Abstract
Background and Aims
Although the causes and consequences of seedling herbivory for plant community composition are well understood, the mechanisms by which herbivores influence plant species recruitment to the established phase remain less clear. The aim was to examine how variation in the intensity of seedling herbivory interacts with growth-defence trade-offs and herbivore feeding preferences to affect plant community development.
Methods
Using 14-d-old seedlings of Trifolium pratense and T. repens, relative growth and susceptibility to herbivory by the snail Helix aspersa was quantified to elucidate putative growth-defence trade-offs for these species. Then mixed assemblages of 14-d-old Trifolium seedlings were exposed to herbivory by zero, two, five or ten snails and determined how variation in the intensity of herbivory affected competitive interactions into the mature phase (as measured by total plant biomass at 120 d old).
Key Results
In the absence of herbivory, communities were dominated by T. pratense; a result expected on the basis that it yielded larger and presumably more competitive seedlings. However, when seedlings were exposed to herbivory, the balance of competition shifted. At low levels of herbivory (two snails), both Trifolium species contributed equally to total plant biomass. More intense herbivory (five snails) resulted in almost total mortality of T. pratense and dominance of the mature community by T. repens. The most intense herbivory (ten snails) effectively removed all seedlings from the experimental community.
Conclusions
The study illustrates a mechanism whereby spatio-temporal fluctuations in seedling herbivory, when coupled with species-specific variation in competitive ability and sensitivity to herbivore attack, can differentially influence plant recruitment into the mature phase. This mechanism may be a key element in our attempts to understand plant species coexistence, since fluctuations in plant recruitment are fundamental to the many theories that view coexistence as a consequence of a spatio-temporal lottery for dominance over regeneration micro-sites.
Key words: Growth-defence trade-off, lottery models, plant–animal interactions, plant size variability, seedling acceptability, seedling defence, spatio-temporal niches, Trifolium pratense, Trifolium repens
INTRODUCTION
Largely due to their small size and total reliance on the nutrient reserves stored within their cotyledons, seedlings represent the most vulnerable stage of the plant life cycle (Hanley et al., 2004; Fenner and Thompson, 2005). Although a number of agents, including disease, competition, nutrient limitation, drought and trampling can often result in the death of entire seedling cohorts, foremost among the factors limiting seedling recruitment is herbivory (Moles and Westoby, 2004; Fenner and Thompson, 2005). In addition to the more obvious effects that herbivores have on seedling demography (Linquist and Carroll, 2004; Maron and Kauffman, 2006), the selective removal of seedlings exerts long-lasting effects on plant community composition through differential recruitment of component species to the established community (Hanley et al., 1995a, 1996a; Howe et al., 2002; Asquith and Mejia-Chang 2005; Beckage and Clarke, 2005).
Although seedling age (Hanley et al., 1995b; Scheidel and Bruelheide, 2004), phenology (Hanley et al., 1996a, b), and neighbour environment (Bergelson, 1990; Hanley, 2004) are important factors, seedling selection is based primarily on the expression of anti-herbivore defences (Hanley and Lamont, 2001; Burt-Smith et al., 2003; Rafferty et al., 2005). Even from a relatively early age, seedlings possess a diverse array of secondary metabolites, although their development and deployment varies between species and ontogenetic stages (Schaffner et al., 2003; Barton, 2007; Elger et al., 2009). Consequently, variation in the expression of anti-herbivore defences may be pivotal in dictating the likelihood of seedling herbivory and, therefore, seedling survival. However, chemical or structural defence is generally predicted to entail some kind of cost to the plant that possesses them (Herms and Matson, 1992; Hanley et al., 2007). Where these costs are apparent they are often manifest in the form of reduced fecundity or growth (Fine et al., 2006; Glynn et al., 2007), although such fitness costs have not been established for all plant species (Koricheva, 2002; Haring et al., 2008) and there is a relative paucity of information regarding growth-defence trade-offs for seedlings (Kelly and Hanley, 2005; Hanley et al., 2007). Nonetheless, even at the seedling stage, plants are assumed to face an allocation choice between investment of resources in anti-herbivore defence, or rapid onward growth (Herms and Mattson, 1992; Boege and Marquis, 2005; Kelly and Hanley, 2005). This trade-off may go a long way towards explaining how selective seedling removal has so marked an effect on plant community composition.
Recent studies in the Amazonian forests of Peru, for example, suggest that a growth-defence trade-off during the recruitment stage influences the structure and composition of mature forest communities (Fine et al., 2004, 2006). Thus, species with relatively well-developed seedling defences are at a significant advantage over faster-growing, but poorly defended species when recruitment coincides with intense seedling herbivory. However, when seedling herbivory is relaxed, the faster-growing species dominates the plant community by virtue of its superior competitive ability. This kind of relationship has also been invoked to explain patterns of seedling recruitment in grassland plant species (Hanley et al., 1995a; Kelly and Hanley, 2005). Although there have been few experimental tests of this hypothesis, when coupled with the inherent unpredictability of seedling herbivory, the growth-defence trade-off clearly has the potential to influence plant community composition via interactions between seedlings vying for dominance of regeneration micro-sites.
