Abstract
Background and Aims
Growth and reproductive strategies of plants are often related to particular, although usually poorly characterized, spatial distributions of shoots within the plant's architecture. In this study it is therefore hypothesized that a close relationship exists between architectural position, axis morphology (length, diameter, leaf area), and functional behaviour (branching, flowering and fruiting). The study focused on the architectural position of mango growth units, defined here as being the relative position, apical or lateral, on the parent growth unit, i.e. growing from the apical or a lateral meristem, respectively.
Methods
Stem length and leaf characteristics (area, dry weight) were measured on apical and lateral growth units of four mango cultivars over two years. Branching, flowering and fruiting were assessed for both growth unit types using an exhaustive description of tree vegetative and reproductive growth over two years. The relationships between growth unit diameter and flowering and fruiting were assessed for one of the four cultivars.
Key Results
A pronounced morphological dimorphism was observed for the four cultivars. Across cultivars, stem length was significantly 1·31–1·34 times longer and total leaf area was 2·54–3·47 times larger in apical compared to lateral growth units. Apical growth units tended to branch, flower and fruit more than lateral growth units. The relationship between growth unit diameter and flowering rate was quadratic and dependent on growth unit position. The relationship between growth unit diameter and fruiting rate was linear and independent of growth unit position.
Conclusions
Morphological traits of mango growth units were clearly involved in the determinism of flowering and fruiting, although in different ways. The results, however, showed that current hypotheses of flowering, such as carbohydrate availability and florigenic promoters, are not sufficient in themselves if they neglect the hierarchical relationships between axes, i.e. their relative position, apical or lateral.
Key words: Axis dimorphism, branching, flowering, fruiting, growth unit, Mangifera indica, mango, Reunion Island
INTRODUCTION
Growth and reproductive strategies of plants are often connected to the morphology of axes and to their position within the plant architecture. The coexistence of axes with different morphological characteristics, hereafter referred to as axis polymorphism, is frequent and plays a role in plant life strategies (Hallé et al., 1978; Suzuki, 2000; Hasegawa and Takeda, 2001; Remphrey et al., 2002; Kawamura and Takeda, 2006). Axis polymorphism results from contrasting meristem expression and activity (Hallé et al., 1978). It is mainly related to axis orientation (vertical or orthotropy vs. horizontal or plagiotropy) or axis length (short axis vs. long axis) (Bell, 1991; Barthélémy and Caraglio, 2007).
In most species, axis polymorphism is a main determinant of plant architectural organization. In particular, differentiation of axis size may affect several variables related to the stem (length, diameter, dry mass), to the leaves (area, dry mass) or both. As an element of plant life strategy, axis polymorphism can express the adaptive behaviour of plants faced with evolutionary constraints. For example, in dioecious species, different reproductive costs between plants that behave as males or as females may lead to dimorphism in secondary sexual characteristics such as leaf area, internode size, the form of the canopy (Bond and Midgley, 1988; Kohorn, 1994), and in primary and secondary growth characteristics (Verdú et al., 2007). Another example is the case of species living in a seasonal climate. Axis dimorphism (i.e. two morphologically distinct kinds of axes) appear in the Mediterranean Cistus incanus subsp. incanus as the result of the seasonal adaptation of axis morphology to the prevailing climatic conditions during the plant's growth: short axes with few, densely packed, small leaves when grown in summer, and long axes with many, large leaves when grown in winter and spring (Aronne and De Micco, 2001).
Axis polymorphism does not concern only axis morphology but axis functioning as well, and especially reproductive traits. Long and short axes are specialized in environmental exploration and exploitation, respectively (Bell, 1991). A main difference concerns flowering and fruiting: short axes generally bear flowers and fruit, and long axes are mainly vegetative (Bell, 1991). This phenomenon has been described and studied in some temperate fruit tree species such as apple (Malus domestica: Johnson and Lakso, 1986; Wünsche et al., 1996; Wünsche and Lakso, 2000; Lauri and Kelner, 2001) and cherry (Prunus avium: Lauri, 1992), as well as in temperate forest tree species (Hasegawa and Takeda, 2001; Remphrey et al., 2002; Kawamura and Takeda, 2006). The location of the different kinds of axes within the canopy layers has been characterized for some species (Hasegawa and Takeda, 2001; Remphrey et al., 2002; Kawamura and Takeda, 2006); however, only a few studies have dealt with their topological location within the tree architecture (Suzuki, 2000).
Rather than the axis length itself, the predominance of stem components over the foliar components, referred to as axialization, determines the vegetative status of the axes in apple, cherry and some tropical species (Lauri and Térouanne, 1991; Lauri, 1992; Lauri and Kelner, 2001). The higher the axialization is, the more vegetative the axis will be. In contrast, the lower the axialization is, the more floriferous the axis will be. This characteristic evolves during the plant's life. As shown in the case of invasive bramble (Rubus alceifolius), axialization is low during the first stages of growth, leading to the rapid autotrophy of the plant, whereas it is high at the adult stage when the plant explores the environment (Baret et al., 2003). In cherry, the axialization progressively decreases as the tree ages, in parallel with the reduction of axis length and the development of flowering on the axes (Lauri, 1992).
Nonetheless, the effects of morphological dimorphism on functional traits other than flowering and fruiting have been poorly investigated. In particular, branching is a point of interest. If branching processes differ between dimorphic axes, the tree architecture and form may be affected by the number and the position of each kind of axis.
The identification, characterization and location of morphologically and functionally different axes are of major importance in fruit tree species. This knowledge provides insights for a better understanding of the determinants of flowering and fruiting, and leads to practical recommendations for tree management in order to optimize fruit production and/or quality (Wünsche et al., 1996; Wünsche and Lakso, 2000; Lauri and Laurens, 2005; Stephan et al., 2007). Although this knowledge has been developed over more than a century for temperate fruit trees, data are lacking for tropical fruit species. Goguey (1997) contributed to this topic with an architectural analysis of mango seedling trees. He identified the respective roles of the architectural units belonging to the Scarrone model, with orthotropic axes, rhythmic branching and terminal flowering (Hallé et al., 1978), and reiteration characterized by a delayed growth of buds within the existing tree architecture.
The present study focuses on the growth unit (GU), an elementary component of the axes forming the architectural unit. We have characterized two architecturally contrasted GUs (Hallé et al., 1978), the apical and the lateral GUs of four mango (Mangifera indica) cultivars, both morphologically (stem length, leaf mass and area) and functionally (branching, flowering and fruiting). We hypothesized that apical GUs will have larger stem and leaf area and, consequently, will be more likely to flower, fruit and branch. This hypothesis was based on the suggested positive role of carbohydrate availability for flowering and fruiting in mango (Suryanarayana, 1978; Pandey, 1988; Chacko, 1991; Davenport and Nuñez-Elisea, 1997); a larger leaf area and stem implies larger carbohydrate synthesis and storage, respectively. The hypothesis rested on two implicit assumptions. First, no trade-off occurs among functions (vegetative growth and reproduction) at the GU level. Second, apical and lateral GUs are not functionally differentiated; they branch, flower and fruit in an equivalent manner, depending only on resource allocation to individual GUs. These assumptions diverged from the knowledge available on the functional differentiation of axes (e.g. Bell, 1991; Hasegawa and Takeda, 2001; Kawamura and Takeda, 2006), but formed a null hypothesis in the case of mango, for which no data was available on the morphology and functional differentiation of GUs. We addressed the following questions. (1) Do apical and lateral GUs of mango have different morphological traits? (2) Are these differences cultivar-dependent? (3) Are these differences related to contrasted flowering, fruiting and branching behaviours of apical and lateral GUs?
