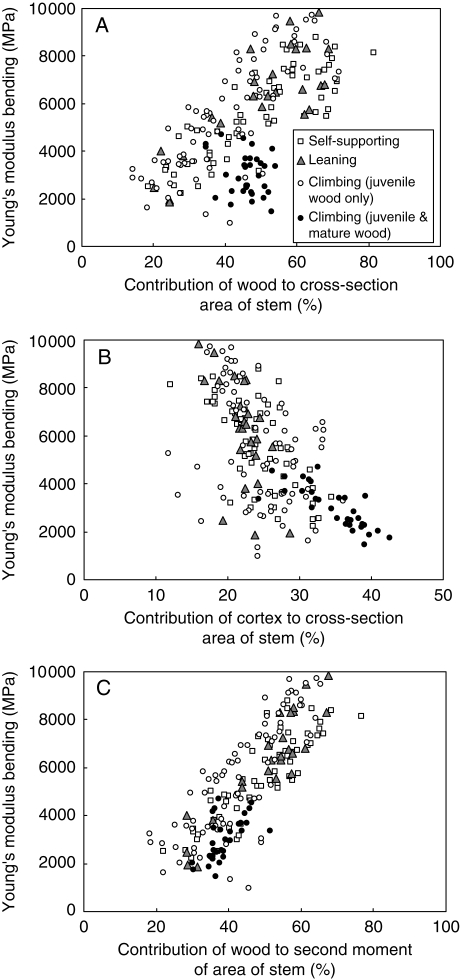
With the exception of climbers that had developed lianoid wood, stems of all growth forms showed an increase in E with increasing cross-sectional area of wood (Fig. 4A). This is concomitant with a decrease in cross-sectional area of the cortex (= secondary phloem, primary cortex and tissues of the periderm) (Fig. 4B). Stems of climbers that had developed lianoid wood showed relatively low values of E that were significantly lower than those for stems with equivalent areas of wood (Fig. 4A). Older climbing stems that had produced lianoid wood also produced proportionally more cortical tissues than treelets, leaning stems or young climbing plants (Fig. 4B).
Fig. 4.
(A) Young's elastic modulus measured in bending plotted against the percentage contribution of the wood cylinder to the total cross-sectional area of each tested stem. Generally, in all growth forms, the modulus increases with increasing proportion of wood surface area, except for a group of older climbing stems between 38 % and 56 % contribution which have relatively low wood contributions coupled with low modulus values. (B) Young's elastic modulus measured in bending plotted against the percentage contribution of the cortex to the total cross-sectional area of each stem tested. There is a general decrease in modulus with increasing proportion of cortical tissues. (C) Young's elastic modulus measured in bending plotted against the percentage contribution of the wood to the total second moment of area of each tested stem. In general, stems increase in stiffness with increasing contribution of the wood to second moment of area (I). Interestingly, for a given value of percentage wood contribution to I, the stiffest stems [the upper cloud of points increasing in stiffness (E) towards the right] belong mostly to young climbing stems (<10 mm in diameter) that are lightly attached to vertical supports.
The contribution of a tissue to the second moment of area (I) of the stem is a geometrical value combining (a) the position of the tissue in the stem (i.e. the square of the distance of each material point of that tissue from the neutral axis, which lies on the centre of gravity of the specimen in bending) and (b) the surface area of the tissue (an externally positioned area of tissue with a high value of I and of given stiffness, E, will resist bending more than a centrally positioned tissue of the same area and stiffness). Figure 4C indicates that, with a few exceptions, climbing stems that had not yet developed lianoid wood (the upper cloud of points of climbing stem segments increasing in E between approx. 6000 MPa and 10 000 MPa) were stiffer than most self-supporting or leaning stems with equivalent values of wood contribution in terms of second moment of area. This suggests that the wood or other tissues of the young stems produce a stiffer stem overall than equivalent sized non-climbing stems.
