Abstract
Background and Aims
Nepenthes pitchers are sophisticated traps that employ a variety of mechanisms to attract, capture and retain prey. The underlying morphological structures and physiological processes are subject to change over the lifetime of a pitcher. Here an investigation was carried out on how pitcher properties and capture efficiency change over the first 2 weeks after pitcher opening.
Methods
Prey capture, trapping efficiency, extrafloral nectar secretion, pitcher odour, as well as pH and viscoelasticity of the digestive fluid in N. rafflesiana pitchers were monitored in the natural habitat from pitcher opening up to an age of 2 weeks.
Key Results
Pitchers not only increased their attractiveness over this period by becoming more fragrant and secreting more nectar, but also gained mechanical trapping efficiency via an enhanced wettability of the upper pitcher rim (peristome). Consistently, natural prey capture was initially low and increased 3–6 d after opening. It was, however, highly variable within and among pitchers. At the same time, the pH and viscoelasticity of the digestive fluid decreased, suggesting that the latter is not essential for effective prey capture.
Conclusions
Prey capture and attraction by Nepenthes are dynamic processes strongly influenced by the changing properties of the pitcher. The results confirm insect aquaplaning on the peristome as the main capture mechanism in N. rafflesiana.
Key words: Carnivorous plants, pitcher development, prey attraction, prey capture, insect aquaplaning, extrafloral nectar, Nepenthes rafflesiana
INTRODUCTION
Ever since the first description of a Nepenthes plant by Etienne de Flacourt in 1658, these plants have fascinated naturalists and researchers with the bizarre shapes of their pitchers and their unusual carnivorous lifestyle. For more than a hundred years, scientists tried to elucidate how the pitcher traps work (e.g. Knoll, 1914; Lloyd, 1942; Juniper and Burras, 1962; Gaume et al., 2002; Bohn and Federle, 2004; Bauer et al., 2008). They found that pitcher plants possess several capture mechanisms, which may act synergistically. The upper pitcher rim, known as the peristome, possesses a specialized surface that is highly wettable and becomes very slippery for insects when wet (Bohn and Federle, 2004; Bauer et al., 2008). In addition, many Nepenthes species have a crystalline wax layer on the inner pitcher wall, which prevents insect adhesion and is reported to contribute towards initial capture and prey retention (Juniper, 1962; Gaume et al., 2002). The digestive fluid, which fills roughly the bottom half of the pitcher, is viscoelastic in some species, including the typical form of N. rafflesiana. This trait has been reported to improve prey retention (Clarke, 2001; Gaume and Forterre, 2007).
Before prey can be captured and digested, visitors have to be lured to the trap. Nepenthes pitcher plants employ several different mechanisms to ensure prey attraction. Ants dominate the prey spectrum in most of the species studied to date (Jebb, 1991; Moran, 1996) and are mainly attracted by extrafloral nectar (Joel, 1988; Merbach et al., 2001). Flying insects, the second largest fraction of the prey spectrum in N. rafflesiana, are attracted to the pitchers by sweet scent (Moran, 1996) and conspicuous UV reflection patterns (Moran et al., 1999).
Starting from a tiny flattened swelling on the tip of the leaf tendril, pitchers grow over several weeks before they inflate, fill partly with fluid and finally break open (Clarke, 1997; Owen and Lennon, 1999). Once open, pitchers stay alive for a period of from a few weeks to up to 9 months, depending on the species and, probably, environmental factors (Clarke, 1997; Osunkoya et al., 2008). Most research on pitcher plants, however, has not considered that the trapping function could change over the lifetime of a pitcher. For example, pitchers continue to change in shape and colour during the first days after opening (see Fig. 1A, B), and this is likely to affect prey attraction and capture success. Nepenthes pitchers are not just simple pitfall traps; instead their trapping ability depends on a number of intrinsic and extrinsic factors such as nectar secretion, odour, coloration and environmental factors (Moran, 1996; Bauer et al., 2008). The capture efficiency of N. rafflesiana is subject to strong diurnal and shorter term variation because the trapping function of the peristome is activated by wetness, i.e. at times of high air humidity or during and shortly after rainfalls (Bauer et al., 2008). As the wetting of the peristome is also enhanced by the secretion of nectar, it is likely that variation will also occur on a larger time scale, over the lifetime of a pitcher. Capture total, as opposed to trapping efficiency, is furthermore dependent on effective attraction and retention of prey, and both might vary with pitcher age as well.
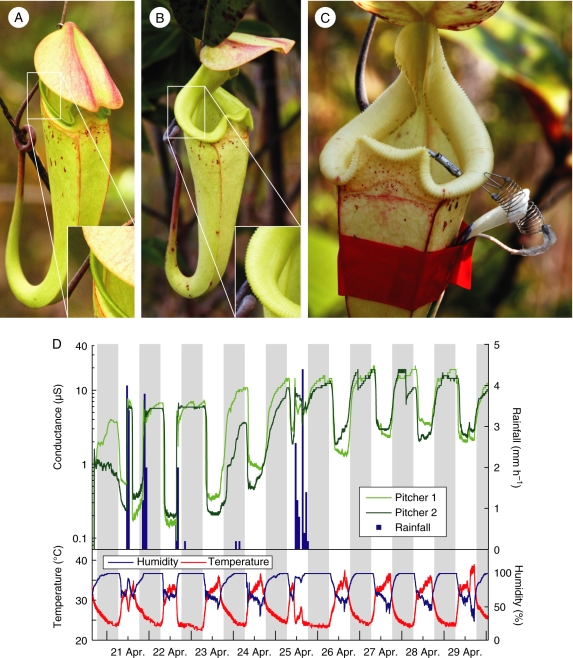
Fig. 1.