MATERIALS AND METHODS
Field site and plant material
The experimental orchard in which the study was conducted was located in a research station of the French Agricultural Research Centre for International Development (CIRAD) in Saint-Pierre, Reunion Island (20°52′S, 55°31′E) at 280 m a.s.l. An automatic weather station located close to the orchard recorded climatic data. Trees of eight mango (Mangifera indica) cultivars grafted onto the same polyembryonic rootstock, ‘Maison Rouge’, were planted in May 2001, about 8 months after grafting. The homogeneity of the nucellar rootstock seedlings was visually assessed at the nursery stage. The orchard was flat and the soil homogenous, but for ease of care among cultivars, planting was designed as plots of seven aligned trees per cultivar, repeated in two blocks, yielding 14 trees per cultivar. Tree spacing was 6 × 4 m, wide enough to avoid interactions between canopies during the study. Trees of a cultivar were therefore genetically similar, and environmental differences between them were minimal. Trees were drip-irrigated. Cultural practices were those recommended by local extension services. Trees were not pruned after planting to enable the natural development of the canopy. The first crop was harvested at the beginning of 2004, two and a half years after planting.
Four of these cultivars, ‘Cogshall’, ‘Irwin’, ‘José’ and ‘Kensington Pride’, were chosen for the study on the basis of their diverse origin, tree size, growth and flowering habits. ‘José’ is an Indian-type mango selected in the middle of the 19th century in Reunion Island and is the main cultivar grown locally (Vincenot, 2004); it is a medium-sized tree with an open canopy. ‘Cogshall’ was selected in Florida (Campbell, 1992) and is grown locally for export; it is a medium-sized tree with a dense, compact canopy. ‘Irwin’ was selected in Florida; it is a small-to-medium-sized tree with an open canopy (Campbell, 1992; Knight, 1997). ‘Kensington Pride’ is a commercial cultivar selected and grown in Australia; it is a large, vigorous tree with a dense spreading canopy (Knight, 1997). ‘Cogshall’ and ‘Irwin’ tend to be regular bearers, whereas ‘Kensington Pride’ and especially ‘José’ exhibit alternate bearing.
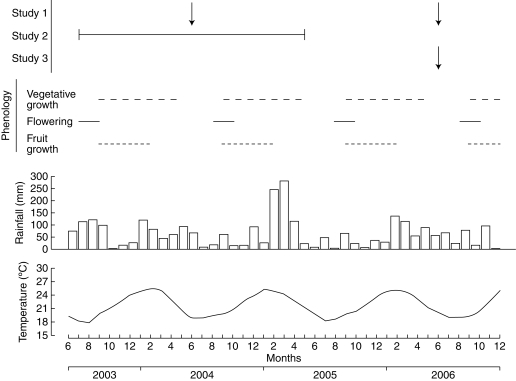
At the study site, mango trees flower from August to October and the harvest extends from the end of December to March (Fig. 1). Vegetative growth begins slowly with flowering, continues during fruit growth, and flushes after harvest during the hot and rainy season, until May. About half of the vegetative growth occurs after harvest. The vegetative resting period separating two growing seasons, each of which includes the reproductive stages and vegetative growth, occurs from June to July, just before flowering of the next growing season. Morphological measurements were performed during the vegetative resting period, after the last GUs had matured and leaves were completely expanded.
Fig. 1.
Time scale of the studies performed, mango phenology, monthly rainfall and mean monthly temperatures at the experimental orchard (20°52′S, 55°31′E) from June 2003 to December 2006.
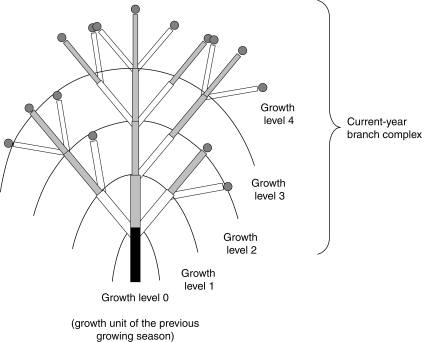
Mango trees have rhythmic and mainly sequential growth (Hallé et al., 1978). A resting period of a few weeks to a few months occurs between the end of the GU extension and the burst of its apical and possibly lateral buds to give new GUs. During the growing season, a terminal GU produces one-to-several GUs, among which can be distinguished an apical GU stemming from the apical bud if present, and none-to-several lateral GUs stemming from lateral buds. These GUs can themselves grow and branch similarly during the same growing season and produce successive growth levels constituting a current-year branch complex. Each generation of GUs during a growing season will hereafter be referred to as a growth level (Fig. 2). By definition, a growth level includes the GUs located at the same distance, expressed in number of GUs, from the terminal GU of the previous growing season. For a given growth level G (G = 1, 2, … ), the corresponding initial growth level G–1 is the preceding growth level at which GUs grew and branched to produce the growth level G. The initial growth level 0 thus corresponds to the terminal GUs of the previous growing season that resulted in the first growth level of the current annual growth (Fig. 2). Inflorescences appear apically on terminal GUs in mango. Consequently, GUs that have already flowered can only produce lateral GUs at the first growth level.
Fig. 2.
Schematic representation of a mango current-year branch complex composed of one apical branch and two lateral branches borne by a growth unit (GU) of the previous year's growing season (in black). Rectangles are GU stems; leaves are not represented. Apical GUs are in grey and lateral GUs are in white. The dark grey circles are the potential apical sites for flowering. Growth levels are illustrated by arcs and numbered according to the order of development from the beginning of the growing season.
A mango inflorescence is composed of hundreds of individual flowers that open successively for a period of about 2 weeks. They are pollinated by insects, in particular flies of several genera and bees. Because of the number of flowers per inflorescence, it is a time-consuming job to calculate a fruit-set rate as the ratio of the number of fruit to the number of flowers per GU. Moreover, natural fruit drop occurs during the month following fruit set. Consequently, and because the individual unit in this work was the GU, we considered that a GU flowered or fruited if it bore at least one inflorescence or if it bore at least one fruit until harvest, respectively.
Effect of GU position on its morphology
The effect of GU position on its morphology was investigated in June 2004 and June 2006 on ten to 15 current-year branches randomly sampled each year on three to five trees per cultivar. These branches were composed of one to 16 GUs arranged in successive (1 to 4) growth levels. The position (apical vs. lateral) of the basal GU of each branch was recorded before sampling. Each GU of these branches was identified as apical or lateral. They were then separated with pruning shears and individually subjected to the measurements detailed below. Overall, 306 GUs were sampled, 174 in 2004 and 132 in 2006. Sample size per cultivar is given in Table 1.
Table 1.