Most climbing stems >10 mm in diameter and all climbing stems >12 mm in diameter have much lower Young's moduli than equivalent-sized self-supporting stems. Mature climbing stems also produced a noticeably higher proportion of cortex (Fig. 4B) than other growth forms. Tissues of the cortex are produced outside the wood cylinder and have a correspondingly high value of I. In terms of I, the relative proportion of wood in mature climbing stems was therefore actually less than in younger stems (Fig. 4C).
Stem anatomy
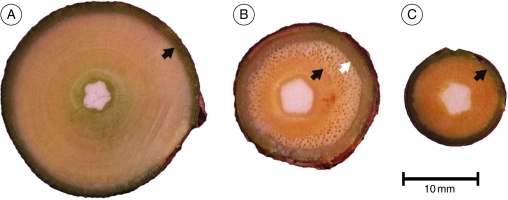
Self-supporting stems had homogeneous, dense wood from the inner to the outer part of the wood cylinder and a narrow band of secondary phloem and cortical tissue outside (Fig. 5A). This contrasts with mature climbing stems, with an inner cylinder of dense juvenile wood and an outer cylinder of lighter-coloured, less-dense wood with numerous large diameter vessels (Fig. 5B). Young stems of climbing individuals, like those of self-supporting plants (Fig. 5C), showed a large proportion of homogeneous dense wood. Tissues comprising the outer area of mature lianoid stems included a wide band of soft secondary phloem tissue and an outer band of periderm.
Fig. 5.
Transverse section surfaces showing macroanatomical development in M. aff. quinquepartita. In all sections the central white area is pith; the black arrow marks the outer limit of the juvenile wood and the white arrow marks the outer limit of the adult lianoid wood. (A) Large, self-supporting stem with narrow pith, homogeneous, dense, stiff wood and narrow band of cortical tissues and bark. This kind of organization is typical of large self-supporting shrub-like stems growing in the absence of supports. (B) Mature climbing stem showing a narrow pith, a band of dense juvenile wood and abrupt transition to less dense wood with abundant large-diameter vessels and a broad band of cortical tissue and bark. This kind of organization is typical of plants that are irreversibly attached to surrounding vegetation by wide-angled branches. (C) Young climbing axis showing narrow pith, large proportion of dense stiff, juvenile wood and narrow band of external cortex. This kind of organization characterizes the stiffest stems of M. aff. quinquepartita, characteristic of vertical stems that are lightly attached to host supports via petiole bases.
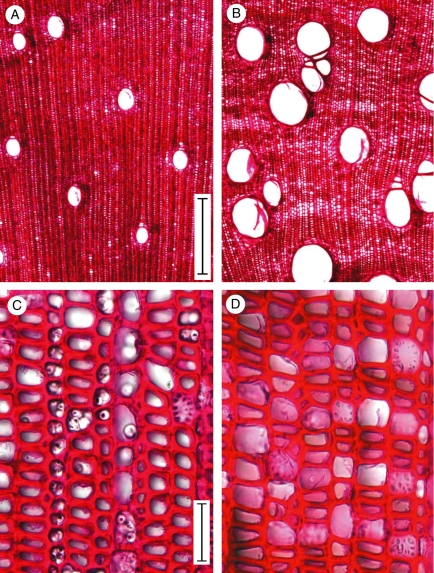
Wood of self-supporting shrubs was dense with relatively few, small diameter vessels (128–170 µm diameter; Fig. 6A), few narrow rays and thick-walled fibres and fibre tracheids (Fig. 6C). Dense wood was observed in the inner wood of mature climbing stems as well as in young stems of climbing axes. Outer, lianoid wood of climbing stems showed many large-diameter vessels up to 321 µm diameter with narrow rays (Fig. 6B) and fibres and fibre tracheids with large lumens and narrow cell walls (Fig. 6D). All stages of growth of all growth forms showed abundant starch bodies of varying amounts in the lumens of perforate fibres, fibre tracheids and vessels when viewed from sections of freshly cut material. These are visible in the stained sections in Fig. 6C but not in Fig. 6D as starch grains are highly mobile during the slide-preparation process.
Fig. 6.