Age-dependent changes in N. rafflesiana pitchers. (A) A freshly opened pitcher. Note that the lid is still pointing downward and the peristome (see inset) is straight and orientated almost vertically. (B) A 1-week-old pitcher. Note that the lid is now horizontal and the peristome rounded and inclined towards its inner margin. (C) A pitcher with attached electrodes for measuring the surface wetness on the peristome. (D) Example conductance recording from two pitchers over the first 9 d after opening, shown together with rainfall, air humidity and air temperature, as indicated. Night-time is marked by a grey background. High conductance indicates a wet peristome.
Investigating the details of these longer term changes is not only crucial for understanding the costs and benefits of carnivory in these plants, but also provides an opportunity to investigate the relevance of different mechanisms of attraction and trapping in a natural experiment, where development-dependent variables change gradually over time. Here pitchers of N. rafflesiana are studied over the first 2 weeks after opening, corresponding to approximately a quarter of their life span (approx. 6–10 weeks, Osunkoya, 2008; U.Bauer, pers. observ.). The investigation focused in particular on how (a) odour and secretion by peristome nectaries, (b) digestive fluid viscoelasticity and pH, (c) wettability and capture efficiency of the peristome and (d) natural prey capture total changed with pitcher age during the early stages of pitcher life.
MATERIALS AND METHODS
All experiments were conducted on aerial pitchers of Nepenthes rafflesiana [formerly named as N. rafflesiana var. typica G. Beck (1895)] in its natural habitat at a site of degraded heath forest (kerangas) in Brunei, NW Borneo, during three different field research trips (March–April 2007, August–September 2007 and March–May 2008). Although rarely present in ground pitchers of N. rafflesiana, all investigated pitchers did not possess a crystalline wax layer on the inner pitcher wall. Several pitcher parameters relevant to attraction, prey capture and retention were recorded over a period of 2 weeks (6 weeks in the case of pH) after pitcher opening. Limiting the observation period to the first 2 weeks of the pitcher's lifetime was necessary to achieve a sufficient sample size of developing pitchers within the available study period. The shorter time also reduced possible negative effects of the experimental manipulations (e.g. regular observations and prey removal).
Natural prey capture
Ten pitchers on ten different plants were marked prior to opening. The bottom end of N. rafflesiana aerial pitchers is strongly tapered and elongated, so that reliable prey sampling would be impossible if prey was not prevented from being lost into the tapered section. Therefore, polyurethane ear plugs (Pura-Fit Moldex 7700, Moldex-Metric, Walddorfhäslach, Germany) were inserted into the bottom end of each pitcher on the day of pitcher opening. Prey was collected daily at 0800 h, using a clean 20 mL syringe with an attached silicon tube to suck out the digestive fluid together with the prey from the pitcher. After removing and counting the prey items, the fluid was returned and the pitcher remained undisturbed until the next sampling.
Nectar production
Ten pitchers that were about to open were enclosed in fine-mesh gauze bags and their tendrils were coated with Tanglefoot® sticky resin (Tanglefoot Corp., Grand Rapids, MI, USA), to exclude visiting insects. In addition, the pitchers were roofed with sheets of stiff transparent plastic foil to protect nectar from being washed off by rain. From the day of pitcher opening onwards, nectar secreted by the peristome nectaries was sampled daily by gently moistening the peristome with a wet, approx. 1-cm2 piece of Kimwipe® (Kimberley-Clark, Reigate, UK) and wiping it thoroughly with Sugi® absorbent swabs (Kettenbach Medical, Eschenburg, Germany), small highly absorbent triangular sponges made from cotton and cellulose. Forceps and latex gloves were used when handling Kimwipes® and Sugi® swabs, which were both collected in Eppendorff tubes. The samples were dried over silica gel (GeeJay Chemicals, Sandy, UK) immediately after collection. The dried nectar was re-diluted in the laboratory in 0·2–0·5 mL of distilled water (the amount depending on the volume of absorptive material). The concentration and hence the total amount of sugar was determined in sucrose equivalents using a temperature-compensated handheld refractometer (ATAGO, L. Kübler, Karlsruhe, Germany).
To find out if nectar secretion was stimulated by prey capture, an additional experiment was performed where ten pairs of Tanglefoot®-coated and gauze-bagged pitchers (prior to opening) were marked on ten different plants. Once open, one pitcher of each pair was left untouched while the other one was ‘fed’ with ten small Crematogaster sp. ants (approx. 3 mm body length) each on the fifth, seventh, ninth and 11th day after opening. On the 11th day, all nectar was completely removed from the peristomes using wet pieces of Kimwipe. On the 13th day, the accumulated nectar from the peristomes was sampled and quantified using the method described above.