F- and P-values of the analyses of variance of the effects of growth unit (GU) position (apical, lateral), cultivar (4) and year (2) and their interactions for six morphological variables describing the growth units of four mango cultivars: growth unit length, number of leaves, leaf dry weight, individual leaf area, growth unit leaf area and axialization index
| Effects | d.f. | Stem length |
No. of leaves |
Leaf d. wt |
Individual leaf area |
GU leaf area |
Axialization index |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | P | F | P | F | P | F | P | F | P | F | P | ||
| Position | 1 | 24·0 | <0·001 | 490·9 | <0·001 | 391·1 | <0·001 | 195·4 | <0·001 | 449·2 | <0·001 | 245·6 | <0·001 |
| Cultivar | 3 | 65·1 | <0·001 | 40·4 | <0·001 | 27·9 | <0·001 | 26·1 | <0·001 | 47·2 | <0·001 | 16·4 | <0·001 |
| Year | 1 | 18·4 | <0·001 | 10·9 | 0·001 | 8·4 | 0·004 | 7·2 | 0·008 | 15·8 | <0·001 | 2·3 | 0·130 |
| Position × cultivar | 3 | 4·6 | 0·003 | 15·4 | <0·001 | 16·7 | <0·001 | 4·2 | 0·006 | 22·8 | <0·001 | 4·8 | 0·003 |
| Position × year | 1 | 0·9 | 0·346 | 16·3 | <0·001 | 14·4 | <0·001 | 6·5 | 0·011 | 22·5 | <0·001 | 3·5 | 0·061 |
| Cultivar × year | 3 | 4·3 | 0·005 | 6·8 | <0·001 | 1·6 | 0·192 | 2·6 | 0·049 | 3·7 | 0·012 | 1·2 | 0·293 |
| Position × cultivar × year | 3 | 0·4 | 0·742 | 5·0 | 0·002 | 1·3 | 0·274 | 0·6 | 0·630 | 2·1 | 0·106 | 0·8 | 0·513 |
| Residuals | 290 | – | – | – | – | – | – | – | – | – | – | – | – |
Sample size per cultivar: ‘Cogshall’, n = 73; ‘Irwin’, n = 64; ‘José’, n = 64; ‘Kensington Pride’, n = 105. d.f., degrees of freedom.
Stem length was measured from the base to the apical bud of the GU stem. The leaves were counted, and individual leaf area was measured with a planimeter (AM200, ADC BioScientific Ltd., Hoddesdon, UK). The leaf area of a GU was the sum of the individual areas of its leaves. The leaves of each GU were then oven-dried at 80 °C for 72 h and weighed. Because of secondary growth in diameter on GUs of branches composed of several growth levels, stem diameter and dry weight were not relevant variables. Thus, it was not possible to calculate the axialization index as the ratio of stem dry weight to leaf dry weight at the end of primary growth (Lauri and Kelner, 2001) for each GU. To overcome this problem, we approximated the axialization index as the ratio of stem length to leaf dry weight, expressed in mm g−1. This value is the inverse of the linear density of leaf dry weight along the stem and is an indicator of the balance between the stem and leaf components of the GU, independent of the stem secondary growth.
Effect of GU position on branching, flowering and fruiting
The effect of GU position on branching, flowering and fruiting was investigated non-destructively using a dataset resulting from an exhaustive topological description of GUs, flowering and fruiting of three trees per cultivar during two growing seasons. During the 2003 rest period, all terminal GUs of the 12 trees studied (three trees × four cultivars) were recorded as apical or lateral and labelled. The occurrence of flowering was recorded on these GUs between July and September 2003. The occurrence of fruiting was recorded from December 2003 to February 2004 during harvest. Vegetative growth was recorded from August 2003 to May 2004. Each new GU was identified as apical or lateral and labelled. The same observations were carried out during the following growing season, from July 2004 to May 2005. Overall, 11 756 GUs were recorded for this study.
Effect of GU position and diameter on flowering and fruiting
In the studies detailed above, morphology and functioning (branching, flowering and fruiting) of apical and lateral GUs were measured and analysed separately on different trees in the same orchard. Therefore, the interpretation of the relationships between morphology and functioning was based on the average behaviour of apical and lateral GUs for each cultivar. To further analyse the relationship between morphology and functioning and, in particular, to determine whether or not the different flowering and fruiting behaviours of apical and lateral GUs were related to their respective size according to our hypothesis, we carried out an additional study in the orchard, on the cultivar ‘Cogshall’ only. The objective was to establish the relationship between the diameter of the GU stem and flowering and fruiting for apical and lateral GUs. GU diameter is allometrically related to leaf mass and area of the GU (Normand et al., 2008) and is therefore an easy and non-destructive estimator of GU morphology.
In June 2006, 1510 terminal GUs (606 apical and 904 lateral) were randomly sampled and labelled on three trees of the cultivar ‘Cogshall’. Their relative position was recorded and their basal diameter was measured with a digital calliper. The occurrence of flowering on these GUs was recorded from July to September 2006. Fruiting (occurrence and number of fruit per GU) was recorded in December 2006, after natural fruit drop and before the beginning of harvest.
Data analysis
Statistical analyses were performed using R software (R Development Core Team, 2006). Morphological data were subjected to a full model of analysis of variance (GU position × cultivar × year). This was possible since the two years could be considered independent because they were not consecutive, and because of the random sampling of branches each year on several trees. The year effect might reflect tree ageing and environmental influences. Although the objective of the study was not to compare GU morphology between cultivars, the cultivar factor was integrated into the model to evaluate the contribution of each factor to explaining the variability of GU morphology.
The effect of GU position on branching, flowering and fruiting was analysed for each year and cultivar with generalized linear models (GLMs). The full model analysis was not performed as previously described: in the second study, years were not independent since the GUs recorded in the second year were the descendants of the GUs recorded in the first year. The cultivar effect could be related to genetic and to uncontrolled endogenous factors (e.g. phenology, previous yield) whose discussion was not the subject of this work. Branching was described by two variables: the occurrence of vegetative growth (Pvg), i.e. the occurrence of a terminal GU to produce at least one new GU, apical or lateral, during a growing season; and branching density (Dbr), i.e. the number of lateral GUs stemming from a GU where vegetative growth occurred. Dbr was estimated separately for GUs bearing or not bearing an apical GU in order to account for apical dominance. The total number of GUs stemming from a GU that branched was therefore Dbr + 1 if an apical GU was present, and Dbr otherwise. Moreover, Pvg and Dbr were estimated and analysed for each growth level to account for possible contrasting branching behaviours between growth levels. Growth units produced later had less time to grow again during the growing season, and we thus expected that Pvg would decrease as the growth levels increased.
GLMs were estimated assuming that the occurrence of vegetative growth (Pvg), flowering (Pflo), and fruiting (Pfru) of a GU followed binomial distributions (Venables and Ripley, 2002). Preliminary analysis of Dbr suggested an over-dispersed Poisson distribution for this parameter, i.e. with variance larger than mean. GLMs were therefore estimated for this parameter with a Poisson distribution and a dispersion parameter larger than 1 to account for over-dispersion (Venables and Ripley, 2002). GLMs were not estimated in the case of very unbalanced samples or when sample size was lower than five GUs for at least one factor level. Estimates of Pvg, Pflo, Pfru and Dbr for apical and lateral GUs and their 95 % confidence interval were calculated from GLMs by Monte Carlo sampling (n = 100 samples of two-thirds of the considered GU's population), followed by jackknifing (Efron, 1982).
Data from the third study, on cultivar ‘Cogshall’ only, were analysed according to Lauri and Trottier (2004). The GU basal diameter was measured with a digital calliper to the nearest 0·1 mm. Consequently, we had classes of GU diameter with a 0·1 mm span, which contained one-to-several GUs, each characterized by the occurrence of flowering and fruiting, and by the number of fruit (for those that bore fruit). For the analyses, only classes with five or more GUs were considered in order to study flowering, and with five or more GUs that flowered in order to study fruiting. In order to have a large sample size, the classes with four or more GUs bearing fruit were considered to study the mean number of fruit per GU. For each class of diameter, the flowering rate was calculated as the relative frequency of terminal GUs that flowered (number of GUs that flowered/total number of GUs), the fruiting rate was calculated as the relative frequency of terminal GUs that flowered and bore at least one fruit until harvest (number of GUs that bore at least one fruit/number of GUs that flowered), and the mean number of fruit per GU was calculated for GUs that bore fruit. The relationships between flowering rate, fruiting rate and mean number of fruit per GU, on the one hand, and GU diameter, on the other hand, were compared for apical and lateral GUs.