(A) Transverse section (TS) of juvenile wood; (B) TS of lianoid wood; (C) TS of dense juvenile wood – note presence of abundant starch bodies; (D) TS of lianoid wood – note larger lumen size and thinner wall thickness of fibre tissue compared with the juvenile wood observed. Scale bars: (A, B) = 500 µm; (C, D) = 50 µm.
DISCUSSION
Like many lianas, Manihot aff. quinquepartita shows an early phase of self-supporting development (Putz, 1984; Putz and Holbrook, 1991; Caballé, 1998; Speck and Rowe, 1999; Rowe et al., 2006). However, the species differs from most lianas studied in its ability to continue growth significantly as a self-supporting individual in the absence of host supports. This type of growth differs radically from lianas that continue growth as procumbent or trailing forms in the absence of supports (Caballé, 1998).
Changes of mechanical properties with life history
In M. aff. quinquepartita, the highest values of E measured were mostly of median parts of slender, climbing stems less than about 10 mm in diameter and up to 5 m length. These high values and their positioning suggest that unstable stems are well adapted mechanically to remain upright while lightly attached via petiole bases and represent an extended ‘searcher’ phase of development. Both genets (seedlings) and ramets become vertically unstable, though lightly attached to surrounding branches and stems via the angles formed by petioles. While this kind of attachment is probably not effective in supporting any considerable weight or movement it effectively ensures that vertically unstable stems can remain upright and continue to grow towards the canopy.
Increased growth and branching of upright and leaning stems eventually produce relatively stiff branches interlocking with the stems and branches of neighbouring trees. Bending properties of stems and branches initially remain stiff, facilitating connection to the canopy and ensuring that the stem will not slip. The results indicated that following anchorage, climbing stems develop flexible lianoid wood and the stiffness of climbing stems greater than 10–12 mm in diameter drops from over 4000 MPa down to below 1800 MPa in stems up to 25 mm diameter. These lower values reflect stems that are not as flexible as those of many other tropical woody lianas, particularly among twiners and tendril climbers, which can show values as little as 200–300 MPa (Rowe et al., 2006). Flexibility in older climbing stems of M. aff. quinquepartita probably results from a combination of traits including reduced fibre tissue density, larger numbers of wide vessels and an increased proportion of flexible phloem and periderm outside of the wood cylinder. The latter feature explains the relatively low proportion of wood to the stem total cross-sectional area and second moment of area in stems which have developed a lianoid wood.
The leaning habit as a ‘step’ towards true lianescence
Many plants tested under experimental conditions show some kind of thigmomorphogenetic response when either staked or mechanically perturbed (Jaffe et al., 2002). These responses can vary between species, but often involve changes in geometry, mechanical properties, height, slenderness and branching. Leaning behaviour is often observed in relatively young stages of growth of tree species, particularly in forest margins and in dense stands. The margins of cleared forest areas in French Guiana readily show young trees that have buckled or collapsed after removal of the neighbouring vegetation (Rowe and Speck, 2005). Interestingly, this kind of behaviour among normally self-supporting plants does not include (a) some kind of attachment organ, even albeit an ‘unspecialized’ one such as branch angles or (b) highly flexible stems in older stages of development (Rowe and Speck, 2005). The lianoid habit evolved in a wide range of woody angiosperm families (Gentry, 1991). One of the factors possibly explaining this is that immature trees and many other forms show leaning behaviour in dense vegetation (Speck and Rowe, 1999; Rowe and Speck, 2005). Potential advantages conferred by this type of mechanical strategy might have led to the appearance of more specialized non-self-supporting growth forms over a wide range of angiosperm groups. It would be of interest to know whether leaning behaviour among essentially self-supporting plants represents a potential niche or prerequisite leading to the appearance of further non-self-supporting traits such as the appearance of (a) attachment organs, (b) specialized stem mechanics and (c) a specialized lianoid climbing habit.