Peristome surface wetness
Surface wetness on the peristomes of 30 freshly opened pitchers was continuously monitored during two different field trips (September 2007 and March–May 2008). The degree of wetting was measured by the electrical resistance between two electrodes attached to opposing margins of the peristome (Fig. 1C), as described in Bauer et al. (2008). In brief, an electrical circuit with a fixed resistor (1 MΩ) potential divider was used to measure the electrical resistance of the peristome as a voltage. Data were recorded from up to eight pitchers simultaneously with a sampling frequency of 1 min−1 using a μLog VL100S datalogger (a.b.i. data, Brussels, Belgium); data were downloaded every 5 d onto a portable computer. Voltage values were converted to conductance, which reflects the degree of surface wetting. The method does not allow conclusions about the absolute amount of fluid present, because the electrical resistance between the electrodes is influenced by the variable electrolyte content of the fluid on the peristome surface and by the non-standardized distance and contact area of the electrodes (cf. Klemm et al., 2002). The position and contact area of the electrodes nevertheless remained constant during the experiment, so that temporal variations of peristome wetness on the same pitcher could be reliably recorded.
In addition to the measurements of peristome wetness, air temperature and relative humidity were continuously recorded using Tinytag® TGP-1500 and Tinytag® TGP-4500 dataloggers (Gemini Data Loggers, Chichester, UK) and rainfall using a tipping bucket rain gauge (Rain Collector II, Davis Instruments Corp., Hayward, CA, USA) connected to a Tinytag® TGPR-1201 datalogger.
To obtain a reproducible and weather-independent measure of the longer-term, developmental change of peristome wetness, for each day and pitcher the value of peristome conductance at one ‘intermediate’ air humidity level was evaluated, when it was reached in the afternoon. The value of ‘intermediate’ humidity was defined as the value halfway in between the minimum and the maximum air humidity on each day, averaged over all days in one experimental period. This value (76·36 ± 2·96 % relative humidity) varied slightly between individual experimental periods depending on weather conditions. By choosing intermediate rather than maximum air humidity as the reference point, it was ensured that the measured wetness level was caused by condensation and not by rain.
Trapping efficiency
Trapping efficiency was measured as in Bauer et al. (2008) by bringing large numbers of ants into contact with a N. rafflesiana pitcher. Partial colonies of ants belonging to the Camponotus (Colobopsis) saundersi group were collected and kept in plastic containers with the walls coated with slippery Fluon® (Whitford, Dietz, Germany). These ants are part of the natural prey spectrum of N. rafflesiana in Brunei. A live pitcher in the field was placed upright into the container with ants, so that the insects had access to the pitcher. The ants immediately started to explore the new object. The pitcher lid was carefully bent upwards, so that it did not obstruct the view, and the peristome was filmed from above for a period of 15 min. The video recordings were analysed by counting the number of captured ants and the number of peristome visitors. Each ant newly entering the peristome with more than three legs in contact with the peristome surface was counted as a ‘visitor’. This experiment was repeated daily at 1900 h, a time of day when the air humidity had risen to a level sufficient to wet the peristomes. Since rain wets peristomes regardless of their hygroscopic surface properties (Bauer et al., 2008), this experiment was only conducted after rainless afternoons. Therefore, data are available for the second, fourth, fifth, sixth and 14th day after the pitcher had opened, but not for the first and third day.
Pitcher odour, fluid viscoelasticity and pH
A total of 21 unopened pitchers were marked and, once they had opened, one of us (U. Bauer) performed qualitative assessments of pitcher odour and fluid viscoelasticity daily between 0730 h and 0830 h. Pitcher odour could be very different from plant to plant: some pitchers of N. rafflesiana emitted a sweet and honey-like scent while others smelled fruity or sourish, and very young pitchers often appeared odourless. Odour was divided into four categories: (0) absent, (1) faint, (2) medium and (3) strong. While the method of assessing odour intensity was clearly subjective, it was sufficient for identifying general trends.
Nepenthes rafflesiana pitchers possess a viscoelastic digestive fluid, which forms long filaments when poured out (Gaume and Forterre, 2007). For a qualitative assessment of the fluid's viscoelasticity, a droplet of fluid was pulled apart between two fingertips. Depending on the maximum length of filaments before breaking apart, the fluid was assigned to one of four categories: (0) no filaments = watery; (1) short filaments <1 cm = slightly viscoelastic; (2) long filaments >1 cm and <30 cm = viscoelastic; and (3) very long filaments >30 cm = highly viscoelastic. Viscoelasticity was not measured in the strict sense but the fluid's capacity to form coherent filaments, which is related to its viscoelastic properties, was tested simply. The term ‘viscoelasticity’ is used in this sense throughout the paper.
In the closely related carnivorous family of Droseraceae, mucus viscosity is strongly pH dependent (Rost and Schauer, 1977). Therefore, the changes of pitcher fluid pH were monitored from the day of pitcher opening. Thirteen pitchers were checked daily at 0830 h using a CyberScan PC300/03K pH meter (Eutech Instruments, Nijkerk, The Netherlands). On the day of pitcher opening, a second measurement was performed 5 h after the first one. pH was also monitored in a second group of seven pitchers that were enclosed in gauze bags to prevent prey capture, to test whether changes are triggered by the presence and the decay of prey items. As a third experimental group, the fluid of 15 fully inflated but still unopened pitchers was collected in 50 mL polypropylene centrifugation tubes (Falcon™) and they were placed open, but roofed, at ground level close to the study plants. The pH of these samples was measured at the same intervals as the pitchers, but only for 2 weeks.