A quadratic adjustment was made to the relationships between the flowering rate and the GU diameter. A comparison of maximum location (i.e. GU diameter at maximum flowering rate) and maximum value (i.e., maximum flowering rate) between curves of apical and lateral GUs was tested. To compare maximum location between curves, a deviance F-test between embedded models was set up, defining the model with constraint of maximum located at the same place as the submodel. For comparison of maximum values, the standard error of the difference was calculated based on the covariance matrix of the model parameters, and a Z-test was then used. Linear adjustments were made to the relationships between the fruiting rate and the GU diameter, and between the mean number of fruit per GU and the GU diameter. To compare slopes and intercepts for apical and lateral GUs, an F-test between three embedded models (different slopes and intercepts, common slope and different intercepts, common slope and intercept) was set up. The effects of GU position on fruiting rate, GU diameter and mean number of fruit per GU were tested with one-way analysis of variance.
RESULTS
Effect of GU position on its morphology
Except for stem length, the GU position was the main factor explaining the variability of GU morphology, well ahead of the cultivar factor (Table 1). These two factors were highly significant for all variables. Year was the less-explanatory single factor of the variability of GU morphology; its effect was not significant for the axialization index. Results are presented in Fig. 3 after pooling data from the two years.
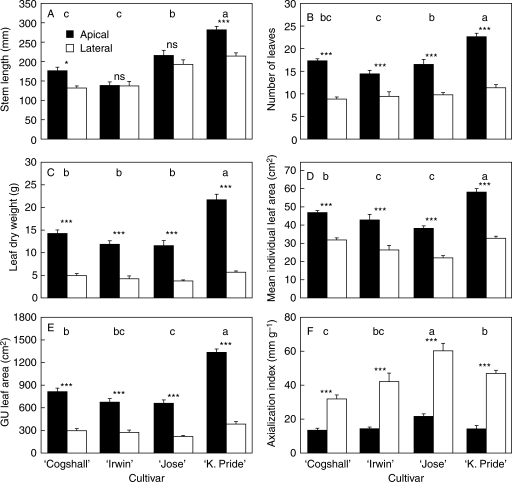
Fig. 3.
Effect of growth unit (GU) position, apical or lateral as indicated, on (A) stem length, (B) number of leaves, (C) leaf dry weight, (D) mean individual leaf area, (E) growth unit leaf area and (F) axialization index for four mango cultivars. Data for two years, 2004 and 2006, are pooled. Bars indicate s.e. For each variable, cultivars with different letters have significantly different means (Tukey's test, P < 0·05). The effect of growth unit position is indicated: ns, P > 0·05; *, P < 0·05; ***, P < 0·001.
The effect of GU position indicated axis dimorphism in these mango cultivars: apical GUs were longer, more leafy, and had a lower axialization index than lateral GUs (Fig. 3). Stem length was the variable the least affected by GU position. On average, apical GUs were 1·31 and 1·34 times longer than lateral GUs for ‘Kensington Pride’ and ‘Cogshall’, respectively. These differences were not significant for ‘Irwin’ and ‘José’. The GU leaf area was 2·54–3·47 times larger in apical GUs than in lateral GUs. Leaf dry weight followed the same pattern because of similar values of specific leaf area (leaf area per unit of leaf dry matter) among GU positions and cultivars (data not shown). These pronounced differences in GU leaf area between apical and lateral GUs were the consequence of not only a larger number of leaves per GU (1·49–1·99 times larger among cultivars), but also of a larger individual leaf area (1·46–1·78 times larger among cultivars). Since the range of leaf area was larger than that of stem length, the axialization index of apical GUs was 0·31–0·41 times that of lateral GUs.
Although the objective of this study was not to compare the morphological attributes of the cultivars, it is interesting to note that the vigorous cultivar ‘Kensington Pride’ (Knight, 1997) had the longest and most leafy GUs, with the largest individual leaves (Fig. 3). The year effect expressed an increase in GU morphological attributes between 2004 and 2006.
The significant interactions between factors were related to different patterns of change of the variables with respect to these factors. They were more significant for variables related to leaves than for stem length and axialization index, in particular for interactions involving GU position. This indicated that the effect of GU position differed among cultivars (Fig. 3) and years. For example, the GU position has the lowest effect on morphological attributes of ‘Irwin’ GUs, and variables related to leaves increased more for apical GUs than for lateral GUs between 2004 and 2006 (data not shown).
Effect of GU position on branching, flowering and fruiting
As a general rule, apical GUs grew and branched more (Table 2), and flowered and fruited more (Table 3) than lateral GUs, indicating contrasting functioning between the two GU types. For branching, results from 2005 are presented in Table 2; similar results and trends were observed in 2004 (data not shown).
Table 2.
Occurrence of vegetative growth (Pvg) and branching density with presence (Dbr A+ ) or absence (Dbr A− ) of apical dominance for apical and lateral growth units of different growth levels for four mango cultivars in 2005, assessed using generalized linear models
| Variable | Initial growth level | ‘Cogshall’ |
‘Irwin’ |
‘José’ |
‘Kensington Pride’ |
||||
|---|---|---|---|---|---|---|---|---|---|
| Apical | Lateral | Apical | Lateral | Apical | Lateral | Apical | Lateral | ||
| Pvg | 0 | 0·70a | 0·47b | 0·83a | 0·45b | 0·43 | 0·33 | 0·61a | 0·35b |
| 1 | 0·71a | 0·09b | 0·74a | 0·32b | 0·51 | 0·55 | 0·41a | 0·05b | |
| 2 | 0·36a | 0·20b | 0·35 | 0·17 | 0·08a | 0·01b | 0·17a | 0·07b | |
| 3 | 0·08a | 0·02b | (0·10) | – | – | – | – | – | |
| Dbr A+ | 0 | 2·60a | 1·09b | 0·15 | 0·22 | 2·67a | 0·80b | 2·37a | 1·39b |
| 1 | 0·85 | 0·69 | 0·15 | 0·00 | 1·20a | 0·44b | 2·27a | 0·75b | |
| 2 | 1·77a | 0·41b | (0·00) | – | (0·21) | – | 1·70 | 0·37 | |
| 3 | (1·90) | – | – | – | – | – | – | – | |
| Dbr A − | 0 | 4·51a | 2·46b | 2·60a | 1·37b | 2·00 | 2·06 | 6·54a | 3·97b |
For each growth level and cultivar, different letters indicate significantly different values (P < 0·05); differences are not significant otherwise. Sample size always >5. Values within parenthesis are estimated from the data but were not tested for the effect of growth unit position (very unbalanced samples). For clarity, 95 % confidence intervals are not shown: their ranges vary between 0·002–0·040 for Pvg; 0·022–0·160 for Dbr A+ ; and 0·029–0·158 for Dbr A− .
Table 3.