A recent synthesis of the changes in stem mechanics over ontogeny in a wide range of scandent plants proposed that the degree of change in flexibility of the stem during ontogeny would vary with type of attachment (Rowe et al., 2006). Lianas and vines with secure attachment, such as stem twiners, branch twiners and woody tendril climbers, would develop highly flexible stems, whereas climbers with less-fixed types of attachment such as open hooks, spines and wide-angle branches would retain relatively stiff characteristics. While these overall trends were indeed generally the case, something of a reversal of the trend was observed in three branch-angle climbers studied including two species of Croton, discussed above, and the phylogenetically distant lycopsid Lycopodiella cernua. All three showed significant reductions in Young's modulus from young to older flexible stages of growth (Rowe et al., 2006).
Modes of attachment
The new findings on Manihot, like those on Croton, emphasize that wide-angle branches can be a highly effective anchoring device for lianoid growth habits. The climber eventually becomes irreversibly attached to the host canopy and only then produces compliant stem properties. Interestingly, mature lianoid stems of both Croton and Manihot show values of E that do not drop as far as in highly flexible species of specialized stem twiners such as Aristolochia brasiliensis (104 ± 72 MPa). However, not all twiners and tendril climbers show very low values of E modulus in older stages of development. In an analysis of over 20 species of twining lianas, about half showed values of E of the same order as that found in M. aff. quinquepartita (Rowe et al., 2006).
These observations at least partially support the hypothesis that flexibility of the stem is potentially linked with type of attachment and, furthermore, is probably fine-tuned during development of the plant so that stiff properties might persist up until such a point that the plant is well attached. In the case of the species of Manihot studied, flexible stem properties – equivalent to those of many other tested tendril climbers and twiners – are not developed before the stem is irreversibly attached to the vegetation. Something of a surprise in the results of this study is that attachment by branches is so effective. One of the factors contributing to this efficiency of attachment is possibly linked to the architectural organization of branches in groups of threes, forming affective grappling hooks.
In Manihot aff. quinquepartita, stiff juvenile wood is produced well into the climbing phase and the transition from self-supporting to climbing growth occurs via a ‘light’ type of attachment – petiole angles – and then by wide-angled branches. Observations of M. aff. quinquepartita suggest that petiole bases are possibly thigmo-sensitive. In lianoid species of Croton (L. Ménard and N. Rowe, pers. obs. 2008) active re-orientations of the petiole are less apparent, though petioles might nevertheless help retain a vertical position (Gallenmüller et al., 2001). Further observations are required in both genera to determine whether petiole orientation really does change in response to contact or whether the phenomenon simply occurs during development. In Croton, rough leaf surfaces are characteristic of many species of the genus and in climbing species could assist in maintaining a vertical orientation (Gallenmüller et al., 2004). Croton and Manihot are relatively closely related and placed within adjacent clades of the Euphorbiaceae (Tokuoka, 2007). Interestingly, the combination of similar biomechanical climbing traits might therefore be phylogenetically linked. However, differences between the two genera appear to include reactions to high light levels, which in Croton nuntians produce a stunted growth form of short unbranched plants (Gallenmüller et al., 2004). This differs from the response observed in Manihot aff. quinquepartita, which develops robust, self-supporting treelets in open conditions.
Evolution and phylogenetic constraints
The ability to grow as either self-supporting or climbing plants, well into the developmental trajectory, might be viewed as a high degree of phenotypic plasticity (Bradshaw, 1965; Sultan, 2003), with shrub or treelet-sized individuals developing similar basal diameters to those of fruit-producing mature lianas, but with different mechanical properties. The species shows features in common with two species of Croton (Euphorbiaceae), C. pullei and C. nuntians (Gallenmüller et al., 2001, 2004; Rowe et al., 2006). Both Croton species, also from French Guiana, climb via wide-angle branches and can show long phases of self-supporting growth to 25 mm in diameter as well as values of E below 1000 MPa in mature lianoid stages. Both Croton species also show transitions from stiff juvenile wood to compliant lianoid wood, with significant cortical and periderm tissue outside of the wood cylinder in C. nuntians (Gallenmüller et al., 2004).