RESULTS
Natural prey capture, trapping efficiency and peristome wetness
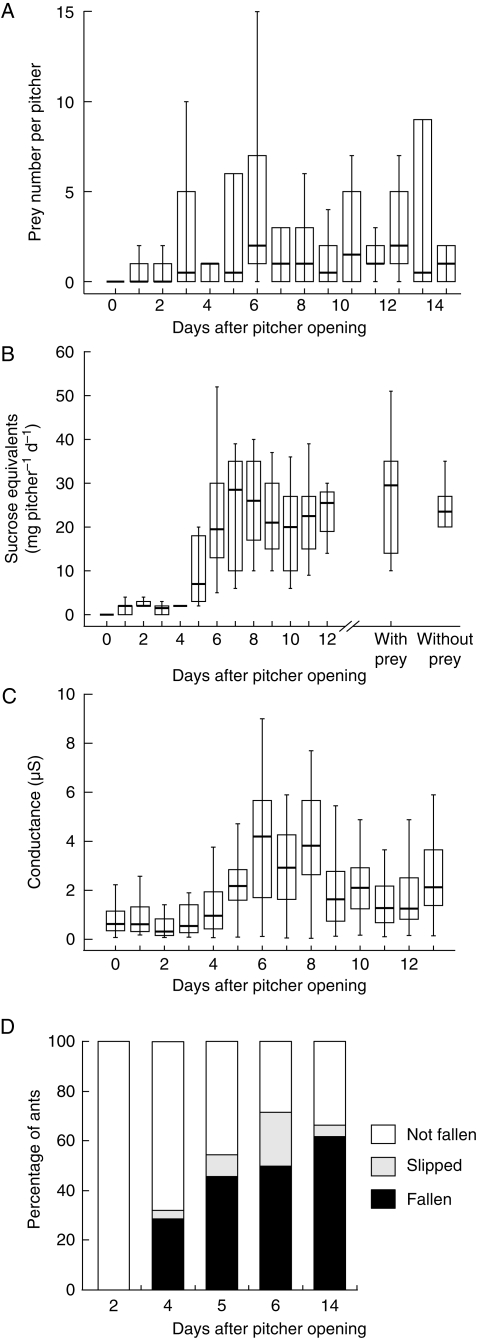
Prey numbers were generally low in the first few days after pitcher opening and increased markedly during the second half of the first week (Fig. 2A). The main increase in capture total took place from day 3 to 6 after pitcher opening. After that, prey numbers levelled out but were generally highly variable. The upward trend of total captured prey over the course of the first 2 weeks after pitcher opening was statistically highly significant (Page's test for trend, n = 10 pitchers, 15 d, L = 10 300·5, P < 0·01). However, if the prey classes of ants and flying insects were analysed separately, the upward trend was only significant for flying insects (Page's test for trend, n = 10 pitchers, 15 d, L = 10 387·0, P < 0·001) but not for ants (Page's test for trend, n = 10 pitchers, 15 d, L = 9731·5, P = 0·29). This may be explained by the fact that the number of captured ants was more variable than that of other prey animals (overall s.d. for ants, 4·14; flying insects, 1·32). The number of captures varied from 0 to 25 ants per pitcher and day, both within pitchers over the whole experiment as well as between pitchers on the same day. In comparison, the number of captured flying insects per pitcher varied from 0 to 8 (again both within and between pitchers). Ants constituted the largest proportion of prey (67·4 % of all captured animals) while flying insects accounted for another 28·2 %. The remaining 4·4 % consisted of other arthropods (flightless insects, spiders, millipedes). The proportion of ants was exceptionally high during the first few days after pitcher opening (mean proportion 84·7 % during the first 6 d, but only 52·5 % during the rest of the experimental period).
Fig. 2.
Age-dependent changes of (A) natural prey capture (n = 10 pitchers); (B) peristome nectar secretion (n = 10 pitchers) – the two box-whiskers on the right show the nectar production of prey-deprived pitchers and of pitchers ‘fed’ with ants (n = 10 pitchers each, 13 d old); (C) peristome wetness (as measured by its electrical conductance), recorded daily at a given intermediate air humidity (n = 30 pitchers, cf. Materials and Methods); and (D) capture efficiency as obtained in running experiments with ants on a single pitcher. Bars represent medians, boxes the inner quartiles and whiskers all data within the 95 % confidence interval.
The calculation of variance/mean ratios as an index of dispersion showed that prey capture events were not randomly distributed in time and across pitchers. Excluding young pitchers of <6 d after opening (to ensure that the pitchers had reached their full trapping capacity), the distribution of ant captures was highly aggregated (variance/mean ratio = 7·75, χ2 = 689·4, d.f. = 89, P < 0·001) while for flying insects the aggregation was less pronounced but still highly significant (variance/mean ratio = 2·47, χ2 = 220·0, d.f. = 89, P < 0·001).
The increase of total captured prey coincided with an increase in trapping efficiency, defined as the number of captured ants per number of peristome visits (Fig. 2D). Between the second and sixth day after pitcher opening, the trapping efficiency of the tested pitcher increased from 0 to 50 % captured ants. A week later, on day 14, the proportion of captured ants had only risen slightly (to 62 %). Therefore, the main increase in trapping efficiency occurred simultaneously with the increase in total captured prey.