Flowering (Pflo) and fruiting (Pfru) occurrence for apical and lateral growth units of four mango cultivars in 2004 and 2005, assessed using generalized linear models
| Variable | Year | ‘Cogshall’ |
‘Irwin’ |
‘José’ |
‘Kensington Pride’ |
||||
|---|---|---|---|---|---|---|---|---|---|
| Apical | Lateral | Apical | Lateral | Apical | Lateral | Apical | Lateral | ||
| Pflo | 2004 | 0·79a | 0·57b | 0·79a | 0·65b | 0·40 | 0·35 | 0·89a | 0·79b |
| 2005 | 0·51 | 0·49 | 0·82a | 0·70b | 0·61a | 0·45b | 0·52 | 0·57 | |
| Pfru | 2004 | 0·52a | 0·24b | 0·45a | 0·27b | 0·69a | 0·39b | 0·71a | 0·44b |
| 2005 | 0·31 | 0·25 | 0·43a | 0·32b | 0·39a | 0·14b | 0·51a | 0·29b | |
For each cultivar and year, different letters indicate significantly different values between the two positions (P < 0·05); differences are not significant otherwise. For clarity, 95 % confidence intervals are not shown; their ranges vary between 0·003–0·016 for Pflo and 0·005–0·020 for Pfru.
The occurrence of vegetative growth, Pvg, decreased from the lower to the higher initial growth levels, justifying a posteriori the analysis of this variable for each growth level (Table 2, statistical tests not shown). This pattern was regular for ‘Irwin’ and ‘Kensington Pride’ for both years, but fluctuated somewhat for ‘Cogshall’ and ‘José’. In general, apical GUs had a significantly higher Pvg than lateral GUs, regardless of the growth level and the year. Some differences were not significant, but this hierarchy was respected in most of the cases. For a given cultivar and growth level, Pvg varied with year.
The branching density, Dbr, also varied among growth levels and with the presence/absence of an apical GU, justifying a posteriori the analysis per level of these factors (Table 2, statistical tests not shown). As a general rule, apical GUs had a higher branching density than lateral GUs. Across all cultivars, growth levels and years, they significantly produced 0·73–1·87 more GUs than lateral GUs with apical dominance, and 0·67–2·57 more GUs otherwise. Apex growth did not strongly repress the simultaneous growth of lateral meristems, indicating a rather mild apical dominance. However, cultivars differed in their sensitivity to apical dominance, e.g. the relative increase of Dbr in the absence of apical dominance was larger for ‘Irwin’ and ‘Kensington Pride’ than for ‘Cogshall’ and ‘José’ (statistical tests not shown). Dbr without apical dominance was calculated for the initial growth level 0 only, where apical flowering on some GUs prevented the development of new apical GUs.
The occurrence of flowering, Pflo, was significantly higher for apical than for lateral GUs in five out of eight cultivar × year combinations, and was not significant in three out of eight combinations (Table 3).
The occurrence of fruiting, Pfru, was significantly higher for apical than for lateral GUs in seven out of eight cultivar × year combinations; it was not significantly higher for ‘Cogshall’ in 2005 (Table 3). These results suggested that the GU position had a greater effect on fruiting than on flowering. This was also supported by the higher mean ratio of apical value to lateral value for Pfru than for Pflo for each cultivar × year combination (mean ± s.e.: 1·79 ± 0·17 for Pfru vs. 1·17 ± 0·06 for Pflo; P < 0·01).
Effect of GU position and diameter on flowering and fruiting
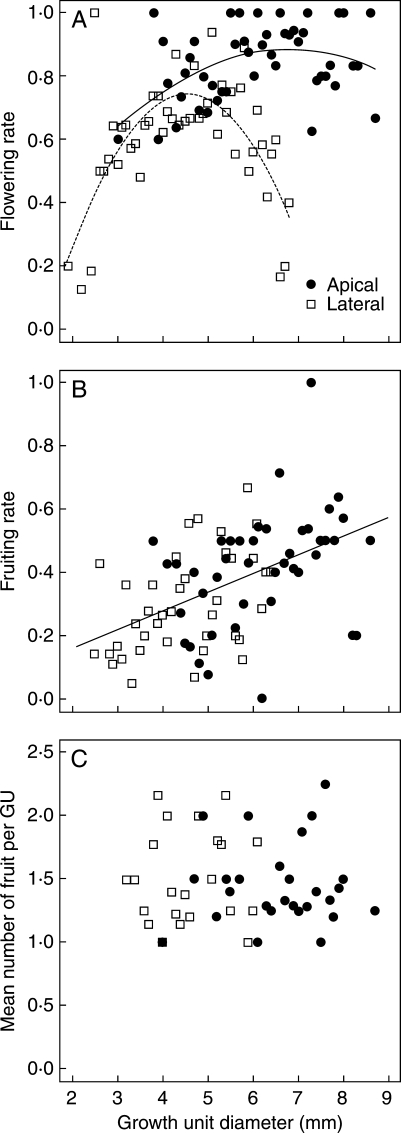
The relationships between GU diameter and flowering rate for apical and lateral GUs were quadratic but differed in their patterns (Fig. 4A). The maximum flowering rates of each curve were significantly different (0·89 and 0·75 for apical and lateral GUs, respectively; P < 0·001), and the position of the maximum also differed (6·8 mm and 4·5 mm for apical and lateral GUs, respectively, P < 0·001). Beyond the maximum value, the flowering rate decreased as the GU diameter increased. This decrease was sharper and affected GUs with a smaller diameter for lateral than for apical GUs. The curve fitted for apical GUs was always above the curve fitted for lateral GUs, indicating that for a given GU diameter, apical GUs were more likely to flower than lateral GUs over the range of GU diameters in our dataset. This was especially true for GU diameters greater than approx. 5 mm.
Fig. 4.
Relationships between (A) flowering rate, (B) fruiting rate and (C) mean number of fruit per growth unit (GU) and growth unit diameter for apical and lateral terminal GUs as indicated for the mango cultivar ‘Cogshall’ in 2006. Quadratic relationships for flowering rate were as follows: apical GUs, y = −0·017x2 + 0·230x + 0·103, n = 47, R2 = 0·19, P = 0·01; lateral GUs, y = −0·076x2 + 0·691x −0·822, n = 47, R2 = 0·49, P < 0·001. The linear relationship for fruiting rate was y = 0·059x + 0·041, n = 82, r2 = 0·24, P < 0·001. No relationship was observed between the mean number of fruit per growth unit and growth unit diameter (n = 49, r2 = 0·001, P = 0·81).
The fruiting rate was linearly related to the GU diameter, independently of the GU position (Fig. 4B). In the embedded linear models tested (different slopes and intercepts, common slope and different intercepts, common slope and intercept), the GU diameter effect was always significant (P < 0·01), whereas the GU position effect and the interaction were not significant. The simpler model with a common slope and intercept was therefore retained (F-test comparison with the different slopes and intercepts model: P = 0·86). The mean fruiting rate was significantly higher for apical than for lateral GUs (0·41 and 0·30, respectively; P < 0·01), in accordance with the results obtained in the second study (Table 3). Since the two types of GUs shared the same relationship between GU diameter and fruiting rate, this was related to the larger mean diameter of apical GUs (6·2 mm and 4·5 mm for apical and lateral GUs, respectively; P < 0·001).
The mean number of fruit per fruiting GU was variable, between 1·0 and 2·2, with a mean value (± s.e.) of 1·48 ± 0·05 (Fig. 4C). It was, however, independent of the GU position and diameter (P = 0·99 for position effect, P = 0·89 for diameter effect, P = 0·89 for position × diameter interaction in the linear model with different slopes and intercepts).