Although herbivory is frequently viewed as a characteristically patchy process (Adler et al., 2001; Maron and Crone, 2006; Johnson et al., 2008), studies on spatio-temporal variation in seedling herbivory are limited. Nevertheless, Izhaki and Ne'eman (1996) and Manzaneda et al. (2005) describe significant spatial variation in seedling losses to porcupines and Lepidoptera, respectively, in Mediterranean pine forests, while several authors (Hanley et al., 1996a, b; Hill and Silvertown, 1997; Scheidel and Bruelheide, 2004) report seasonal variation in seedling attack by molluscs. In addition, Barnes and Weil (1944) and Symondson et al. (2002) demonstrate important year-to-year changes in mollusc populations. As the principal seedling herbivore in temperate ecosystems (Jennings and Barkham, 1975; Crawley, 1997), spatio-temporal fluctuation in mollusc abundance may be particularly important in shaping interactions between temperate grassland plant species. Weiner's glasshouse study (Weiner, 1993), for example, showed how increasing snail number (a surrogate for variation in the intensity of herbivore pressure) acted to increase plant size variability (PSV) in Hypochaeris radicata. Variation in plant size, brought about in this case via the interaction between snail herbivory and plant density, is fundamental to plant competition (Weiner, 1985; Wiegand et al., 2008) and can significantly alter the balance of plant competition at the regeneration stage (Hanley and Groves, 2002).
Nevertheless, while there are clear conceptual grounds to suppose that spatio-temporal variation in the intensity of mollusc herbivory influences patterns of plant community composition, this interaction is poorly understood. The aim of this study was to examine how competition between two closely related, sympatric chalk grassland plant species was influenced by variation in snail herbivore pressure during the seedling stage. The first objective was to determine whether a growth-defence trade-off existed for the study species, since the effect of snail herbivory on between-species competition would be dictated by the balance between competitive ability and susceptibly to herbivore attack. The second objective was to determine whether selective removal of a more acceptable, but superior competitor by snails could allow the subordinate species to dominate the experimental plant community by virtue of having more resistance to herbivore attack. As part of this second objective, the following hypotheses were tested: (1) that when combined with the growth-defence trade-off, variation in herbivore pressure imposed on competing seedling species can significantly alter patterns of plant species composition into the mature stage, and (2) that variation in seedling herbivory will influence plant competition via changes to seedling size hierarchies.
MATERIALS AND METHODS
Seed collection and germination
Seeds of Trifolium repens L. and T. pratense L. were collected from over 20 maternal plants growing in the same chalk grassland community at Weather Hill (51°15′N, 1°42′W), Salisbury Plain, southern England during September 2004. A sympatric, congeneric pair was selected on the basis that their shared evolutionary history confers a fundamental physiological similarity, increasing the likelihood and intensity of competition (Kelly and Bowler, 2005; Kelly and Hanley, 2005). The study by Hulme (1994) also suggests that the two species vary in their susceptibility to mollusc attack at the seedling stage (T. pratense being more vulnerable than T. repens). Seeds were set to germinate in 90-mm-diameter plastic Petri dishes containing two layers of 90-mm-diameter Whatman No. 1 filter paper and 5 mL of distilled water. The dishes were maintained in a dark incubator set at 15 °C.
Relative seedling acceptability and growth
Immediately following radicle appearance, seedlings were transferred to 50-mm-diameter plastic plant pots containing rendzina soil collected from Weather Hill and sieved through a 15-mm2 mesh prior to use. Two newly germinated, conspecific Trifolium seedlings were planted 45 mm apart and grown in glasshouse conditions (mean daily temperature: minimum, 17·3 °C ± 0·2 °C, maximum, 22·6 °C ± 0·2 °C; 12-h day:night) for 7 d. At this time two newly emerged lettuce seedlings (‘Tom Thumb’) were planted 45 mm apart in the same pot, perpendicular to the Trifolium seedlings (such that all four seedlings were arranged in a square). Lettuce seedlings, cultivated simultaneously in large plastic trays containing commercial potting compost, were used to ascertain the relative acceptability of the ‘test’ species and allow comparison between Trifolium congeners with reference to the same ‘index’ species (Fenner et al., 1999). Rapid development of lettuce seedlings compared with the test species meant that 7-d-old seedlings were at approximately the same ontogenetic stage as 14-d-old test seedlings. When the test seedlings were 14 d old they were exposed to herbivory by snails (Helix aspersa). Five replicate pots for each Trifolium species were sunk into large plastic propagator trays (350 × 215 × 70 mm deep) filled with commercial potting compost, such that the top of each pot was flush with the level of the compost. One pot was placed into the centre of each tray, with the remaining four pots located in the tray corners. This arrangement was replicated ten times for each Trifolium species. Four snails (Helix aspersa) of uniform size (approx. 3 cm diameter) were then added to each tray and retained overnight (approx. 16 h) using a clear plastic propagator lid (350 × 215 × 115 mm deep). The total number of Trifolium test species and lettuce index seedlings attacked by snails was determined for each replicate tray (all attacked seedlings suffered 100 % above-ground tissue loss). These values were used to calculate an acceptability index (AI) for Trifolium seedlings within individual trays, based on the formula given by Fenner et al. (1999):
| 1 |
Average AI for T. pratense and T. repens was then calculated across all ten replicate trays for each species.