Peristome surface conductance as a measure of wetness oscillated with a diurnal rhythm, in phase with the changes of air humidity (cf. Bauer et al., 2008). Superimposed on this oscillation was an increase from a lower (first 3 d) to a higher conductance (after day 6). This effect can also be seen in the degree of peristome surface wetness measured for each day at a defined ‘intermediate’ air humidity (Fig. 2C, see Materials and methods). After day 8, the median conductance decreased slightly but still remained on a higher level than at the beginning of the experiment. As with the data on total captured prey, the variance within and between pitchers was high (Fig. 2C). Nevertheless, the upward trend of peristome wetness over the course of the first 2 weeks after pitcher opening was statistically highly significant (Page's test for trend, n = 30 pitchers, 14 d, L = 22 046·5, P < 0·001).
Nectar secretion and pitcher odour
Nectar secretion was initially very low (0–2 mg sucrose equivalents pitcher−1 d−1 during the first 3 d) and increased approx. 10-fold from day 4 to 7 after pitcher opening. After that, the rate of sugar secretion fluctuated around 25 mg pitcher−1 d−1 (Fig. 2B, left). The increase over the course of the experiment was highly significant (Page's test for trend, n = 10 pitchers, 13 d, L = 7814·0, P < 0·001). Nectar secretion was not influenced by prey capture and digestion, as simulated by ‘feeding’ the pitchers with ants (Fig. 2B, right; paired t-test, d.f. = 9, t = 0·67, P = 0·52). Freshly collected nectar samples emanated a sweet, honey-like scent, suggesting that the characteristic pitcher odour is based on volatiles contained in the nectar.
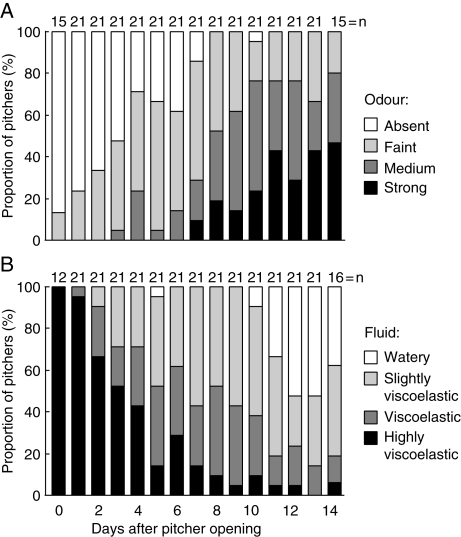
Newly opened pitchers were virtually odourless. The intensity of pitcher odour increased gradually over the first 13 d after pitcher opening (Page's test for trend, n = 21 pitchers, 13 d, L = 16 063·0, P < 0·001). The most notable increase took place from day 6 to 8 after pitcher opening (Fig. 3A).
Fig. 3.
Increase of pitcher odour (A) and decrease of digestive fluid viscoelasticity (B) over the first 2 weeks after pitcher opening. Both trends were highly significant (see text).
Pitcher fluid viscoelasticity and pH
Digestive fluid viscoelasticity showed an opposite trend compared with odour intensity, decreasing significantly over the course of the experiment (Page's test for trend, n = 21 pitchers, 13 d, L = 16 253·5, P < 0·001) and most obviously from day 2 to 10 after pitcher opening (Fig. 3B). Ten days after the pitchers had opened, the fluid of about half of them had lost its viscoelastic properties completely. The nature of the viscoelastic fluid was investigated by adding a small quantity of it to pure ethanol. This led to a white precipitate that could be slowly re-dissolved in water, re-producing a slightly viscoelastic fluid. In contrast, boiling the pitcher fluid reduced the viscoelasticity irreversibly without leading to any precipitation.
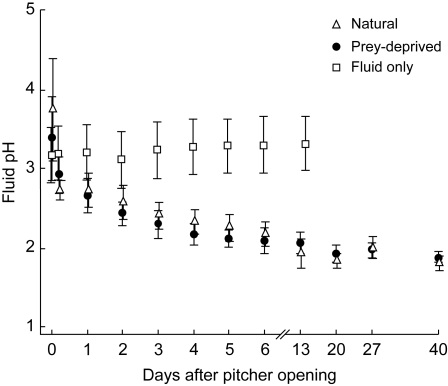
The fluid of freshly opened pitchers had a mean pH of 3·74 ± 0·66. Fluid pH decreased rapidly during the first few hours after pitcher opening, and continued to decrease moderately for about 1 week. After that, pH values remained largely constant at a mean of 1·95 ± 0·13 (Fig. 4). The results show that the decrease in pH was not induced by prey capture: pitchers that were kept in tight gauze bags exhibited a similar pH change. In contrast, isolated pitcher fluid exposed to the air in open plastic vials did not show any decrease in pH: the mean initial pH of 3·16 ± 0·34 even increased marginally to 3·31 ± 0·34 after 2 weeks. The higher variance of the first two pH measurements (Fig. 4) suggests that slightly different times might have elapsed in each pitcher between the opening and the first measurement. This again illustrates that the fluid pH changes very rapidly during the first hours after pitcher opening, probably triggered by some type of signal caused by the opening.
Fig. 4.
Development of pitcher fluid pH after pitcher opening. Under natural conditions and in prey-deprived pitchers the pH decreased rapidly during the first hour after opening. In contrast, the pH of the isolated pitcher fluid exposed to the air in open plastic vials stayed approximately constant.