This kind of woody architecture differs from many other so called ‘herbaceous’ lianoid architectures (Rowe and Speck, 2005) where the transition from stiff juvenile to compliant adult properties occurs via the rupture of a stiff band of fibrous primary tissue and where observations in many species show only one kind of compliant wood that is produced throughout development (Isnard et al., 2003b; Speck et al., 2003). We have proposed elsewhere that this kind of developmental difference is possibly linked to different phylogenetic constraints and putative ancestral groups that were either herbaceous or woody in organization (Isnard et al., 2003b; Rowe and Speck, 2005). Rowe and Speck (2005) suggested that such herbaceous organizations were possibly limited in terms of the degree of ‘reach’ of searcher branches prior to the rupture of the outer tissues and differed from woody patterns of growth where the transition from juvenile to adult stems possibly favoured longer ‘searcher’ branches and a longer self-supporting phase. The latter could also explain why this species of Manihot is capable of producing treelet-sized phenotypes. Further studies should investigate to what extent herbaceous or woody organizations influence growth form plasticity and stem biomechanics during development.
Conclusions
Climbing plants are notoriously difficult to study accurately in terms of life form, growth form and exact habitat preference and life history (Putz and Mooney, 1991). Ongoing interest in the ecology and growth dynamics of climbers and lianas, particularly with reference to potential human action and/or global environmental change (Phillips et al., 2002), requires a precise understanding of their biology, diversity and complex development. A clear result that has emerged from the work on Manihot aff. quinquepartita is that the shift to flexible lianoid properties occurs late in the developmental trajectory because of the nature of the late-developed close connection with the host plants. Modification of juvenile, mature and aged phases of growth is possibly a key factor in varying plant growth-form variation. Further studies should investigate how key developmental transitions such as shifts from juvenile to adult growth are mediated and to what extent such changes are inevitably ‘programmed’ by intrinsic ontogenetic processes or modulated by environmental factors. Such an understanding might help us to explain how some plant families such as the Euphorbiaceae are incredibly diverse in terms of growth form and what developmental factors, both biomechanical and otherwise, underlie this diversity.
ACKNOWLEDGEMENTS
We gratefully acknowledge funding to N.R. from the national ANR research foundation for a project (2006–2008) entitled ‘WOODIVERSITY’, and to D.M. from the French government [Contrats Projets Etat Région (CPER) Guyane; Programme ‘Ecosystèmes Tropicaux’ from the French Ministry of Ecology and Sustainable Development] for projects (2006–2008) on diversity of Manihot in French Guiana. We thank two anonymous reviewers for their valuable comments and we are also grateful to members of the Scientific Campus at Kourou, French Guiana, for assistance and the people of Säul, central French Guiana, for their help. AMAP (Botany and Computational Plant Architecture) is a joint research unit which associates CIRAD (UMR51), CNRS (UMR5120), INRA (UMR931), IRD (R123) and Montpellier 2 University (UM27); http://amap.cirad.fr/
LITERATURE CITED
- Bradshaw AD. Evolutionary significance of phenotypic plasticity in plants. Advances in Genetics. 1965;13:115–155. [Google Scholar]
- Caballé G. Liana structure, function and selection: a comparative study of xylem cylinders of tropical rainforest species in Africa and America. Botanical Journal of the Linnean Society. 1993;113:41–60. [Google Scholar]
- Caballé G. Le port autoportant des lianes tropicales: une synthèse des stratégies de croissance. Canadian Journal of Botany. 1998;76:1703–1716. [Google Scholar]