Contemporaneously with the seedling acceptability trial, seedling growth was quantified by planting one newly germinated seedling into the centre of a 50-mm-diameter pot containing rendzina. Twelve seedlings of each Trifolium species were grown in the same glasshouse conditions until 14 d old before being removed from the pots, cleaned of any adhering soil and oven-dried for 24 h at 60 °C. Dry weight biomass was then quantified for each seedling. Following the reasoning of Kelly and Hanley (2005), absolute size at 14 d old was used rather than other commonly employed measures of plant growth such as relative growth rate to compare seedling competitive ability. Although absolute size naturally incorporates differences in relative growth rate between species, it also allows for the effects of initial seed mass on seedling competitive ability: larger seeded species often produce larger, more competitive seedlings (Westoby et al., 1996).
Snail herbivory and Trifolium seedling competition
Trifolium seedlings were germinated in Petri dishes as described above. Immediately following appearance of the radicle, seedlings were transferred to 110-mm-diameter pots containing rendzina. Eleven seedlings of each species were planted together into a regular hexagonal array, such that each was 20 mm away from its closest neighbour. Seedlings were also positioned in the same stratified, random configuration in each of the 24 pots. By using this arrangement, it was ensured that patterns of association between the two species which might otherwise affect seedling selection by molluscs (Hanley, 2004), or competitive interactions between neighbouring seedlings (Hanley and Groves, 2002), were held constant between treatments. The seedlings were grown in glasshouse conditions (mean daily temperature: minimum, 18·1 °C ± 0·2 °C, maximum, 22·1 °C ± 0·1 °C; 12-h day:night) for 14 d. At this time each of the 24 pots was sunk into a plastic box (390 × 273 × 90 mm deep) such that top of the pot was flush with the compost that filled it. Variation in the intensity of herbivore pressure was simulated by allowing two, five or ten snails (Helix aspersa) to graze the seedlings in each pot overnight, with snails being retained by means of a plastic propagator lid (350 × 215 × 115 mm deep). There were six replicates of each snail density treatment along with a similar number of ungrazed controls. Following snail removal, the number of surviving seedlings was noted before pots were randomly arranged on a glasshouse bench and the remaining plants cultivated for a further 106 d (mean daily temperature: minimum, 18·2 °C ± 0·2 °C; maximum, 22·0 °C ± 0·2 °C; 12-h day:night). At this time all plants were harvested, cleaned of any adhering soil and oven-dried for 24 h at 60 °C before being weighed.
Estimating plant size variation
The most commonly employed method for quantifying PSV is the Gini coefficient (Weiner and Solbrig, 1984; Damgaard and Weiner, 2000; Hanley and Groves, 2002). In this approach, individuals are ranked according to biomass and the cumulative percentage of biomass is plotted against the cumulative percentage of the population. Perfect equality results in a diagonal line from the origin to the upper right-hand corner; deviation from the diagonal represents inequality in size distribution, quantified as the ratio of the area between the diagonal and the curve – the Gini coefficient (Weiner and Solbrig, 1984). Thus, G has a maximum value of 1·0 in an infinite population where all individuals except one have zero biomass, and 0, where all individuals have exactly the same biomass. In studies such as the present one where the analysis of PSV is confounded by mortality, Weiner (1993) suggests allocating plants killed by herbivory a size equal to zero. Given the likelihood that snails would be expected to chose seedlings based on size (Hanley et al., 1995b) thus further confounding the effect of herbivory on PSV as smaller seedlings would be selectively killed, Weiner's suggestion (Weiner, 1993) was followed here.
Size inequality for whole -plant dry weight biomass for n plants having a mean weight was determined by calculating the Gini coefficient:
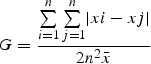 |
2 |
G values were multiplied by n/(n – 1) to provide unbiased values (G'; Weiner and Solbrig, 1984). Mean G' was calculated for both Trifolium species across the six replicate pots within each snail treatment.
RESULTS
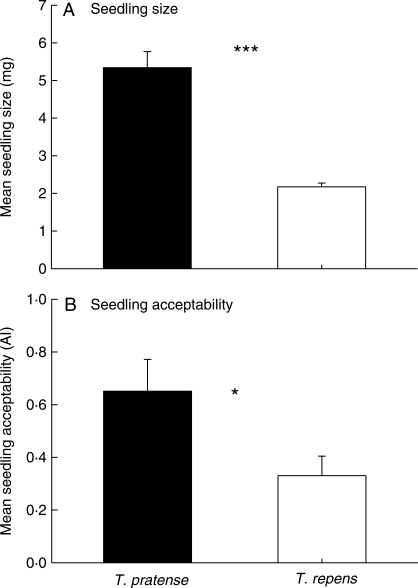
There were clear differences in relative seedling size and seedling acceptability between the two Trifolium species (Fig. 1). Following data transformation [ln(x + 1) ‘seedling size’, and arcsine ‘seedling acceptability’] and application of a Cochran test to ensure homogeneity of variances (Underwood, 1997), one-way ANOVA revealed that T. pratense seedlings were much larger than T. repens seedlings (F1,22 = 76·26 P < 0·0001), but also more susceptible to snail herbivory (F1,18 = 5·22, P = 0·035). These results provide evidence for a trade-off between seedling defence and competitive ability in the congeneric pair; i.e. the larger, dominant competitor is also more susceptible to herbivore attack.