DISCUSSION
The present results show that Nepenthes pitchers are dynamic structures whose properties change over the first 2 weeks after opening. Pitchers of N. rafflesiana reached full functionality as traps about 6 d after they had opened. Natural capture total increased synchronously with experimentally measured trapping efficiency, peristome wettability and nectar secretion. The correlation of natural capture total with peristome wettability and trapping efficiency confirms that insect aquaplaning on the peristome is the main capture mechanism in N. rafflesiana. The data suggest that the increased wettability of the peristome is mediated by nectar secretion. The presence of hygroscopic nectar has been shown to enhance peristome wetness and therefore trapping efficiency (Bauer et al., 2008). However, extrafloral nectar also plays a key role in attracting ants and other insects to the pitchers (Merbach et al., 2001). Ants constitute the majority of prey caught by N. rafflesiana (Moran, 1996; and this study), and thus nectar secretion is expected to increase prey capture rate by attracting more ants. Surprisingly, the results failed to show a significant increase in captured ants. While peristome wettability and slipperiness, total prey number and number of flying prey increased significantly with nectar secretion, the number of captured ants merely showed a weak upward trend. Several explanations are possible for this finding.
First, natural prey capture was highly variable, with the number of captured ants being even more variable than that of other prey. This is due to the fact that many foraging ants are captured batch-wise while most flying insects forage solitarily and are captured individually. Being social insects, ants typically forage in large numbers and move about on highly frequented trails (Hölldobler and Wilson, 1990). This pattern of temporary mass recruitment to different pitchers results in highly variable numbers of ant visitors and aggregated occurrence of capture events.
A second explanation is given by the fact that pitcher plants have extrafloral nectaries (EFNs) not only at the inner margin of the peristome but also scattered on the outside of the pitcher and on the tendril, the broadened leaf base and young stems (Merbach et al., 2001). These EFNs are morphologically different from the peristome nectaries, and they are already active on developing pitchers (Merbach et al., 2007). Therefore, foraging trails of ants can be established well before the pitchers open, with the site of major nectar secretion only shifting from the outside of the pitcher to the peristome once the pitcher opens. Consistent with this explanation, the prey caught in the first few days after opening were almost exclusively ants.
In contrast to ants, flying insects are attracted to N. rafflesiana pitchers mainly by flower-like visual and olfactory cues (Moran, 1996; Moran et al., 1999). The number of flying prey captured by the pitchers sharply increased from the fourth to the sixth day after pitcher opening. Is this mainly due to increased attraction, or to a higher trapping efficiency? Pitcher coloration changes during the first week, but it remains unclear if UV reflection patterns change as well. In freshly opened pitchers, though, the lid is still close to the pitcher mouth, and the peristome is still folded downward. Therefore, it seems unlikely that UV contrasts between the peristome and main pitcher body can be perceived well by potential prey until the lid is fully open and the peristome has assumed its final shape. This development takes about 1–2 d (pers. observ.). Therefore, the increase in captured flying prey coincides better with the increase in trapping efficiency than with the development of optical cues.
The effect of pitcher odour on prey attraction was not explicitly tested, but it appears likely that stronger attraction by enhanced fragrance partly accounts for the observed increase of flying insects trapped by the pitchers. Different sources of pitcher odour have been proposed in earlier publications. Moran (1996) reported for N. rafflesiana that the prey-attracting fragrance originates from the pitcher fluid. In contrast, di Guisto (2008) described the peristome as the most fragrant pitcher part and suggested that the nectar might be the source of the scent. We also observed that peristome nectar emitted a honey-like scent similar to that of whole pitchers and, consistently, pitcher fragrance and nectar secretion increased at approximately the same time. It is nevertheless possible that volatiles from other parts of the pitcher or from the fluid add to the bouquet. Detailed chemical analyses of peristome nectar and of the pitchers' fragrance are required to confirm these observations and to establish whether pitchers are indeed using flower scents to attract insects. The close link between fragrance (attraction) and wetness (capture efficiency) that is mediated by the dual capacity of peristome nectar makes perfect sense for the plant because it ensures that investment in nectar for prey attraction is maximally rewarded.
The observed decrease in fluid pH after pitcher opening is consistent with earlier findings on Nepenthes pitcher fluid (Nakayama and Amagase, 1968; Tökés et al., 1974; Heslop-Harrison, 1975). Morrissey (1955) found that the pH of the digestive fluid decreased by approximately two units after pitcher opening, and concluded that the pitcher secretes H+ ions along with proteolytic enzymes to provide an acidic environment near the pH optimum of the enzymes. Juniper et al. (1989) report that the drop in pH can be induced by adding nitrogenous solution (mimicking prey input) to the fluid. In this study, however, it was found that the decrease of fluid pH in the field was independent of prey input, though induced by the plant and not a simple chemical effect due to the exposure to air (cf. Fig. 4). We propose that the pH decrease is triggered by an unknown signal when the pitcher opens, but not by prey capture.
Not only fluid pH, but also fluid viscoelasticity decreased markedly over time. The presence of a redissolvable ethanol precipitate and the heat sensitivity suggest that the fluid's viscoelasticity is based on a polysaccharide (cf. Gaume and Forterre, 2007), like in the mucin of the sundew Drosera capensis (Rost and Schauer, 1977). The use of polysaccharides in the fluids of Nepenthes and Drosera could represent a plesiomorphic trait, as the Droseraceae and Nepenthaceae are members of the same clade within the Caryophyllidae (Nandi et al., 1998; Meimberg et al., 2000; Soltis et al., 2000). Rost and Schauer (1977) found that mucin viscosity was strongly pH dependent, being maximal at pH 5 and decreasing irreversibly for lower or higher pH values. Likewise, the decreasing viscoelasticity of N. rafflesiana fluid could at least partly be a consequence of the decreasing fluid pH. Rheological measurements at different pH levels are required to elucidate in detail the influence of acidity on viscoelasticity. Alternative reasons for the loss of viscoelasticity could be a gradual decomposition of the polysaccharide (cf. Rost and Schauer, 1977) by the infauna or by microbial digestion.