DISCUSSION
Morphological variability between apical and lateral growth units
The results revealed GU dimorphism in the four mango cultivars studied in relation to the relative position, apical or lateral, of the GU. Growth unit dimorphism was more conspicuous for leaf characteristics than for stem length. As a consequence, the axialization index was lower on apical GUs (Fig. 3), indicating a higher linear density of leaf dry weight on these GUs. The four cultivars exhibited the same pattern of differences between apical and lateral GUs, but with a cultivar effect on GU morphology and on dimorphism intensity (interaction between GU position and cultivar; Table 1).
Because of GU secondary growth in diameter for branches composed of several growth levels, stem diameter was not considered as a pertinent morphological variable. On these four cultivars, the total leaf area and dry weight of a branch are allometrically related to the branch basal cross-sectional area or to the basal diameter (Normand et al., 2008). This was true in particular for branches composed of a single GU, i.e. before secondary growth. This suggests that apical GUs have a larger initial basal diameter (before secondary growth) than lateral GUs, in relation to their larger leaf area. This was supported for ‘Cogshall’ by the results of the third study on terminal GUs (Fig. 4). Consequently, the stem of apical GUs was longer, at least for ‘Cogshall’ and ‘Kensington Pride’, and thicker than the stem of lateral GUs, indicating a larger GU volume.
The year effect could reflect ontogenetic development of the tree and environmental influences. The weather – mean temperature, rainfall (Fig. 1) and global solar radiation (data not shown) – was relatively similar during the 2004 and the 2006 growing seasons. The larger GU morphological attributes during the 2006 growing season than during the 2004 one suggested that these young trees (3–5-years old) were still in a phase of vegetative development of the canopy, and that tree ageing had not yet occurred.
Effect of growth unit position on branching
Besides their morphological differences, the two types of GUs behaved differently with respect to branching. The occurrence of vegetative growth, Pvg, and branching density, Dbr, were both affected by GU position, independently of the growth level (Table 2). Apical GUs tended to branch more than lateral GUs. Significant differences were more numerous in 2005 than in 2004, suggesting a year effect, which was probably a combination of climate (there was more rainfall during the 2005 growing season than the 2004 one: 901 vs. 665 mm from August 1 to May 31, respectively; Fig. 1) and of fruit load (heavier in the 2005 growing season for the four cultivars). Differences between the two years were nevertheless difficult to explain with our data alone. The differences were more consistently significant for ‘Cogshall’ for the two years, suggesting a cultivar effect.
The occurrence of vegetative growth tended to decrease with increasing growth level. Pvg variability across years suggested that factors other than GU position and growth level affected this parameter. For the initial growth level 0 (i.e. GUs of the previous growing season), the occurrence of flowering and fruiting on some GUs at the beginning of the growing season might have affected their subsequent growth. For the initial growth levels 1, 2 and 3, Pvg variability could have been linked to the fact that Pvg was probably more closely related to the date of appearance of each GU than to the growth level itself. Growth units that appeared early in the growing season were more likely to grow during the same season than late GUs. The initial growth levels 2 and 3 were mainly composed of GUs that appeared late in the growing season, and consequently had lower Pvg as growth level increased. In contrast, the initial growth level 1 was a mixture of GUs that appeared early and late (and which did not grow further) in the growing season. Pvg then depended on the balance between early and late GUs. Although the effect of GU position was demonstrated with our data, further studies are required to investigate the effect of other factors on branching.
At the GU level, apical dominance appeared mild and cultivar-dependent in mango. Differences in branching density between apical and lateral GUs could be viewed as an example of apical control at the branch level (Cline, 1997), where branching of an apical GU has a depressive effect on branching of the lateral GUs of the same growth level (Fig. 2). As for flowering and fruiting, it would be interesting to investigate the relationship between GU diameter, GU position and branching, in particular to clarify if there is a functional differentiation of apical and lateral GUs for this process.
Relationships between growth unit position, morphology, and flowering and fruiting
Flowering and fruiting rates were both affected by GU position, and they were higher for apical than for lateral GUs. In general, short axes bear flowers and fruit, and long axes are more vegetative (Bell, 1991); this phenomenon is well known in apple (Wünsche et al., 1996; Lauri and Kelner, 2001; Webster, 2005) and in cherry (Lauri, 1992; Thompson, 1996). In contrast, if we consider mango apical GUs as long GUs, and lateral GUs as short GUs, our results showed that the long GUs had the higher capacity to flower and especially to fruit. In that case, could apical (long) GUs and lateral (short) GUs of mango be related to exploration and exploitation structures, respectively (Bell, 1991)? The answer is probably no; first, because of their reproductive behaviour and, second, because of the small differences in stem length between apical and lateral GUs of mango (Fig. 3) compared to the large differences generally observed between long and short axes. This indicated a lack of functional differentiation between apical and lateral GUs with respect to exploration and exploitation. From an evolutionary point of view, the longer stem of the most floriferous apical GUs might be an adaptive advantage since it can improve the floral display of mango apical inflorescences.
On the other hand, the lower axialization index of apical GUs in mango was linked to a higher occurrence of flowering (Fig. 3, Table 3), consistent with similar results in temperate and tropical species (Lauri and Térouanne, 1991; Lauri, 1992; Lauri and Kelner, 2001). In mango, a negative relationship between the axialization index and fruiting was observed. It therefore seems that the predominance of the foliar components over the stem components in mango is related to increased flowering and fruiting as well.
The analysis of architectural development of mango seedlings allowed Goguey (1997) to identify five types of axes in the mango canopy. Among them, the delayed proleptic axes, which appear on trees older than 4 years old, are more floriferous than the others. To link these results to our study is difficult because of methodological differences (climatic conditions, study unit) and differences in plant material (cultivar, young trees and sequential growth in our study); however, it would be interesting to study the within-canopy variability of functional behaviour of apical and lateral GUs in relation to the architectural structures of old mango trees.
Our hypothesis, based on the suggested role of carbohydrate availability for flowering and fruiting in mango (Suryanarayana, 1978; Pandey, 1988; Chacko, 1991; Davenport and Nuñez-Elisea, 1997), predicted a positive relationship between GU diameter, an estimator of leaf area and stem size (Normand et al., 2008), and flowering and fruiting: larger leaf area indicates a larger photosynthetic surface, and larger stems represent a larger local storage capacity for carbohydrates and a higher capacity for assimilate transport. The results for the average morphology and the average behaviour of apical and lateral GUs (first and second studies) supported this hypothesis. Results of the third study, at the GU level for the cultivar ‘Cogshall’, supported this prediction for fruiting but not for flowering (Fig. 4). The quadratic relationship between GU diameter and flowering showed that there was an optimal diameter corresponding to the maximal flowering rate. Consistent with results from the second study, the optimal diameter and maximum flowering rate were different for apical and lateral GUs. The flowering rate of lateral GUs was more sensitive to GU diameter, as indicated by the sharper curvature of the relationship.
Similar non-linear relationships between shoot morphology and flowering have been observed for several temperate forest tree species: Alnus hirsuta var. sibirica (Hasegawa and Takeda, 2001), Fraxinus pennsylvanica var. subintegerrima (Remphrey et al., 2002), Vaccinium hirtum (Kawamura and Takeda, 2006) and apple (Lauri and Trottier, 2004). Shoot morphology was represented by shoot length in the first three species, and by the number of leaves of the shoot in the latter species. Kawamura and Takeda (2006) proposed a hypothesis for this non-linear pattern based on size-related changes in shoot-level resource availability and costs of flowering on subsequent vegetative growth. The lower degree of flowering on small and, to a lesser extent, on large shoots could then be explained in different ways (Hasegawa and Takeda, 2001; Kawamura and Takeda, 2006). Within the framework of this hypothesis, small shoots would have a shortage of resources for reproduction and large shoots would have too high a flowering cost in terms of the maximization of their life-time reproductive success. Further studies on the costs of reproduction in mango are needed to determine if this hypothesis is supported by our results.