Fig. 1.
Mean (±s.e.) size (A) and acceptability (B) of 14-d-old Trifolium pratense and T. repens seedlings. Seedling acceptability was determined following exposure to snail (Helix aspersa) herbivory overnight; seedling size was quantified as dry weight biomass. Differences between treatment means for the two species following one-way ANOVA are shown as *, P < 0·05; ***, P < 0·001.
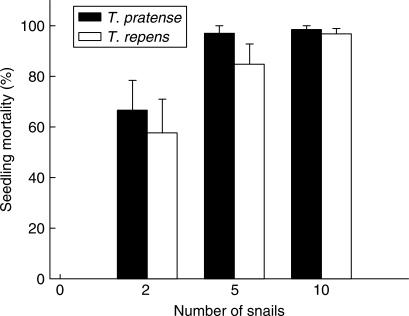
Following application of a Cochran test and data transformation where necessary, two-way ANOVA was used to examine the interactive effects of snail number and Trifolium species identity on seedling mortality at 14 d old, mean plant dry weight biomass, and PSV in the mixed plantings. Seedling mortality at 14 d old (Fig. 2) increased with snail number (F3,40 = 103·77, P < 0·0001 – after arcsine transformation), but did not vary between species (F1,40 = 3·02, P = 0·09), nor was there any significant interaction (F3,40 = 0·79, P = 0·51) between factors. No seedling deaths were recorded in the ungrazed treatment, while mortality exceeded 97 % for both species when pots were exposed to ten snails. Seedling mortality was also high for both species in the five-snail treatment. All but a single T. repens individual was eaten in four of the six replicate pots, and all but two T. pratense seedlings (within one replicate pot) were consumed by snails. For plants initially subject to snail herbivory (data not shown), remarkably few plants died over the next 106 d. However, mortality increased from zero at 14 d old to 7·6 % (±4·3 s.e.) for T. pratense and 16·7 % (±6·8 s.e.) for T. repens plants in the established, ungrazed pots.
Fig. 2.
Mean percentage seedling mortality of Trifolium pratense and T. repens following exposure to snails (Helix aspersa). Eleven seedlings of each species were planted together in hexagonal arrays in 110-mm-diameter pots and at 14 d old subjected to herbivory by zero, two, five or ten snails overnight.
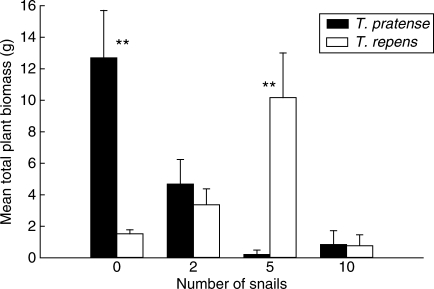
Variation in snail number at 14 d old had a significant effect on total mean plant dry weight biomass (F3,40 = 6·93, P = 0·007) for mature plants (Fig. 3). Although the ‘species’ effect was negligible (F1,40 = 0·02, P = 0·564), there was a highly significant interaction between factors (F3,40 = 15·1, P < 0·001). When differences were compared between treatment means for the two Trifolium species using S-N-K tests (Underwood 1997), significant (P < 0·01) differences were found between the total biomass of T. pratense and T. repens plants in both the ungrazed and five snail treatments. However, the relative composition of the two Trifolium species varied dramatically between these two treatment groups. In the ungrazed treatment, pots were almost totally dominated by T. pratense, while the composition of the five snail treatment pots was heavily biased towards T. repens.
Fig. 3.
Mean total dry weight biomass of Trifolium pratense and T. repens plants 106 d following seedling exposure to snails (Helix aspersa). Eleven seedlings of each species were planted together in hexagonal arrays in 110-mm-diameter pots and at 14 d old subjected to herbivory by zero, two, five or ten snails overnight. Results of S-N-K tests showing differences between treatment means for the two Trifolium species following two-way ANOVA are shown as **, P < 0·01.