- Dewalt SJ, Schnitzer SA, Denslow JS. Density and diversity of lianas along a chronosequence in a central Panamanian lowland forest. Journal of Tropical Ecology. 2000;16:1–19. [Google Scholar]
- Ewart AJ. On contact irritability. Annales du Jardin Botanique de Buitzenzorg. 1898;15:187–242. [Google Scholar]
- Gallenmüller F, Müller U, Rowe NP, Speck T. The growth form of Croton pullei (Euphorbiaceae) – functional morphology and biomechanics of a neotropical liana. Plant Biology. 2001;3:50–61. [Google Scholar]
- Gallenmüller F, Rowe NP, Speck T. Development and growth form of the neotropical liana Croton nuntians: the effect of light and mode of attachment on the biomechanics of the stem. Journal of Plant Growth Regulation. 2004;23:83–97. [Google Scholar]
- Gartner BL. Is the climbing habit of poison oak ecotypic? Functional Ecology. 1991;5:696–704. [Google Scholar]
- Gentry AG. The distribution and evolution of climbing plants. In: Putz FE, Mooney HA, editors. The biology of vines. Cambridge: Cambridge University Press; 1991. [Google Scholar]
- Gillespie LJ. Euphorbiaceae (spurge family) In: Mori SA, Cremers G, Gracie CA, et al., editors. Guide to the vascular plants of central French Guyana. New York, NY: New York Botanical Garden Press; 2002. Part 2. Dicotyledons. [Google Scholar]
- Hegarty EE. Vine–host interactions. In: Putz FE, Mooney HA, editors. The biology of vines. Cambridge: Cambridge University Press; 1991. [Google Scholar]
- van der Heijden GMF, Phillips OL. What controls liana success in neotropical forests? Global Ecology and Biogeography. 2008;17:372–383. [Google Scholar]
- Isnard S, Rowe NP. The climbing habit in palms: biomechanics of the cirrus and flagellum. American Journal of Botany. 2008;95:1538–1547. doi: 10.3732/ajb.0700005. [DOI] [PubMed] [Google Scholar]
- Isnard S, Rowe NP, Speck T. Growth habit and mechanical architecture of the sand dune-adapted climber Clematis flammula var. maritima L. Annals of Botany. 2003;a 91:407–417. doi: 10.1093/aob/mcg044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isnard S, Speck T, Rowe NP. Mechanical architecture and development in Clematis: implications for canalised evolution of growth forms. New Phytologist. 2003;b 158:543–559. doi: 10.1046/j.1469-8137.2003.00771.x. [DOI] [PubMed] [Google Scholar]
- Jaffe MD, Leopold AC, Staples RC. Thigmo responses in plants and fungi. American Journal of Botany. 2002;89:375–382. doi: 10.3732/ajb.89.3.375. [DOI] [PubMed] [Google Scholar]
- Lahaye R, Civeyrel L, Speck T, Rowe NP. Evolution of shrub-like growth forms in the lianoid subfamily Secamonoideae (Apocynaceae s.l.) of Madagascar: phylogeny, biomechanics and development. American Journal of Botany. 2005;92:1381–1396. doi: 10.3732/ajb.92.8.1381. [DOI] [PubMed] [Google Scholar]
- Londré RA, Schnitzer SA. The distribution of lianas and their change in abundance in temperate forests over the past 45 years. Ecology. 2006;87:2973–2978. doi: 10.1890/0012-9658(2006)87[2973:tdolat]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Mohan JE, Ziska LH, Schlesinger WH, et al. Biomass and toxicity responses of poison ivy (Toxicodendron radicans) to elevated atmospheric CO2. Proceedings of the National Academy of Sciences of the USA. 2006;103:9086–9089. doi: 10.1073/pnas.0602392103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori SA, Cremers G, Gracie CA, de Granville J-J, Hoff M, Mitchell JD. New York, NY: New York Botanical Garden Press; 1997. Guide to the vascular plants of central French Guyana. Part 1. Pteridophytes, gymnosperms and monocotyledons. [Google Scholar]
- Peñalosa J. Morphological specialization and attachment success in two twining lianas. American Journal of Botany. 1982;69:1043–1045. [Google Scholar]
- Phillips OL, Martínez RV, Arroyo L, et al. Increasing dominance of large lianas in Amazonian forests. Nature. 2002;418:770–774. doi: 10.1038/nature00926. [DOI] [PubMed] [Google Scholar]