The linear relationship between fruiting rate and GU diameter, independent of the GU position, indicated that morphological differences between apical and lateral GUs significantly contributed to explaining the differences in fruiting rate. This result was in accordance with the hypothesis that carbohydrates play a major role in mango fruiting (Davenport and Nuñez-Elisea, 1997). However, differences in GU diameter can also positively affect stem hydraulic characteristics (Tyree and Zimmermann, 2002), and consequently fruit set and retention. Further analyses would be necessary to clarify the role of GU size on GU photosynthesis, carbohydrate content and hydraulic characteristics in relation to fruiting. In addition to this initial hypothesis, another one related to allometry could be proposed. Corner's rule on axis conformity (Corner, 1949; Hallé et al., 1978) states that ‘the stouter, or more massive the axis in a given species, the larger and more complicated its appendages’ (leaves, inflorescences, fruit). In particular, the relationship between stem diameter and inflorescence size have been verified with different Leucadendron species (Midgley and Bond, 1989). Normand et al. (2008) showed that the relationship between stem size and leaf size remained valid at the cultivar level in mango. Assuming that Corner's rule in relation to reproductive organs remains valid at the cultivar level, then GUs with larger diameters would have a larger inflorescence (our unpublished experimental results support this hypothesis), i.e. an inflorescence with more flowers and a longer display for pollinators. Consequently, their fruiting rate would be improved, which was consistent with our results. The variability around the fitted line (Fig. 4B) suggested, however, that factors other than GU diameter affected fruiting.
In contrast to previous research that has focused mainly on the relationship between shoot morphology and flowering (Remphrey et al., 2002; Kawamura and Takeda, 2006), we simultaneously investigated the relationships between shoot morphology and flowering and fruiting. The contrasting relationships between GU diameter on the one hand, and flowering and fruiting on the other, showed that if vegetative features were clearly involved in the determinism of flowering and fruiting, the underlying rules were not the same. Fruiting was linearly size-related to the GU, whereas flowering was not, which could explain the greater impact of GU diameter on fruiting than on flowering observed in the second study. With reference to the assumptions supporting our general hypothesis, this suggested that apical and lateral GUs were functionally differentiated for flowering, but not for fruiting. The mango tree has high direct costs of reproduction because of large and heavy fruit (Hasegawa and Takeda, 2001). The linear, non-limiting, positive relationship between GU diameter and fruiting could be detrimental to tree development and survival because of excessive carbohydrate reserve exhaustion, for example if growing conditions favoured large GUs. In this context, the quadratic relationship between GU diameter and flowering appeared as a means for the tree to limit, at an earlier phenological stage, direct reproduction costs by limiting the occurrence of flowering on large GUs.
Factors associated with GU architectural position were clearly involved in mango flowering. Two related questions could therefore be raised. What are these factors? And why do the thickest GUs have a lower flowering rate? Specific studies, for example on the costs of reproduction (Kawamura and Takeda, 2006), are necessary to investigate these questions. However, one factor could be eliminated from the potential candidates: the florigenic promoter. It has been hypothesized that the vegetative or reproductive fate of buds in mango involves the interaction of a florigenic promoter and a vegetative promoter (Reece et al., 1949; Kulkarni, 1986, 1988; Davenport and Nuñez-Elisea, 1997; Davenport et al., 2006; Wilkie et al., 2008). The florigenic promoter is synthesized in leaves in the presence of light and cool temperatures, and moves through the phloem to buds where it contributes to floral induction. A larger leaf area in apical GUs might suggest that a larger amount of florigenic promoter is synthesized, consequently leading to a higher flowering rate. Our results did not support this hypothesis because of the decrease in the flowering rate for larger GU diameters, i.e. larger GU leaf area. This hypothesis is no longer supported by recent results that show that only one quarter of a leaf is sufficient to synthesize florigenic promoter and induce flowering on a terminal GU, and that the florigenic promoter can be translocated as far as 1 m away from a leaf and induce flowering (Davenport et al., 2006). Consequently, the amount of florigenic promoter in our non-defoliated apical and lateral GUs was largely sufficient to ensure maximal flowering on both kinds of GUs under our climatic conditions.
Conclusions
A pronounced GU dimorphism in relation to the relative position of the GU was observed in four mango cultivars. Besides morphological differences, apical and lateral GUs of the four cultivars behaved differently with respect to branching, flowering and fruiting. However, exploration and exploitation functions (Bell, 1991) could not be assigned to apical and lateral GUs, respectively. The quadratic relationship between GU diameter and flowering was dependent on the GU position and suggested that factors other than carbohydrates, in relation to the GU relative position, regulated flowering. This relationship, which is cultivar-dependent in apple (Lauri and Trottier, 2004), should be investigated on different cultivars and might offer new ways to study mango flowering. Fruiting was linearly related to the GU diameter, irrespective of GU position. The results thus suggest a functional differentiation of apical and lateral GUs for flowering, but not for fruiting, in these four mango cultivars. It was also demonstrated that routine and easy-to-score parameters such as leaf area or stem length and diameter were not sufficient to infer the flowering behaviour of a GU. The balance between vegetative branch components, quantified by the axialization index, is likely to play a significant role in reproductive behaviour in mango. These results constitute basic knowledge that can be used to further study functional differentiation of growth units, not only in mango but also on virtually all temperate and tropical fruit species, as well as the trade-offs between reproductive and vegetative functions.
ACKNOWLEDGEMENTS
We would like to thank Clarisse Magne, Armelle Renard, Soizick Josse, Claire Bissery, Doralice Jessu and Philippe Cabeu for their helpful contributions to field and laboratory work, as well as two anonymous referees for their valuable comments on a previous version of the manuscript.