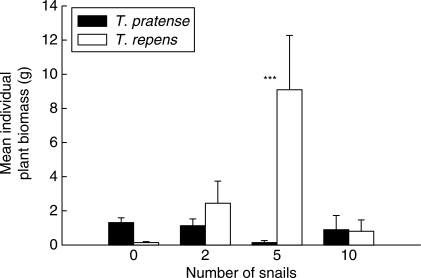
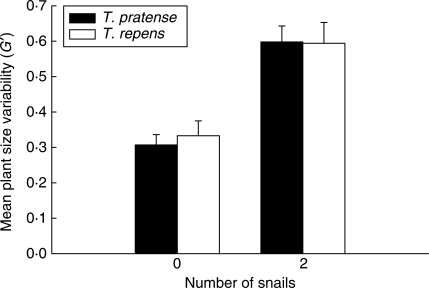
Individual plant biomass (Fig. 4) was greatly affected by snail number [F3,40 = 44·11, P = 0·003 – after ln(x + 0·001) transformation], and showed significant variation between species (F1,40 = 5·05, P = 0·03). There was also a significant interaction between factors (F3,40 = 4·34, P = 0·01), although S-N-K tests revealed a significant (P < 0·001) difference between individual T. pratense and T. repens plant biomass in the five-snail treatment only. While a relatively large number of T. pratense plants dominated the biomass of ungrazed pots, their individual size was small relative to the few very large T. repens plants that dominated the five snail treatment pots. This variability in plant size is further highlighted in the G'-values derived for plants in these treatments (Fig. 5). Due to high plant mortality and many replicate pots having no individuals present at harvest (thus G' could not be calculated), mean G' was compared for ungrazed and two-snail treatments only. However, PSV was reduced when seedlings were exposed to snail herbivory (F1,17 = 20·94, P < 0·0001 after arcsine transformation), an effect consistent for both species (‘species’ F1,17 = 0·07, P = 0·790; ‘snail number’ × ‘species’ interaction F1,17 = 0·165, P = 0·690).
Fig. 4.
Mean individual dry weight biomass of Trifolium pratense and T. repens plants 106 d following seedling exposure to snails (Helix aspersa). Eleven seedlings of each species were planted together in hexagonal arrays in 110-mm-diameter pots and at 14 d old subjected to herbivory by zero, two, five or ten snails overnight. Results of S-N-K tests showing differences between treatment means for the two Trifolium species following two-way ANOVA are shown as ***, P < 0·001.
Fig. 5.
The effects of snail herbivory (Helix aspersa) on mean plant size variability (unbiased Gini coefficient G') of Trifolium pratense and T. repens. Eleven seedlings of each species were planted together in hexagonal arrays in 110-mm-diameter pots and at 14 d old subjected to herbivory by two snails overnight or left ungrazed (zero snails). Mature plants were harvested at 120 d old.
DISCUSSION
Having found evidence for a growth-defence trade-off for the sympatric Trifolium seedlings, our hypotheses predicted that: (1) variation in the intensity of seedling herbivore pressure would differentially affect the recruitment success of the two species into the mature phase, and (2) seedling herbivory would influence recruitment through its effect on plant size hierarchies. Despite small differences in mortality suffered by the two species at 14 d old, shifts in community composition from dominance in ungrazed pots by T. pratense (more competitive but herbivore-sensitive), through species co-dominance at intermediate herbivory (two-snails), to dominance by T. repens (weaker competitor, but less herbivore-sensitive) in the more intensively grazed (five-snail) treatment, offers strong support for hypothesis 1.
Dominance of the five-snail treatment by T. repens was inevitable given that all except two T. pratense seedlings (present in one replicate pot) had been consumed by snails. However, in accordance with the earlier study by Weiner (1993), we also found that herbivory increased PSV between the zero- and two-snail treatments. Weiner (1993) ascribed this effect to preferential snail selection of small plants; thus large plants were left intact to grow alongside the remaining small plants, an initial discrepancy in size which was amplified as competition proceeded. Indeed, selective herbivory upon small seedlings has been observed (Hanley et al., 1995b), an effect that may be ascribed in part to an increase in chemical defence with seedling ontogeny (Boege and Marquis, 2005; Elger et al., 2009). Consequently similar intra-specific size-based selection by snails was likely here, although initial differences in size for 14-d-old seedlings would have been much less than those in Weiner's study where older plants were used. Moreover in the present study, size-based selection is partly confounded by the fact that snails showed a general preference for larger (more acceptable) T. pratense seedlings. It is probable, however, that having opted to feed on one or other Trifolium species, snails preferentially selected the smaller seedlings of each. However, since PSV increased simultaneously and by about the same magnitude for both Trifolium species when seedlings were exposed to herbivory, the present data do not support hypothesis 2; i.e. variation in PSV does not explain how seedling herbivory influences competition between two establishing species.
Despite the fact that the wholesale removal of T. pratense seedlings by snails played a more prominent role in inter-specific interactions than their influence on PSV, this study nonetheless highlights an important interaction between seedling herbivory and plant competition. Although many studies have shown how selective seedling herbivory impacts upon plant community composition (Hanley et al., 1995a, 1996a; Howe et al., 2002; Asquith and Mejia-Chang, 2005; Beckage and Clarke, 2005), the precise mechanism underpinning this process has more often been assumed than demonstrated. It is shown here how an interaction between a growth-defence trade-off at the seedling stage and variation in the intensity of herbivore pressure (snail number), conspires to dictate which of two sympatric competitors dominated a synthesized plant community. Moreover, although based on a relatively short-term glasshouse study involving only two species, this study also addresses concepts relevant to species coexistence in natural plant communities.