Two recent studies have shown experimentally that the fluid's viscoelasticity contributes to the retention efficiency of the trap. Di Guisto (2008) measured fluid viscosity and retention efficiency (with Drosophila melanogaster) of upper and lower pitchers of N. rafflesiana and found that both parameters were higher for upper pitchers. Gaume and Forterre (2007) performed detailed rheological measurements and demonstrated that the fluid's extensional viscosity was critical for prey retention. They also compared the retention efficiency between fluid from freshly opened N. rafflesiana pitchers and water (using flies and ants) and found pitcher fluid to be 100 % retentive while all test animals but one ant escaped from water. However, the effect of the fluid's viscoelasticity on natural prey capture has not been investigated previously. The present data show opposing trends of fluid viscosity and peristome trapping efficiency over time. This might suggest a scenario of alternative capture strategies at different stages in a pitcher life, with peristome ‘aquaplaning’ gradually replacing fluid viscoelasticity. However, with both mechanisms equally important, it would be expected for prey total to be more or less constant over time. In contrast, the present data show a clear and synchronous increase of peristome capture efficiency and prey numbers over the first week after pitcher opening. This suggests that, despite the positive effect of fluid viscoelasticity on prey retention, nectar secretion and peristome wetting play a more important role for prey capture under natural conditions.
The present findings of age-dependent changes in prey attraction and trapping closely resemble the results of Fish and Hall (1978) on Sarracenia purpurea. As with N. rafflesiana, the pitchers of this species still changed in coloration after opening, and reached peak capture rates only about 2 weeks after opening. The similarities in the development of trapping efficiency add to a number of parallels in the means of attraction and trapping employed by both species, such as UV absorption patterns, nectar secretion on the peristome and a similar peristome microstructure, providing a striking example of closely convergent evolution (Juniper et al., 1989).
In contrast to the present study, Fish and Hall (1978) were able to collect data over the whole life span of S. purpurea pitchers. They found that after the first 2 weeks, capture rates gradually decreased. Although not quantified in the present study, it is likely that capture efficiency will decay in a similar way in older pitchers of N. rafflesiana. For a species with short-lived pitchers such as N. rafflesiana, it will be best to capture most of the prey while a pitcher is still young, to ensure that the remaining lifetime of the pitcher is sufficient to break down the prey and take up the nutrients completely. In this context, it appears significant that N. rafflesiana pitchers in the field die ‘progressively’: while the upper half of the pitcher withers about 6–10 weeks after pitcher opening, the lower half often stays alive for twice as long. We believe that pitchers in this condition are no longer functional traps, but they continue to digest prey.
Recognizing Nepenthes pitchers as dynamic structures is important for understanding the detailed mechanisms of prey attraction and trapping. The present results show that nectar secretion and peristome wettability are the main determinants of capture success in N. rafflesiana. Long-term field studies on prey attraction, trapping and nutrient uptake over the entire pitcher life span are required to achieve a more comprehensive understanding of the ecology of prey capture in pitcher plants.
ACKNOWLEDGEMENTS
We thank Universiti Brunei Darussalam (UBD) and Brunei Forestry Department for kind permission to conduct field work. Olusegun Osunkoya and Ulmar Grafe of UBD have provided invaluable help with the logistics of this research. Konrad Öchsner developed and built the circuits for measuring peristome surface conductance. Ingolf Karl and Katja Rembold assisted with experiments in the field. This work was supported by an external research studentship of Trinity College Cambridge to U.B. and an equipment grant of the Isaac Newton Trust Cambridge [Minute 4·43 (v)] to W.F. The Mark Pryor Fund (Trinity College Cambridge) and the Balfour Trust (Department of Zoology, University of Cambridge) funded the expenses of the 2008 field trip.