LITERATURE CITED
- Aronne G, De Micco V. Seasonal dimorphism in the Mediterranean Cistus incanus L. subsp. incanus. Annals of Botany. 2001;87:789–794. [Google Scholar]
- Baret S, Nicolini E, Le Bourgeois T, Strasberg D. Developmental patterns of the invasive bramble (Rubus alceifolius Poiret, Rosaceae) in Reunion Island: an architectural and morphometric analysis. Annals of Botany. 2003;91:1–10. doi: 10.1093/aob/mcg006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthélémy D, Caraglio Y. Plant architecture: a dynamic, multilevel and comprehensive approach to plant form, structure and ontogeny. Annals of Botany. 2007;99:375–407. doi: 10.1093/aob/mcl260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell A. Plant form. An illustrated guide to flowering plant morphology. Oxford: Oxford University Press; 1991. [Google Scholar]
- Bond WJ, Midgley JJ. Allometry and sexual differences in leaf size. The American Naturalist. 1988;131:901–910. [Google Scholar]
- Campbell RJ. Guide to mangos in Florida. Miami, FL: Fairchild Botanical Garden; 1992. [Google Scholar]
- Chacko EK. Mango flowering – still an enigma. Acta Horticulturae. 1991;291:12–21. [Google Scholar]
- Cline MG. Concepts and terminology of apical dominance. American Journal of Botany. 1997;84:1064–1069. [PubMed] [Google Scholar]
- Corner EJH. The Durian theory or the origin of the modern tree. Annals of Botany. 1949;13:367–414. [Google Scholar]
- Davenport TL, Nuñez-Elisea R. Reproductive physiology. In: Litz RE, editor. The Mango. Botany, production and uses. Wallingford, UK: CAB International; 1997. pp. 69–146. [Google Scholar]
- Davenport TL, Ying Z, Kulkarni V, White TL. Evidence for a translocatable florigenic promoter in mango. Scientia Horticulturae. 2006;110:150–159. [Google Scholar]
- Efron B. The jackknife, the bootstrap and other resampling plans. Philadelphia, PA: Society for Industrial and Applied Mathematics; 1982. [Google Scholar]
- Goguey T. Architectural approach of the mechanisms of canopy growth and flowering of mango trees. Acta Horticulturae. 1997;455:124–131. [Google Scholar]
- Hasegawa S, Takeda H. Functional specialization of current shoots as a reproductive strategy in Japanese alder (Alnus hirsuta var. sibirica) Canadian Journal of Botany. 2001;79:38–48. [Google Scholar]
- Hallé F, Oldeman RAA, Tomlinson PB. Tropical trees and forests. An architectural analysis. Berlin: Springer-Verlag; 1978. [Google Scholar]
- Johnson RS, Lakso AN. Carbon balance model of a growing apple shoot. II. Simulated effects of light and temperature on long and short shoots. Journal of the American Society for Horticultural Science. 1986;111:164–169. [Google Scholar]
- Kawamura K, Takeda H. Costs and probability of flowering at the shoot level in relation to variability in shoot size within the crown of Vaccinium hirtum (Ericaceae) New Phytologist. 2006;171:69–80. doi: 10.1111/j.1469-8137.2006.01737.x. [DOI] [PubMed] [Google Scholar]
- Knight RJ., Jr . Important mango cultivars and their descriptors. In: Litz RE, editor. The Mango, botany, production and uses. Wallingford, UK: CAB International; 1997. pp. 545–565. [Google Scholar]
- Kohorn LU. Shoot morphology and reproduction in jojoba: advantages of sexual dimorphism. Ecology. 1994;75:2384–2394. [Google Scholar]
- Kulkarni VJ. Graft-induced off-season flowering and fruiting in the mango (Mangifera indica L.) Journal of Horticultural Science. 1986;61:141–145. [Google Scholar]
- Kulkarni VJ. Further studies on graft-induced off-season flowering and fruiting in mango (Mangifera indica L.) Journal of Horticultural Science. 1988;63:361–367. [Google Scholar]
- Lauri PÉ. Données sur le contexte végétatif lié à la floraison chez le cerisier (Prunus avium) Canadian Journal of Botany. 1992;70:1848–1859. [Google Scholar]
- Lauri PÉ, Kelner JJ. Shoot type demography and dry matter partitioning: a morphometric approach in apple (Malus × domestica) Canadian Journal of Botany. 2001;79:1270–1273. [Google Scholar]
- Lauri PÉ, Laurens F. Architectural types in apple (Malus × domestica Borkh) In: Ramdane D, editor. Crops: growth, quality and biotechnology. Helsinki: World Food Limited; 2005. pp. 1300–1313. [Google Scholar]
- Lauri PÉ, Térouanne E. Eléments pour une approche morphométrique de la croissance végétale et de la floraison: le cas d'expèces tropicales du modèle de Leeuwenberg. Canadian Journal of Botany. 1991;69:2095–2112. [Google Scholar]
- Lauri PÉ, Trottier C. Patterns of size and fate relationships of contiguous organs in the apple (Malus domestica) crown. New Phytologist. 2004;163:533–546. doi: 10.1111/j.1469-8137.2004.01136.x. [DOI] [PubMed] [Google Scholar]
- Midgley JJ, Bond WJ. Leaf size and inflorescence size may be allometrically related traits. Oecologia. 1989;78:427–429. doi: 10.1007/BF00379120. [DOI] [PubMed] [Google Scholar]
- Normand F, Bissery C, Damour G, Lauri PÉ. Hydraulic and mechanical stem properties affect leaf-stem allometry in mango cultivars. New Phytologist. 2008;178:590–602. doi: 10.1111/j.1469-8137.2008.02380.x. [DOI] [PubMed] [Google Scholar]
- Pandey RM. Physiology of flowering in mango. Acta Horticulturae. 1988;231:361–380. [Google Scholar]
- R Development Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2006. http://www.R-project.org . [Google Scholar]
- Reece PC, Furr JR, Cooper WC. Further studies of floral induction in the Haden mango (Mangifera indica L.) American Journal of Botany. 1949;36:734–740. [Google Scholar]
- Remphrey WR, Bartlett GA, Davidson CG. Shoot morphology and fate of buds in relation to crown location in young Fraxinus pennsylvanica var. subintegerrima. Canadian Journal of Botany. 2002;80:1274–1282. [Google Scholar]
- Stephan J, Lauri PÉ, Dones N, Haddad N, Talhouk S, Sinoquet H. Architecture of the pruned tree: impact of contrasted pruning procedures over 2 years on shoot demography and spatial distribution of leaf area in apple (Malus domestica) Annals of Botany. 2007;99:1055–1065. doi: 10.1093/aob/mcm049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryanarayana V. Seasonal changes in sugars, starch, nitrogen and carbon-nitrogen in mango shoots in relation to flowering. Plant Biochemistry Journal. 1978;5:108–117. [Google Scholar]
- Suzuki A. Patterns of vegetative growth and reproduction in relation to branch orders: the plant as a spatially structured population. Trees. 2000;14:329–333. [Google Scholar]
- Thompson M. Flowering, pollination and fruit set. In: Webster AD, Looney NE, editors. Cherries – crop physiology, production and uses. Wallingford, UK: CAB International; 1996. pp. 223–241. [Google Scholar]
- Tyree MT, Zimmermann MH. Xylem structure and the ascent of sap. 2nd edn. Berlin: Springer-Verlag; 2002. [Google Scholar]
- Venables WN, Ripley BD. Modern applied statistics with S. 4th edn. New York: Springer; 2002. [Google Scholar]
- Verdú M, Spanos K, Čaňová I, Slobodník B, Paule L. Similar gender dimorphism in the costs of reproduction across the geographic range of Fraxinus ornus. Annals of Botany. 2007;99:183–191. doi: 10.1093/aob/mcl241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincenot D. Mangues de La Réunion – origines, histoire, caractéristiques, usages culinaires. Saint-Denis, La Réunion: Océan Editions; 2004. [Google Scholar]
- Webster AD. Shoot growth. In: Tromp J, Webster AD, Wertheim SJ, editors. Fundamentals of temperate zone tree fruit production. Leiden, The Netherlands: Backhuys Publishers; 2005. pp. 120–135. [Google Scholar]
- Wilkie JD, Sedgley M, Olesen T. Regulation of floral initiation in horticultural trees. Journal of Experimental Botany. 2008;59:3215–3228. doi: 10.1093/jxb/ern188. [DOI] [PubMed] [Google Scholar]
- Wünsche JN, Lakso AN. The relationship between leaf area and light interception by spur and extension shoot leaves and apple orchard productivity. HortScience. 2000;35:1202–1206. [Google Scholar]
- Wünsche JN, Lakso AN, Robinson TL, Lenz F, Denning SS. The bases of productivity in apple production systems: the role of light interception by different shoot types. Journal of the American Society for Horticultural Science. 1996;121:886–893. [Google Scholar]