Alongside environmental variation in resource availability and species-specific differences in resource requirements, plant coexistence is widely believed to depend upon environmental fluctuations that permit spatially and temporally segregated establishment of species with different sensitivities to factors causing juvenile mortality (Connell, 1971; Chesson, 1986; Tilman, 1994; Pacala and Tilman, 1994; Kelly and Bowler, 2002). It is also well established that plant survival during the regeneration phase is an important bottle-neck for recruitment to the adult stage; a combination of disease, herbivory, nutrient limitation, competition and other stresses limit early growth and often result in exceptionally high mortality for entire seedling cohorts (Grubb, 1977; Moles and Westoby, 2004; Fenner and Thompson, 2005). As the primary agent of mortality for most seedlings (Moles and Westoby, 2004) it is not unreasonable therefore to assume that spatial, seasonal or yearly shifts in herbivore pressure are a prime cause of fluctuation in plant regeneration success. However, while spatio-temporal variation in predation is firmly encapsulated within mainstream species-coexistence theory (Caswell, 1978; Chase et al., 2002; Kuang and Chesson, 2009), only the Janzen–Connell hypothesis (Janzen, 1970; Connell, 1971) specifically considers the role of spatio-temporal fluctuation in seedling predation as a foundation for plant coexistence.
The present study shows how a growth-defence trade-off and simulated spatio-temporal variation in the intensity of seedling herbivory can significantly influence the outcome of seedling regeneration success. At the ecosystem-scale we suggest that where spatio-temporal variation in seedling herbivory is common, one might consequently expect to find high plant species diversity. This hypothesis is all the more compelling given the considerable variation in seedling growth and defence traits (Grime et al., 1997; Elger et al., 2009) and recent support for inter-specific growth-defence trade-offs at the seedling stage (Fine et al., 2004, 2006; Kelly and Hanley, 2005). We note, however, that in some instances the interaction between predation and competition may be more complex than the relationship demonstrated here. Recent work, for example, has shown that natural enemies such as herbivores can undermine species coexistence as well as promote it (Chesson and Kuang, 2008; Kuang and Chesson, 2009). Nevertheless, it is illustrated here how spatio-temporal variation in herbivory and an ecophysiological link between seedling competition and anti-herbivore defence can be integrated to explain how seedling herbivory impacts on plant species coexistence. The task now remains for ecologists working in natural plant communities to demonstrate whether this interaction can result in the stable equilibrium of plant species within the established plant community.
ACKNOWLEDGEMENTS
We thank Dr Colleen Kelly for her helpful comments and suggestions throughout the project, Emma Fegan and Clare Phillips for technical assistance, and Dr Miguel Franco and two anonymous referees for comments on an earlier draft of this manuscript. This research was partly funded by a British Ecological Society, Small Ecological Project Grant (No. 2240) to R.J.S.
LITERATURE CITED
- Adler PB, Raff DA, Laurenroth WK. The effect of grazing on the spatial heterogeneity of vegetation. Oecologia. 2001;128:465–479. doi: 10.1007/s004420100737. [DOI] [PubMed] [Google Scholar]
- Asquith NM, Mejia-Chang M. Mammals, edge effects, and the loss of tropical forest diversity. Ecology. 2005;86:379–390. [Google Scholar]
- Barnes HF, Weil JW. Slugs in gardens their numbers, activities and distribution: Part I. Journal of Animal Ecology. 1944;13:140–175. [Google Scholar]
- Barton KE. Early ontogenetic patterns in chemical defense in Plantago (Plantaginaceae): genetic variation and trade-offs. American Journal of Botany. 2007;94:56–66. doi: 10.3732/ajb.94.1.56. [DOI] [PubMed] [Google Scholar]
- Beckage B, Clark JS. Does predation contribute to tree diversity? Oecologia. 2005;143:458–469. doi: 10.1007/s00442-004-1815-9. [DOI] [PubMed] [Google Scholar]
- Bergleson J. Spatial patterning in plants: opposing effects of herbivory and competition. Journal of Ecology. 1990;78:937–948. [Google Scholar]
- Boege K, Marquis RJ. Facing herbivory as you grow up: the ontogeny of resistance in plants. Trends in Ecology and Evolution. 2005;88:441–448. doi: 10.1016/j.tree.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Burt-Smith GS, Grime JP, Tilman D. Seedling resistance to herbivory as a predictor of relative abundance in a synthesised prairie community. Oikos. 2003;101:345–353. [Google Scholar]
- Caswell H. Predator-mediated coexistence: a nonequilibrium model. American Naturalist. 1978;112:127–154. [Google Scholar]