LITERATURE CITED
- Bauer U, Bohn HF, Federle W. Harmless nectar source or deadly trap: Nepenthes pitchers are activated by rain, condensation and nectar. Proceedings of the Royal Society B, Biological Science. 2008;275:259–265. doi: 10.1098/rspb.2007.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn HF, Federle W. Insect aquaplaning: Nepenthes pitcher plants capture prey with the peristome, a fully wettable water-lubricated anisotropic surface. Proceedings of the National Academy of Sciences of the USA. 2004;101:14138–14143. doi: 10.1073/pnas.0405885101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke C. Nepenthes of Borneo. Kota Kinabalu: Natural History Publications; 1997. [Google Scholar]
- Clarke C. Nepenthes of Sumatra and Peninsular Malaysia. Kota Kinabalu: Natural History Publications; 2001. [Google Scholar]
- Fish D, Hall DW. Succession and stratification of aquatic insects inhabiting the leaves of the insectivorous pitcher plant. Sarracenia purpurea. American Midland Naturalist. 1978;99:172–183. [Google Scholar]
- de Flacourt E. Histoire de la grande isle Madagascar. Paris: G. de Lvyne; 1658. [Google Scholar]
- Gaume L, Forterre Y. A viscoelastic deadly fluid in carnivorous pitcher plants. PLoS ONE. 2007;2:e1185. doi: 10.1371/journal.pone.0001185. doi:10.1371/journal.pone.0001185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaume L, Gorb S, Rowe N. Function of epidermal surfaces in the trapping efficiency of Nepenthes alata pitchers. New Phytologist. 2002;156:479–489. doi: 10.1046/j.1469-8137.2002.00530.x. [DOI] [PubMed] [Google Scholar]
- di Guisto B, Grosbois V, Fargeas E, Marshall DJ, Gaume L. Contribution of pitcher fragrance and fluid viscosity to high prey diversity in a Nepenthes carnivorous plant from Borneo. Journal of Bioscience. 2008;33:121–136. doi: 10.1007/s12038-008-0028-5. [DOI] [PubMed] [Google Scholar]
- Heslop-Harrison Y. Enzyme release in carnivorous plants. In: Dingle JT, Dean RT, editors. Lysozymes in biology and pathology. Amsterdam: North Holland Publishing Company; 1975. pp. 525–578. [PubMed] [Google Scholar]
- Hölldobler B, Wilson EO. The ants. Cambridge, MA: Harvard University Press; 1990. [Google Scholar]
- Jebb M. An account of Nepenthes in New Guinea. Science in New Guinea. 1991;17:7–54. [Google Scholar]
- Joel DM. Mimicry and mutualism in carnivorous pitcher plants (Sarraceniaceae, Nepenthaceae, Cephalotaceae, Bromeliaceae) Biological Journal of the Linnean Society. 1988;35:185–197. [Google Scholar]
- Juniper BE, Burras JK. How pitcher plants trap insects. New Scientist. 1962;13:75–77. [Google Scholar]
- Juniper BE, Robins RJ, Joel DM. The carnivorous plants. Diego: Academic Press; 1989. [Google Scholar]
- Klemm O, Milford C, Sutton MA, Spindler G, van Putten E. A climatology of leaf surface wetness. Theoretical and Applied Climatology. 2002;71:107–117. [Google Scholar]
- Knoll F. Über die Ursache des Ausgleitens der Insektenbeine an wachsbedeckten Pflanzenteilen. Jahrbücher für wissenschaftliche Botanik. 1914;54:448–497. [Google Scholar]
- Lloyd FE. The carnivorous plants. Waltham, MA: Chronica Botanica; 1942. [Google Scholar]
- Meimberg H, Dittrich P, Bringmann G, Schlauer J, Heubl G. Molecular phylogeny of Caryophyllidae s.l. based on MatK sequences with special emphasis on carnivorous taxa. Plant Biology. 2000;2:218–228. [Google Scholar]
- Merbach MA, Zizka G, Fiala B, Maschwitz U, Booth WE. Patterns of nectar secretion in five Nepenthes species from Brunei Darussalam, Northwest Borneo, and implications for ant–plant relationships. Flora. 2001;196:153–160. [Google Scholar]
- Merbach MA, Zizka G, Fiala B, Merbach D, Booth WE, Maschwitz U. Why a carnivorous plant cooperates with an ant – selective defense against pitcher-destroying weevils in the myrmecophytic pitcher plant Nepenthes bicalcarata Hook. f. Ecotropica. 2007;13:45–56. [Google Scholar]
- Moran JA. Pitcher dimorphism, prey composition and the mechanism of prey attraction in the pitcher plant Nepenthes rafflesiana in Borneo. Journal of Ecology. 1996;84:515–525. [Google Scholar]
- Moran JA, Booth WE, Charles JK. Aspects of pitcher morphology and spectral characteristics of six Bornean Nepenthes pitcher plant species: implications for prey capture. Annals of Botany. 1999;83:521–528. [Google Scholar]
- Morrissey S. Chloride ions in the secretion of the pitcher plant. Nature. 1955;176:1220–1221. [Google Scholar]
- Nakayama S, Amagase S. Acid protease in Nepenthes: partial purification and properties of the enzyme. Proceedings of the Japan Academy. 1968;44:358–362. [Google Scholar]
- Nandi O, Chase MW, Endress PK. A combined cladistic analysis of angiosperms using rbcL and non-molecular data sets. Annals of the Missouri Botanical Garden. 1998;79:249–265. [Google Scholar]
- Osunkoya OO, Daud SD, Wimmer FL. Longevity, lignin content and construction cost of the assimilatory organs of Nepenthes species. Annals of Botany. 2008;102:845–853. doi: 10.1093/aob/mcn162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen TP, Lennon KA. Structure and development of the pitchers from the carnivorous plant Nepenthes alata (Nepenthaceae) American Journal of Botany. 1999;86:1382–1390. [PubMed] [Google Scholar]
- Rost K, Schauer R. Physical and chemical properties of the mucin secreted by Drosera capensis. Phytochemistry. 1977;16:1365–1368. [Google Scholar]
- Soltis DE, Soltis PS, Chase MW, et al. Angiosperm phylogeny inferred from 18S rDNA, rbcL, and atpB sequences. Botanical Journal of the Linnean Society. 2000;133:381–461. [Google Scholar]
- Tökés ZA, Woon WC, Chambers SM. Digestive enzymes secreted by the carnivorous plant Nepenthes macfarlanei L. Planta. 1974;119:39–46. doi: 10.1007/BF00390820. [DOI] [PubMed] [Google Scholar]