- Putz FE. The natural history of lianas on Barro Colorado Island, Panama. Ecology. 1984;65:1713–1724. [Google Scholar]
- Putz FE. Vines in treetops: consequences of mechanical dependence. In: Lowman MD, Nadkarni NM, editors. Forest canopies. San Diego, CA: Academic Press; 1995. [Google Scholar]
- Putz FE, Chai P. Ecological studies of lianas in Lambir National Park, Sarawak, Malaysia. Journal of Ecology. 1987;775:523–531. [Google Scholar]
- Putz FE, Holbrook NM. Biomechanical studies of vines. In: Putz FE, Mooney HA, editors. The biology of vines. Cambridge: Cambridge University Press; 1991. [Google Scholar]
- Putz FE, Mooney HA. The biology of vines. Cambridge: Cambridge University Press; 1991. [Google Scholar]
- Rowe NP, Speck T. Biomechanical characteristics of the ontogeny and growth habit of the tropical liana Condylocarpon guianense (Apocynaceae) International Journal of Plant Science. 1996;157:406–417. [Google Scholar]
- Rowe NP, Speck T. Biomechanics of plant growth forms: the trouble with fossil plants. Review of Palaeobotany and Palynology. 1998;102:43–62. [Google Scholar]
- Rowe NP, Speck T. Hydraulics and mechanics of plants: novelty, innovation and evolution. In: Poole I, Hemsley AR, editors. The evolution of plant physiology. Kew: Elsevier Academic Press; 2004. [Google Scholar]
- Rowe NP, Speck T. Plant growth forms: an ecological and evolutionary perspective. New Phytologist. 2005;166:61–72. doi: 10.1111/j.1469-8137.2004.01309.x. [DOI] [PubMed] [Google Scholar]
- Rowe NP, Isnard S, Gallenmüller F, Speck T. Diversity of mechanical architectures in climbing plants: an ecological perspective. In: Herrel A, Speck T, Rowe NP, editors. Ecology and biomechanics: a mechanical approach to the ecology of animals and plants. Boca Raton, FL: Taylor & Francis; 2006. [Google Scholar]
- Schnitzer SA. A mechanistic explanation for global patterns of liana abundance and distribution. The American Naturalist. 2005;166:262–276. doi: 10.1086/431250. [DOI] [PubMed] [Google Scholar]
- Schnitzer SA, Bongers F. The ecology of lianas and their role in forests. Trends in Ecology and Evolution. 2002;17:223–230. [Google Scholar]
- Speck T, Rowe NP. A quantitative approach for analytically defining, growth form and habit in living and fossil plants. In: Kurmann MH, Hemsley AR, editors. The evolution of plant architecture. London: Royal Botanic Gardens, Kew; 1999. [Google Scholar]
- Speck T, Rowe NP, Civeyrel L, Classen-Bockhoff R, Neinhuis C, Spatz H-C. The potential of plant biomechanics in functional biology and systematics. In: Stuessy TF, Mayer V, Hörandl E, editors. Deep morphology: toward a renaissance of morphology in plant systematics. Königstein: Koeltz; 2003. [Google Scholar]
- Spector T, Putz FE. Biomechanical plasticity facilitates invasion of maritime forests in the southern USA by Brazilian pepper (Schinus terebinthifolius) Biological Invasions. 2006;8:255–260. [Google Scholar]
- Sultan SE. Phenotypic plasticity in plants: a case study in ecological development. Evolution and Development. 2003;5:25–33. doi: 10.1046/j.1525-142x.2003.03005.x. [DOI] [PubMed] [Google Scholar]
- Tokuoka T. Molecular phylogenetic analysis of Euphorbiaceae sensu stricto based on plastid and nuclear DNA sequences and ovule and seed character evolution. Journal of Plant Research. 2007;120:511–522. doi: 10.1007/s10265-007-0090-3. [DOI] [PubMed] [Google Scholar]
- Vincent JFV. Plants. In: Vincent JFV, editor. Biomechanics – materials, a practical approach. a. Oxford: IRL Press at Oxford University Press; 1990. [Google Scholar]
- Vincent JFV. Structural biomaterials. b second edn. Princeton, NJ: Princeton University Press; 1990. [Google Scholar]
- Wright SJ, Calderón O, Hernandéz A, Paton S. Are lianas increasing in importance in tropical forests? A 17 year record from Panama. Ecology. 2004;85:484–489. [Google Scholar]