- Chase JM, Abrams PA, Grover JP, et al. The interaction between predation and competition: a review and synthesis. Ecology Letters. 2002;5:302–315. [Google Scholar]
- Chesson PL. Environmental variation and the coexistence of species. In: Diamond J, Case TJ, editors. Community ecology. New York, NY: Harper and Row; 1986. pp. 240–256. [Google Scholar]
- Chesson P, Kuang JJ. The interaction between predation and competition. Nature. 2008;456:235–238. doi: 10.1038/nature07248. [DOI] [PubMed] [Google Scholar]
- Connell JH. On the role of natural enemies in preventing competitive exclusion in some marine animals and rainforest trees. In: den Boer BJ, Gradwell GR, editors. Dynamics of populations. Wageningen: Centre for Agricultural Publishing and Documentation; 1971. pp. 298–310. [Google Scholar]
- Crawley MJ. Plant–herbivore dynamics. In: Crawley MJ, editor. Plant ecology. 2nd edn. Oxford: Blackwell; 1997. pp. 401–474. [Google Scholar]
- Damgaard C, Weiner J. Describing inequality in plant size or fecundity. Ecology. 2000;81:1139–1142. [Google Scholar]
- Elger A, Lemoine DG, Fenner M, Hanley ME. Plant ontogeny and chemical defence: older seedlings are better defended. Oikos. 2009;118:767–773. [Google Scholar]
- Fenner M, Thompson K. The ecology of seeds. 2nd edn. Cambridge: Cambridge University Press; 2005. [Google Scholar]
- Fenner M, Hanley ME, Lawrence R. Comparison of seedling and adult palatability in annual and perennial plants. Functional Ecology. 1999;13:546–551. [Google Scholar]
- Fine PVA, Mesones I, Coley PD. Herbivores promote habitat specialization by trees in Amazonian forests. Science. 2004;305:663–665. doi: 10.1126/science.1098982. [DOI] [PubMed] [Google Scholar]
- Fine PVA, Miller ZJ, Mesones I, et al. The growth-defense trade-off and habitat specialization by plants in Amazonian forests. Ecology. 2006;87:S150–S162. doi: 10.1890/0012-9658(2006)87[150:tgtahs]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Glynn C, Herms DA, Orians CM, Hansen RC, Larsson S. Testing the growth-differentiation balance hypothesis: dynamic responses of willows to nutrient availability. New Phytologist. 2007;176:623–634. doi: 10.1111/j.1469-8137.2007.02203.x. [DOI] [PubMed] [Google Scholar]
- Grime JP, Thompson K, Hunt R, et al. Integrated screening validates primary axes of specialisation in plants. Oikos. 1997;79:259–281. [Google Scholar]
- Grubb PJ. The maintenance of species-richness in plant communities: the importance of the regeneration niche. Biological Reviews. 1977;52:107–145. [Google Scholar]
- Hanley ME. Seedling herbivory and the influence of plant species richness in seedling neighbourhoods. Plant Ecology. 2004;170:35–42. [Google Scholar]
- Hanley ME, Groves RH. Effect of the rust fungus Puccinia chondrillina TU 788 on plant size and plant size variability in Chondrilla juncea. Weed Research. 2002;42:370–376. [Google Scholar]
- Hanley ME, Lamont BB. Herbivory, serotiny and seedling defence in Western Australian Proteaceae. Oecologia. 2001;126:409–417. doi: 10.1007/s004420000538. [DOI] [PubMed] [Google Scholar]
- Hanley ME, Fenner M, Edwards PJ. An experimental field study of the effects of mollusc grazing on seedling recruitment and survival in grassland. Journal of Ecology. 1995;a 83:621–627. [Google Scholar]
- Hanley ME, Fenner M, Edwards PJ. The effect of seedling age on the likelihood of herbivory by the slug Deroceras reticulatum. Functional Ecology. 1995;b 9:754–759. [Google Scholar]
- Hanley ME, Fenner M, Edwards PJ. The effect of mollusc grazing on seedling recruitment in artificially created grassland gaps. Oecologia. 1996;a 106:240–246. doi: 10.1007/BF00328604. [DOI] [PubMed] [Google Scholar]
- Hanley ME, Fenner M, Edwards PJ. Mollusc grazing and seedling survivorship: the role of gap size, species identity and season. Acta Oecologica. 1996;b 17:331–341. [Google Scholar]
- Hanley ME, Fenner M, Whibley H, Darvil B. Early plant growth: identifying the end point of the seedling phase. New Phytologist. 2004;163:61–66. doi: 10.1111/j.1469-8137.2004.01094.x. [DOI] [PubMed] [Google Scholar]
- Hanley ME, Lamont BB, Fairbanks MM, Rafferty CM. Plant structural traits and their role in anti-herbivore defence. Perspectives in Plant Ecology, Evolution and Systematatics. 2007;8:157–178. [Google Scholar]
- Haring DA, Huber MJ, Suter D, Edwards PJ, Luescher A. Plant enemy-derived elicitors increase the foliar tannin concentration of Onobrychis viciifolia without a trade-off to growth. Annals of Botany. 2008;102:979–987. doi: 10.1093/aob/mcn189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herms DA, Mattson WJ. The dilemma of plants: to grow or to defend. Quarterly Review of Biology. 1992;67:283–335. [Google Scholar]
- Hill BHC, Silvertown J. Higher-order interaction between molluscs and sheep affecting seedling numbers in grassland. Acta Oecologica. 1997;18:587–596. [Google Scholar]
- Howe HF, Brown JS, Zorn-Arnold B. A rodent plague on prairie diversity. Ecology Letters. 2002;5:30–36. [Google Scholar]
- Hulme PE. Seedling herbivory in grassland: relative impact of vertebrate and invertebrate herbivores. Journal of Ecology. 1994;82:873–880. [Google Scholar]
- Izhaki I, Ne'eman G. The effect of porcupine and bast scale on Aleppo pine recruitment after fire. Acta Oecologica. 1997;17:97–107. [Google Scholar]
- Janzen DH. Herbivores and the number of tree species in tropical forests. American Natuarlist. 1970;104:501–508. [Google Scholar]
- Jennings TJ, Barkham JP. Foods of slugs in mixed deciduous woodland in Norfolk, England. Oikos. 1975;26:211–221. [Google Scholar]
- Johnson MP, Hanley ME, Frost NJ, Moseley MWJ, Hawkins SJ. The persistent spatial patchiness of limpet grazing. Journal of Experimental Marine Biology and Ecology. 2008;365:136–141. [Google Scholar]
- Kelly CK, Bowler MG. Coexistence and relative abundance in forest tree species. Nature. 2002;417:437–440. doi: 10.1038/417437a. [DOI] [PubMed] [Google Scholar]
- Kelly CK, Bowler MG. A new application of storage dynamics: differential sensitivity, diffuse competition and temporal niches. Ecology. 2005;86:1012–1022. [Google Scholar]
- Kelly CK, Hanley ME. Juvenile growth and palatability in congeneric British herbs. American Journal of Botany. 2005;92:1586–1589. doi: 10.3732/ajb.92.9.1586. [DOI] [PubMed] [Google Scholar]
- Koricheva J. Meta-analysis of sources of variation in fitness costs of plant antiherbivore defences. Ecology. 2002;83:176–190. [Google Scholar]
- Kuang JJ, Chesson P. Coexistence of annual plants: generalist seed predation weakens the storage effect. Ecology. 2009;90:170–182. doi: 10.1890/08-0207.1. [DOI] [PubMed] [Google Scholar]
- Lindquist ES, Carroll CR. Differential seed and seedling predation by crabs: impacts on tropical coastal forest composition. Oecologia. 2004;141:661–671. doi: 10.1007/s00442-004-1673-5. [DOI] [PubMed] [Google Scholar]
- Manzaneda AJ, Sperens U, García MB. Effects of microsite disturbances and herbivory on seedling performance in the perennial herb Helleborus foetidus (Ranunculaceae) Plant Ecology. 2005;179:73–82. [Google Scholar]
- Maron JL, Crone E. Herbivory: effects on plant abundance, distribution and population growth. Proceedings of the Royal Society Series B. 2006;22:2575–2584. doi: 10.1098/rspb.2006.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maron JL, Kauffman MJ. Habitat-specific impacts of multiple consumers on plant population dynamics. Ecology. 2006;87:113–124. doi: 10.1890/05-0434. [DOI] [PubMed] [Google Scholar]
- Moles AT, Westoby M. What do seedlings die from and what are the implications for evolution of seed size? Oikos. 2004;106:193–199. [Google Scholar]
- Pacala SW, Tilman D. Limiting similarity in mechanistic and spatial models of plant competition in heterogeneous environments. American Naturalist. 1994;143:222–257. [Google Scholar]
- Rafferty C, Lamont BB, Hanley ME. Selective feeding by western grey kangaroos on seedlings of Hakea species varying in morphology and chemistry. Plant Ecology. 2005;177:201–208. [Google Scholar]
- Schaffner U, Vrieling K, van der Meijden E. Pyrrolizidine alkaloid content in Senecio: ontogeny and developmental constraints. Chemoecology. 2003;13:39–46. [Google Scholar]
- Scheidel U, Bruelheide H. Age-specific and season-specific mollusk damage to seedlings of grassland Asteraceae. Journal of the Torrey Botanical Society. 2004;131:140–149. [Google Scholar]
- Symondson WOC, Glen DM, Ives AR, Langdon CJ, Wiltshire CW. Dynamics of the relationship between a generalist predator and slugs over five years. Ecology. 2002;83:137–147. [Google Scholar]
- Tilman D. Competition and biodiversity in spatially structured habitats. Ecology. 1994;75:2–16. [Google Scholar]
- Underwood AJ. Experiments in ecology. Cambridge: Cambridge University Press; 1997. [Google Scholar]
- Weiner J. Size hierarchies in experimental populations of annual plants. Ecology. 1985;66:743–752. [Google Scholar]
- Weiner J. Competition, herbivory and plant size variability: Hypochoeris radicata grazed by snails (Helix aspersa) Functional Ecology. 1993;7:47–53. [Google Scholar]
- Weiner J, Solbrig OT. The meaning and measurement of size hierarchies in plant populations. Oecologia. 1984;61:334–336. doi: 10.1007/BF00379630. [DOI] [PubMed] [Google Scholar]
- Westoby M, Leishman MR, Lord J. Comparative ecology of seed size and dispersal. In: Silvertown J, Franco M, Harper JL, editors. Plant life histories: ecology, phylogeny and evolution. Cambridge: Cambridge University Press; 1996. pp. 143–162. [Google Scholar]
- Wiegand K, Saltz D, Ward D, Levin SA. The role of size inequality in self-thinning: a pattern-oriented simulation model for arid savannas. Ecological Modelling. 2008;210:431–445. [Google Scholar]